Abstract
Organochalcogens have been widely studied given their antioxidant activity, which confers neuroprotection, antiulcer and antidiabetic properties. Given the complexity of mammalian models, understanding the cellular and molecular effect of organochalcogens has been hampered. The nematode worm Caenorhabditis elegans (C. elegans) is an alternative experimental model that affords easy genetic manipulations, green-fluorescent protein tagging and in vivo live-analysis of toxicity. We previously showed that manganese (Mn)-exposed worms exhibit oxidative-stress-induced neurodegeneration and lifespan reduction. Here we use Mn-exposed worms as a model for an oxidatively challenged organism to investigate the underlying mechanisms of organochalcogen antioxidant properties. First, we recapitulate in C. elegans the effects of organochalcogens formerly observed in mice, including their antioxidant activity. This is followed by studies on the ability of these compounds to afford protection against Mn-induced toxicity. Diethyl-2-phenyl-2-tellurophenyl vinylphosphonate (DPTVP) was the most efficacious compound, fully reversing the Mn-induced reduction in survival and lifespan. Ebselen was also effective reversing the Mn-induced reduction in survival and lifespan, but to a lesser extent compared with DPTVP. DPTVP also lowered Mn-induced increases in oxidant levels, indicating that the increased survival associated with exposure to this compound is secondary to a decrease in oxidative stress. Furthermore, DPTVP induced nuclear translocation of the transcriptional factor DAF-16/Foxo, which regulates stress responsiveness and aging in worms. Our findings establish that the organochalcogens DPTVP as well as ebselen act as anti-aging agents in a model of Mn-induced toxicity and aging by regulating DAF-16/Foxo signaling and attenuating oxidative stress.
Keywords: manganese, organochalcogens, tellurium, selenium, oxidative stress, toxicity, C. elegans, FOXO
Introduction
The reactivity of organoselenium and organotelurium compounds [1–3], characterized by high nucleophilicity and antioxidant potential, provides the basis for their pharmacological activities in mammalian models. This class of compounds possesses anti-inflammatory, anti-ulcer, anticancer, hepato- and neuro-protective properties [1, 4–7]. These pharmacological properties inherent to ebselen, diphenyl diselenide (PhSe)2) and diethyl-2-phenyl-2-tellurophenyl vinylphosphonate (DPTVP) are attributed to their ability to scavenge reactive oxygen species and nitrogen species (ROS and RNS, respectively) [1, 5, 8–9]. It has been also shown that toxicity in cancer and neuroblastoma cell lines, caused by high doses of these compounds is modulated by activation of apoptotic pathways [9–11]. Nevertheless, because of the complexity of mammalian models, it has been difficult to determine the molecular pathways and specific proteins that are modulated in response to treatments with these compounds.
Accordingly, we used a simpler genetic model, the nematode worm Caenorhabiditis elegans (C. elegans). C. elegans is a useful experimental model given its high degree of orthology between the worm and human genomes, its ease of handling, the relatively straightforward generation of knockout strains for genes of interest as well as transgenic worms expressing green fluorescent protein (GFP)-tagged genetic reporters for tissue-expression or protein localization studies [12–13]. Thanks to the large brood size and short reproductive cycle of C. elegans, low and high throughput screens for toxicants have been established [14–15]. The evaluation of several metals in this model has contributed to novel information on their toxic mechanisms. For instance, manganese (Mn) toxicity in C. elegans has uncovered the role of dopamine and ROS in mediating Mn-induced dopaminergic (DAergic) neurodegeneration [16–17]
Mn-induced neurodegeneration is modulated by activation of several transcription factors. For example, Benedetto et al. demonstrated that the expression of SKN-1, the worm’s orthologue of the human transcription factor nuclear erythroid-derived 2-like 2 (NRF2) is altered by Mn exposure [16]. SKN-1 activation, in turn, leads to transcription of antioxidant genes, conferring protection against ROS. In parallel, DAF-16, a transcription factor and the worm’s orthologue of mammalian FOXO (forkhead box O), activates genes that codify antioxidant enzymes, such as superoxide dismutase (SOD) and E3 ubiquitin ligase [18]. These antioxidants neutralize oxygen reactive species, which are also involved in the aging process. Consequently, this pathway has been implicated in the regulation of stress resistance and lifespan [18–19]. Notably, DAF-16 belongs to the DAF-2 insulin-like cascade, which is negatively regulated by phosphorylation. Inhibition of this phosphorylation cascade causes the translocation of DAF-16 into the nucleus, where it binds to a DAF-16 binding element (a core sequence TTGTTTAC of the DNA), thus increasing transcriptional activation of antioxidants, in turn, reducing oxidative stress and slowing down the aging process [20].
