Abstract
Background
Both HIV infection and antiretroviral therapy are associated with dyslipidemias in adults but there are fewer data on outcomes in young children. Here we examined lipid profile changes in a cohort of young children before and after suppression on an initial ritonavir-boosted lopinavir (LPV/r)-based regimen and after switch to a nevirapine (NVP)-based regimen.
Methods
195 HIV-infected children who initiated LPV/r-based therapy when <24 months of age at one site in Johannesburg, South Africa, and who achieved viral suppression (<400copies/ml sustained for ≥ 3 months) were randomised to either continue on the LPV/r-based regimen (n=99) or to switch to a NVP-based regimen (n=96). Non-fasting concentrations of total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL) and triglycerides (TG) were measured pre-treatment, at randomization when suppressed, and at 9, 20 and 31 months post-randomization.
Results
Median age at treatment initiation was 9 months and the initial regimen was maintained for an average of 9 months before randomization. TC, LDL and HDL increased from pre-treatment to randomization (p<0.0001) and TC/HDL ratio and TG decreased (p<0.0001). After switching to NVP, HDL was significantly higher (p<0.02) and TC/HDL and TG significantly lower (p<0.0001) through 31 months post-switch relative to remaining on the LPV/r-based regimen.
Conclusion
Initiating antiretroviral therapy was associated with changes to a more favorable lipid profile in young children. Switching from a LPV/r-based regimen to a NVP-based regimen accentuated and continued these improvements. Investigation of safe and effective methods for managing dyslipidemias in children of different ages in resource-limited settings is warranted.
Keywords: perinatal HIV infection, dyslipidaemia, nevirapine, ritonavir boosted lopinavir
Introduction
Lipid abnormalities are reported in association with HIV infection as well as with its treatment. Dyslipidemias occur in treatment-naive HIV-infected persons, indicating that HIV infection per se may affect fatty acid metabolism.1 Furthermore, despite clinical and virologic benefits of antiretroviral therapy (ART), therapy itself is associated with metabolic derangements, including dyslipidemias.2 ART-related dyslipidemias, even in children, are characterised by elevated total cholesterol (TC), low density lipoprotein cholesterol (LDL) and plasma triglyceride (TG) levels and lower high density lipoprotein cholesterol (HDL) levels.3-10 Protease inhibitor (PI)-containing regimens, particularly those including ritonavir, have been strongly implicated as a cause of dyslipidemias, including hypercholesterolemia and hypertriglyceridemia.2 Other antiretroviral drug classes have also been implicated.11
The limited study of lipid metabolism in infants and young children is of particular concern since they are exposed to ART during developmentally-critical periods and will likely have longer cumulative exposure to ART. Current HIV treatment guidelines recommend routine use of PI-based regimens for first-line treatment of young children because of exposure to nevirapine (NVP) to prevent mother-to-child HIV transmission (PMTCT).12 Concerns are heightened by accumulating evidence of the importance of childhood metabolic parameters in long-term development of atherosclerosis in adults13,14 as well as increasing recognition of the role of early exposures in shaping development of these metabolic pathways.15 Dyslipidemias have been described in 50-70% of children receiving ART.16-20 But studies of young children and infants are limited, and generally have not followed children prospectively or compared different treatment regimens.
In the context of a trial evaluating re-use of NVP for children exposed to this agent at birth when it was used as prophylaxis,21 we examined lipid profiles of HIV-infected South African children initiating PI-based ART when less than two years of age. We assessed changes from pre-treatment to the time of viral suppression and then subsequent changes when children were either continued on their primary PI-based regimen or were switched to a NVP-based regimen. The randomized design allowed us to investigate whether switching from a PI-sparing regimen to a NVP-based regimen would result in measurable changes in young children’s lipid profiles.
Methods
Study design
We report results of serum lipoprotein and triglyceride measurements from 195 HIV-infected infants and young children enrolled in an ART strategies trial before and after they were suppressed on their primary regimen containing ritonavir-boosted lopinavir (LPV/r).21 Children initiated treatment between April 2005 and July 2007 at a single secondary-level hospital in Johannesburg, South Africa (Rahima Moosa Mother and Child Hospital). Children were referred from inpatient wards, as well as nearby hospitals and surrounding clinics. Participants lived mainly in the urban neighbourhoods surrounding the health facility and came from poorer socioeconomic backgrounds. All children were exposed to single-dose NVP prophylaxis at birth and were younger than 24 months of age when they initiated PI-based ART. Those who achieved and sustained plasma HIV-1 RNA <400 copies/ml for at least 3 months within the first 12 months of treatment were eligible for randomization to either continue on the LPV/r-based regimen or to switch to a NVP-based regimen. Here we describe the lipid profiles of the children prior to starting any therapy, at the time of randomization when suppressed, and at 9, 20 and 31 months post-randomization. The study was approved by the Institutional Review Boards of Columbia University (New York, NY) and the University of the Witwatersrand (Johannesburg, South Africa). Signed informed consent was obtained from the child’s parent or guardian.
Treatment regimens
As per South African Department of Health guidelines in place at the time,22 children >6 months were started on ritonavir-boosted lopinavir-230mg/m2 (LPV/r), lamivudine-4mg/kg (3TC) and stavudine–1mg/kg (d4T) taken 12 hourly. In children <6 months of age, or those receiving concomitant rifampicin-based tuberculosis (TB) treatment, LPV/r was replaced with ritonavir-450mg/m2 (RTV). Once older than 6 months and at the completion of TB treatment, RTV was replaced with LPV/r. All children were receiving a LPV/r-based regimen at the time of randomization. Dosages were recalculated monthly according to weight. All medications were in liquid formulation.
