Abstract
Black cohosh preparations are popular dietary supplements among women seeking alternative treatments for menopausal complaints. For decades, triterpene glycosides and phenolic acids have dominated the phytochemical and biomedical research on this plant. In this study, we provide evidence that black cohosh contains an unexpected and highly diverse group of secondary nitrogenous metabolites previously unknown to exist in this plant. Using a dereplication approach that combines accurate mass measurements, database searches and general knowledge of biosynthetic pathways of natural products, we identified or tentatively identified 73 nitrogen-containing metabolites, many of which are new natural products. The identified compounds belong to several structural groups including alkaloids, amides or esters of hydroxycinnamic acids and betains. Among the alkaloids, several classes such as guanidino alkaloids, isoquinolines and β-carbolines were identified. Fragmentation patterns for major compound classes are discussed, which provides a framework for the discovery of these compounds from other sources. Identification of alkaloids as a well-known group of bioactive natural products represents an important advance in better understanding of the pharmacological profile of black cohosh.
Keywords: black cohosh, alkaloids, cinnamides, metabolomics, dereplication, mass spectrometry
1. Introduction
The roots/rhizomes of black cohosh (Cimicifuga racemosa (L.) Nutt., syn. Actaea racemosa L.) have traditionally been used by Native Americans for treating a variety of medical conditions such as colds, rheumatism as well as for alleviating menopausal symptoms such as hot flashes [1]. Because of the risks associated with hormone replacement therapy, black cohosh preparations have become popular dietary supplements among women seeking alternative treatments for menopausal complaints [2]. Extensive preclinical and clinical investigations have provided conflicting evidence regarding the efficacy of black cohosh [3]. Early studies suggested that black cohosh extracts were effective in reducing the frequency and intensity of hot flashes among perimenopausal and postmenopausal women [4–7], while several recent trials including double-blind placebo-controlled studies demonstrated no vasomotor symptom benefits [8–11].
In terms of the chemical composition of black cohosh, triterpene glycosides and phenolic acids represent the major constituents of black cohosh extracts and interest in them has dominated the phytochemical and biomedical research on this plant for decades [12]. Abundant triterpenes such as actein and 23-epi-26-deoxyactein are often used as markers for the standardization of black cohosh preparations. The major phenolic constituents are hydroxycinnamic acids (caffeic, ferulic and isoferulic acid) and their condensation products with glycoloyl phenylpropanoids, commonly known as cimicifugic acids [13]. A third group of black cohosh constituents that has received far less attention is the alkaloids. We recently described the isolation and identification of several guanidine alkaloids from black cohosh including cimipronidine, cyclocimipronidine and dopargine as well as salsolinol, a member of the tetrahydroisoquinoline (THIQ) group of alkaloids [14, 15]. Apart from these compounds, little is known about the presence of nitrogen-containing compounds in black cohosh, which prompted us to explore further this part of the black cohosh secondary metabolome. In this study, we carried out a detailed mass spectrometric investigation of the nitrogen-containing metabolome of a 75% ethanolic extract of black cohosh roots/rhizomes. The results revealed that black cohosh contains an unexpected and remarkably diverse group of nitrogenous metabolites previously unknown to exist in this plant. These results may provide important insights into the future investigation and understanding of the biological activities of this popular botanical dietary supplement.
2. Experimental
2.1 Chemicals
All organic solvents were HPLC-grade or better and were purchased from Fisher Scientific (Fair Lawn, NY). All chemicals used for synthesis were purchased from Sigma-Aldrich (St. Louis, MO). Authentic standards for compound identification were either commercially available, synthesized in-house or previously isolated from other plants. All of the commercial standards were purchased from Sigma-Aldrich except allocryptopine and protopine which were purchased from MP Biosciences (San Diego, CA) and ChemDiv (San Diego, CA), respectively. Magnoflorine, menisperine, magnocurarine, reticuline, laurotetatine, and laurolitsine were kind gifts from Drs. Jan Glinski (Planta Analytica), Yimin Zhao (Beijing Institute of Pharmacology) and Shoei-Sheng Lee (National Taiwan University).
2.1.1 Synthesis of ferulic and isoferulic acid amides
Small-scale synthesis of ferulic and isoferulic acid amides was carried out using routine synthetic coupling reactions utilizing 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI) as the activating agent.
2.1.2 Synthesis of feruloyl and isoferuloyl choline
Feruloyl (47) and isoferuloyl choline (48) were synthesized according to the protocol of Boettcher et al. [16].
2.1.3 Synthesis of N-formyl arginine
N-formyl arginine (25) was prepared by treating ariginine with formic acid at elevated temperature according to the method of Karapetyan et al. [17].
2.1.4 Pictet Spengler adducts
2(N)-methyl-6-hydroxy-1,2,3,4-tetrahydro-β-carboline (58) and cimitrypazepine (59) were synthesized by condensation of Nω-methylserotonin and formaldehyde according to the method of Somei et al. [18]. (3S)-1,2,3,4-Tetrahydro-β-carboline-3-carboxylic acid (46) was synthesized by condensation of tryptophan and formaldehyde under acidic conditions as described by Brossi et al. [19]. N(2)-methyl-6-hydroxy-3,4-dihydro- β-carboline (53) was prepared by treating Nω-methylserotonin with glyoxylic acid under alkaline conditions according to the protocol of Yamano et al. [20].
Cimitrypazepine (59) 1H-NMR (CD3OD) δ: 2.75 (3H, s), 3.16 (2H, m), 3.31 (2H, m), 4.34 (2H, s), 7.02 (1H, brs), 6.68 (1H, d, J= 8.6 Hz), 7.1 (1H, d, J= 8.6 Hz).
N(2)-methyl-6-hydroxy-3,4-dihydro-β-carboline (53) 1H-NMR (CD3OD) δ: 7.42 (d, J = 9.2 Hz, 1H), 6.94 (brs, 1H), 6.33 (d, J = 9.2 Hz, 1H), 3.78 (m, 2H), 3.63 (s, 3H), 3.11 (m, 2H).
2.2 Plant material
The raw plant material and the corresponding 75% ethanolic extract used in this study were identical to the materials used in our recent Phase IIb clinical trial and were described previously [11, 21–23]. Briefly, the plant material was acquired from Naturex (previously Pure World, South Hackensack, NJ) and botanically authenticated using PCR and microscopy [24]. Milled roots/rhizomes were extracted with 75% ethanol by large-scale percolation, vacuum-dried at 45 °C and milled though a 60-mesh screen to yield a powdered extract.
2.3 Fractionation
The 75% ethanolic extract of black cohosh roots/rhizome was partitioned between water and ethyl acetate. The water partition was further fractionated on a column filled with Amberlite XAD-2 resin to yield water and methanol-soluble fractions. The methanol fraction was then subjected to pH-zone refinement fast centrifugal partition chromatography (FCPC) using water/butanol/ethyl acetate (5:4:1) as the solvent system. This approach yielded six chemically distinct fractions labeled FCPC 1–6. More detailed description of the fractionation procedure has been published elsewhere [13, 21].
2.4 Dereplication
We followed a standard dereplication approach used in mass spectrometry-based metabolomics studies beginning with the determination of elemental composition by accurate mass measurement, followed by the acquisition of product ion tandem mass spectra. Since product ion mass spectra were acquired using accurate mass measurement, the elemental composition of the fragment ions could also be determined. The validity of the molecular formula obtained from accurate mass measurements was established using additional criteria such as isotope pattern, elemental composition of fragment ions as well as general plausibility of the formula based on general knowledge of natural product chemistry. The elemental composition was then searched in SciFinder and Beilstein CrossFire Commander databases of natural products as well as in the MassBank (www.massbank.jp) database of tandem mass spectra [25]. If a match was obtained in the MassBank database, final confirmation of compound identity was obtained by comparing the retention time and fragmentation pattern with those of authentic standards. This was a necessary precaution due to well-known differences in appearance of product ion spectra obtained using different instruments [26]. For compounds for which there were no spectra in the MassBank database, the hits obtained in the SciFinder or Beilstein databases provided clues as to possible structure. Based on the interpretation of product ion spectra, a plausible structure was proposed which was tested by comparison with an authentic standard. This iterative process was repeated until a conclusive assignment could be made.
2.4.1 LC-MS
Reversed-phase separations were carried out on a Hypersil GOLD (Thermo Fisher) 2.1 × 150 mm column (5 µm particle size) using a mobile phase consisting of 0.1% formic acid (solvent A) and 95% acetonitrile/0.1% formic acid (solvent B) and the following gradient program: 6–36% B over 30 min and then 36–100% B over 10 min. The flow rate was 0.2 ml/min, and the column was thermostated at 30°C. HILIC separations were carried out using a Waters (Milford, MA) XBridge™ Amide 2.1 × 150 mm column (3.5 µm particle size) using a mobile phase consisting of 10 mM ammonium formate with 0.1% formic acid (solvent A) and acetonitrile (solvent B) and a linear gradient from 95%-65% B over 30 min at a flow rate of 0.2 ml/min. The column was thermostated at 30°C. Typically, 2–5 µL of a 0.2–0.3 mg/ml test solution was injected for LC-MS analysis.
Mass spectrometric data were acquired using a Waters (Milford, MA) SYNAPT hybrid quadrupole/time-of-flight mass spectrometer with positive ion electrospray. Data were acquired at 10,000 FWHM resolution using Leu-enkephalin as the lock mass, which was introduced via a separate sprayer. Peak centroiding was carried out during data acquisition using the extended dynamic range option available in the MassLynx software. To confirm certain fragmentation pathways, MSn measurements were carried out using a Shimadzu IT-TOF hybrid ion trap/time-of-flight mass spectrometer.
3. Results and Discussion
3.1 LC-MS analyses
During partitioning of the black cohosh ethanolic extract, most of the compounds of interest were found in the water phase, while the ethyl acetate partition contained primarily triterpene glycosides. Due to complexity of the metabolome contained in the water partition, LC-MS dereplication was carried out on individual fractions rather than on the entire partition. This way, many low abundance compounds whose signals might have been masked by the more abundant ones could be detected, characterized and identified. Due to the range of polarity among even the compounds in the water partition, both reversed phase and HILIC separations were employed. The XAD water fraction (Figure 1a) and FCPC fractions 1–2 were comprised of polar compounds that could be best separated using a HILIC column, while fractions 3–6 contained more hydrophobic compounds that were more suitable for reversed phase chromatography (Figure 1b). In general, there was only a small degree of chemical overlap among fractions indicating that the fractionation procedure provided excellent group separation. Since compounds of interest contained nitrogen, positive ion electrospray using an acidified mobile phase was found to be the optimum ionization method.
Figure 1.
Positive ion electrospray LC-MS chromatograms of black cohosh fractions: (a) HILIC separation of XAD water fraction. This fraction contained primarily small, highly polar primary and secondary metabolites; (b) Reversed phase separation of FCPC fraction 6.
3.2 Compound identification
Analytical data for all of the structurally identified compounds and those with proposed chemical structures are listed in Table 1, and their chemical structures are shown in Figure 2. Most of the compounds described in this study were identified at the annotation levels 1 and 2 according to nomenclature by Sumner et al. [27]. Identification at level 1 is established by comparing the retention time and fragmentation pattern of an unknown with those of an authentic standard. This level of evidence provides the highest degree of confidence in the assignment and is a widely accepted criterion for positive identification of compounds not only in the research domain but also in forensic and regulatory areas. Some compounds were identified at level 2 by comparing their product ion tandem mass spectra either with published spectra or with tandem mass spectra of close structural analogs. Comparison of product ion spectra of close chemical analogs is a viable approach commonly used in drug metabolism or chemical degradation studies. Finally, for level 3 characterization only a chemical class of the unknown could be ascertained based on the similarity of tandem mass spectra with known compounds belonging to the same class.
Table 1.