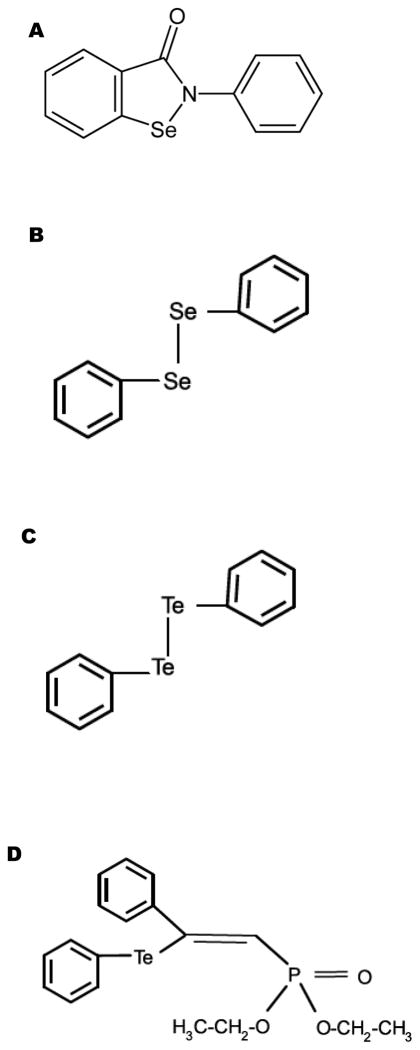
To address the antioxidant mechanisms of organotellurium and organoselenium, we characterized the effects of four organochalcogens (ebselen, diphenyl diselenide [PhSe)2], diphenyl diteluride [PhTe)2] and DPTVP (see Figure 1) on C. elegans survival and lifespan. We hypothesized that these compounds (1) act as antioxidants, decreasing the levels of ROS and carbonylated proteins or (2) attenuate Mn uptake in the worms, thus diminishing its toxic effects. Our results establish that DPTVP has the greatest antioxidant activity vs. the other tested compounds. The attenuation of Mn toxicity by DPTVP is associated with modulation of the DAF-16 pathway, absent changes in Mn uptake in the worms.
Figure 1.
Chemical structure of A) ebselen; B) (PhSe)2; C) (PhTe)2; D) DPTVP.
Materials and Methods
Chemicals
Ebselen, diphenyl diselenide (PhSe)2), diphenyl diteluride (PhTe)2) and DPTVP (Figure 1A–D) were synthesized according to previously described methods [21–23]. Oxyblot protein oxidation analyses kits were purchased from Millipore (S7150- Billerica, CA). All other reagents were obtained from Sigma (St Louis, MO).
C. elegans strains and handling of the worms
C. elegans Bristol N2 (wild type) and TJ356 (DAF-16::GFP) were handled and maintained at 20°C on E. coli OP50/NGM (nematode growth media) plates as previously described [24]. All strains were provided by the Caenorhabditis Genetics Center (CGC, Minnesota). Synchronous L1 population were obtained by isolating embryos from gravid hermaphrodites using bleaching solution (1% NaOCl; 0.25M NaOH), followed by floatation on a sucrose gradient to segregate eggs from dissolved worms and bacterial debris, accordingly to standard procedures previously described [25]. All experiments were carried out at 22°C in humidified controlled environment.
Dose-response curves and acute Mn exposure treatments
The lethal dose 50% (LD50) of organotellurium and organoselenium compounds in C. elegans was determined with doses ranging from 0.01 to 5 mM. Five thousand synchronized L1 worms per dose were treated at 22°C for 30 min by constant agitation in a rotator with each of the compounds, followed by three washes with 85 mM NaCl solution at the end of the incubation. Worms were placed on OP50 seeded NGM plates and the dose-response curves were plotted from scoring the number of surviving worms on each dish at 24h post-exposure. LD50 values were obtained from those curves. We chose doses of organochalcogens below the LD05 (5% lethality) for subsequent experiments. To assess the effect of organochalcogens on Mn-exposed animals, we pre-treated worms for 30 min at 22°C with non-lethal doses of each of the compounds, and subsequently washed the worms 3 times in NaCl 85 mM. Worms were then exposed for 30 min at 22°C to 35 mM manganese chloride (MnCl2), which corresponds to the LD25 for MnCl2 as previously reported by Benedetto et al [16]. Next, the worms were washed and placed on OP50 seeded NGM plates. Scoring of surviving worms was performed 24 hours (h) after MnCl2 exposure. For all dose-response curves, scores were normalized to percent control (0 mM organochalcogens/0 mM MnCl2 exposure).
Lifespan experiments
Synchronized L1 worms were acutely exposed to the organochalcogens compounds and/or MnCl2 as described earlier. Live and healthy-looking worms (around 30 per condition; in duplicates) were collected on the same day at the late L4 stage and transferred every four days to new OP50-seeded NGM plates. Survival was assessed each day until all the worms died. All tested C. elegans strains were assessed in parallel and each experiment was performed three times. Plotted curves represent averages of those three independent experiments.
