Summary
The pathogenesis of canine mast cell tumour (MCT) remains unknown. Moreover, therapeutic options are limited and resistance to targeted drugs and recurrences are common, necessitating the identification of additional cellular targets for therapy. In this study we investigated the expression of phosphorylated AKT protein in 25 archival canine MCT samples by immunohistochemistry and examined the correlation between the immunohistochemical scores and histopathological tumour grades. AKT protein was detected in all of the samples and 24 of the 25 samples expressed the phosphorylated form of the protein, albeit with variable intensity. However, when the immunohistochemical scores of weak, intermediate and strong labelling were compared with the histopathological grades of I to III, there was no strong correlation. This study suggests that canine MCT cells have activated AKT and indicates the need for further research on the role of the AKT protein and the possibility of targeting the AKT signalling pathway in MCTs.
Keywords: dog, mast cell tumour, AKT protein
Mast cell tumours (MCTs) are among the most common cutaneous tumours in dogs, representing 16–21% of all skin tumours (Finnie and Bostock, 1979; Bostock et al., 1989; O'Keefe, 1990; Goldschmidt and Hendrick, 2002). Treatment of MCTs involves radical surgery and chemotherapy with various drugs. Current chemotherapeutic options for MCTs are limited and recurrences are common. The most common chemotherapeutic drugs used against MCTs include cyclophosphamide, lomustine (nitrosourea compound), vincristine and vinblastine (both vinca alkaloids). More recent therapeutic options include molecularly targeted tyrosine kinase inhibitors (e.g. Toceranib™) (Kobie et al., 2007; Hahn et al., 2008).
The c-kit gene is mutated to generate tandem internal duplications or functional activation of the protein in about 40% of canine MCTs. The molecular pathology in the remaining proportion of cases remains largely unknown (Welle et al., 2008). The AKT protein (originally described from an AKR mouse T-cell lymphoma virus), also known as protein kinase B (PKB), is a signalling molecule involved in a number of cellular functions (Vivanco and Sawyers, 2002; Meier et al., 2005; Carnero, 2010). There are three family members of AKT (AKT1–3) in cells, but AKT1 is the most important and the most studied member of the family. The human AKT1 protein is activated by phosphorylation of threonine 308 and serine 473 residues. Phosphorylated AKT mediates several pathways that ultimately aid in cell survival and evasion of apoptosis (Vivanco and Sawyers, 2002; Meier et al., 2005; Carnero, 2010). Since AKT phosphorylation is a survival pathway for tumour cells, the use of AKT inhibitors could be considered as an alternative or adjunct option for the treatment of MCTs. Several inhibitors of the AKT protein or pathway are currently undergoing clinical trials in man (Yap et al., 2008; Engelman, 2009). The aim of this study was to examine immunohistochemically the expression and activation of the AKT1 protein (hereafter referred to as AKT) in canine MCTs and to determine whether there is a correlation between AKT activation and histopathological grade of MCT.
Twenty-five formalin-fixed and paraffin wax-embedded samples were obtained from the archives of the Department of Pathobiology, Tuskegee University School of Veterinary Medicine. The MCT cases including tumour location, grade, as well as breed, sex and age of the patient are shown in Table 1. Prior to the use of these samples for this study, the tumours had been diagnosed, confirmed and histopathologically graded as MCTs (Goldschmidt and Hendrick, 2002) by veterinary pathologists at Tuskegee School of Veterinary Medicine. For antigen retrieval, dewaxed slides were drained onto a paper towel and placed into a staining dish containing 250ml of Target Retrieval Solution (Dako, Santa Barbra, California, USA). A temperature-monitoring strip was placed across the staining dish and the dish was then placed into a pressure cooker set on high for 40 min to reach a maximum temperature of 120°C. The slides were allowed to cool for 20 min. The slides were then rinsed with 4–5 changes of distilled water to remove any residual Target Retrieval Solution. Immunohistochemistry (IHC) for detection of AKT was performed using antibodies specific for pan-AKT (C67E7) and phospho-AKT (D9E), both obtained from Cell Signaling Technologies (Boston, Massachusetts, USA), at a 1 in 50 dilution in commercial antibody diluent (Cell Marque®, Rocklin, California, USA). Sections were incubated with antibody overnight using Autostainer Plus® (Dako). Polyscan DAB (Cell Marque) was used as a substrate and the slides were counterstained with haematoxylin. Control slides were incubated without primary antibody and processed similarly to the other samples. Additionally one slide from each of the three stages was incubated with non-immune rabbit serum as a negative control. Evaluation of the IHC was performed subjectively. Sections stained by haematoxylin and eosin (HE) were examined in parallel. All slides subjected to IHC were examined at the same time to identify those with the weakest and the most intense labelling. Tumour tissue on the entire slide was examined and scored for intensity. As all of the samples expressed AKT protein, no scoring was done for total AKT protein expression. For slides incubated with the anti-phopho-AKT antibody, a score of zero was assigned to slides with no identifiable labelling. Based on the overall intensity, weak positive, intermediate positive and strong positive labelling was identified by two investigators (SR and TS), and assigned scores of +1, +2 and +3, respectively. Correlation analyses were performed with SAS statistical software.