Randomization divided the cohort into two groups. The control group underwent no regimen change and continued with the LPV/r-containing regimen. The switch group substituted NVP for LPV/r. Both groups continued d4T and 3TC. If children in either group were diagnosed with TB post-randomisation, they commenced anti-tuberculous therapy and their regimen was modified according to South African guidelines. For the switch group this entailed discontinuation of NVP. In the event of viral failure that did not respond to adherence counselling, children in the switch group were returned to the LPV/r-based regimen. None of the children received any lipid lowering agents.
Laboratory analysis
TC, LDL, HDL and TG concentrations were measured at five time points: pre-treatment (Time 0), randomization (Time 0R) and 9, 20 and 31 months post-randomization. Due to the young age of the cohort, children were not fasted prior to the blood draw. One child in each group was still breastfeeding at the time of enrolment into the study. Due to enrolment procedures, pre-treatment blood samples were only available for 151/195 children who had been randomized. Quantitative determination of the serum lipogram was performed using the Roche COBAS®INTEGRA 400 system. Lipogram values were all reported in mmol/l. For ease of interpretation we present key findings also in mg/dl. For these calculations we multiplied lipid values by 39 and triglyceride values by 89. Hypercholesterolemia was defined as TC ≥5.13 mmol/l (≥200mg/dl). LDL was classified as borderline high if between 2.82-3.31 mmol/l (110-129 mg/dl) and high if ≥3.31 (≥130mg/dl). HDL was considered low if <1.03 mmol/l (<40 mg/dl). Hypertriglyceridemia was defined as TG ≥1.69mmol//l (≥150mg/dl).23 The ratio of TC:HDL was also calculated.
CD4 T-cell counts and percentages were measured pre-treatment and every 3 months during follow-up. For this analysis, we selected the CD4 determinations done pre-treatment, closest to the time of randomization and closest to the follow-up lipogram measurements. CD4 cell counts and percentages were obtained using the Beckman Coulter FlowCARE™ PLG CD4 Reagent system. HIV-1 RNA quantity (viral load) was measured pre-treatment and 3-monthly until randomization, and at 1, 4, 6 months post-randomization and every 3 months thereafter. The standard assay was used for the sample collected pre-treatment (quantification range 400-750 000 copies/ml) and the ultrasensitive assay (quantification range 50-150 000 copies/ml) for samples collected after ART initiation (Roche Amplicor Assay, version 1.5, Branchburg, NJ).
Clinical Monitoring
Clinical evaluations were performed at regular intervals and included anthropometric measurements (height, weight and head circumference), consultation with study physicians, phlebotomy, adherence assessments, including one and two-day recall and reconciliation of returned medications, and dispensing of medications.
Statistical methods
Paired t-tests were used to compare lipid values before and after treatment initiation and McNemar tests to compare changes when classifying lipid abnormalities in categories. Treatment groups were compared as randomized (intent-to-treat). Wilcoxon rank-sum tests were used to compare continuous variables between groups and Chi-squared or Fisher’s exact tests for categorical variables. Kaplan-Meier methods were used to describe virologic endpoints. Associations on the continuous scale were examined using Spearman Rank Order correlations or using chi-squared tests if categorical. Weight-for-age Z (WAZ)-scores and height-for-age Z (HAZ)-scores were calculated using WHO software.24 All p-values are two-tailed and p-values <0.05 were considered statistically significant. Data analysis was performed using SAS software (Cary, NC).
Results
Study population
The median age when treatment was started was 10 months (range 2-24 months) in the whole cohort of 195 HIV-infected children who achieved viral suppression and were randomized, and 47% were female. Prior to starting therapy, 55% had HIV RNA quantity >750,000 copies/ml, the median CD4 percentage was 18.5 and the mean weight-for age Z-score was −2.18. The characteristics of the 151 children with pre-treatment samples available for lipid measurements are shown in Table 1. Those missing pre-treatment samples did not differ significantly from those with available samples in age, sex, CD4 percentage or weight-for-age. Pre-treatment viral load results were missing for most of those missing pre-treatment samples. In those with pre-treatment samples, the median age at treatment initiation was 9.3 months and by the time of randomization, after a mean 9.4 months on PI-based ART, all children had viremia <400 copies/ml (by definition), 70.9% were suppressed <50 copies/ml, the median CD4 percentage had risen to 29.8, and the mean weight-for-age Z-score was −0.54.
Table 1.
Characteristics of 151 HIV-infected children with available lipid measurements before starting treatment (pre-treatment) with ritonavir-boosted lopinavir (LPV/r)-based therapy and at the time of randomization after achieving virologic suppression (suppressed) on this regimen.