Analytical data for compounds identified in 75% ethanolic extract of black cohosh
| No. | tR (min) |
Fractio n |
m/z [M+H]+ |
Formula | Error (ppm) |
Major fragmentsc | Identification | Lev el |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.1a | H2O | 170.0809 | C8H11NO3 | −4.7 | 152.0702 (10); 134.0604(100); 124.0758(9); 106.0659(10); 79.0541(9); 77.0395(10) |
Pyridoxine | 1 |
| 2 | 8.8 | H2O | 136.0620 | C5H5N5 | −2.2 | 136.0620(48); 119.0350(100); 109.0517(5);94.0401(6); 92.0222(15); 67.0286(6); 65.0413(6) |
Adenine | 1 |
| 3 | 8.9 | H2O | 220.1178 | C9H17NO5 | −3.2 | 220.1178(8); 142.0858(10); 124.0712(37); 103.0721(12); 98.0217(51); 90.0529(100); 85.0547 (35); 72.0417(69); 70.0295(18) |
Panthotenic acid | 1 |
| 4 | 9.4 | H2O | 186.1236 | C8H15N3O2 | −3.8 | 186.1236(60); 169.1000(35); 154.0996(75); 144.1032(100); 137.0715(35); 112.0782(20); 95.0529 (30); 70.0656(70) |
Cimipronidine methyl ester |
1 |
| T5 | 9.6 | H2O | 168.1128 | C8H13N3O | −5.4 | 168.1128(100); 112.0768(13); 94.0658(6);70.0678(12); 67.0512(4) |
N-methyl cyclocimipronidin e |
2d |
| 6 | 11.5 | H2O | 154.0978 | C7H11N3O | −1.3 | 154.0978(100); 112.0768(20); 95.0547(6);94.0656(16); 70.0676(16); 67.0512(10) |
Cyclocimipronidi ne |
1 |
| 7 | 11.7 | H2O | 104.1068 [M+] |
C5H14NO+ | −6.7 | 104.1068 (100); 60.0818(14) | Choline | 1 |
| T8 | 12.9 | H2O | 130.0971 | C5H11N3O | −6.9 | 130.0971(60); 112.0872(30); 71.0502(12); 70.0660 (100); 60.0570(7) [15eV] |
γ-Guanidino butyraldehyde |
2 |
| 9 | 13.3 | H2O | 180.1017 | C10H13NO2 | −4.4 | 180.1017(3); 163.0752(12); 151.0727(10); 145.0651 (46); 117.0698(100); 115.0540(95); 105.0962(8); 91.0541(20); |
Salsolinol | 1 |
| 10 | 13.9 | H2O | 266.0747 | C9H13N3O5 | −2.3 | 266.0747(25); 134.0334(100) | Cytidine | 1 |
| T11 | 14.2 | H2O | 132.1127 | C5H13N3O | −7.6 | 132.1127(100); 90.0960(15); 73.0617(12); 60.0570(7); 55.0575(8) |
γ-Guanidino butanol |
2 |
| 12 | 14.3 | H2O | 166.0869 | C9H11NO2 | 0.6 | 166.0869(18); 149.0608(18); 137.0618(100); 121.0641 (17); 121.0649(90); 103.0530(28); 91.0560(37); 77.0390(12) |
Norsalsolinol | 1 |
| 13 | 15.5 | H2O | 166.0870 | C9H11NO2 | 1.2 | 120.0824(100); 103.0565(55); 93.0727(6); 91.0570(7); 77.0418(18) |
Phenylalanine | 1 |
| 14 | 15.8 | H2O | 306.0815 [M+Na+] |
C10H13N5 O5 |
2.0 | Guanosine | 1 | |
| T15 | 16.2 | H2O | 332.1340 | C14H21NO8 | −1.5 | 332.1340(6); 314.1237(29); 152.0704(100); 136.0765(16); 134.0602(28); 124.0764(26); 108.0816 (55); 106.0651(10) |
5'-O-(β-D- glucopyranosyl) Pyridoxine |
3 |
| 16 | 16.5 | H2O | 118.0872 [M+] |
C5H12NO2+ | 3.4 | 118.0872(100); 59.0711(10) | Glycine betaine | 1 |
| 17 | 16.6 | H2O | 144.1018 [M+] |
C7H14NO2+ | −4.9 | 144.1018(100); 84.0823(10); 58.0672(10) | Proline betaine | 1 |
| 18 | 17.4 | H2O | 138.0547 [M+] |
C7H8NO2+ | −5.8 | 138.0547(100);136.0394(5);110.0587(6);94.0647( 30)92.0494(24);65.0377(6) |
Trigonelline | 1 |
| T19 | 17.7 | H2O | 160.1077 | C6H13N3O2 | −5.4 | 160.1077 (10); 101.0026(100); 100.0532(88) | δ- guanidinovaleric acid |
2 |
| 20 | 18.9 | H2O | 172.1086 | C7H13N3O2 | 0.0 | 172.1086(100); 154.0972(64); 137.0704(18); 130.0863(70); 119.0611(15); 112.0762(25);95.0568 (21); 94.0538(16); 70.0657(60) |
Cimipronidine | 1 |
| 21 | 19.1 | H2O | 146.0925 | C5H11N3O2 | −3.4 | 146.0969(100); 128.0856(20); 111.0585(12); 104.0720 (12); 87.0440(40); 86.0601(35); 69.0307(7); 60.0570(6)[15eV] |
γ-Guanidino butyric acid |
1 |
| 22 | 19.6 | H2O | 130.0860 | C6H11NO2 | −6.1 | 84.0823(100) | Pipecolic acid | 1 |
| 23 | 20.7 | H2O | 162.1122 [M+] |
C7H16NO3+ | −4.9 | 162.1122(100); 103.0406(20); 102.0930(8);85.0300(7); 60.0829(8) [15eV] |
L- Carnitine | 1 |
| 24 | 23.1 | H2O | 217.1297 | C8H16N4O3 | −1.8 | 217.1297(9); 175.1200(22); 158.0935(100); 130.0978 (17);116.0693(60);115.0881(35);112.0840(50);74. 0231 (18);71.0505(32);70.0653(90);60.0565(5) |
α-N-acetyl arginine |
1 |
| 25 | 23.4 | H2O | 203.1144 | C7H15N4O3 | 0.0 | 203.1144(100); 186.0820; 175.1172(50); 158.0940(30); 144.0661(45); 143.0809(10); 130.1028 (10);116.0690(20);112.0845(15);98.0640(18);71.0 493 (10);70.0653(30); 60.0660(6) [15eV] |
N-formyl arginine | 1 |
| 26 | 24.1 | H2O | 130.0503 | C5H7NO3 | −0.8 | 84.0449(100) | Pyroglutamic acid | 1 |
| T27 | 24.3 | H2O | 198.1237 [M+] |
C9H16N3O2 + |
−3.0 | 198.1237(14);154.1338(100);95.0619(60); 68.0517(6); 60.0829(8) [15eV] |
Histidine betaine | 2 |
| T28 | 24.4 | H2O | 266.1617 [M+] |
C11H23NO6 + |
4.9 | 266.1617(95);104.1073(100);60.0838(13) | Choline hexoside | 3 |
| 29 | 29.7 | H2O | 175.1191 | C6H14N4O2 | −1.5 | 175.1191(12);158.0938(12);130.0978(75);116.069 3 (35); 112.0840(18); 71.0493(25); 70.0653(100); 60.0565(10) |
Arginine | 1 |
| 30 | 2.7b | 3 | 268.1036 | C10H13N5O 4 |
−3.7 | 136.0623(100); 119.0352(10) | Adenosine | 1 |
| 31 | 3.0 | 3 | 160.1081 | C6H13N3O2 | −3.1 | 160.1098(70);128.0857(5);118.0834(5);101.0598( 100);86.0602(5);59.0504(9) |
γ-Guanidino butyric acid methylester |
1 |
| 32 | 4.9 | 3 | 337.1514 | C15H20N4O 5 |
0.6 | 337.1514(6);278.1035(6);175.1211(15);163.0399( 100);158.0932(10);145.0291(30);135.0449(20);11 7.0340 (19);89.0396(10);70.0672(6) |
Caffeoyl arginine | 2 |
| T33 | 8.0 | 3 | 332.1257 | C16H18N3O 5 |
3.3 | 177.0551(100); 163.0395(3); 149.0597(16); 145.0283(21); 117.0346(20); 110.0744(5);89.0395(10); |
N-isoferuloyl histidine |
2 |
| 34 | 8.7 | 3 | 351.1664 | C16H22N4O 5 |
−1.1 | 351.1678(6);292.1192(5);177.0554(100);175.1200 (16); 158.0932(8);149.0603(8);145.0282(83);130.0983( 5); 117.0342(32);116.0710(5);89.0396(11);70.0671(7 ); 60.0572(5) |
N-feruloyl arginine |
1 |
| 35 | 10.1 | 3 | 351.1664 | C16H22N4O 5 |
−1.1 | 351.1678(6);292.1192(5);177.0554(100);175.1200 (11); 163.0394(8);158.0932(8);149.0603(17);145.0282( 24); 130.0983(5);117.0342(18);116.0710(5);89.0396(1 1); 70.0671(5);60.0572(4) |
N-isoferuloyl arginine |
1 |
| 36 | 12.5 | 3 | 342.1705 [M+] |
C20H24NO4 + |
0.0 | 342.1725(28); 299.1301(11); 297.1129(84);282.0892 (34); 279.1039(8); 265.0876(100); 237.0920(30); 222.0700(10); 219.0804(14); 209.0981(8);207.0806(8); 191.0882(10) |
Magnoflorine | 1 |
| T37 | 13.1 | 3 | 342.1705 [M+] |
C20H24NO4 + |
0.0 | 342.17105(5); 192.1018 (100); 177.0802(10) | Phellodendrine or cyclanoline |
3e |
| 38 | 4.5b | 4 | 174.1244 | C7H15N3O2 | 0.6 | 174.1244(100);146.0953(9);132.1046(9);128.0857 (11);115.0753(73);87.0432(85);86.0611(12)[15eV ] |
γ-Guanidino butyric acid ethylester |
1 |
| T39 | 5.1 | 4 | 174.1243 | C7H15N3O2 | 0.0 | 174.1244(100); 132.1034(8); 115.0762(78); 100.0771(8); 73.0659(18); 55.0568(15) |
δ- guanidinovaleric acid methyl ester |
2 |
| T40 | 5.3 | 4 | 282.1183 | C11H15N5O 4 |
−6.7 | 136.0622(100); 119.0360(10) | 2’-O- methyladenosine |
2 |
| T41 | 6.9 | 4 | 282.1202 | C11H15N5O 4 |
2.1 | 150.0783(100) | N- methyladenosine |
2 |
| 42 | 8.5 | 4 | 265.1543 | C14H20N2O 3 |
−3.4 | 177.0558(90); 149.0595(18); 145.0300(100);117.0347 (90); 89.0390(55) |
Feruloyl putrescine |
1 |
| 43 | 9.6 | 4 | 265.1543 | C14H20N2O 3 |
−3.4 | 177.0553(100); 163.0356(4); 149.0590(22); 145.0289 (31); 134.0355 (7); 117.0343(30); 89.0385(25) |
Isoferuloyl putrescine |
1 |
| 44 | 9.6 | 4 | 280.1341 [M+] |
C12 H18NO2+ |
1.4 | 208.1341(6); 149.0584(90);105.0357(100);77.0374(18) |
Benzoyl choline | 1 |
| 45 | 9.7 | 4 | 314.1749 [M+] |
C19H24NO3 + |
−2.2 | 314.1749(100);271.1338(10);269.1193(50);239.10 40 (10);237.0931(46);211.1048(15);209.0978(28);19 2.1030(15);175.0763(35);151.0752(10);145.0646( 18);143.0534(18);137.0611(18);107.0484(40) |
Magnocurarine | 1 |
| 46 | 10.6 | 4 | 217.0981 | C12H12N2O 2 |
1.8 | 144.0806(100); 143.0723(12);130.0654(6);117.0685(8) |
1,2,3,4,- tetrahydro-β- carboline-3- carboxylic acid |
1 |
| 47 | 10.9 | 4 | 280.1554 [M+] |
C15 H22NO4+ |
1.8 | 221.0815(100); 206.0581 (20); 177.0550(60); 149.0604(8); 145.0325(30); 117.0372(20); 89.0386(8); |
Feruloyl choline | 1 |
| 48 | 12.2 | 4 | 280.1554 [M+] |
C15 H22NO4 |
1.8 | 221.0813(100); 206.0578(20); 177.0544(60); 163.0358(5); 162.0318(5); 149.0605(10);145.0320(14); 134.0369(6);117.0341(10);89.0388(10) |
Isoferuloyl choline |
1 |
| T49 | 15.9 | 4 | 492.1878 | C24H29NO1 0 |
1.6 | 321.0971(20); 177.0558(100); 149.0657(6); 145.0328 (65); 137.0607(28); 119.0501(6); 117.0375(18); 91.0558(7); 89.0421(6) |
N-feruloyl dopamine-4’-O- hexoside |
2 |
| T50 | 16.5 | 4 | 492.1878 | C24H29NO1 0 |
1.6 | 321.0980(15); 177.0558(100); 163.0390(3); 149.0657 (10);145.0328(17);137.0606(22);119.0501(5);117. 0375(10); 91.0558(7); 89.0421(6) |
N-isoferuloyl dopamine-4’-O- hexoside |
2 |
| 51 | 3.4b | 5 | 178.0882 | C10H11NO2 | 7.9 | 178.0882(100); 176.0704(5); 163.0645(44); 162.0566 (54);160.0765(5);137.0614(11);135.0453(16);117. 0354 (8); 115.0569(8); 89.0410(4) |
1,2- Dehydrosalsolinol |
1 |
| T52 | 7.0 | 5 | 178.0875 | C10H11NO2 | 3.9 | 178.0879(20); 119.0476(40); 91.0547(100) | N-phenylacetyl acetamide |
3 |
| T53 | 7.1 | 5 | 201.1031 [M+] |
C12H13N2O + |
1.5 | 201.1031(100); 186.0808(29); 185.0730(6); 172.0771 (11); 171.0578(40); 170.0608(52); 160.0777(12); 142.0662(12); 115.0558(15) |
N(2)-methyl-6- hydroxy-3,4- dihydro-β- carboline |
1 |
| T54 | 11.3 | 5 | 338.1234 | C16H19NO7 | −1.8 | 338.1234(20); 177.0558(100); 163.0390(6); 149.0600 (21); 145.0286(30); 117.0350(40); 89.0401(25) |
N-isoferuloyl glutamic acid |
2 |
| T55 | 14.2 | 5 | 342.1702 [M+] |
C20H24NO4 + |