Table 1.
Description of cases evaluated in this study
| Identification | Location | Grade | Breed | Age (yr) | Sex |
|---|---|---|---|---|---|
| B08-026 | Hip | I | Labrador | 3 | M |
| B08-058 | Ventral Abdomen | I | Labrador | 10 | FN |
| B08-068 | Eyelid | II | Rottweiler | 12 | FN |
| B08-077 | Right hindlimb | I | Mixed breed | 7 | F |
| B08-097 | Left hind digit | I | Mixed breed | 5 | MN |
| B08-100 | Metacarpal pad | III | Pit-bull terrier | 11 | M |
| B08-124 | Ventral abdomen | I | Mixed breed | 10 | MN |
| B08-128 | Ventral neck | I | Terrier mixed | 3 | MN |
| B09-004 | Cranial abdomen | II | Mixed breed | 8 | FN |
| B09-006 | Perianal region | I | Boxer | 7 | FN |
| B09-018 | Ventral perianal region | II | Pug | 11 | MN |
| B09-030 | Skin unspecified | II | Pit-bull terrier | 10 | MN |
| B09-051 | Preputial skin | II | Basset hound | 11 | M |
| B09-058 | Right thigh | I | Pit-bull terrier | 4 | MN |
| B09-063 | Hock | III | Mixed breed | 7 | MN |
| B09-077 | Left caudal flank | I | Basset hound | 11 | FN |
| B09-093 | Neck | II | Boxer | 6 | MN |
| B10-003 | Thoracic inlet | II | Boxer | 8 | FN |
| B10-021 | Right thigh | III | Toy poodle | 10 | FN |
| B10-046 | Dorsal thorax | II | Mixed breed | 8 | FN |
| B10-079 | Ventral thorax | II | Boxer | 6 | MN |
| B10-090 | Dorsolateral abdomen | II | Boxer | 3 | FN |
| B10-094 | Dorsolateral thorax | II | Bernese Mountain dog | 3 | F |
| B10-110 | Ventral abdomen | II | Labrador | 13 | FN |
| B10-126 | Dorsal pelvic area | III | Mixed breed | 8 | FN |
F, female; FN, female neutered; M, male; MN, male neutered
Nine of the 25 samples were of histological grade I, 12 were of grade II and four were Grade III. The age of the affected dogs ranged from 3–13 years (mean age 7.52 years). Thirteen dogs were female and 11 of these were neutered. Twelve dogs were male and eight of these were neutered. Seven of the dogs were of mixed breed and there were five boxers in the study group.