| Pre-treatment (Time 0) | Suppressed (Time 0R) | p-value* | |||
|---|---|---|---|---|---|
| Sex | |||||
| Male | 78 | (51.7) | 78 | (51.7) | |
| Female | 73 | (48.3) | 73 | (48.3) | |
|
| |||||
| Age in months | |||||
| <6 | 41 | (27.2) | - | - | |
| 6 to 11 | 58 | (38.4) | 12 | (8.0) | |
| 12 to 17 | 33 | (21.9) | 60 | (39.7) | |
| 18 to 23 | 19 | (12.6) | 39 | (25.8) | |
| ≥24 | - | - | 40 | (26.5) | |
|
| |||||
| Median age in months | 9.3 | 18.5 | |||
| Range | 2.2-24 | 9.1-35.5 | |||
|
| |||||
| CD4 percent | |||||
| Median | 19.5 | 29.8 | <0.0001 | ||
| Range | 2.6-41.8 | 7.3-55.7 | |||
|
| |||||
| CD4 count cells/mm3 | |||||
| Median | 936 | 1869 | <0.0001 | ||
| Range | 10-3762 | 104-5423 | |||
|
| |||||
| HIV RNA copies per ml † | |||||
| <50 | - | - | 107 | (70.9) | |
| 50-399 | - | - | 44 | (29.1) | |
| 400-99,999 | 13 | (9.0) | - | ||
| 100,000-749,999 | 46 | (31.7) | - | ||
| ≥750 000 | 86 | (59.3) | - | ||
|
| |||||
| Weight for age Z-score | |||||
| Mean [std] | −2.07 [1.65] | −0.54 [1.12] | <0.0001 | ||
|
| |||||
| Height for age Z-score | |||||
| Mean [std] | −3.17 [1.63] | −3.04 [1.55] | >0.10 | ||
Paired t-tests were used to compare appropriate parameters pre- and post-treatment
Pre-treatment measurements missing for 6 children
Changes in lipids with treatment initiation
There were significant increases in TC, LDL and HDL and significant decreases in TC:HDL ratio and TG from pre-treatment to the time of randomization when viral suppression was attained (p<0.0001) (Table 2). Prior to starting therapy, TC, LDL and HDL were low, with no children with high levels of TC or LDL and 93.1% of children with HDL values considered low. TG above the threshold considered high were observed in 63.3% of children (Table 2). On average 9 months later, after viral suppression had been attained, TC increased by an average of 1.09 mmol/l and LDL by 0.86 mmol/l and 5.6% and 7.0% of children were classified as having high TC and LDL measurements, respectively. Although HDL increased on average 0.44 mmol/l, by the time of suppression, 59.6% were still considered to have low HDL. TG decreased 0.39 mmol/l with 37.7% remaining elevated at randomization. Only results for the 151 children who had samples available pre-treatment are shown. Those missing pre-treatment samples had similar post-treatment levels to those with both time points (data not shown).
Table 2.
Changes in lipids among 151 HIV-infected children before starting treatment (pre-treatment) with ritonavir-boosted lopinavir (LPV/r)-based therapy and at the time of randomization after achieving virologic suppression (suppressed) on this regimen.
| Pre-treatment (Time 0) | Suppressed (Time 0R) | ||||
|---|---|---|---|---|---|
| Mean [standard deviation] | Mean [standard deviation] | ||||
| Continuous variable | mmol/l | mg/dL | mmol/l | mg/dL | p-value* |
| Total Cholesterol (TC) | 2.62 [0.78] | 102 [30.3] | 3.71 [0.97] | 145 [38.0] | <0.0001 |
| Low density lipoprotein (LDL) | 1.11 [0.71] | 43 [27.7] | 1.98 [0.85] | 77 [33.3] | <0.0001 |
| High Density lipoprotein (HDL) | 0.59 [0.33] | 23 [13.0] | 1.03 [0.34] | 40 [13.3] | <0.0001 |
| Triglycerides (TG) | 2.02 [0.86] | 180 [76.2] | 1.64 [0.87] | 146 [77.6] | <0.0001 |
| TC:HDL ratio | 6.35 [5.08] | 3.90 [1.37] | |||
| Categorical classification | n/N† (%) | n/N (%) | |
|---|---|---|---|
|
| |||
| Total cholesterol | |||
| Borderline high 170-199 mg/dL | 3/144 (2.1) | 26/144 (18.1) | |
| High ≥ 200 mg/dL | 0 | 8/144 (5.6) | <0.0001 |
|
| |||
| Low density lipoprotein (LDL) | |||
| Borderline high 110-129 mg/dL | 2/144 (1.4) | 7/143 (4.9) | |
| High ≥ 130 mg/dL | 0 | 10/143 (7.0) | 0.003 |
|
| |||
| High density lipoprotein (HDL) | |||
| Low < 40 mg/dL | 134/144 (93.1) | 87/146 (59.6) | <0.0001 |
|
| |||
| Triglycerides | |||
| High >150 mg/dl | 93/147 (63.3) | 55/146 (37.7) | <0.0001 |
Paired t-tests were used for continuous variables and McNemar tests for categorical variables
There were no significant differences by gender. Lipid changes observed with treatment occurred in both girls and boys. HDL was lower and LDL higher among children older at the time of treatment initiation but, at the time of suppression, no age associations were observed. As a result, the magnitude of the LDL increase was larger and the change in HDL smaller in children who initiated therapy younger than a year of age. Higher pre-treatment HDL was associated with higher CD4 percentage. Lower LDL and higher TG were associated with higher pre-treatment viral loads. There were no associations at the time of randomization.