ROS measurement
Synchronized L1 were acutely pre-treated for 30 minutes with ebselen, (PhSe)2 or DPTVP followed by 30 min exposure to MnCl2 (0 or 35 mM) as earlier described. Next, the worms were washed 2 additional times in M9 buffer. 2′7′-dichlorodihydrofluorescein diacetate (DCF-DA) at a final concentration of 1 mM was added for one hour and the worms were maintained in the dark. Worms were then washed 4 times in M9 buffer. Worms were frozen and thawed twice and homogenized by sonication and then centrifuged. The supernatants were transferred to a 96-well plate and their fluorescence levels (excitation: 485 nm; emission: 535 nm) were detected with a FLEXstation III (Molecular Devices, Sunnyvale, California) pre-heated to 37°C. The fluorescence from each well was measured for 40 min (at 10 min intervals). Fluorescence measurements were normalized to time zero values and rates of increased fluorescence (reflecting ROS levels) were expressed as percent control. The experiments were repeated 3 times in triplicate independent worm preparations [16].
Protein oxidation determination
Twenty-thousand worms were pre-treated for 30 minutes with DPTVP (0, 1 or 10 μM) and then exposed for 30 minutes to MnCl2 (35 mM), as previously described. Next, the worms were homogenized by sonication in a lysis buffer containing 85 mM sodium chloride, 1% Triton X-100, 10 mM Tris Buffer (pH 6.8), 1x protease inhibitor and 50 mM dithiotreitol (DTT). After centrifugation (11,000×g for 1 min), the supernatant was isolated and the protein concentration was determined with the Bradford method [26]. One-hundred micrograms of proteins were derivatized with 2,4, dinitrophenylhydrazine (DNPH), which is converted to 2,4, dinitrophenylhydrazone (DNP) in the presence of carbonyls from oxidized proteins. The carbonyls were detected by western blotting with a commercial antibody directed against derivatized carbonyl groups (anti 2,4- DNP, rabbit IgG) and visualized by horseradish peroxidase conjugated secondary antibody following the manufacturer’s instructions (Oxyblot analysis kit, Millipore). Purified β-actin (A1978, Sigma, St. Louis, MO) was used as a control and the bands’ density was acquired with Image J (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997–2011).
Mn content measurement by atomic absorption spectrophotometry
Triplicates of 10,000 L1 worms per condition were treated with MnCl2 as previously described. The samples were washed 8 times with NaCl and subsequently dehydrated in a vacuum oven at 65°C for 2 hours, and further digested in 200 μl ultrapure nitric acid for 24 hours in a sand bath (60°C). A 20 μl aliquot was brought to a 1 ml total volume with 2% nitric acid and analyzed for Mn content using graphite furnace atomic absorption spectroscopy (GFAAS) (Varian AA240, Varian, Inc USA). Bovine liver digested in ultrapure nitric acid was used as internal standard for analysis (NBS Standard Reference Material, USDC, Washington, DC, diluted at 5 μg Mn/L).
Epifluorescence microscopy
For each slide, a minimum of 20 worms were mounted on 4% agarose pads in M9 and anaesthetized with 0.2% tricaine/0.02% tetramisole in M9. Fluorescence was acquired with an epifluorescence microscope (Nikon Eclipse 80i, Nikon Corporation, Tokyo, Japan) equipped with a Lambda LS Xenon lamp (Sutter Instrument Company) and Nikon Plan Fluor 20x dry and Nikon Plan Apo 60× 1.3 oil objectives. Microscopes were housed in air-conditioned rooms (20–22°C).
Statistics
Dose-response lethality curves, longevity curves and ROS content and oxyblot analysis were generated with GraphPad Prism (GraphPad Software Inc.). We used a sigmoidal dose-response model with a top constraint at 100% to draw the curves and determine the LD50 or the average lifespan values. Statistical analysis of significance was carried out by one-way analysis of variance (ANOVA) for the dose-response curves, longevity curves and ROS content, followed by post-hoc Bonferroni test when the overall p value was < 0.05. Unpaired two-tailed T-test was used to assess statistical differences in mean values. In all figures, error bars represent the standard errors of the means (SEM).