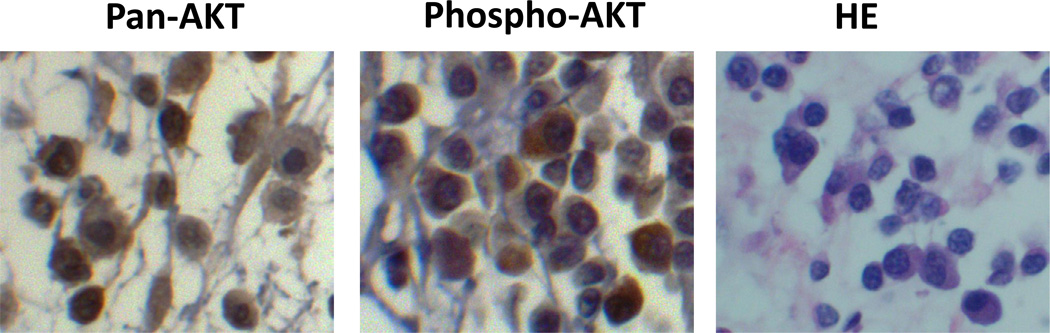
Immunohistochemical labelling intensity was generally uniform for the entire tumour section on each slide, although individual cells within the tumour showed some variation in the degree of labelling. AKT expression was both cytoplasmic and nuclear, while phospho-AKT labelling was predominantly cytoplasmic (Fig. 1). Labelling intensity scores are summarized in Table 2. All of the 25 samples were strongly labelled for the AKT protein, and therefore samples were not scored for AKT expression. Since the AKT protein may be present in a cell without significant activation, we mainly focused on the status of active AKT as indicated by S473 phosphorylation. Only a single sample did not express phospho-AKT. Of the remaining 24 samples, five had an intensity score of +3, eight had an intensity score of +2 and 11 had a score of +1.
Fig. 1.
Immunohistochemical localization of the AKT and phospho-AKT proteins in MCT cells. Left and middle panels show MCT cells labelled with anti-pan-AKT antibody (left) or with anti-phospho-AKT antibody (middle). A microphotograph from a section of the same tumour stained with HE is shown in the right panel. ×400.
Table 2.
Phospho-AKT immunohistochemical intensity scores
| Identification | MCT grade (at diagnosis) |
IHC score (current study) |
|---|---|---|
| B09-006 | I | 0 |
| B08-026 | I | +1 |
| B08-058 | I | +1 |
| B09-018 | II | +1 |
| B09-030 | II | +1 |
| B09-058 | I | +1 |
| B09-063 | III | +1 |
| B09-077 | I | +1 |
| B09-093 | II | +1 |
| B10-003 | II | +1 |
| B10-079 | II | +1 |
| B10-094 | II | +1 |
| B08-077 | I | +2 |
| B08-097 | I | +2 |
| B08-124 | I | +2 |
| B09-051 | II | +2 |
| B10-021 | III | +2 |
| B10-041 | II | +2 |
| B10-090 | II | +2 |
| B10-110 | II | +2 |
| B08-068 | II | +3 |
| B08-100 | III | +3 |
| B08-128 | I | +3 |
| B09-004 | II | +3 |
| B10-126 | III | +3 |
MCT, mast cell tumour; IHC, immunohistochemistry
There was no significant correlation between the IHC score and the MCT grade (Pearson correlation coefficient = 0.30). Similarly, no correlation was found between the IHC score and the age of the dog at the time of clinical presentation (Pearson correlation coefficient= −0.07). An increased sample size may be needed to identify such correlations more definitively.
The results of this study demonstrate that most canine MCTs express active AKT. Since activated AKT provides survival signals to cells, we hypothesize that MCT cell survival may be partially dependent on this signal transduction pathway. Studies have shown that the c-KIT pathway is involved in the pathogenesis of MCTs (London et al., 1996, 1999). KIT is a member of the tyrosine kinase family of growth factor receptors and is expressed by a variety of haemopoietic cells including mast cells. Stem cell factor (SCF)-dependent activation of KIT is critical for mast cell homeostasis and function (Kent et al., 2008). However, when KIT is activated inappropriately, survival, multiplication and accumulation of mast cells in tissues results in disorders such as mastocytosis in man and MCT in animals (Akin and Metcalfe, 2004; Jensen et al., 2008).
Up to 40% of canine MCTs express a mutated or internally tandem duplicated form of KIT (London et al., 1999; Ma et al., 1999; Downing et al., 2002; Zemke et al., 2002; Jones et al., 2004; Riva et al., 2005; Webster et al., 2006) and some of these mutant forms can be treated by targeted drugs like tyrosine kinase inhibitors (London and Seguin, 2003; Gleixner et al., 2007; Kobie et al., 2007; Peter et al., 2010; Yamada et al., 2011). However, the molecular mechanisms behind the pathology of a larger proportion of MCTs (more than 60% of them) remain unknown (Welle et al., 2008). It can be assumed that signalling mechanisms other than the KIT pathway may be active in a portion of MCTs (Gleixner et al., 2011) and the AKT pathway may be an alternative candidate. Moreover, cancer cells may not rely entirely on one pathway and may have alternative networks of survival signalling. Currently, to what extent activated phospho-AKT supports the survival of MCT cells is unknown. Studies are needed to examine the role AKT signalling pathway in MCT cell survival and to determine whether AKT inhibitory agents can be considered for mainstream or adjuvant treatment of canine MCT.