Post-randomization changes in lipids
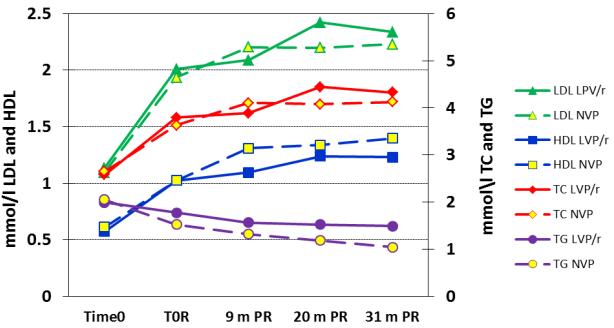
Post-randomization, lipid changes in both groups displayed similar trends to that observed after treatment initiation, namely increases in TC, LDL and HDL and decreases in TC:HDL ratio and TG. However, increases in HDL and decreases in TG were greater in the switch group (Figure 1). The switch group had significantly higher HDL compared to the control group at 9, 20 and 31 months post-randomization (Table 3). The switch group had continued increases in HDL after randomization with slower increases in the control group. By 31 months post-randomization, 19.0% of children in the switch group and 40.3% of children in control group had persistently low HDL (p=0.01) (Table 4). The switch group also had more pronounced declines in TG and in TC:HDL ratio (Table 3). By 31 months post-randomization, 10.5% of children in the switch group and 35.3% of children in control group had persistently high TG (p=0.001) (Table 4). There were no consistent differences between the groups in LDL or TC levels post-randomization. At the time of randomization, there were no significant differences between the groups in HDL, LDL, TC or TC:HDL ratio. There was a borderline difference in TG levels, but the later larger differences between the groups remained significant after adjusting for TG levels at the time of randomization.
Figure 1.
Mean lipid values among 195 HIV-infected children who achieved viral suppression with ritonavir-boosted lopinavir (LPV/r)-based treatment and then were randomized to either continue on the LPV/r-based regimen (control group) or to switch to a NVP-based regimen (switch group). Mean values are shown prior to starting any treatment (time 0), at the time of randomization when suppressed and at nine (time 0R), 20 and 31 months post-randomization (PR).
Table 3.
Mean lipid values among 195 HIV-infected children suppressed on ritonavir-boosted lopinavir (LPV/r)-based treatment and randomized to either continue on the LPV/r-based regimen (control group) or to switch to a NVP-based regimen (switch group) at the time of randomization (Time 0R) and at 9, 20 and 31 months post-randomization (PR).
| Control group | Switch group | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | Mean [standard deviation] |
N | Mean [standard deviation] |
p-value* | |||
| mmol/l | mg/dL | mmol/l | mg/dL | ||||
|
| |||||||
| Total Cholesterol | |||||||
| Time 0R | 93 | 3.79 (1.08) | 148 (42.1) | 95 | 3.63 (0.94) | 142 (36.6) | >0.10 |
| 9 months PR | 93 | 3.89 (0.97) | 152 (37.8) | 88 | 4.10 (0.87) | 160 (34.0) | >0.10 |
| 20 months PR | 79 | 4.44 (0.86) | 173 (33.7) | 74 | 4.08 (0.80) | 159 (31.1) | 0.007 |
| 31 months PR | 67 | 4.32 (0.84) | 169 (33.0) | 55 | 4.12 (0.77) | 161 (30.1) | 0.07 |
|
| |||||||
| Low Density Lipoprotein | |||||||
| Time 0R | 93 | 2.01 (0.91) | 78 (35.3) | 94 | 1.94 (0.82) | 75 (31.8) | >0.10 |
| 9 months PR | 93 | 2.09 (0.79) | 81 (31.0) | 87 | 2.20 (0.77) | 86 (30.1) | >0.10 |
| 20 months PR | 72 | 2.42 (0.84) | 94 (32.9) | 72 | 2.20 (0.75) | 86 (29.2) | >0.10 |
| 31 months PR | 67 | 2.34 (0.80) | 91 (31.1) | 58 | 2.23 (0.76) | 87 (29.7) | >0.10 |
|
| |||||||
| High Density Lipoprotein | |||||||
| Time 0R | 95 | 1.03 (0.39) | 40 (15.2) | 95 | 1.02 (0.35) | 40 (13.7) | >0.10 |
| 9 months PR | 93 | 1.09 (0.37) | 43 (14.4) | 87 | 1.31 (0.44) | 51 (17.1) | 0.0006 |
| 20 months PR | 73 | 1.24 (0.58) | 48 (22.6) | 73 | 1.34 (0.39) | 52 (15.2) | 0.01 |
| 31 months PR | 67 | 1.23 (0.54) | 48 (20.9) | 58 | 1.40 (0.48) | 54 (18.8) | 0.02 |
|
| |||||||
| TC/HDL ratio | |||||||
| Time 0R | 92 | 4.14 (1.89) | 95 | 3.83 (1.44) | >0.10 | ||
| 9 months PR | 93 | 3.95 (1.77) | 87 | 3.42 (1.22) | 0.03 | ||
| 20 months PR | 71 | 3.98 (1.28) | 69 | 3.24 (0.95) | 0.0003 | ||
| 31 months PR | 64 | 3.87 (1.46) | 55 | 3.18 (0.89) | 0.0096 | ||
|
| |||||||
| Triglycerides | |||||||
| Time 0R | 95 | 1.78 (0.93) | 158 (82.9) | 95 | 1.52 (0.76) | 136 (67.9) | 0.05 |
| 9 months PR | 93 | 1.56 (0.73) | 139 (64.6) | 87 | 1.32 (0.65) | 118 (58.1) | 0.03 |
| 20 months PR | 70 | 1.52 (0.71) | 136 (63.2) | 73 | 1.18 (0.65) | 105 (58.2) | 0.0009 |
| 31 months PR | 68 | 1.49 (0.71) | 133 (63.2) | 57 | 1.04 (0.59) | 93 (52.7) | <0.0001 |
Groups are compared using Wilcoxon rank-sum tests
Table 4.