Results
Survival rate and life span after organochalcogens exposure
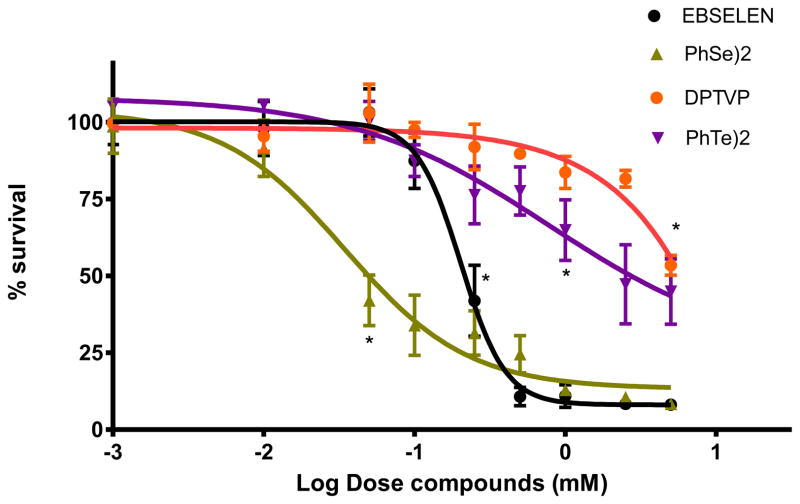
As shown in Figure 2, the LD50 for (PhSe)2 and ebselen corresponded to 0.05 and 0.21 mM, respectively. Worms showed higher tolerance to organotellurium vs. organoselenium compounds, with LD50 of 2.69 and ~10 mM for (PhTe)2 and DPTVP, respectively. Due to its high toxicity in rodent models, (PhTe)2 was used in this study only to obtain the LD50 for comparison with DPTVP. Analogous to a previous rodent study, (PhTe)2 was significantly more toxic vs. DPTVP [27].
Figure 2.
Dose-response curves for LD50 determination after acute treatment (30 min): black line depicts ebselen, yellow line (PhSe)2, purple line (PhTe)2 and orange line DPTVP. Data are expressed as mean± SEM. * indicates the lowest dose where the effect is statistically significant (p<0.05) vs. the control group.
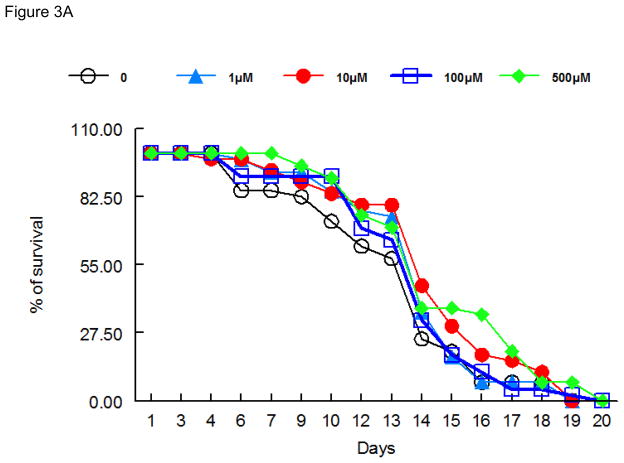
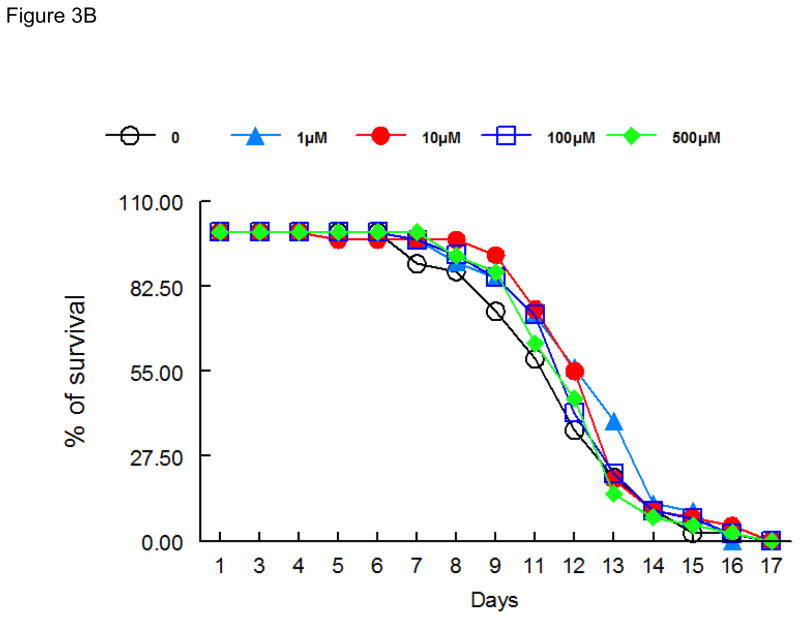
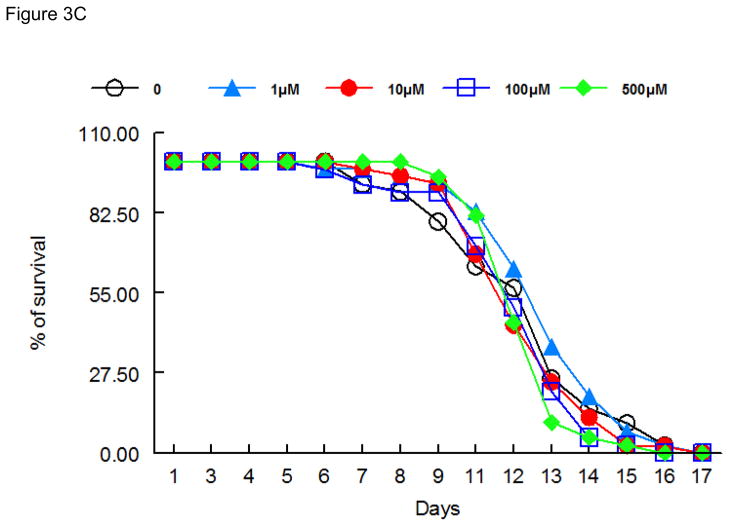
As shown in Figure 3, lifespan after 48 h exposure to ebselen (Figure 3A), (PhSe)2 (Figure 3B) or DPTVP (Figure 3C) treatment, at all tested doses, was indistinguishable from controls, indicating that these compounds do not increase worms’ mortality.