Prior to this study, the AKT signal transduction pathway had not been studied in mast MCTs. Therefore, it was not known to what extent the AKT protein contributes to MCT pathology. Moreover, the KIT signalling pathway may also converge and crosstalk with the AKT pathway (Serve et al., 1995; Vosseller et al., 1997; Timokhina et al., 1998; Kent et al., 2008; Brandwein et al., 2011), raising the possibility that the latter may also play a role in MCT pathobiology.
Overall, despite the consistent presence of abundant AKT in MCT cells in all of the samples examined, the intensity of the phospho-AKT IHC labelling appeared to be low. Some factors to be considered may be the suitability of the antibody for paraffin wax-embedded tissues and the compatibility of antigen retrieval procedure with the antibody. Since the pan-AKT antibody does not differentiate between inactive and active AKT, it labels all AKT proteins in the cells. The discord between pan-AKT and phospho-AKT IHC labelling intensities should be examined further, because as a signalling protein kinase, abundantly available AKT may be readily activated under circumstances that initiate an upstream signalling cascade.
It is also possible that MCT cell survival mechanisms involve complex signalling events that not only use the AKT pathway, but perhaps also involve multiple hallmarks of cancer and the supportive microenvironment (Hanahan and Weinberg, 2011). Hence, it will be interesting to examine the sensitivity of MCT cells treated with an AKT inhibitor in vitro and in vivo. The outcome of such studies would dictate whether inhibition of the AKT pathway would benefit patients with mast cell disorders. Although the possibility exists that MCT cells use various mechanisms for survival, it will be of interest to determine whether disruption of the AKT pathway would force MCT cells to undergo apoptosis. Moreover, the AKT pathway has recently been considered as one of the key targets for cancer therapy and many studies are currently being conducted on how to inhibit this signalling pathway (Yap et al., 2008; Engelman, 2009). Currently, there are no studies or clinical trials that target MCTs using AKT inhibitors.
Although there was no correlation between MCT histological grade and phospho-AKT IHC scores, the degree of AKT-phosphorylation may be better correlated with other parameters such as cell proliferation, mitotic index or other cell proliferation indices. These parameters should to be evaluated to determine the correlation of phospho-AKT with MCT pathology. In conclusion, the results of the present study have suggested that the AKT signalling pathway may be active in canine MCTs. While further in-vitro studies are needed to corroborate the results, the current data suggest that using an AKT inhibitor could be a future option in the treatment of canine MCTs that are not dependent on KIT mutations for pathogenesis.
Acknowledgments
We thank Dr E. Graham for technical help and review of the manuscript and Mrs. P. Adams and Mrs. E. Williams for IHC. Research in the laboratory of TS is supported by NIH/NCI/NIGMS grant number SC2CA138178. The NCRR/RCMI imaging core lab facility at Tuskegee University is supported by NCRR/RCMI grant #G12RR003059. The funding agencies had no role in the initiation, execution or analysis of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no conflict of interest.