−0.9 | 297.1237(28); 282.1075(10); 265.1015(100); 237.0991 (40); 250.0738(10); 237.0991(42); 233.0677(22); 205.0789(20) |
Laurifoline | 2e |
| T56 | 15.1 | 5 | 504.1869 | C25H29NO1 0 |
−0.2 | 321.0974(5); 177.0558(100); 166.0851(60); 149.0654 (10); 145.0238(6);137.0539(6);120.0821(5);89.0414(6) |
N-feruloyl phenylalanine-4’- O hexoside |
2 |
| T57 | 15.9 | 5 | 206.1550 | C13H19NO | 2.4 | 107.0504(100);100.1135(5);79.0544(18);77.0405( 16) |
N-cyclohexyl-4- hydroxy benzylamine |
3 |
| 58 | 3.3b | 6 | 203.1185 | C12H14N2O | 0.5 | 160.0758(100); 159.0696(6); 132.0865(6); 117.0614(6) |
N(2)-methyl-6- hydroxy-1,2,3,4- tetrahydro-β- carboline |
1 |
| 59 | 3.8 | 6 | 203.1185 | C12H14N2O | 0.5 | 188.0953(5); 174.0938(100); 162.0894(30); 160.0758 (20); 159.0696(50); 147.0688(46); 146.0600(22); 131.0760(12); 130.0651(10); 129.0706(6) |
Cimitrypazepine | 1 |
| T60 | 7.4 | 6 | 272.1287 | C16H17NO3 | 0.0 | 255.0926(20); 237.0890(40); 161.0591(30); 143.0712 (30); 115.0583(37); 107.0503(100); 77.0449(18) |
Norcoclaurine | 2e |
| 61 | 11.6 | 6 | 314.1392 | C18H19NO4 | 1.3 | 297.1128(18); 282.0860(25); 265.0874(78); 237.0914 (100); 222.0710(15); 205.0646(50); 177.0729(15) |
Laurolitsine | 1 |
| 62 | 13.6 | 6 | 330.1719 | C19H23NO4 | 4.2 | 330.1720(6); 299.1310(6); 267.1067(6);192.1037(100); 177.0803(11); 175.0783(20); 143.0502(20); 137.0599 (24); 115.0526(10) |
Reticuline | 1 |
| T63 | 14.5 | 6 | 314.1750 [M+] |
C19H24NO3 + |
−1.9 | 314.1767(100); 271.1332(10); 269.1196(50); 239.1010 (10);237.0925(25);211.1153(5);209.0938(20);192. 1030(18);175.0801(15); 145.0646(15); 143.0513(14); 137.0603(10); 115.0569(20); 107.0506(40) |
Isomer of magnocuranine (oblongine) |
3 |
| 64 | 14.6 | 6 | 365.1840 | C17H24N4O 5 |
4.1 | 365.1840(7); 306.1232(5); 177.0558(100);189.1389(6); 163.0385(7);149.0609(9);145.0282(18);117.0336( 10); 89.0380(8);70.0673(5) |
N-isoferuloyl arginine methylester |
1 |
| 65 | 16.5 | 6 | 356.1861 [M+] |
C21H26NO4 + |
−0.3 | 356.1865(30); 313.1440(8); 311.1273(38); 296.1041 (35); 281.0831(17); 279.1023(100); 280.1116(30); 265.0888(15); 251.1107(21); 264.0786(42); 248.0844 (40); 236.0838(16) |
Menisperine | 1 |
| T66 | 17.5 | 6 | 476.1929 | C24H29NO9 | 1.7 | 314.1398(100); 177.0558(90); 149.0607(6);145.0282 (45); 121.0617(3); 117.0363(10); 89.0380(6) |
N-feruloyl tyramine-4”’-O- hexoside |
2 |
| 67 | 17.6 | 6 | 379.1989 | C18H26N4O 5 |
2.1 | 379.1989(10); 203.1508(6); 186.1271(7); 177.0558 (100); 163.0385(6); 149.0609(10); 145.0282(20); 117.0336(10); 89.0414(7); 70.0672(4); 60.0575(4) |
N-isoferuloyl arginine ethylester |
1 |
| 68 | 18.1 | 6 | 328.1556 | C19H21NO4 | 2.1 | 328.1556(5);311.1280(20);296.1084(50);281.0759 (55);280.1108(100); 265.0848(60); 237.0956(10) |
Laurotetanine | 1 |
| T69 | 18.2 | 6 | 506.2026 | C25H31NO1 0 |
2.4 | 344.1525(12); 177.0558(100); 149.0609(5); 145.0282 (62); 117.0336(15); 89.0414(6) |
N-feruloyl-3”’- methoxytyramine -4”’-O-hexoside |
2 |
| T70 | 18.5 | 6 | 356.1870 [M+] |
C21H26NO4 + |
2.2 | 356.1870(5); 192.1016(100); 177.0803(10) | N-methyl tetrahydrocolumb amine or isomer |
3 |
| T71 | 18.6 | 6 | 356.1874 [M+] |
C21H26NO4 + |
3.4 | 356.1865(6); 311.1273(40); 296.1041(60); 281.0831(16); 280.1116(100); 265.0868(13) |
Xanthoplanine | 2e |
| 72 | 19.4 | 6 | 354.1359 | C20H19NO5 | 5.1 | 354.1359(90); 336.1168(20); 323.0955(9); 275.0661(20); 247.0757(15); 206.0807(15); 189.0783 (64); 188.0702(85); 149.0609(25); 119.0476(6); 91.0582(6) |
Protopine | 1 |
| 73 | 20.9 | 6 | 370.1653 | C21H23NO5 | −0.3 | 370.1653(100); 352.1522(42); 290.0963(42); 206.0807 (34); 189.0783(38); 188.0702(100); 165.0932(10); 149.0609 (10) |
Allocryptopine | 1 |
Retention time obtained using HILIC separation;
Retention times obtained using reversed phase separation;
Spectra at 25eV except where noted;
Most compounds annotated at levels 2 or 3 were tentatively identified by comparison of their fragmentation patterns with those of structural analogs;
Identification based on published tandem mass spectra
Figure 2.
Chemical structures of the nitrogenous metabolites from black cohosh identified ot tentatively identified in the present study. Structures of some compounds not shown here appear in the corresponding tandem mass spectra. For clarity, structures of well-known primary metabolites are omitted.
Due to lack of authentic standards, identifications at levels 2 and 3 are considered tentative and that fact is acknowledged by labeling the corresponding chromatographic peaks with the letter T (see Table 1). It should be noted that annotation levels primarily reflect the strength of analytical evidence rather than novelty of the compound; indeed, many compounds identified at level 1 were new natural products. The identified compounds will be discussed below based on their chemical class rather than on their appearance in individual fractions or elution order during LC-MS. In this manner, the mass spectrometric evidence is easier to follow since fragmentation patterns of chemical analogs are closely related.
3.2.1 Guanidino compounds
Several compounds containing either an acylic or cyclic guanidino group were identified. During collision-induced dissociation (CID), acyclic guanidines displayed a characteristic loss of neutral guanidine (−59 Da; CH5N3). Accurate mass measurements were particularly useful to distinguish this loss from other isobaric losses of 59 Da such as acetamide (CH3CONH2) originating from an acetylated amino group or trimethylamine originating from a quaternary nitrogen, both of which were observed during this study (see below). In addition to the loss of neutral guanidine, protonated guanidine (CH6N3) of m/z 60 was usually observed. Formation of protonated guanidine is thought to proceed via an ion-neutral complex and its abundance strongly depends on the applied collision energy with lower collision energy increasing the abundance of this ion [28].
The prototype acyclic guanidino compound and a biosynthetic precursor of these compounds is the amino acid arginine identified as compound 29 in the XAD water fraction (Figure 3a). The fragmentation pattern of arginine has been previously described in detail [28–30]. The product ion tandem mass spectrum of 24 (Figure 3b) contained many of the same fragment ions as arginine. The short retention of this compound during HILIC indicated that it is less polar than arginine, while its elemental composition indicated addition of a C2H2O2 moiety to arginine. These data are consisted with the acetylation of arginine. Since an ion of m/z 60 was present, this indicated that the guanidino group was free and that acetylation occurred on the amino group. Identification of 24 as α-N-acetyl arginine was confirmed by comparison with an authentic standard. α-N-acetyl arginine is an intermediate in arginine metabolism and is found both in plants and animals, although we are not aware of reports describing the actual isolation of this compound from plants.
Figure 3.
Product ion tandem mass spectra of (a) arginine, (b) N-acetyl arginine, and (c) N-formyl arginine.
The product ion spectrum of compound 25 (Figure 3c) was also similar to that of arginine. The elemental composition of C7H15N4O3 suggested an addition of a carbonyl group to arginine. Since the ion of m/z 60 was present, this indicated that the carbonyl group is most likely attached to the primary amino group. The identification of 25 as N-formyl arginine was confirmed by comparison with an authentic standard. Since N-formyl arginine can be produced by incubation of arginine with formic acid at elevated temperatures it is not clear if this compound is an artifact of isolation or a genuine natural product. At this point there have been no reports related to the isolation of this compound from plant sources.
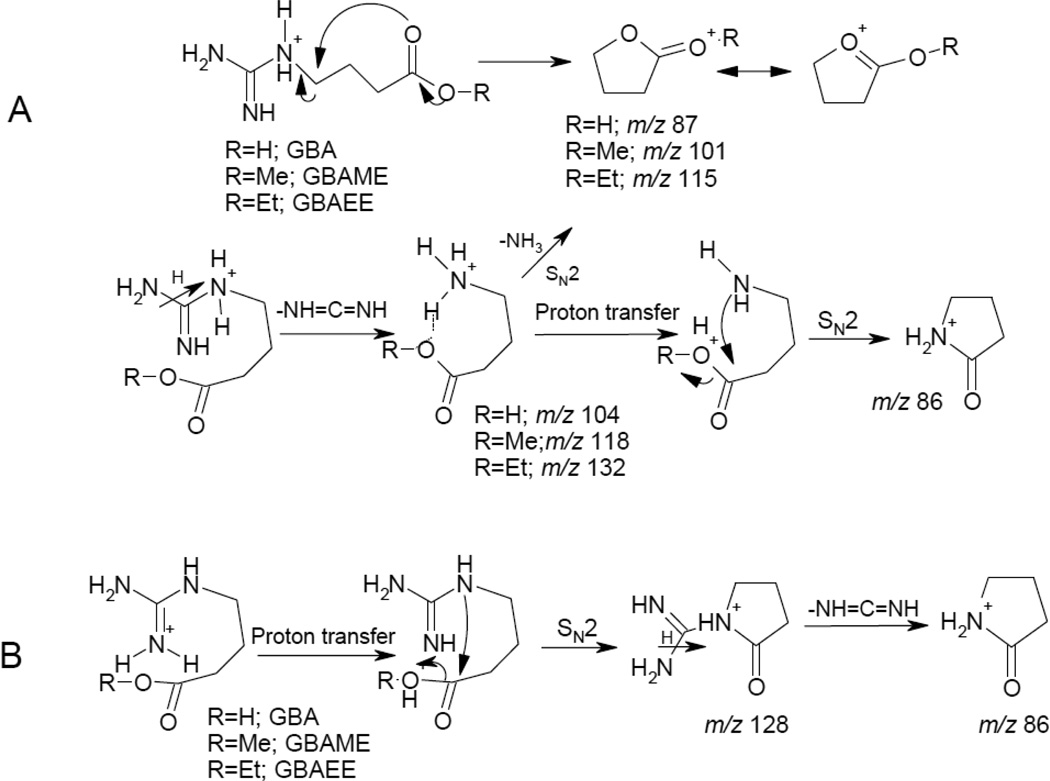
The product ion spectrum of compound 21 showed a loss of 59 Da (m/z 87) characteristic of acyclic guanidines (Figure 4a). By a combination of database searching and comparison with an authentic standard, this compound was identified as γ-guanidino butyric acid (GBA). The product ion of m/z 87 is likely to be protonated butyrolactone, and its formation from γ-guanidino butyric acid can be rationalized by an SN2 attack of the carbonyl oxygen on the carbon atom bearing the guanidino group (Scheme 1A). Direct attack of the carbonyl oxygen is supported by observation of protonated guanidine at m/z 60, which is formed by proton transfer in the ion-neutral complex between guanidine and protonated butyrolactone. Formation of an ion-neutral complex is further supported by observation that, at higher collision energies, protonated guanidine is not observed due to insufficient survival time of the ion-neutral complex. An additional minor pathway for formation of the ion of m/z 87 is by elimination of ammonia from protonated γ-aminobutyric acid (GABA) (m/z 104) as determined in separate ion-trap experiments (data not shown). Similarly, the product ion of m/z 86 has an elemental composition of C4H8NO corresponding to protonated butyrolactam. Ion-trap experiments indicated that the main pathways for formation of this ion are a loss of methylene diamine (NH=C=NH) from the ion of m/z 128 [MH-H2O]+ and a loss of water from protonated GABA (Scheme 1B).