Figure 3.
Lifespan of worms exposed to organochalogens A) ebselen; B) (PhSe)2; C) DPTVP. Values are expressed as mean and * indicates p<0.05 as compared to control group.
Effects of sublethal doses of ebselen and DPTVP on Mn-induced toxicity
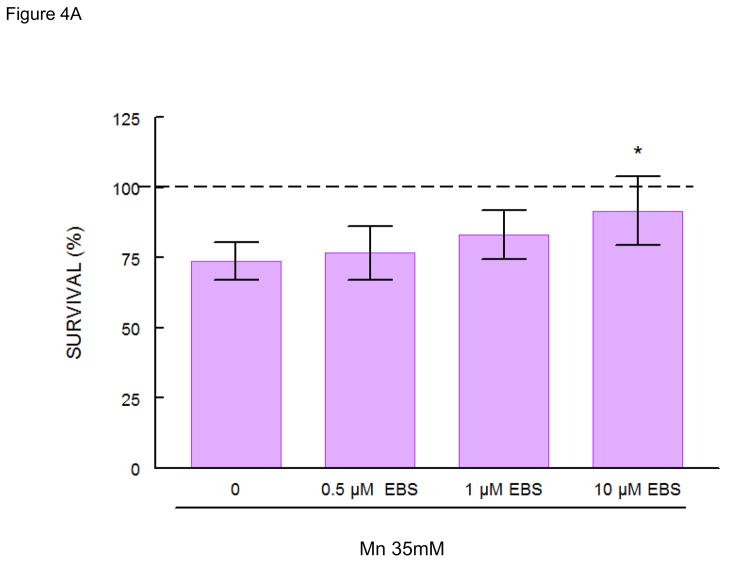
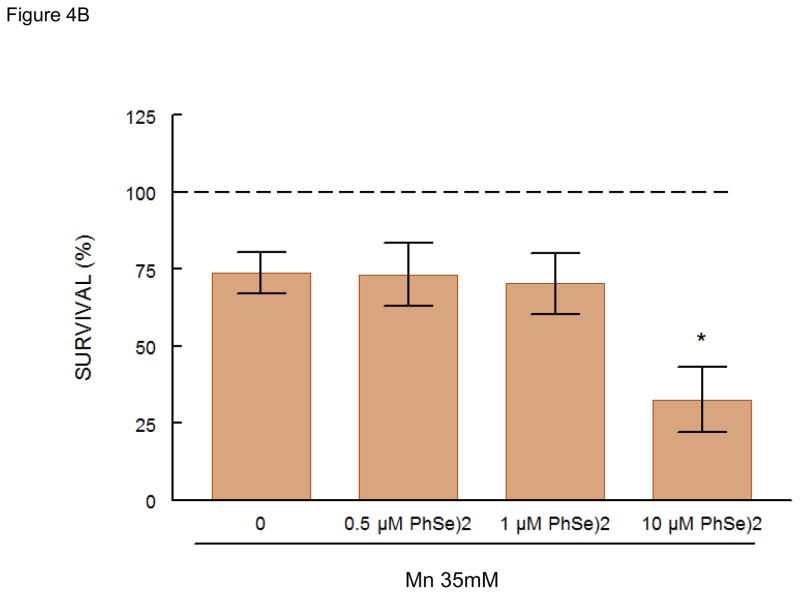
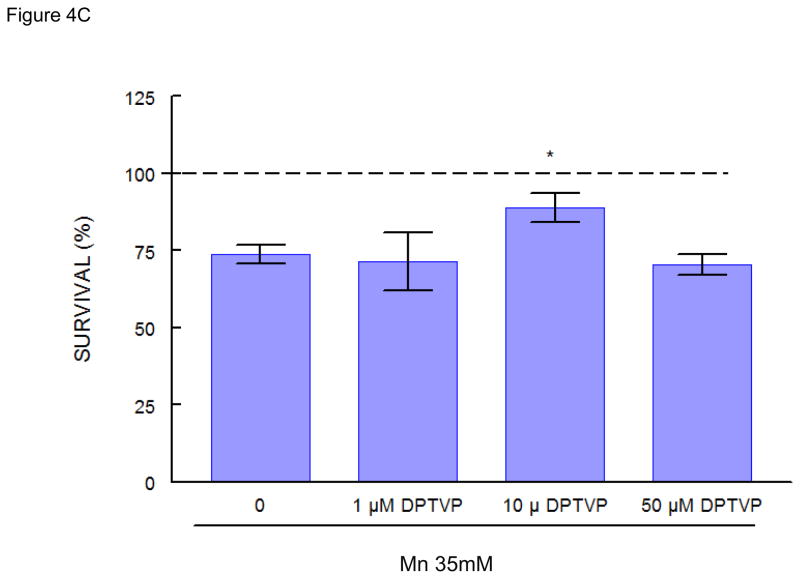
Mn (35 mM) caused ~25% decrease in worm survival. Pretreatment with sublethal doses of DPTVP (10 μM) protected the worms, increasing the survival rate to levels indistinguishable from the control group (Figure 4C, p<0.05 when compared to the Mn-treated group). Ebselen (10 μM) also protected against Mn-induced toxicity (Figure 4A, p<0.05 when compared to the Mn-treated group). Higher doses of ebselen caused an increase in Mn-induced toxicity, whereas higher DPTVP doses did not affect the worms’ survival (data not shown). In contrast, (PhSe)2 (10 μM) failed to protect against Mn-induced toxicity, causing a significant increase in the worms’ mortality (Figure 4B, p<0.05). Based on these data, for the next series of assays we chose to use the lower Mn doses which did not cause any toxic effects.