Proportions of children with lipid abnormalities 9, 20 and 31 months after randomization to either remain on ritonavir-boosted lopinavir (control group) or switch to NVP-based therapy (switch group)
| Control group | Switch group | ||
|---|---|---|---|
|
| |||
| n/N (%) | n/N (%) | p value* | |
|
| |||
| 9 months post-randomization | |||
|
| |||
| Total cholesterol | |||
| Borderline high 170-199 mg/dL | 14/93 (15.1) | 20/88 (22.7) | |
| High ≥ 200 mg/dL | 13/93 (14.0) | 11/88 (12.5) | >0.10 |
|
| |||
| Low density lipoprotein | |||
| Borderline high 110-129 mg/dL | 8/93 (8.6) | 8/87 (9.2) | |
| High ≥ 130 mg/dL | 8/93 (8.6) | 9/87 (10.3) | >0.10 |
|
| |||
| High density lipoprotein | |||
| Low < 40 mg/dL | 43/93 (46.2) | 22/87 (25.3) | 0.004 |
|
| |||
| Triglycerides | |||
| High >150 mg/dl | 35/93 (37.6) | 25/87 (28.7) | >0.10 |
|
| |||
| 20 months post-randomization | |||
|
| |||
| Total cholesterol | |||
| Borderline high 170-199 mg/dL | 27/79 (34.2) | 18/74 (24.3) | |
| High ≥ 200 mg/dL | 16/79 (20.3) | 8/74 (10.8) | 0.05 |
|
| |||
| Low density lipoprotein | |||
| Borderline high 110-129 mg/dL | 15/72 (20.8) | 9/72 (12.5) | |
| High ≥ 130 mg/dL | 7/72 (9.7) | 4/72 (5.6) | >0.10 |
|
| |||
| High density lipoprotein | |||
| Low < 40 mg/dL | 29/73 (39.7) | 17/73 (23.3) | 0.03 |
|
| |||
| Triglycerides | |||
| High >150 mg/dl | 22/70 (31.4) | 16/73 (21.9) | >0.10 |
|
| |||
| 31 months post-randomization | |||
|
| |||
| Total cholesterol | |||
| Borderline high 170-199 mg/dL | 28/67 (41.8) | 12/55 (21.8) | |
| High ≥ 200 mg/dL | 11/67 (16.4) | 7/55 (12.7) | 0.03 |
|
| |||
| Low density lipoprotein | |||
| Borderline high 110-129 mg/dL | 8/67 (11.9) | 10/58 (17.2) | |
| High ≥ 130 mg/dL | 8/67 (11.9) | 5/58 (8.6) | >0.10 |
|
| |||
| High density lipoprotein | |||
| Low < 40 mg/dL | 27/67 (40.3) | 11/58 (19.0) | 0.01 |
|
| |||
| Triglycerides | |||
| High >150 mg/dl | 24/68 (35.3) | 6/57 (10.5) | 0.001 |
Groups are compared using chi-square tests
There were no significant differences in lipid concentrations by sex within treatment groups at any time point. However, differences between the groups in HDL were more marked among boys and TG declines were stronger among girls. There were no consistent associations between age at starting therapy or at randomization and lipid concentrations.
As we have previously reported, children randomized to the switch group were significantly more likely to meet the virological endpoint used in the trial to consider regimen change (defined as confirmed viremia >1000 copies/ml) than children randomized to the LPV/r group. By 9 months, 17.2% of children in switch group had confirmed viremia >1000 copies/ml compared to 2.2% in the control group. By 20 and 31 months respectively, these proportions were 23.9% and 23.9% in the switch group; and 9.6% and 11.1% in the control group (p=0.009). There were no significant differences in lipids in those who failed virologically overall or within treatment group but numbers of failures were small. The associations between group assignment and HDL, TC:HDL ratio and TG persisted if the analysis was restricted only to those who did not fail therapy.
Discussion
In this randomized clinical trial, young HIV-infected children had low TC, HDL and LDL and high plasma TG prior to starting therapy. Initiation of a PI-based regimen resulted in significant increases in TC, LDL and HDL and decreases in TC:HDL ratio and TG. This pattern corresponds with prior descriptions of viremia-associated dyslipidaemia and changes after therapy.4,25 However, even when virologically-suppressed, a large proportion of children still had low HDL and high TG. After randomization, only those switched from their LPV/r-based regimen to the NVP-based regimen showed further significant improvements in HDL, TC:HDL ratio and TG levels.
While metabolic abnormalities have been well described among HIV-infected children and youth on treatment,2-9,16,20,25 fewer studies have characterized patterns of dyslipidemia among young children prior to ART. Among untreated adults, there is an early decrease in HDL followed by decreases in LDL and increases in TG.26 Chantry et al. reported that, in a cohort of ART-naïve children, 30% had HDL abnormalities compared with 4% among a matched comparison group.18 Among 103 ART-naïve children in London with mild/moderate HIV disease, median age 7.1 years, lipid levels were normal with the exception of below normal HDL.20 In the British cohort no association was found between lipids and viral load or CD4+ cell count.20 In our young cohort, where most children had advanced disease, 93% had low HDL and 63% had elevated TG prior to ART initiation. Lower LDL and higher TG were associated with higher HIV RNA while higher HDL was associated with higher CD4 percentage. The high rate of lipid abnormalities pre-treatment may be related to the young age and advanced state of disease among children in our cohort. We hypothesize that the initial improvements in lipids may be secondary to ART-mediated control of viral replication and restoration of immune function.