References
- Akin C, Metcalfe DD. The biology of Kit in disease and the application of pharmacogenetics. Journal of Allergy and Clinical Immunology. 2004;114:13–19. doi: 10.1016/j.jaci.2004.04.046. [DOI] [PubMed] [Google Scholar]
- Bostock DE, Crocker J, Harris K, Smith P. Nucleolar organiser regions as indicators of post-surgical prognosis in canine spontaneous mast cell tumours. British Journal of Cancer. 1989;59:915–918. doi: 10.1038/bjc.1989.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandwein JM, Hedley DW, Chow S, Schimmer AD, Yee KW, et al. A phase I/II study of imatinib plus reinduction therapy for c-kit-positive relapsed/refractory acute myeloid leukemia: inhibition of Akt activation correlates with complete response. Leukemia. 2011;25:945–952. doi: 10.1038/leu.2011.34. [DOI] [PubMed] [Google Scholar]
- Carnero A. The PKB/AKT pathway in cancer. Current Pharmaceutical Design. 2010;16:34–44. doi: 10.2174/138161210789941865. [DOI] [PubMed] [Google Scholar]
- Downing S, Chien MB, Kass PH, Moore PE, London CA. Prevalence and importance of internal tandem duplications in exons 11 and 12 of c-kit in mast cell tumors of dogs. American Journal of Veterinary Research. 2002;63:1718–1723. doi: 10.2460/ajvr.2002.63.1718. [DOI] [PubMed] [Google Scholar]
- Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nature Reviews Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- Finnie JW, Bostock DE. Skin neoplasia in dogs. Australian Veterinary Journal. 1979;55:602–604. doi: 10.1111/j.1751-0813.1979.tb07068.x. [DOI] [PubMed] [Google Scholar]
- Gleixner KV, Mayerhofer M, Cerny-Reiterer S, Hormann G, Rix U, et al. KIT-D816V-independent oncogenic signaling in neoplastic cells in systemic mastocytosis: role of Lyn and Btk-activation and disruption by dasatinib and bosutinib. Blood. 2011;118:1885–1898. doi: 10.1182/blood-2010-06-289959. [DOI] [PubMed] [Google Scholar]
- Gleixner KV, Rebuzzi L, Mayerhofer M, Gruze A, Hadzijusufovic E, et al. Synergistic antiproliferative effects of KIT tyrosine kinase inhibitors on neoplastic canine mast cells. Experimental Haematology. 2007;35:1510–1521. doi: 10.1016/j.exphem.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Goldschmidt MH, Hendrick MJ. Tumors of the skin and soft tissues. In: Meuten DJ, editor. Tumors in Domestic Animals. Ames: Iowa State Press; 2002. pp. 105–107. [Google Scholar]
- Hahn KA, Ogilvie G, Rusk T, Devauchelle P, Leblanc A, et al. Masitinib is safe and effective for the treatment of canine mast cell tumors. Journal of Veterinary Internal Medicine. 2008;22:1301–1309. doi: 10.1111/j.1939-1676.2008.0190.x. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Jensen BM, Akin C, Gilfillan AM. Pharmacological targeting of the KIT growth factor receptor: a therapeutic consideration for mast cell disorders. British Journal of Pharmacology. 2008;154:1572–1582. doi: 10.1038/bjp.2008.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CL, Grahn RA, Chien MB, Lyons LA, London CA. Detection of c-kit mutations in canine mast cell tumors using fluorescent polyacrylamide gel electrophoresis. Journal of Veterinary Diagnostic Investigation. 2004;16:95–100. doi: 10.1177/104063870401600201. [DOI] [PubMed] [Google Scholar]
- Kent D, Copley M, Benz C, Dykstra B, Bowie M, et al. Regulation of hematopoietic stem cells by the steel factor/KIT signaling pathway. Clinical Cancer Research. 2008;14:1926–1930. doi: 10.1158/1078-0432.CCR-07-5134. [DOI] [PubMed] [Google Scholar]
- Kobie K, Kawabata M, Hioki K, Tanaka A, Matsuda H, et al. The tyrosine kinase inhibitor imatinib [STI571] induces regression of xenografted canine mast cell tumors in SCID mice. Research in Veterinary Science. 2007;82:239–241. doi: 10.1016/j.rvsc.2006.06.006. [DOI] [PubMed] [Google Scholar]
- London CA, Galli SJ, Yuuki T, Hu ZQ, Helfand SC, et al. Spontaneous canine mast cell tumors express tandem duplications in the proto-oncogene c-kit. Experimental Haematology. 1999;27:689–697. doi: 10.1016/s0301-472x(98)00075-7. [DOI] [PubMed] [Google Scholar]