Figure 4.
Product ion tandem mass spectra of (a) γ-guanidino butyric acid, (b) γ-guanidino butanal, and (c) γ-guanidino butanol.
Scheme 1.
Proposed fragmentation pathways for γ -guanidino butyric acid and its esters.
Using the fragmentation pattern of GBA as a model, several analogs of this compound were identified. Compounds 19 and 31 had elemental compositions corresponding to an addition of an extra methylene unit to GBA. In the product ion spectrum of 19, both the base peak at m/z 101 and the second most abundant fragment ion of m/z 100 were shifted by 14 Da compared to those of GBA, indicating that the extra carbon atom is located in the carbon chain and not on the carboxylic or guanidino group. Based on these considerations, 19 is tentatively identified as δ-guanidinovaleric acid. In contrast to 19, the base peak but not the ion of m/z 86 is shifted by 14 Da in the product ion tandem mass spectrum of 31. This indicates that the extra carbon is located on the carboxylic group of 31 in the form of a methyl ester (Scheme 1). Based on these considerations, 31 was identified as the methyl ester of GBA, and this assignment was confirmed by comparison with an authentic standard. Similarly, 38 was identified as the ethyl ester of GBA. Note that in the product ion tandem mass spectra of 31 and 38, no protonated guanidine was observed, since the acidic proton of GBA was replaced with an alkyl group, and there were no other acidic protons available for transfer. Both 31 and 38 are most likely artifacts of sample isolation and handling as is commonly the case for methyl and ethyl esters.
Using similar spectral arguments, compounds 8 and 11 were tentatively identified as γ-guanidino butyraldehyde (4-guanidinobutanal) and γ-guanidino butyl alcohol (4-guanidino-1-butanol), respectively (Figure 4b and 4c). The product ion tandem mass spectrum of 11 can be rationalized in the same manner as described above for GBA in that the hydroxyl group participates in the SN2 attack on the carbon-bearing guanidino group (Scheme 2A). Again, the two products, protonated tetrahydrofuran (THF) and guanidine, likely remain in an ion-neutral complex, which can either dissociate to produce neutral guanidine and protonated THF, or proton transfer can occur leading to formation of protonated guanidine. On the other hand, the product ion mass spectrum of 8 can be explained as being derived from both the cyclic and acyclic forms of this aldehyde (Scheme 2B). The presence of the cyclic form can explain the ready loss of water, as well as explain the base peak of m/z 70 which has the structure of protonated dihydropyrrole. All of the preceeding compounds are products of normal cellular catabolism of arginine [14, 31]. Representing an intermediate in the catabolism of arginine, 8 can either be further oxidized to GBA or reduced to alcohol 11. Compound 8 was postulated in our previous publication as a building block in the formation of dopargine [14]. Compound 11 was previously isolated from various Leonorus species [32].
Scheme 2.
Proposed fragmentation pathways for γ-guanidinobutanal and γ-guanidinobutanol.
In contrast to acyclic guanidines, the predominant fragmentation pathway of cyclic guanidines is a loss of methylenediamine (−42 Da; NH=C=NH). The two prototype compounds from this class are cyclocimipronidine and cimipronidine identified as compounds 6 and 20, respectively. In addition, a methyl ester of cimipronidine was identified as compound 4. The isolation and full structural characterization of these compounds was reported previously [14]. By analogy to the fragmentation patterns of 6 and 20, two additional congeners were identified. Compound 5 had an elemental composition corresponding to one CH2 unit more than cyclocimipronidine. The product ion mass spectrum of 5 contained the same fragment ions as that of cyclocimipronidine, indicating that the extra methyl group was lost during fragmentation. In the spectrum of cyclocimipronidine, the elemental composition of the fragment ion of m/z 112 indicated a loss of NH=C=NH, which suggested that methylation of 5 occurred on the guanidino group, making the tentative assignment of this compound as N-methyl cyclocimipronidine, a new natural product. In the absence of additional structural data, the position of the methyl group could not be determined at this time.
3.2.2 Compounds containing quaternary nitrogen
As a prototype member of this group, the quaternary amino alcohol choline (7) was identified by mass spectra database searching and confirmed by comparison with an authentic standard. In addition to 7, several derivatives of this alcohol were identified. Compound 44 present in FCPC fraction 4 displayed a characteristic loss of trimethylamine from the precursor ion of m/z 208 to form an ion at m/z 149, which can further fragment to lose CO2 and produce an ion of m/z 105. These data are consistent with the identification of 44 as benzoyl choline, an ester of benzoic acid and choline. This assignment was confirmed by comparison with an authentic standard. Elemental composition and product ion tandem mass spectrometric analysis of 28 indicated that it also contains a choline moiety, since elemental composition of the major fragment ions of m/z 104 and 60 corresponded to those of choline. The remainder of the molecule corresponded to that of a hexose sugar, which suggests a tentative assignment of this compound as O-hexosyl choline. This identification is also consistent with late elution of 28, indicative of a highly polar molecule. This is the first report of such class of compounds occurring in plants.
In addition to choline derivatives, several betaines were identified. Wood et al. [33] described fragmentation patterns of simple betaines, which were used as the basis for discovery of this class of compounds in black cohosh in the present study. A combination of database and literature searching led to the identification of glycine betaine (16), proline betaine (17), L-carnitine (23) and tentative identification of histidine betaine (hercynine) (27). L-carnitine is widely distributed throughout the plant and animal kingdoms. In living cells, it plays an important role in energy production since it helps transport fatty acids from the cytoplasm into the mitochondria where their degradation takes place. Although many plants contain L-carnitine, meat products are the main source of L-carnitine in the human diet. L-carnitine is also sold as a dietary supplement for its purported beneficial role in cardiovascular disease, diabetes or weight loss. In contrast to L-carnitine, histidine betaine is mostly found in fungi and rarely in higher plants. Curiously, it was first isolated from the latex of the Brazilian rubber tree (Hevea brasiliensis) [34]. At this point, there is little known about the biological role or activities of this compound. Finally, trigonelline (18), a widespread plant alkaloid formed by methylation of nicotinic acid was also identified in the XAD water fraction. Among other plants, trigonelline has been identified in coffee and is thought to have antioxidant and other health-promoting properties [35]. Interestingly, a recent study identified trigonelline as a new phytoestrogen capable of stimulating growth of MCF-7 breast cancer cell line at very low doses [36].
3.2.3 Hydroxycinnamic acid amides and esters
The most abundant hydroxycinnamic acids in black cohosh are caffeic, ferulic and isoferulic acid. The identification of amides and esters of ferulic/isoferulic acid was enabled by their characteristic fragmentation pattern dominated by fragment ions of m/z 177, 149, 145, 117, and 89 originating from the ferulic/isoferulic acid portion of the amide. Caffeic acid amides produce a similar ion series at m/z 163, 145, 135, 117, and 89. Fragment ions corresponding to the protonated amine may also be observed, but their abundance is usually lower and strongly depends on the type of amine. Whether the carboxylic acid portion is ferulic or isoferulic acid can be determined based on the presence of a low abundance but diagnostic fragment ion of m/z 163 with the elemental composition of C9H7O3, which was observed only during fragmentation of protonated isoferulic acid, but not ferulic acid ([37] and data not shown). This fragment ion originates as a product of ion-molecule reaction in the collision cell and is formed by addition of water to the ion of m/z 145 (manuscript in preparation). In addition, isoferulic acid amides tend to produce secondary fragment ions of lower abundance (m/z 149, 145 and 117) that originate from losses of CO or methanol from the primary acylium ion of m/z 177 (see below).
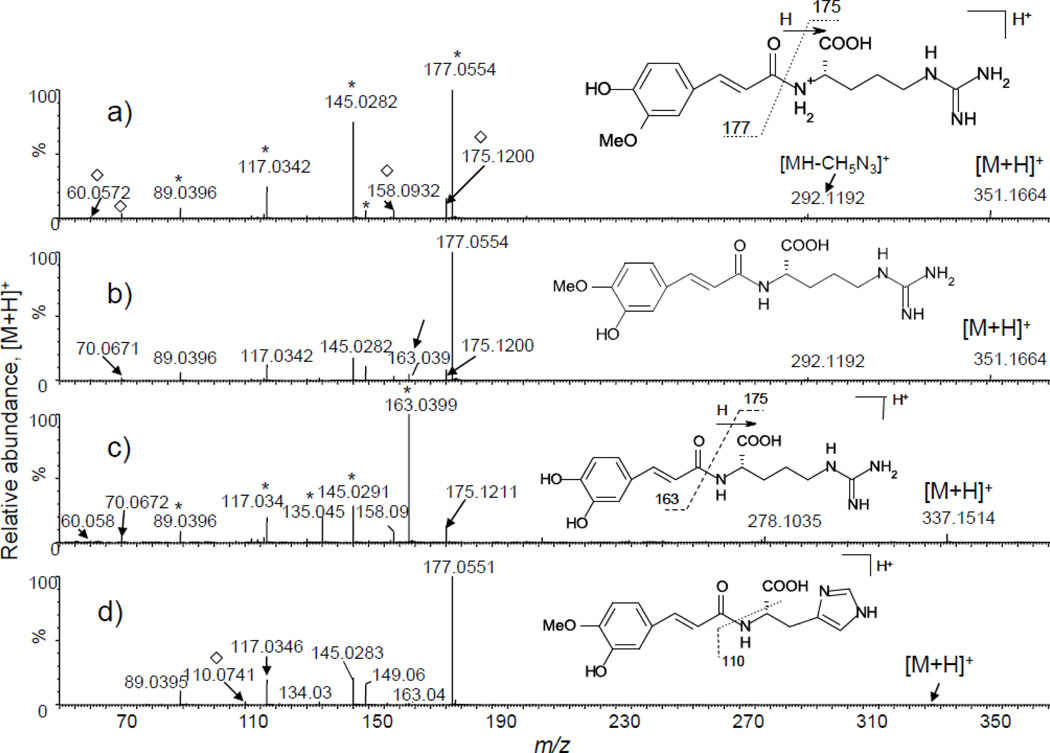
Once the diagnostic ions from ferulic/isoferulic acid are observed in the product ion spectrum of an unknown compound, the amine portion of the amide can be deduced based on database searching of the elemental composition of the remainder of the molecule. As an example of this identification strategy, Figures 5a and 5b show product ion spectra of compounds 34 and 35 with elemental composition of C16H22N4O3. Both spectra show a typical ferulic/isoferulic acid amide fragmentation pattern with 35 showing an additional peak at m/z 163, suggesting that 34 is an amide of ferulic while 35 is an amide of isoferulic acid. Database searching of the composition of the remainder of the molecule (C6H14N4O2) suggested that the amine portion is the amino acid arginine: protonated arginine was observed at m/z 175, along with other ions originating from fragmentation of arginine such as ions of m/z 158, m/z 70 and m/z 60 (see Figure 3a). The presence of the latter two ions indicated a free guanidino group and confirmed that the carboxylic acid was attached to the amino and not the guanidino nitrogen. Based on these data, compounds 34 and 35 were identified as N-feruloyl and N-isoferuloyl arginine, respectively. These assignments were confirmed by synthesis and comparison with authentic standards.
Figure 5.
Product ion tandem mass spectra of amides of hydroxycinnamic acids amides with amino acids; (a) feruloyl arginine, (b) isoferuloyl arginine, (c) caffeoyl arginine, and (d) isoferuloyl histidine. Ion series corresponding to the acid portion of the amide are labeled “*” for ferulic and caffeic acid in (a) and (c), respectively, while those corresponding to the amine portion are labeled “◊” for arginine and histidine in (a) and (d), respectively. Note the diagnostic but low abundance fragment ion of m/z 163 [(b) and (d)], which is formed by amides of isoferulic acid but not ferulic acid.
The product ion tandem mass spectrum of 32 (Figure 5c) was dominated by the fragment ion series originating from caffeic acid (m/z 163, 145, 135, 117, 89), indicating that this is a caffeic acid amide. Since the product ion spectrum of this compound was similar to those of 34 and 35 in that it also contained ions originating from fragmentation of protonated arginine, 32 was also an arginine derivative. Combined with the elemental composition data (Table 1), the most plausible structure of 32 is caffeoyl arginine, a new natural product.