Those children randomized to remain on the LPV/r-based regimen continued to have increases in TC, LDL and HDL and declines in TC:HDL ratio and TG. Despite 31 months of treatment, high rates of abnormalities persisted in this group including high TC (16%), LDL (12%), TG (36%) and low HDL (40%). Among children randomized to switch to NVP-based treatment, the pattern of improvement in lipids (HDL increase and TC:HDL and TG decline) was more pronounced. In comparison to those remaining on LPV/r-based therapy, HDL was significantly higher and TC/HDL and TG significantly lower through 31 months among those switched to the NVP-based regimen.
Several studies have documented lipid abnormalities among children on treatment but most of these studies have been observational and included older children on a variety of different ART regimens. Our study is randomized and focuses only on young children under 2 years of age when initiating ART. In a large multi-site cross-sectional study in the U.S., Aldrovandi et al. found a high prevalence of lipid abnormalities among treated children compared with seronegative controls: among 161 children receiving PI-based ART, 52% had elevated TG, 29% high TC, 19% high LDL and 10% low HDL (< 35 mg/dl.)8 Each year of ritonavir use was associated with increases in TC, TG and LDL while NVP and efavirenz were associated with increases in HDL.8 Among 441 children followed in London, Rhoads et al. evaluated changes in lipid levels and associations with individual antiretroviral drugs.20 All lipids rose over a period of 4.5 years of observation. LPV/r was associated with increases in non-HDL of 0.43 millimoles per year in the first 0-1 year and 0.8 millimoles per year at >4-year exposure compared with a more modest effect of NVP with increases of 0.2-0.39 millimoles per year.
Our findings highlight the subtleties of the metabolic effects of different antiretroviral agents. It is likely that the early impact of ART initiation which leads to correction of HIV-related dyslipidemia (even with a LPV/r-based regimen) is balanced, over time, by the specific drug-related lipid abnormalities associated with this regimen. By comparison, in the switch cohort, the additional improvements in lipid profile can be attributed to the particular characteristics of NVP which have been associated with a more favourable lipid profile and lower long-term cardiovascular risk.27 Several substitution studies in adults where a PI was switched to NVP resulted in significant improvements in dyslipidemia changes which are likely due to both the discontinuation of the PI as well as the specific drug substitution.27-30 A pediatric study also observed significant improvements in lipid profile with switch to efavirenz.31
Recent developments in antiretroviral therapies offer an increasingly large array of treatment options for adults with HIV infection. Many of the newer agents have better toxicity profiles and, in well-resourced settings, drug regimens can be individualized to improve efficacy, facilitate adherence and minimize both short and long term side effects. Unfortunately, for children, particularly infants and young children, treatment options remain profoundly limited. LPV/r-based regimens are currently recommended for children who have been exposed to nevirapine used as part of PMTCT.12 The comparative virologic efficacy of different regimens is important, but other short- and long-term impacts on other disease parameters also need to be studied in order to understand how to optimize use of the available drugs.
The long-term consequence of abnormal lipids in infants and young children with HIV infection is unknown. Several studies have identified early atherosclerotic changes with carotid artery imaging studies among youth with perinatal HIV infection and long term ART exposure.32,33 With increased availability of ART in high HIV prevalence settings, increasingly large numbers of infants and young children are initiating treatment, with either NVP- or LPV/r-based regimens. The long-term consequences of ART starting early in life and extending through childhood and adolescence are unknown but warrant careful study. Childhood metabolic parameters are associated with atherosclerosis and cardiovascular disease in adulthood. Current guidelines in the US recommend biannual monitoring of fasting lipids in children on ART but such tests are generally not available in resource-constrained settings.34 Recommendations for management of lipid abnormalities include switching antiretroviral drugs, exercise and dietary interventions and, for older children, use of lipid-lowering agents but are not widely implemented.19,34 Most commercially-available lipid-lowering medications have not been studied in children with HIV.
There are several limitations of our study. Due to the young age of the cohort, fasting blood samples were not obtained. While most normative lipoprotein reference data are based on fasting samples, sample timing with respect to feedings does not significantly influence results.35 Our approach is also similar to many of the published pediatric studies and our findings appear comparable. Moreover, since the same protocols were followed for the collection of samples pre- and post-treatment and between the groups, any measurement error introduced is likely to be non-differential resulting in weaker associations. We note that our reference levels are based on data obtained from children in North America as there are no population-specific lipid reference levels for South African children. No information on the children’s diet or family history of lipid disorders was recorded but since the study is randomized this is unlikely to have any consequence. Since all children received d4T, the individual impact of this agent could not be discerned.36
We have previously reported on the virologic constraints of re-using nevirapine in nevirapine-exposed children as well as on the benefits of switching for CD4 response and growth.21 These new results demonstrate the potential value of switching from an initial LPV/r-based regimen to a NVP-based regimen in young HIV-infected children on select metabolic parameters. Those children who were switched had a more favorable lipid profile with higher HDL and lower TC:HDL ratio and TG concentrations. Although the long-term clinical implications of these changes are unknown, our results raise concern about the long-term risk of cardiovascular disease with prolonged use of LPV/r-based therapy initiated in infants and young children. Our study reinforces the need for ongoing monitoring of the lipid profile of treated children, especially those receiving boosted PI. Investigations of safe and effective methods for managing dyslipidemias in children of different ages in resource-limited settings are warranted.