- London CA, Kisseberth WC, Galli SJ, Geissler EN, Helfand SC. Expression of stem cell factor receptor (c-kit) by the malignant mast cells from spontaneous canine mast cell tumours. Journal of Comparative Pathology. 1996;115:399–414. doi: 10.1016/s0021-9975(96)80074-0. [DOI] [PubMed] [Google Scholar]
- London CA, Seguin B. Mast cell tumors in the dog. Veterinary Clinics of North America: Small Animal Practice. 2003;33:473–489. doi: 10.1016/s0195-5616(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Ma Y, Longley BJ, Wang X, Blount JL, Langley K, et al. Clustering of activating mutations in c-KIT's juxtamembrane coding region in canine mast cell neoplasms. Journal of Investigative Dermatology. 1999;112:165–170. doi: 10.1046/j.1523-1747.1999.00488.x. [DOI] [PubMed] [Google Scholar]
- Meier F, Schittek B, Busch S, Garbe C, Smalley K, et al. The RAS/RAF/MEK/ERK and PI3K/AKT signaling pathways present molecular targets for the effective treatment of advanced melanoma. Frontiers in Biosciences. 2005;10:2986–3001. doi: 10.2741/1755. [DOI] [PubMed] [Google Scholar]
- O'Keefe DA. Canine mast cell tumors. Veterinary Clinics of North America: Small Animal Practice. 1990;20:1105–1115. doi: 10.1016/s0195-5616(90)50087-x. [DOI] [PubMed] [Google Scholar]
- Peter B, Hadzijusufovic E, Blatt K, Gleixner KV, Pickl WF, et al. KIT polymorphisms and mutations determine responses of neoplastic mast cells to bafetinib (INNO-406) Experimental Haematology. 2010;38:782–791. doi: 10.1016/j.exphem.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Riva F, Brizzola S, Stefanello D, Crema S, Turin L. A study of mutations in the c-kit gene of 32 dogs with mastocytoma. Journa of Veterinary Diagnostic Investigation. 2005;17:385–388. doi: 10.1177/104063870501700416. [DOI] [PubMed] [Google Scholar]
- Serve H, Yee NS, Stella G, Sepp-Lorenzino L, Tan JC, et al. Differential roles of PI3-kinase and Kit tyrosine 821 in Kit receptor-mediated proliferation, survival and cell adhesion in mast cells. EMBO Journal. 1995;14:473–483. doi: 10.1002/j.1460-2075.1995.tb07023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timokhina I, Kissel H, Stella G, Besmer P. Kit signaling through PI 3-kinase and Src kinase pathways: an essential role for Rac1 and JNK activation in mast cell proliferation. EMBO Journal. 1998;17:6250–6262. doi: 10.1093/emboj/17.21.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nature Reviews Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Vosseller K, Stella G, Yee NS, Besmer P. c-kit receptor signaling through its phosphatidylinositide-3'-kinase-binding site and protein kinase C: role in mast cell enhancement of degranulation, adhesion, and membrane ruffling. Molecular Biology of the Cell. 1997;8:909–922. doi: 10.1091/mbc.8.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JD, Kiupel M, Yuzbasiyan-Gurkan V. Evaluation of the kinase domain of c-KIT in canine cutaneous mast cell tumors. BMC Cancer. 2006;6:85. doi: 10.1186/1471-2407-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle MM, Bley CR, Howard J, Rufenacht S. Canine mast cell tumours: a review of the pathogenesis, clinical features, pathology and treatment. Veterinary Dermatology. 2008;19:321–339. doi: 10.1111/j.1365-3164.2008.00694.x. [DOI] [PubMed] [Google Scholar]
- Yamada O, Kobayashi M, Sugisaki O, Ishii N, Ito K, et al. Imatinib elicited a favorable response in a dog with a mast cell tumor carrying a c-kit c.1523A>T mutation via suppression of constitutive KIT activation. Veterinary Immunology and Immunopathology. 2011;142:101–106. doi: 10.1016/j.vetimm.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Yap TA, Garrett MD, Walton MI, Raynaud F, de Bono JS, et al. Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and promises. Current Opinion in Pharmacology. 2008;8:393–412. doi: 10.1016/j.coph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Zemke D, Yamini B, Yuzbasiyan-Gurkan V. Mutations in the juxtamembrane domain of c-KIT are associated with higher grade mast cell tumors in dogs. Veterinary Pathology. 2002;39:529–535. doi: 10.1354/vp.39-5-529. [DOI] [PubMed] [Google Scholar]