Product ion mass spectra of compounds 64 and 67 were also very similar to those of 35. Based on the elemental composition of 64 (C17H24N4O5), the amine portion contained an additional CH2 unit compared to 35, which could be either in the form of an ester of arginine or in the form of homoarginine. The detection of a product ion of m/z 70, which requires four carbon atoms connected to the nitrogen, suggests that 64 is a methyl ester of 35. Similarly, 67 was determined to be an ethyl ester of 35. Compounds 64 and 67 are likely formed during extraction and fractionation and can be considered artifacts rather than novel natural products. Although none of these arginine amides have been reported previously, their presence in black cohosh is not surprising given that both the hydroxycinnamic acids and arginine are abundant constituents in the extracts of this plant.
The product ion tandem mass spectrum of 33 contained a small but discernible fragment ion of m/z 163, suggesting an isoferulic acid amide (Figure 5d). The elemental composition of the amine portion was determined to be C6H9N3O2. Database searching of this formula indicated that the amine portion is likely histidine. Therefore, 33 is tentatively determined to be N-isoferuloyl histidine. This assignment is supported by the observation of a fragment ion of m/z 110, corresponding to an immonium ion from histidine which is typically used to identify the presence of this amino acid in peptides. To our knowledge, this is the first report of this compound occurring in plants. Using a similar approach, compound 54 was determined to be N-isoferuloyl glutamic acid. Compounds 42 and 43 were identified as N-feruloyl putrescine and N-isoferuloyl putrescine, respectively by using similar reasoning in addition to comparison with authentic standards.
In addition to the cinnamides described above, several glycosidated analogs were found in black cohosh. For example, compound 66 (see product ion tandem mass spectrum in Figure 6a) had an elemental composition of C24H29NO9. The product ion tandem mass spectrum of 66 contained a fragment ion of m/z 314 with a formula of C18H19NO4, which when formed using in-source fragmentation and then characterized using MS-MS with collision-induced dissociation, produced a tandem mass spectrum that was identical to that of synthetic N-feruloyl tyramine (data not shown). These data indicate that 66 is a hexoside of N-feruloyl tyramine. By analogy, hexosides of several other amides such as N-feruloyl dopamine (49) and N-isoferuloyl dopamine (50), N-feruloyl phenylalanine (56), and N-feruloyl methoxytyramine (69) were partially characterized. The structure of the sugar in these compounds could not be determined from these data and will require additional investigation.
Figure 6.
Product ion tandem mass spectra of glycosidated amides of ferulic acid with (a) tyramine, (b) O-methyldopamine, (c) dopamine, and (d) and phenylalanine. The position of glycosidation could be determined based on the presence of a fragment ion corresponding to the glycosidated ferulic acid (m/z 321). Note the absence of the diagnostic ion of m/z 163, strongly suggesting that these are amides of ferulic acid and not isoferulic acid.
However, the position of glycosidation in 49, 50, 56, 66, and 69 could be determined based on the presence of a fragment ion corresponding to the loss of the amino moiety and generation of the corresponding hexosylated acylium ion of ferulic/isoferulic at m/z 339. Although the ion of m/z 339 was observed at low collision energies (data not shown), at higher collision energies it eliminated water to produce an ion of m/z 321 (Figure 6c and 6d). In addition, complementary fragmentation pathways in which charge was retained on the amino group (m/z 154, 137, 119, and 91 for dopamine, or m/z 166 and 120 for phenylalanine) were also observed, which would be possible only if there was no sugar attached to the amino group. Based on these considerations, 49 and 50 were tentatively assigned as N-feruloyl and N-isoferuloyl dopamine-4’-O-hexoside, respectively, while 56 was tentatively assigned as N-feruloyl phenylalanine-4’-O-hexoside. In contrast, tandem mass spectra of 66 and 69 showed loss of hexose (−162 Da) with no fragment ions corresponding to the amine moiety, indicating that the hexose was attached to the phenol group on the tyramine/methoxytyramine portion. Thus, 66 can be tentatively assigned as N-feruloyl tyramine-4”’-O-hexoside, while 69 can be tentatively assigned as N-feruloyl-3”’-methoxytyramine-4”’-O-hexoside. It is noteworthy that in all of the above cases, the configuration of the double bond of the ferulic/isoferulic acid could not be determined. The majority of known phenylpropanoic acids has been described having trans configuration, although there are examples of cis configurated compounds. [38]
Hexosides of ferulic/isoferulic acid amides are relatively rare in the plant kingdom. To the best of our knowledge, there has been only one other report describing identification of N-feruloyl tyramine glucosides from the unrelated plant, Stephania hispidula [38], from which both 4’-O and 4”’-O- glucosides of N-feruloyl tyramine were described. In the case of N-feruloyl methoxytyramine, a 4’-O-glucoside as well as a 4’-O-galactoside have been identified. [38, 39] The galactoside analog, known as cimicifugamide, was identified in a related plant Cimicifuga dahurica [39], making it likely that 69 is a galactoside based on chemotaxonomic considerations. However, the position of glycosidation for these compounds is different from the proposed structure of 69. If proven correct by more detailed spectroscopic analysis, 69 would represent a new natural product. Similarly, we are not aware of reports concerning the identification of 49, 50 and 56, which makes this the first description of these compounds in plants.
Similar to the compounds described above, 47 and 48, with the elemental composition of C15H22NO4, produced product ion tandem spectra that contained fragment ions characteristic of ferulic/isoferulic acid derivatives. An additional fragment ion corresponding to a loss of trimethylamine (−59 Da) was observed at m/z 221, indicating that these compounds are not amides but contain nitrogen in the form of a quaternary amine. This information, combined with the elemental composition, enabled us to identify these analogs as feruloyl (47) and isoferuloyl choline (48) with isoferuloyl choline being the more abundant analog. These assignments were confirmed by comparison with authentic standards. Fragmentation patterns of feruloyl choline and choline esters with other phenolic acids have been described in detail elsewhere [16].
3.2.4 Dihydro and tetrahydro isoquinoline alkaloids
The isoquinoline alkaloids represent a large group of alkaloids that are biosynthetically derived from Pictet-Spengler condensation of dopamine with various aldehydes. The simplest sub-group of the isoquinoline alkaloids is the tetrahydroisoquinoline alkaloids. Compounds 9 and 12 are two prototype compounds of this class identified in black cohosh. Compound 9 was initially identified as salsolinol based on spectral database searching and subsequently confirmed by comparison with an authentic standard. Compound 9, formed by condensation of dopamine with acetaldehyde, is widely distributed in the plant kingdom. It can also be synthesized endogenously in dopaminergic neurons of mammals including humans [40]. Compound 9 has been studied for its neuropharmacological effects such as modulation of catecholaminergic transmission as well as for a possible role in the etiology of alcoholism. [41] Dietary sources of 9 include alcoholic beverages, bananas, cheese, beef, milk, and cocoa [42–44]. Compound 9 is orally absorbed but it does not cross the blood-brain barrier [45]. Thus it is likely that exogenously administered 9 does not exhibit CNS activities but may have peripheral activities mediated by dopamine D2 receptors [46].
Careful analysis of the product ion tandem mass spectrum of 12 (Table 1) indicated that most of the fragment ions weighed 14 Da less than the corresponding ions of salsolinol, which suggested that 12 is norsalsolinol (6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline), a Pictet-Spengler adduct of dopamine and formaldehyde. This assignment was confirmed by comparison with an authentic standard. Compound 12 has also been identified as a dopamine metabolite in the brain [47] and in urine [48]; however, this is the first report of the occurrence of this compound in higher plants. However, it is not clear weather 12 is an artifact of isolation or a genuine natural product. Traces of formaldehyde might have been present in the extraction solvent, which could lead to a non-enzymatic condensation with dopamine.
Compound 51 had an elemental composition of C10H11NO2, which corresponded to a structure containing two hydrogens less than salsolinol. The product ion tandem mass spectrum (Table 1) indicated a facile loss of a methyl radical (m/z 163.0645) along with a loss of methane (m/z 162.0566). Loss of a methyl radical is typically observed in structures that can stabilize the resulting cation radical, such as for example methoxy groups. The loss of two hydrogens (m/z 176) suggested a structure that can obtain additional stabilization resulting from such a loss. This feature suggests a dihydroisoquinoline ring that can become fully aromatic after a loss of two hydrogens. Within constraints of the elemental composition, the fragmentation pattern can be explained by either 6(7)-methoxy dehydronorsalsolinol or a dehydrosalsolinol. By comparison with an authentic standard, 51 was identified as 6,7-dihydroxy-1-methyl-3,4-dihydroisoquinoline, also known as 1,2-dehydrosalsolinol. Database searches revealed that this compound has not been previously reported in plants. Along with norsalsolinol and salsolinol, 51 is also a product of dopamine metabolism in the brain and has been detected in urine [40].
3.2.5 Benzylisoquinoline alkaloids
A large group of the isoquinoline alkaloids is derived from condensation of dopamine and 4-hydroxyphenylacetaldehyde to form a benzyl tetrahydroisoquinoline skeleton that can be further coupled into a plethora of alkaloids including aporphines, protoberberines and protopines [49]. Compound 60 was tentatively identified as norcoclaurine, which is the prototype molecule of this group of alkaloids, based on comparison with the published product ion tandem mass spectra [50, 51]. Another critical molecule in the biosynthetic pathways of isoquinoline alkaloids is reticuline (62), which was detected in Fraction 6 and identified by comparison with an authentic standard.
Compounds 45 and 63 produced nearly identical product ion spectra (Table 1) but had different retention times suggesting two isomeric structures. Loss of dimethylamine (m/z 271) suggested that these compounds contain quaternary nitrogen (see below). Database searching with this structural constraint revealed that these compounds are likely analogs of the alkaloid magnocurarine. Comparison with authentic magnocurarine led to assignment of 45 as magnocurarine, whereas 63 is likely one of the known positional isomers of magnocurarine such as lotusine.
3.2.6 Aporphine alkaloids
Fragmentation patterns of aporphine alkaloids have been studied in detail previously [52, 53], and we used this information as a basis for identification of this class of compound during this study. Due to the rigid structure of aporphines, their product ion spectra are characterized by a series of small molecule losses such as water, CO or CO2, with cation radical fragment ions frequently present. The degree of substitution on the nitrogen can be easily distinguished based on the loss of nitrogen in the form of ammonia (secondary nitrogen), methylamine (tertiary nitrogen) or dimethylamine (quaternary nitrogen). For example, in the product ion spectrum of 36, loss of dimethylamine (−45 Da) was the second most abundant peak indicating that this compound is a quaternary alkaloid. When the elemental composition of this compound was searched in the Beilstein database, the most plausible hit was the aporphine alkaloid magnoflorine. Compound 36 was then identified by comparison with an authentic standard.
Compound 65 produced a product ion tandem mass spectrum that was very similar to that of magnoflorine with most peaks shifted by 14 mass units, indicating a methylated analog. Compound 65 was then identified as menisperine by comparison with an authentic standard. Compound 55 had the same elemental composition as magnoflorine and produced a very similar product ion tandem mass spectrum (Table 1) that contained the same fragment ions but in different abundances, indicating that this compound is a positional isomer of magnoflorine. Since no authentic standard was available, the product ion tandem spectrum was compared to the published spectra of quaternary aporphine analogs [53], which lead to tentative identification of this compound as laurifoline. In the same manner, compound 71 was tentatively identified as xanthoplanine [53]. In contrast to these quaternary alkaloids, compound 61 showed a loss of 17 Da indicating that it contains a secondary nitrogen in the ring (Figure 7a). Small loses of methyl radicals (m/z 282 and 267) are consistent with a compound with at least two methoxy groups in the aporphine ring. The product ion spectrum of this compound was very similar to another well-known aporphine alkaloid, boldine, with most of the product ions shifted by 14 mass units. Based on these considerations, a structure of laurolitsine (also known as norboldine) was proposed for 61, which was confirmed by comparison with an authentic standard. Similarly, the product ion tandem mass spectrum of 68 (Figure 7b) also showed loss of ammonia, as well as multiple losses of methyl radicals (m/z 296, 281 and 265) indicative of a molecule with multiple methoxy groups. By comparison with an authentic standard, 68 was identified as laurotetanine.
Figure 7.
Product ion tandem mass spectra of aporphine alkaloids (a) laurolitsine and (b) laurotetanine. Loss of ammonia from these compounds indicates a secondary nitrogen in the aporphine ring.
3.2.7 Protoberberine alkaloids
Database and literature searches of the molecular composition of compounds 37 and 70 suggested that these compounds most likely belong to the tetrahydroprotoberberine class of alkaloids. This group of alkaloids contains a dibenzo[a,g]quinolizidine tetracyclic ring system. The tandem mass spectra of 37 and 70 (Table 1) were relatively simple, with base peaks of m/z 192 corresponding to an elemental composition of C11H14NO2 (m/z 192). Loss of a methyl radical from this ion (m/z 177) suggested either a quaternary nitrogen or a 2,3-dimethoxy substitution pattern. The 2,3-dimethoxy substitution pattern was excluded based on analysis of product ion tandem mass spectra of several 2,3-dimethoxy tetrahydroprotoberberine alkaloids including corydaline and tetrahydropalmatine, which showed loss of methane in addition to the loss of a methyl radical (data not shown). Based on these analyses, we concluded that 37 and 70 were N-methyl tetrahydroprotoberbrine alkaloids. A literature search for known spectra of this class revealed that the alkaloid phellodendrine produced an identical product ion tandem mass spectrum to that of 37 [54]. However, given the simplicity of the spectrum, an unequivocal assignment of 37 as phellodendrine is not possible since the alkaloid cyclanoline, which is the 9-hydroxy-10-methoxy analog of phellodendrine, could also produce a similar product ion tandem mass spectrum. Compound 70, which a methylated analog of 37, produced a product ion spectrum similar to that of 37, suggesting that this compound is likely to be N-methyl tetrahydrocolumbamine or a 10,11-dimethoxy isomer thereof [55].