Acknowledgements
We thank the study participants and caregivers, as well as the clinical and administrative team for their continued dedication and support.
Sources of funding: The study was supported in part by grants from the National Institutes of Child Health and Human Development (NICHD) HD 47177 and Secure the Future Foundation RES 219
Footnotes
Potential conflicts of interest. All authors: no conflicts.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- (1).Grunfeld C, Kotler DP, Hamadeh R, Tierney A, Wang J, Pierson RN. Hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med. 1989;86:27–31. doi: 10.1016/0002-9343(89)90225-8. [DOI] [PubMed] [Google Scholar]
- (2).Tassiopoulos K, Williams PL, Seage GR, III, Crain M, Oleske J, Farley J. Association of hypercholesterolemia incidence with antiretroviral treatment, including protease inhibitors, among perinatally HIV-infected children. J Acquir Immune Defic Syndr. 2008;47:607–614. doi: 10.1097/QAI.0b013e3181648e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Farley J, Gona P, Crain M, et al. Prevalence of elevated cholesterol and associated risk factors among perinatally HIV-infected children (4-19 years old) in Pediatric AIDS Clinical Trials Group 219C. J Acquir Immune Defic Syndr. 2005;38:480–487. doi: 10.1097/01.qai.0000139397.30612.96. [DOI] [PubMed] [Google Scholar]
- (4).Melvin AJ, Lennon S, Mohan KM, Purnell JQ. Metabolic abnormalities in HIV type 1-infected children treated and not treated with protease inhibitors. AIDS Res Hum Retroviruses. 2001;17:1117–1123. doi: 10.1089/088922201316912727. [DOI] [PubMed] [Google Scholar]
- (5).Mulligan K, Grunfeld C, Tai VW, et al. Hyperlipidemia and insulin resistance are induced by protease inhibitors independent of changes in body composition in patients with HIV infection. J Acquir Immune Defic Syndr. 2000;23:35–43. doi: 10.1097/00126334-200001010-00005. [DOI] [PubMed] [Google Scholar]
- (6).Carter RJ, Wiener J, Abrams EJ, et al. Dyslipidemia among perinatally HIV-infected children enrolled in the PACTS-HOPE cohort, 1999-2004: a longitudinal analysis. J Acquir Immune Defic Syndr. 2006;41:453–460. doi: 10.1097/01.qai.0000218344.88304.db. [DOI] [PubMed] [Google Scholar]
- (7).Jaquet D, Levine M, Ortega-Rodriguez E, et al. Clinical and metabolic presentation of the lipodystrophic syndrome in HIV-infected children. AIDS. 2000;14:2123–2128. doi: 10.1097/00002030-200009290-00008. [DOI] [PubMed] [Google Scholar]
- (8).Aldrovandi GM, Lindsey JC, Jacobson DL, et al. Morphologic and metabolic abnormalities in vertically HIV-infected children and youth. AIDS. 2009;23:661–672. doi: 10.1097/QAD.0b013e3283269dfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Vink NM, van Rossum AM, Hartwig NG, de GR, Geelen S. Lipid and glucose metabolism in HIV-1-infected children treated with protease inhibitors. Arch Dis Child. 2002;86:67. doi: 10.1136/adc.86.1.67-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Taylor P, Worrell C, Steinberg SM, et al. Natural history of lipid abnormalities and fat redistribution among human immunodeficiency virus-infected children receiving long-term, protease inhibitor-containing, highly active antiretroviral therapy regimens. Pediatrics. 2004;114:e235–e242. doi: 10.1542/peds.114.2.e235. [DOI] [PubMed] [Google Scholar]
- (11).Fontas E, van LF, Sabin CA, et al. Lipid profiles in HIV-infected patients receiving combination antiretroviral therapy: are different antiretroviral drugs associated with different lipid profiles? J Infect Dis. 2004;189:1056–1074. doi: 10.1086/381783. [DOI] [PubMed] [Google Scholar]
- (12).World Health Organization WHO Antiretroviral Therapy for Infants and Children: Report of the WHO Technical Reference Group, Paediatric HIV/ART Care Guideline Group Meeting; 2010; http://whqlibdoc.who.int/publications/2010/9789241599801_eng.pdf. [Google Scholar]
- (13).Li S, Chen W, Srinivasan SR, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290:2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- (14).Raitakari OT, Juonala M, Kahonen M, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- (15).Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA. 2009;301:2234–2242. doi: 10.1001/jama.2009.761. [DOI] [PubMed] [Google Scholar]
- (16).Lainka E, Oezbek S, Falck M, Ndagijimana J, Niehues T. Marked dyslipidemia in human immunodeficiency virus-infected children on protease inhibitor-containing antiretroviral therapy. Pediatrics. 2002;110:e56. doi: 10.1542/peds.110.5.e56. [DOI] [PubMed] [Google Scholar]
- (17).Gonzalez-Tome MI, Amador JT, Pena MJ, Gomez ML, Conejo PR, Fontelos PM. Outcome of protease inhibitor substitution with nevirapine in HIV-1 infected children. BMC Infect Dis. 2008;8(144):144. doi: 10.1186/1471-2334-8-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Chantry CJ, Hughes MD, Alvero C, et al. Lipid and glucose alterations in HIV-infected children beginning or changing antiretroviral therapy. Pediatrics. 2008;122:e129–e138. doi: 10.1542/peds.2007-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Jacobson DL, Williams P, Tassiopoulos K, Melvin A, Hazra R, Farley J. Clinical Management and Follow-up of Hypercholesterolemia Among Perinatally HIV-Infected Children Enrolled in the PACTG 219C Study. J Acquir Immune Defic Syndr. 2011;57:413–420. doi: 10.1097/QAI.0b013e31822203f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Rhoads MP, Lanigan J, Smith CJ, Lyall EG. Effect of Specific ART Drugs on Lipid Changes and the Need for Lipid Management in Children With HIV. J Acquir Immune Defic Syndr. 2011;57:404–412. doi: 10.1097/QAI.0b013e31821d33be. [DOI] [PubMed] [Google Scholar]
- (21).Coovadia A, Abrams EJ, Stehlau R, et al. Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: a randomized controlled trial. JAMA. 2010;304:1082–1090. doi: 10.1001/jama.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).South African National Department of Health South African Guidelines, Factsheet Section 10, Antiretroviral. 2007 http://www.doh.gov.za/docs/factsheets/guidelines/hiv/part5.pdf.