Figure 4.
C. elegans survival rates after 30 min Mn exposure (35 mM) and previous 30 min pre-treatment with organochalcogens A) Ebselen (1 μM); B) PhSe)2 (1 μM); C) DPTVP (10 μM). Data are expressed as mean± SEM. * indicates p<0.05 as compared to control group and # indicates difference from Mn- treated group.
Effects of ebselen, (PhSe)2 and DPTVP on Mn-induced aging
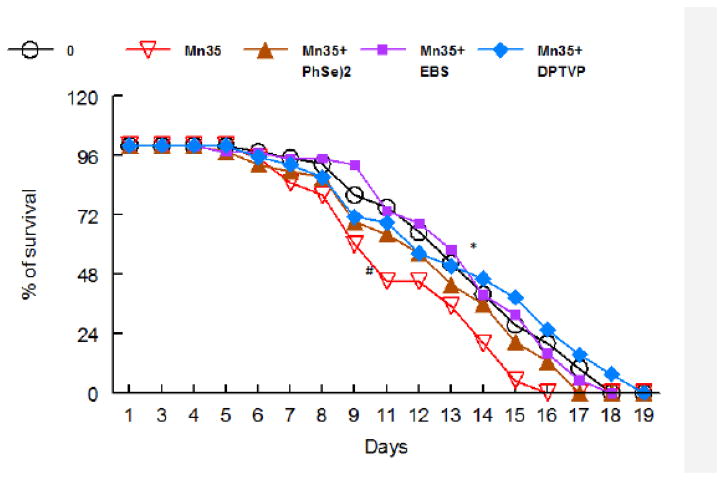
As previously noted [16], high Mn doses decreased the lifespan of exposed worms vs. controls (Figure 5, p<0.05). Ebselen, (PhSe)2 or DPTVP pre-treatments prolonged the lifespan in Mn–exposed worms (Figure 5, p<0.05 vs. the 35 mM Mn group).
Figure 5.
Ebselen (1 μM); B) PhSe)2 (1 μM); C) DPTVP (10 μM) restore C. elegans longevity upon Mn exposure (35 mM). Data are expressed as mean± SEM and statistical significance was assessed using Bonferroni test * indicates a significance of p<0.05 as compared to control group and # indicates difference from Mn- treated group.