3.2.8 Protopine-type alkaloids
The product ion tandem mass spectra of 72 and 73 indicated that these two compounds are structural analogs of each other. MassBank database searching indicated that 72 and 73 were probably the alkaloids protopine and its demethylenated analog protopine, respectively. Both of these assignments were confirmed by comparison with authentic standards. The fragmentation pattern of this type of compound has been discussed in detail elsewhere [51].
3.2.9 Pictet-Spengler adducts with tryptamine derivatives
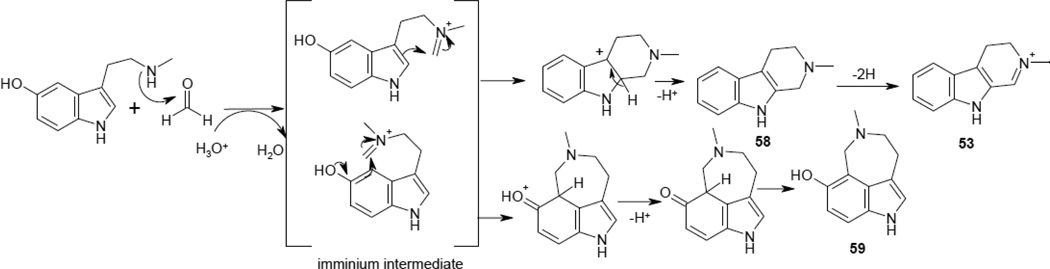
Compounds 58 and 59 eluted at 3.3 and 3.8 min, respectively, during the LC-MS analysis of Fraction 6 and had the same elemental composition (C12H14N2O) but very different fragmentation patterns (Figure 8a and 8b). The elemental compositions of several key fragment ions such as m/z 160, 159, 132, and 117 were the same as those observed in the product ion tandem mass spectra of Nω-methyl serotonin and serotonin [56], suggesting that 58 and 59 are related to these biogenic amines. The elemental composition and double bond equivalents imply that, in 58 and 59, the two nitrogen atoms are in the form of a ring structure. Based on these considerations, Pictet-Spengler adducts of serotonin and Nω-methyl serotonin with formaldehyde or acetaldehyde were prepared, and their fragmentation patterns compared with 58 and 59. Results of these experiments indicated that both 58 and 59 are Pictet-Spengler adducts of Nω-methyl serotonin and formaldehyde. 58 was identified as 6-hydroxy-2-methyl-1,2,3,4-tetrahydro-β-carboline, while 59 was identified as 3,4,5,6-tetrahydro-7-hydroxy-5-methyl-1H-azepino[5,4,3-cd]indole, heretofore named cimitrypazepine, a new natural product.
Figure 8.
Product ion tandem mass spectra of Pictet-Spengler adducts of Nω-methylserotonin and formaldehyde. Scheme 3 provides the proposed mechanism of formation of these compounds.
Fragmentation of 58 is dominated by retro Diels-Alder fragmentation to form the ion of m/z 160 (Figure 8a). Since retro Diels-Alder fragmentation is not possible in 59, it fragments instead by opening of the azepine ring followed by elimination of methylene imine (CH2=NH) to form a base peak of m/z 174.0938 (Figure 8b). Compound 58 has been reported previously only in the unrelated plant Evodia fargesii [57]. Both of these compounds likely originate from the same precursor species that can cyclize into either a six or seven-membered ring (Scheme 3), as has been demonstrated in studies of the reactions of serotonin and Nω-methyl serotonin with various aldehydes [18].
Scheme 3.
Proposed mechanism of formation of compounds 53, 58 and 59. An imminium ion intermediate can attack possible nucleophilic sites on the indole ring to form 58 and 59. 53 is likely formed by dehydrogenation of 58.
Given that both 58 and 59 can be formed during chemical reaction between formaldehyde and Nω-methylserotonin, it is unclear whether they represent artifacts of isolation or genuine natural products. Nω-methylserotonin is a genuine constituent of black cohosh [56], and formaldehyde can be formed as an impurity in organic solvents, thus this reaction can conceivably occur during sample processing. Alternatively, Pictet-Spengler reaction is a proven biosynthetic pathway of natural products, so it is possible that formation of the azepine ring can also be catalyzed by enzymes, although no such reaction has yet been demonstrated. This may represent an interesting area of future research.
Compound 53 had an elemental composition containing two hydrogens less than 58 and 59 (C12H12N2O), suggesting a dihydro-β-carboline structure. The ready loss of a methyl radical (m/z 186), along with the fragment ion of m/z 170, [MH-CH3NH2]+, indicated that the N(2) nitrogen on the β-carboline ring was methylated. Biosynthetic considerations were used to deduce the position of the double bond on the β-carboline ring. Accordingly, the most likely position of the double bond is 1,2 which was confirmed by comparison of retention time and fragmentation pattern with authentic N(2)-methyl-6-hydroxy-3,4-dihydro-β-carboline. Biosynthetically, this compound is likely formed by dehydrogenation of 58 and represents a new natural product. It should be noted that dihydro-β-carbolines are often by-products of Pictet-Spengler condensation [58]. Thus, it is possible that 58 is an isolation artifact.
The product ion spectrum of compound 46 eluting at 10.6 min during LC-MS of fraction 4 was dominated by an ion of m/z 144 with the elemental composition (C10H13N2), corresponding to protonated tryptamine. In-source fragmentation followed by MS-MS product ion analysis of m/z 144 showed a fragmentation pattern identical to authentic tryptamine, suggesting that this compound is a tryptamine derivative. The neutral loss of iminoacetic acid (C2H3NO2) combined with database searching suggested that 46 might be a tetrahydro-β-carboline carboxylic acid. Since two positional isomers (1 and 3-substituted) are known, both analogs were synthesized and compared with 46. These experiments identified 46 as 1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid, a Pictet-Spengler condensation product of tryptophan and formaldehyde. This compound has been found in various dietary products such as fruits, wine, beer, soy sauce, cheese, and raisins [59, 60]. It also occurs in considerable amounts in smoked meat products [61].
3.2.10 Nucleobases and nucleosides
Nucleobases and nucleosides are constituents of normal cellular metabolism, and most of the compounds in this class were identified by spectral database searching and comparison with authentic standards. Of particular interest were 40 and 41, which had the same elemental composition (C11H15N5O4), but different product ion tandem mass spectra (Table 1). The only fragment ions in the tandem mass spectrum of 40 corresponded to protonated adenine (m/z 136) and loss of ammonia from adenine (m/z 119). The elemental composition of 40 indicates that, compared with adenosine (30), this compound has an extra CH2 unit in the sugar moiety. Based on literature searching, the most likely candidate for 40 is 2’-O-methyladenosine, which has been identified in the RNA of various species.
In contrast, the product ion tandem mass spectrum of 41 exhibited a fragment ion of m/z 150 with an elemental composition corresponding to methylated adenine (C11H15N5O4). Database searching revealed that methylation most likely occurred on the amino group, which led to tentative identification of this compound as N-methyladenosine. As stated earlier, mass spectrometry data alone cannot unequivocally determine the stereochemistry of sugar and exclude other possible structures such as 3’-O-methyladenosine.
3.2.11 Miscellaneous primary and secondary metabolites
Examination of the product ion tandem mass spectra of 1 and 15 indicated that they are close structural analogs. Compound 1 was identified as pyridoxine by spectral database searching. The elemental composition of 15 (C14H21NO8) indicated attachment of a hexose sugar to the pyridoxine moiety. The most likely structure of 15 that is consistent with these data is 5'-O-(β-D-glucopyranosyl)pyridoxine. Pipecolic acid (22) and pyroglutamic acid (26) have the same elemental composition and fragmentation pattern but can be distinguished based on the elemental composition of the base peak of m/z 84. These assignments were confirmed by comparison with authentic standards. Finally, panthotenic acid (3), also known as vitamin B5, was identified by spectral database searching and comparison with an authentic standard.
In addition to these common primary metabolites, the unusual compounds 52 and 57 were tentatively identified. Compound 52, with elemental composition of C10H11NO2, showed a simple product ion spectrum dominated by the highly stable benzylium ion at m/z 91 (Table 1). Accurate mass measurement indicated that the loss of 59 Da corresponded to CH3CONH2 indicating that 52 contains an acetyl amide. Abundant loss of CO from the ion of m/z 119 to produce an ion of m/z 91 can be best explained by a benzyl acylium structure for the ion of m/z 119. Based on these considerations, 52 was tentatively indentified as N-phenylacetyl acetamide (Scheme 4A).
Scheme 4.
Proposed fragmentation pathways of unusual secondary metabolites 52 and 57.
Similarly, the product ion tandem mass spectrum of 57 contained a base peak of m/z 107 with an elemental composition of C7H7O. The neutral species lost to produce this fragment ion had an unusual composition of C6H13N, which suggested a cyclohexylamine. An ion of low abundance corresponding to protonated cyclohexylamine was also observed at m/z 100. Ions of m/z 79 and 77 were formed by losses of CO and CO+2H from the ion of m/z 107. This fragmentation pattern is consistent with a structure of N-cyclohexyl-4-hydroxy benzylamine (Scheme 4B). The group of ions of m/z 107, 79 and 77 was also observed for other compounds possessing the 4-hydroxybenzyl moiety such as 4-hydroxyphenyl acetamide (data not shown) further supporting the proposed assignment. Cyclohexyl amines are rather unusual in the plant kingdom, although cyclohexyl urea derivatives have been described in the literature [62]. Therefore, the proposed structure of 57 represents a new natural product.
3.3 Discussion
As indicated in the introduction, triterpene glycosides and phenolic constituents have dominated research on black cohosh. The abundance of these compounds in black cohosh naturally led researchers to seek active compounds among these constituents. However, these compounds have not exhibited potent activities in relevant bioassays such as serotonergic, dopaminergic or opioid assays, suggesting that other classes of compounds might be responsible for the observed CNS activities. Recently, our group began investigating the nitrogen-containing metabolome of black cohosh using a tailored fractionation protocol designed to explore this aspect of black cohosh chemical diversity [13, 14, 22], which resulted in identification of several guanidine-type alkaloids [14, 15]. In addition, Nω-methylserotonin was identified as an active ligand of the serotonin 5-HT7 receptor [56].
The present study reveals that in addition to guanidine alkaloids, black cohosh contains a wide array of other types of nitrogenous metabolites many of which are alkaloids. The discovery of several classes of isoquinoline and tetrahydro-β-carboline alkaloids is perhaps the most significant result of this investigation. These alkaloids are well-known natural bioactive constituents with a wide range of pharmacological activities. Their presence in black cohosh provides significant new results that might explain the bioactivity profile of black cohosh. A recent study of black cohosh identified many of the enzymes involved in the biosynthesis of alkaloids, providing strong evidence that alkaloids are indeed an integral part of the black cohosh metabolome [63]. It is important to keep in mind that the MS-based approach discussed here is not quantitative, which means that congeners of the types of alkaloids identified here might be present at lower or higher abundance. This implies that it is very possible that significant amounts of other bioactive alkaloids can be isolated and/or detected in future studies.
For example, the alkaloid protopine has been reported to have benzodiazepine-like, analgesic, antidepressant, and anticholinergic activities in vitro and in animal studies [64–66]. The presence of this alkaloid might explain anecdotal reports of “vivid dreams” and opioid-like activities observed in patients taking black cohosh [67], as well as explain its observed in vitro opioid activity [68]. Aporphine alkaloids exhibit strong serotonergic activity against the 5-HT1A receptor [69, 70]. For example, N-methyllaurotetanine, which is an N-methyl analog of laurotetanine identified in this study, is a potent ligand for the 5-HT1A receptor [70]. Although it has been shown that small structural changes in the aporphine ring can lead to a large change in pharmacological activity, it is likely that some of the aporphines identified in this study contribute to the serotonergic activity of black cohosh [71]. In addition, aporphine and benzylisoquinoline alkaloids possess vasorelaxing, anti-spasmodic and anti-nociceptive activities, which could explain traditional uses of black cohosh for alleviation of menstrual complaints [72–74]. Further detailed biological studies are necessary to elucidate the bio-activties of the nitrogenous compound fraction in black cohosh preparations, but it should be noted that the 5-HT7 active compounds of the plant have been found present in the FCPC fraction 6, including the previously reported Nω-methylserotonin [56].