- (23).Neal WA. Kliegman: Nelson Textbook of Pediatrics. 18th ed. Saunders; 2007. Disorders of Lipoprotein Metabolism and Transport. [Google Scholar]
- (24).WHO Child Growth Standards (2005) and the WHO Anthro 2005 software and macros. 2005 http://www.who.int/childgrowth/software/en/
- (25).Sztam KA, Jiang H, Jurgrau A, Deckelbaum RJ, Foca MD. Early increases in concentrations of total, LDL, and HDL cholesterol in HIV-infected children following new exposure to antiretroviral therapy. J Pediatr Gastroenterol Nutr. 2011;52:495–498. doi: 10.1097/MPG.0b013e3181f5e9d4. [DOI] [PubMed] [Google Scholar]
- (26).Grunfeld C, Feingold KR. Metabolic disturbances and wasting in the acquired immunodeficiency syndrome. N Engl J Med. 1992;327:329–337. doi: 10.1056/NEJM199207303270506. [DOI] [PubMed] [Google Scholar]
- (27).Clotet B, van d V, Negredo E, Reiss P. Impact of nevirapine on lipid metabolism. J Acquir Immune Defic Syndr. 2003;34(Suppl 1):S79–84. S79–S84. doi: 10.1097/00126334-200309011-00012. [DOI] [PubMed] [Google Scholar]
- (28).Negredo E, Ribalta J, Paredes R, et al. Reversal of atherogenic lipoprotein profile in HIV-1 infected patients with lipodystrophy after replacing protease inhibitors by nevirapine. AIDS. 2002;16:1383–1389. doi: 10.1097/00002030-200207050-00010. [DOI] [PubMed] [Google Scholar]
- (29).Negredo E, Cruz L, Paredes R, et al. Virological, immunological, and clinical impact of switching from protease inhibitors to nevirapine or to efavirenz in patients with human immunodeficiency virus infection and long-lasting viral suppression. Clin Infect Dis. 2002;34:504–510. doi: 10.1086/324629. [DOI] [PubMed] [Google Scholar]
- (30).Martinez E, Arnaiz JA, Podzamczer D, et al. Substitution of nevirapine, efavirenz, or abacavir for protease inhibitors in patients with human immunodeficiency virus infection. N Engl J Med. 2003;349:1036–1046. doi: 10.1056/NEJMoa021589. [DOI] [PubMed] [Google Scholar]
- (31).McComsey G, Bhumbra N, Ma JF, Rathore M, Alvarez A. Impact of protease inhibitor substitution with efavirenz in HIV-infected children: results of the First Pediatric Switch Study. Pediatrics. 2003;111:e275–e281. doi: 10.1542/peds.111.3.e275. [DOI] [PubMed] [Google Scholar]
- (32).McComsey GA, O’Riordan M, Hazen SL, et al. Increased carotid intima media thickness and cardiac biomarkers in HIV infected children. AIDS. 2007;21:921–927. doi: 10.1097/QAD.0b013e328133f29c. [DOI] [PubMed] [Google Scholar]
- (33).Bonnet D, Aggoun Y, Szezepanski I, Bellal N, Blanche S. Arterial stiffness and endothelial dysfunction in HIV-infected children. AIDS. 2004;18:1037–1041. doi: 10.1097/00002030-200404300-00012. [DOI] [PubMed] [Google Scholar]
- (34).Ross AC, McComsey GA. Cardiovascular Disease Risk in Pediatric HIV: The Need for Population-Specific Guidelines. J Acquir Immune Defic Syndr. 2011;57:351–354. doi: 10.1097/QAI.0b013e318227b016. [DOI] [PubMed] [Google Scholar]
- (35).Steiner MJ, Skinner AC, Perrin EM. Fasting Might Not Be Necessary Before Lipid Screening: A Nationally Representative Cross-sectional Study. Pediatrics. 2011;128:463–470. doi: 10.1542/peds.2011-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Domingo P, Labarga P, Palacios R, et al. Improvement of dyslipidemia in patients switching from stavudine to tenofovir: preliminary results. AIDS. 2004;18:1475–1478. doi: 10.1097/01.aids.0000131343.53419.04. [DOI] [PubMed] [Google Scholar]