Effects of ebselen and DPTVP on Mn-induced oxidative stress
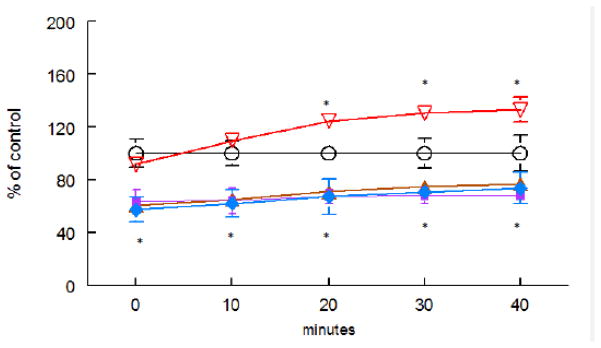
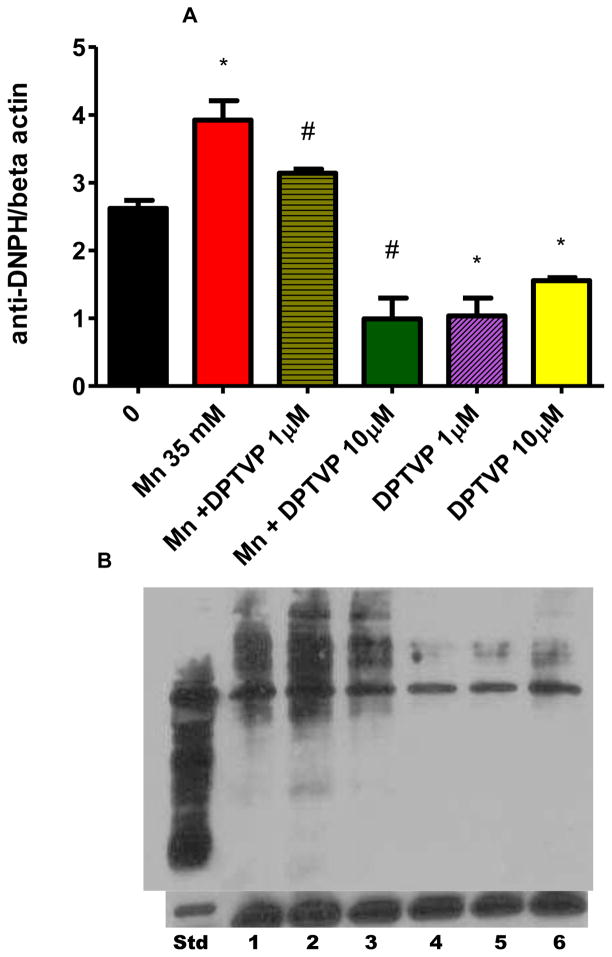
Since oxidative stress is associated with aging, next we characterized parameters of oxidative stress. ROS levels were determined with the DCF-DA dye, which is oxidized to the DCF-fluorophore in the presence of free radicals. Mn exposure caused a significant increase in DCF-DA oxidation from t=20 min, reflecting the generation of ROS (Figure 6, p<0.05). Pre-treatments with ebselen, (PhSe)2 or DPTVP significantly decreased ROS levels even in the control, Mn-untreated animals (Figure 6, p<0.01), resulting in a per se effect. Since DPTVP showed reduced toxic effects vs. ebselen and (PhSe)2, (Figure 2), we further characterized the antioxidant efficacy of DPTVP in Mn exposed animals by determining protein oxidation as a biochemical parameter. In Mn-treated worms, carbonyl levels were significantly increased compared to untreated worms (Figure 7, p<0.05). This increase was abolished by pre-treatment of the worms with 1 or 10 μM DPTVP (Figure 7, p<0.05, vs. the 35 mM Mn group). DPTVP alone showed a per se effect, given that the DPTVP group alone (1 and 10 μM) showed significantly lower carbonyl levels than control group (Figure 7, p< 0.05).
Figure 6.
Ebselen (1 μM); B) PhSe)2 (1 μM); C) DPTVP (10 μM) pre-treatment protects against Mn-induced ROS production in C. elegans. ROS was measured by DCF-DA. Black line depicts control values, red line Mn (35 mM), brown line Mn vs. (PhSe)2 (1 μM), purple line Mn vs. ebselen (1 μM), blue line Mn vs. DPTVP (10 μM). * indicates statistical difference (p<0.01) from the Mn (35 mM) group.
Figure 7.
DPTVP protects against Mn-induced protein carbonylation in C. elegans. DPTVP was administered 30 min prior to acute Mn exposure (30 min). Panel A shows the plotted values. Data are expressed as mean± SEM. * indicates p<0.05 as compared to control group and # indicates p<0.05 vs. the Mn-treated group. Panel B shows an example of a blot obtained in this experiment with β-actin as a control for protein content in each well and the standard (Std) from the kit to show the oxidized proteins. Lane 1- control; lane 2- Mn 35 mM; lane 3- Mn + DPTVP 1 μM; lane 4- Mn + DPTVP 10 μM; lane 5- DPTVP 1 μM; lane 6- DPTVP 10 μM.