In addition to potentially contributing to the biological actions of black cohosh, alkaloids might be involved in drug-herb interactions. For example, we found that protopine and allocryptopine are potent inhibitors of CYP2D6 and may be involved in potential interactions of black cohosh with drugs metabolized by this isoform such as tamoxifen [75].
Another interesting discovery resulting from this investigation was the identification of amides of ferulic/isoferulic acid and their glycosidated analogs in black cohosh. Biosynthetically, these compounds are formed by transfer of an acyl group from feruloyl-S-CoA onto the corresponding amine, catalyzed by feruloyl-CoA acyltransferases. Cinnamate conjugates with amines and amino acids are widely distributed throughout the plant kingdom, with coffee being the major dietary source [76, 77]. This study identified several new members such as feruloyl and isoferuloyl arginine as well as isoferuloyl glutamic acid. At this point, it is unclear whether any of these compounds are unique to the genus Cimicifuga (Actaea). Since the conjugates identified in this study are either new or rare in botanicals, their biological activities are largely unknown. However, most of the known cinnamate conjugates show antioxidant activity derived from the ferulic/isoferulic acid portion [78].
The identification of numerous quaternary amines in black cohosh offers a potential explanation for the biological role of phenolic acids as counter ions for positively charged alkaloids. In plant tissues, alkaloids are typically stored as salts with organic acids, and in black cohosh, this role is likely fulfilled by the abundant phenolic acids. Formation of strong ion pairs between organic acids and quaternary alkaloids needs to be taken into account during isolation of both the acids and the alkaloids. As demonstrated in our earlier work [13], such complexes may lead to isolation of impure compounds that mislead interpretation of bioassay results.
It is important to note that since alkaloids are minor but potent constituents, small differences in their quantity can lead to large differences in the observed activities of crude extracts. Because alkaloids are bioactive natural products, it is reasonable to propose the inclusion of some of these alkaloids in future standardization of black cohosh preparations.
4. Conclusions
This study represents the most comprehensive investigation of the nitrogen-containing metabolome of black cohosh thus far. A total of 73 mostly secondary metabolites were identified or tentatively indentified by employing a dereplication strategy that relies on the combination of accurate mass measurements and database searches supported by the general knowledge of biosynthetic pathways of natural products. Although some compounds such as amino acids, nucleosides or vitamins represent common primary plant metabolites, none of the compounds identified in this study has been previously reported from black cohosh. Several reported compounds are new natural products. Of particular significance for future research of black cohosh is the discovery of various classes of alkaloids, most notably the isoquinoline and β-carboline classes. Alkaloids are well-known bioactive plant constituents with well-established pharmacological activities and their discovery in black cohosh provides an important new direction for research on this popular plant which is used as source material for widely used botanical dietary supplements.
Acknowledgements
This work was supported by grant P50AT00155 from the Office of Dietary Supplements, the National Institute of General Medical Sciences, the Office for Research on Women’s Health and the National Center for Complementary and Alternative Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McKenna DJ, Jones K, Humphrey S, Hughes K. Black cohosh: efficacy, safety, and use in clinical and preclinical applications. Altern Ther Health Med. 2001;7:93–100. [PubMed] [Google Scholar]
- 2.Mahady GB, Parrot J, Lee C, Yun GS, Dan A. Botanical dietary supplement use in peri- and postmenopausal women. Menopause. 2003;10:65–72. doi: 10.1097/00042192-200310010-00011. [DOI] [PubMed] [Google Scholar]
- 3.Borrelli F, Ernst E. Black cohosh (Cimicifuga racemosa) for menopausal symptoms: a systematic review of its efficacy. Pharmacol Res. 2008;58:8–14. doi: 10.1016/j.phrs.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Osmers R, Friede M, Liske E, Schnitker J, Freudenstein J, Henneicke-von Zepelin HH. Efficacy and safety of isopropanolic black cohosh extract for climacteric symptoms. Obstet Gynecol. 2005;105:1074–1083. doi: 10.1097/01.AOG.0000158865.98070.89. [DOI] [PubMed] [Google Scholar]
- 5.Wuttke W, Seidlova-Wuttke D, Gorkow C. The Cimicifuga preparation BNO 1055 vs. conjugated estrogens in a double-blind placebo-controlled study: effects on menopause symptoms and bone markers. Maturitas. 2003;44(1):S67–S77. doi: 10.1016/s0378-5122(02)00350-x. [DOI] [PubMed] [Google Scholar]
- 6.Frei-Kleiner S, Schaffner W, Rahlfs VW, Bodmer C, Birkhäuser M. Cimicifuga racemosa dried ethanolic extract in menopausal disorders: a double-blind placebo-controlled clinical trial. Maturitas. 2005;51:397–404. doi: 10.1016/j.maturitas.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Kronenberg F, Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: a review of randomized, controlled trials. Ann Intern Med. 2002;137:805–813. doi: 10.7326/0003-4819-137-10-200211190-00009. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson JS, Troxel AB, Evans J, Klaus L, Vahdat L, Kinne D, Lo KM, Moore A, Rosenman PJ, Kaufman EL, Neugut AI, Grann VR. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol. 2001;19:2739–2745. doi: 10.1200/JCO.2001.19.10.2739. [DOI] [PubMed] [Google Scholar]
- 9.Newton KM, Reed SD, LaCroix AZ, Grothaus LC, Ehrlich K, Guiltinan J. Treatment of vasomotor symptoms of menopause with black cohosh, multibotanicals, soy, hormone therapy, or placebo: a randomized trial. Ann Intern Med. 2006;145:869–879. doi: 10.7326/0003-4819-145-12-200612190-00003. [DOI] [PubMed] [Google Scholar]
- 10.Liske E, Hanggi W, Henneicke-von Zepelin HH, Boblitz N, Wustenberg P, Rahlfs VW. Physiological investigation of a unique extract of black cohosh (Cimicifugae racemosae rhizoma): a 6-month clinical study demonstrates no systemic estrogenic effect. J Womens Health Gend Based Med. 2002;11:163–174. doi: 10.1089/152460902753645308. [DOI] [PubMed] [Google Scholar]
- 11.Geller SE, Shulman LP, et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: a randomized controlled trial. Menopause. 2009;16:1156–1166. doi: 10.1097/gme.0b013e3181ace49b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li JX, Yu ZY. Cimicifugae rhizoma: from origins, bioactive constituents to clinical outcomes. Curr Med Chem. 2006;13:2927–2951. doi: 10.2174/092986706778521869. [DOI] [PubMed] [Google Scholar]
- 13.Gödecke T, Nikolic D, Lankin DC, Chen SN, Powell SL, Dietz B, Bolton JL, van Breemen RB, Farnsworth NR, Pauli GF. Phytochemistry of cimicifugic acids and associated bases in Cimicifuga racemosa root extracts. Phytochem Anal. 2009;20:120–133. doi: 10.1002/pca.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gödecke T, Lankin DC, Nikolic D, Chen SN, van Breemen RB, Farnsworth NR, Pauli GF. Guanidine alkaloids and Pictet-Spengler adducts from black cohosh (Cimicifuga racemosa) J Nat Prod. 2009;72:433–437. doi: 10.1021/np8006952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabricant DS, Nikolic D, Lankin DC, Chen SN, Jaki BU, Krunic A, van Breemen RB, Fong HH, Farnsworth NR, Pauli GF. Cimipronidine, a cyclic guanidine alkaloid from Cimicifuga racemosa. J Nat Prod. 2005;68:1266–1270. doi: 10.1021/np050066d. [DOI] [PubMed] [Google Scholar]
- 16.Bottcher C, von Roepenack-Lahaye E, Schmidt J, Clemens S, Scheel D. Analysis of phenolic choline esters from seeds of Arabidopsis thaliana and Brassica napus by capillary liquid chromatography/electrospray- tandem mass spectrometry. J Mass Spectrom. 2009;44:466–476. doi: 10.1002/jms.1522. [DOI] [PubMed] [Google Scholar]
- 17.Karapetyan HA, Antipin MY, Sukiasyan RP, Petrosyan AM. Formyl-L-arginine monohydrate. J Mol Struct. 2007;831:90–96. [Google Scholar]
- 18.Somei M, Teranishi S, Yamada K, Yamada F. The chemistry of indoles. CVII. A novel synthesis of 3,4,5,6-tetrahydro-7-hydroxy-1H-azepino[5,4,3-cd]indoles and a new finding on Pictet-Spengler reaction. Chem Pharm Bull. 2001;49:1159–1165. doi: 10.1248/cpb.49.1159. [DOI] [PubMed] [Google Scholar]
- 19.Brossi A, Focella A, Teitel S. Alkaloids in mammalian tissues. 3. Condensations of L-tryptophan and L-5-hydroxytryptophan with formaldehyde and acetaldehyde. J Med Chem. 1973;16:418–420. doi: 10.1021/jm00262a027. [DOI] [PubMed] [Google Scholar]
- 20.Yamano T, Miura R, Kanashiro M, Uemura T. Possible occurrence of a β-carboline pathway in the oxidative catabolism of 5-hydroxytryptamine: chemical approach and structure determination of a yellow substance and related β-carboline derivatives. Bioorg Chem. 1988;16:189–205. [Google Scholar]
- 21.Powell SL, Godecke T, Nikolic D, Chen SN, Ahn S, Dietz B, Farnsworth NR, van Breemen RB, Lankin DC, Pauli GF, Bolton JL. In vitro serotonergic activity of black cohosh and identification of N(omega)-methylserotonin as a potential active constituent. J Agric Food Chem. 2008;56:11718–11726. doi: 10.1021/jf803298z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabricant DS. Ph.D. Thesis. Chicago: The University of Illinois at Chicago; 2006. Pharmacognostic investigation of black cohosh (Cimicifuga racemosa, L. Nutt.) [Google Scholar]
- 23.van Breemen RB, Liang W, et al. Pharmacokinetics of 23-epi 26-deoxyactein in women after oral administration of a standardized extract of black cohosh. Clin Pharmacol Ther. 2010;87:219–225. doi: 10.1038/clpt.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H, Fabricant DS, Piersen CE, Bolton JL, Pezzuto JM, Fong H, Totura S, Farnsworth NR, Constantinou AI. A preliminary RAPD-PCR analysis of Cimicifuga species and other botanicals used for women's health. Phytomedicine. 2002;9:757–762. doi: 10.1078/094471102321621403. [DOI] [PubMed] [Google Scholar]
- 25.Horai H, Arita H, et al. MassBank: a public repository for sharing mass spectral data for life sciences. J Mass Spectrom. 2010;45:703–714. doi: 10.1002/jms.1777. [DOI] [PubMed] [Google Scholar]
- 26.Bristow AW, Webb KS, Lubben AT, Halket J. Reproducible product-ion tandem mass spectra on various liquid chromatography/mass spectrometry instruments for the development of spectral libraries. Rapid Commun Mass Spectrom. 2004;18:1447–1454. doi: 10.1002/rcm.1492. [DOI] [PubMed] [Google Scholar]
- 27.Sumner LW, Amberg A, et al. Proposed minimum reporting standards for chemical analysis. Metabolomics. 2007;3:211–221. doi: 10.1007/s11306-007-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dookeran N, Yalcin T, Harrison AG. Fragmentation reactions of protonated alfa-amino acids. J Mass Spectrom. 1996;31:500–508. [Google Scholar]
- 29.Vishwanathan K, Tackett RL, Stewart JT, Bartlett MG. Determination of arginine and methylated arginines in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Biomed Sci Appl. 2000;748:157–166. doi: 10.1016/s0378-4347(00)00399-6. [DOI] [PubMed] [Google Scholar]
- 30.Csonka IP, Paizs B, Suhai S. Modeling of the gas-phase ion chemistry of protonated arginine. J Mass Spectrom. 2004;39:1025–1035. doi: 10.1002/jms.660. [DOI] [PubMed] [Google Scholar]
- 31.Matsuda H, Suzuki Y. gamma-guanidinobutyraldehyde dehydrogenase of Vicia faba leaves. Plant Physiol. 1984;76:654–657. doi: 10.1104/pp.76.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reuter G, Diehl HJ. Guanidine derivatives in Leonurus sibiricus L. Pharmazie. 1971;26:777. [PubMed] [Google Scholar]
- 33.Wood KV, Bonham CC, Miles D, Rothwell AP, Peel G, Wood BC, Rhodes D. Characterization of betaines using electrospray MS/MS. Phytochemistry. 2002;59:759–765. doi: 10.1016/s0031-9422(02)00049-3. [DOI] [PubMed] [Google Scholar]
- 34.Tan CHA BG. Ergothioneine and hercynine in Hevea brasiliensis latex. Phytochemistry. 1968;7:109–118. [Google Scholar]
- 35.Stennert A, Maier HG. Trigonelline in coffee II. Content of green, roasted and instant coffee. Z Lebensm Unters Forsch. 1994;199:198–200. doi: 10.1007/BF01193443. [DOI] [PubMed] [Google Scholar]
- 36.Allred KF, Yackley KM, Vanamala J, Allred CD. Trigonelline is a novel phytoestrogen in coffee beans. J Nutr. 2009;139:1833–1838. doi: 10.3945/jn.109.108001. [DOI] [PubMed] [Google Scholar]
- 37.Kuhnert N, Jaiswal R, Matei MF, Sovdat T, Deshpande S. How to distinguish between feruloyl quinic acids and isoferuloyl quinic acids by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2010;24:1575–1582. doi: 10.1002/rcm.4537. [DOI] [PubMed] [Google Scholar]
- 38.Yahagi T, Yamashita Y, Daikonnya A, Wu JB, Kitanaka S. New feruloyl tyramine glycosides from Stephania hispidula Yamamoto. Chem Pharm Bull. 2010;58:415–417. doi: 10.1248/cpb.58.415. [DOI] [PubMed] [Google Scholar]
- 39.Li C-J, Chen D-H, Xiao P-G, Hong S-L. Chemical constituents of traditional Chinese drug "Sheng-ma" (Cimicifuga dahurica) Acta Pharm Sin. 1994;52:296–300. [PubMed] [Google Scholar]
- 40.Dostert P, Benedetti MS, Bellotti V, Allievi C, Dordain G. Biosynthesis of salsolinol, a tetrahydroisoquinoline alkaloid, in healthy subjects. J Neural Transm Gen Sect. 1990;81:215–223. doi: 10.1007/BF01245043. [DOI] [PubMed] [Google Scholar]
- 41.Mravec B. Salsolinol, a derivate of dopamine, is a possible modulator of catecholaminergic transmission: a review of recent developments. Physiol Res. 2006;55:353–364. doi: 10.33549/physiolres.930810. [DOI] [PubMed] [Google Scholar]
- 42.Duncan MW, Smythe GA, Nicholson MV, Clezy PS. Comparison of high-performance liquid chromatography with electrochemical detection and gas chromatography-mass fragmentography for the assay of salsolinol, dopamine and dopamine metabolites in food and beverage samples. J Chromatogr. 1984;336:199–209. doi: 10.1016/s0378-4347(00)85142-7. [DOI] [PubMed] [Google Scholar]
- 43.Riggin RM, Kissinger PT. Identification of salsolinol as a phenolic component in powdered cocoa and cocoa-based products. J Agric Food Chem. 1976;24:900. doi: 10.1021/jf60206a043. [DOI] [PubMed] [Google Scholar]
- 44.Riggin RM, McCarthy MJ, Kissinger PT. Identification of salsolinol as a major dopamine metabolite in the banana. J Agric Food Chem. 1976;24:189–191. doi: 10.1021/jf60203a027. [DOI] [PubMed] [Google Scholar]
- 45.Lee J, Ramchandani VA, Hamazaki K, Engleman EA, McBride WJ, Li TK, Kim HY. A critical evaluation of influence of ethanol and diet on salsolinol enantiomers in humans and rats. Alcohol Clin Exp Res. 34:242–250. doi: 10.1111/j.1530-0277.2009.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melzig MF, Putscher I, Henklein P, Haber H. In vitro pharmacological activity of the tetrahydroisoquinoline salsolinol present in products from Theobroma cacao L. like cocoa and chocolate. J Ethnopharmacol. 2000;73:153–159. doi: 10.1016/s0378-8741(00)00291-9. [DOI] [PubMed] [Google Scholar]
- 47.Musshoff F, Lachenmeier DW, Kroener L, Schmidt P, Dettmeyer R, Madea B. Simultaneous gas chromatographic-mass spectrometric determination of dopamine, norsalsolinol and salsolinol enantiomers in brain samples of a large human collective. Cell Mol Biol. 2003;49:837–849. [PubMed] [Google Scholar]
- 48.Musshoff F, Daldrup T, Bonte W, Leitner A, Lesch OM. Salsolinol and norsalsolinol in human urine samples. Pharmacol Biochem Behav. 1997;58:545–550. doi: 10.1016/s0091-3057(97)00251-7. [DOI] [PubMed] [Google Scholar]
- 49.Facchini PJ. Alkaloids biosynthesis in plants: biochemistry, cell Biology, molecular regulation, and metabolic engineering applications. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:29–66. doi: 10.1146/annurev.arplant.52.1.29. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt J, Raith K, Boettcher C, Zenk MH. Analysis of benzylisoquinoline-type alkaloids by electrospray tandem mass spectrometry and atmospheric pressure photoionization. Eur J Mass Spectrom. 2005;11:325–333. doi: 10.1255/ejms.745. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt J, Boettcher C, Kuhnt C, Kutchan TM, Zenk MH. Poppy alkaloid profiling by electrospray tandem mass spectrometry and electrospray FT-ICR mass spectrometry after [ring-13C6]-tyramine feeding. Phytochemistry. 2007;68:189–202. doi: 10.1016/j.phytochem.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 52.Stevigny C, Jiwan JL, Rozenberg R, de Hoffmann E, Quetin-Leclercq J. Key fragmentation patterns of aporphine alkaloids by electrospray ionization with multistage mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:523–528. doi: 10.1002/rcm.1343. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Shi Q, Shi P, Zhang W, Cheng Y. Characterization of isoquinoline alkaloids, diterpenoids and steroids in the Chinese herb Jin-Guo-Lan (Tinospora sagittata and Tinospora capillipes) by high-performance liquid chromatography/electrospray ionization with multistage mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:2328–2342. doi: 10.1002/rcm.2593. [DOI] [PubMed] [Google Scholar]
- 54.Henion JD, Mordehai AV, Cai J. Quantitative capillary electrophoresis-ion spray mass spectrometry on a benchtop ion trap for the determination of isoquinoline alkaloids. Anal Chem. 1994;66:2103–2109. [Google Scholar]
- 55.Guo Y, Kojima K, Lin L, Fu X, Zhao C, Hatano K, Chen Y, Ogihara Y. A new N-methyltetrahydroprotoberberine alkaloid from Tinospora hainanesis. Chem Pharm Bull. 1999;47:287–289. [Google Scholar]
- 56.Powell SL, Gödecke T, Nikolic D, Chen SN, Ahn S, Dietz B, Farnsworth NR, van Breemen RB, Lankin DC, Pauli GF, Bolton JL. In vitro serotonergic activity of black cohosh and identification of N(omega)-methylserotonin as a potential active constituent. J Agric Food Chem. 2008;56:11718–11726. doi: 10.1021/jf803298z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quan-Wen LC-H T, Shi-Jin Q, Xiao F, Da-Yuan Z. Chemical constituents of Evodia fargesii Dode. Zhongguo Tianran Yaowu. 2004;4:25–29. [Google Scholar]
- 58.Bjorklund A, Falck B, Lindvall O, Svensson LA. New aspects on reaction mechanisms in the formaldehyde histofluorescence method for monoamines. J Histochem Cytochem. 1973;21:17–25. doi: 10.1177/21.1.17. [DOI] [PubMed] [Google Scholar]
- 59.Yu AM, Idle JR, Herraiz T, Kupfer A, Gonzalez FJ. Screening for endogenous substrates reveals that CYP2D6 is a 5-methoxyindolethylamine O-demethylase. Pharmacogenetics. 2003;13:307–319. doi: 10.1097/01.fpc.0000054094.48725.b7. [DOI] [PubMed] [Google Scholar]
- 60.Herraiz T. Identification and occurrence of beta-carboline alkaloids in raisins and inhibition of monoamine oxidase (MAO) J Agric Food Chem. 2007;55:8534–8540. doi: 10.1021/jf0719151. [DOI] [PubMed] [Google Scholar]
- 61.Herraiz T. Tetrahydro-beta-carboline-3-carboxylic acid compounds in fish and meat: possible precursors of co-mutagenic beta-carbolines norharman and harman in cooked foods. Food Addit Contam. 2000;17:859–866. doi: 10.1080/026520300420439. [DOI] [PubMed] [Google Scholar]
- 62.Tsai I-LF S-C, Ishikawa T, Chang C-T, Chen I-S. N-cyclohexyl amides and a dimeric coumarin from formosan Todalia asiatica. Phytochemistry. 1997;44:1383–1386. [Google Scholar]
- 63.Spiering MJ, Urban LA, Nuss DL, Gopalan V, Stoltzfus A, Eisenstein E. Gene identification in black cohosh (Actaea racemosa L.): expressed sequence tag profiling and genetic screening yields candidate genes for production of bioactive secondary metabolites. Plant Cell Rep. 2011;30:613–629. doi: 10.1007/s00299-010-0979-5. [DOI] [PubMed] [Google Scholar]
- 64.Xu LF, Chu WJ, Qing XY, Li S, Wang XS, Qing GW, Fei J, Guo LH. Protopine inhibits serotonin transporter and noradrenaline transporter and has the antidepressant-like effect in mice models. Neuropharmacology. 2006;50:934–940. doi: 10.1016/j.neuropharm.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Xu Q, Jin RL, Wu YY. Opioid, calcium, and adrenergic receptor involvement in protopine analgesia. Zhongguo Yao Li Xue Bao. 1993;14:495–500. [PubMed] [Google Scholar]
- 66.Ustunes L, Laekeman GM, Gozler B, Vlietinck AJ, Ozer A, Herman AG. In vitro study of the anticholinergic and antihistaminic activities of protopine and some derivatives. J Nat Prod. 1988;51:1021–1022. doi: 10.1021/np50059a043. [DOI] [PubMed] [Google Scholar]
- 67.Gurley B, Hubbard MA, Williams DK, Thaden J, Tong Y, Gentry WB, Breen P, Carrier DJ, Cheboyina S. Assessing the clinical significance of botanical supplementation on human cytochrome P450 3A activity: comparison of a milk thistle and black cohosh product to rifampin and clarithromycin. J Clin Pharmacol. 2006;46:201–213. doi: 10.1177/0091270005284854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rhyu MR, Lu J, Webster DE, Fabricant DS, Farnsworth NR, Wang ZJ. Black cohosh (Actaea racemosa, Cimicifuga racemosa) behaves as a mixed competitive ligand and partial agonist at the human mu opiate receptor. J Agric Food Chem. 2006;54:9852–9857. doi: 10.1021/jf062808u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cannon JG, Flaherty PT, Ozkutlu U, Long JP. A-ring ortho-disubstituted aporphine derivatives as potential agonists or antagonists at serotonergic 5-HT1A receptors. J Med Chem. 1995;38:1841–1845. doi: 10.1021/jm00011a002. [DOI] [PubMed] [Google Scholar]
- 70.Gafner S, Dietz BM, McPhail KL, Scott IM, Glinski JA, Russell FE, McCollom MM, Budzinski JW, Foster BC, Bergeron C, Rhyu MR, Bolton JL. Alkaloids from Eschscholzia californica and their capacity to inhibit binding of [3H]8-hydroxy-2-(di-N-propylamino)tetralin to 5-HT1A receptors in vitro. J Nat Prod. 2006;69:432–435. doi: 10.1021/np058114h. [DOI] [PubMed] [Google Scholar]
- 71.Burdette JE, Liu J, Chen SN, Fabricant DS, Piersen CE, Barker EL, Pezzuto JM, Mesecar A, Van Breemen RB, Farnsworth NR, Bolton JL. Black cohosh acts as a mixed competitive ligand and partial agonist of the serotonin receptor. J Agric Food Chem. 2003;51:5661–5670. doi: 10.1021/jf034264r. [DOI] [PubMed] [Google Scholar]
- 72.Zhao Q, Zhao Y, Wang K. Antinociceptive and free radical scavenging activities of alkaloids isolated from Lindera angustifolia Chen. J Ethnopharmacol. 2006;106:408–413. doi: 10.1016/j.jep.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 73.Chen KS, Ko FN, Teng CM, Wu YC. Antiplatelet of vasorelaxing actions of some benzylisoquinoline and phenanthrene alkaloids. J Nat Prod. 1996;59:531–534. doi: 10.1021/np960354x. [DOI] [PubMed] [Google Scholar]
- 74.Chen KS, Ko FN, Teng CM, Wu YC. Antiplatelet and vasorelaxing actions of some aporphinoids. Planta Med. 1996;62:133–136. doi: 10.1055/s-2006-957835. [DOI] [PubMed] [Google Scholar]
- 75.Li J, Gödecke T, Chen SN, Imai A, Lankin DC, Farnsworth NR, Pauli GF, van Breemen RB, Nikolic D. In vitro metabolic interactions between black cohosh (Cimicifuga racemosa) and tamoxifen via inhibition of cytochromes P450 2D6 and 3A4. Xenobiotica. 2011 doi: 10.3109/00498254.2011.603385. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clifford MN. Chlorogenic acids and other cinnamates-nature, occurrence, dietary burden, absorption and metabolism. J Sci Food Agric. 2000;80:1033–1043. [Google Scholar]
- 77.Clifford MN, Knight S. The cinnamoyl-amino acid conjugates from green robusta coffee beans. Food Chem. 2004;87:457–463. [Google Scholar]
- 78.Shahidi F, Chandrasekara A. Hydroxycinnamates and their in vitro and in vivo antioxidant activities. Phytochem Rev. 2010;9:147–170. [Google Scholar]