Effects of DPTVP on DAF-16 translocation
Next, to better understand the mechanisms by which DPTVP mediates its antioxidant and anti-aging mechanisms, we investigated DAF-16::GFP subcellular localization upon DPTVP pre-treatment. The worm DAF-16 is orthologous to the Foxo transcription factor in humans, and plays a key role in regulating longevity. As shown in Figure 8A, DPTVP pre-treatment (1 or 10 μM) led to DAF-16::GFP translocation from the cytoplasm to the nucleus (diffuse staining in Figure 8A), consistent with the well-defined circular staining observed in treated worms [28] (Figures 8B–C).
Figure 8.
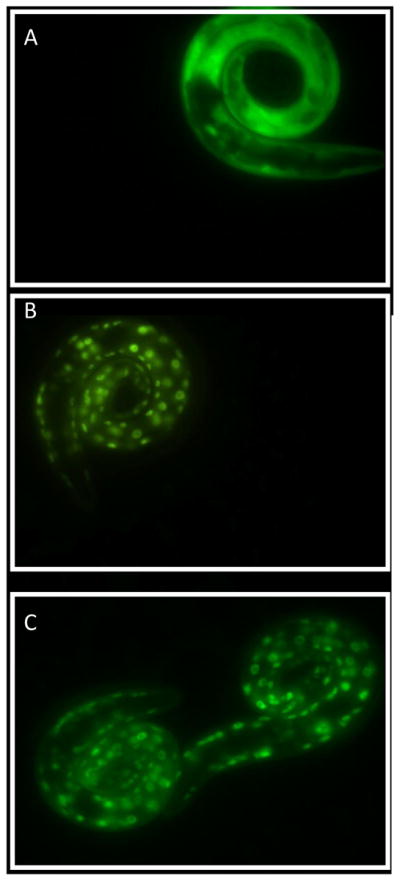
DPTVP causes nuclear migration of transcriptional factor DAF-16 in C. elegans. A) control; B) DPTVP 1 μM; C) DPTVP 10 μM.
Effects of organotellurium compounds on Mn levels
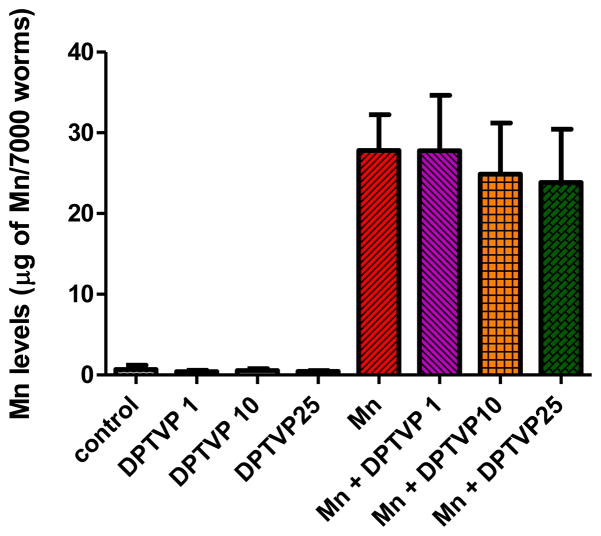
To determine whether DPTVP affects Mn levels in wild type worms, we measured Mn concentrations in whole animals. We observed no change in Mn levels upon DPTVP pretreatment compared to worms treated with Mn alone, indicating that DPTVP does not affect Mn uptake or release (Figure 9).
Figure 9.
DPTVP treatment does not alter Mn levels in N2 wild type worms. Data are expressed as mean± SEM.
Discussion
In the present study, we used the C. elegans model to investigate the antioxidant properties of organochalcogens within the context of a known environmental insult, Mn. We established that ebselen, and to a greater extent DPTVP, confers protection against Mn-induced aging and toxicity. Our results suggest that these effects are due to reduction in oxidative stress associated with the nuclear translocation of the transcription factor Foxo/DAF-16. Furthermore, they occur in the absence of significant changes in whole worm Mn concentrations.
Oxidative stress is associated with the pathogenesis of several diseases, is a sequelae of multiple chemical exposures and is known to modulate longevity in several experimental models [29–31]. Using mammalian models, researchers have studied the molecular basis of ROS generation, its cellular effects and the efficacy of various antioxidants in mitigating ROS-induced cellular damage. The potential for organochalcogens to offer efficacious treatment for disease models associated with oxidative stress has been of interest to several research groups [1–2, 23, 32–34]. The ROS scavenging activity and glutathione peroxidase mimetic property of the organochalcogens likely accounts for their efficacy in attenuating oxidative stress both in in vitro and in vivo rodent models [8, 32, 34–36]. Notably, ebselen was deemed a promising molecule and it entered clinical trials [37]. Its failure in these trials has led to the design and synthesis of novel organoselenium compounds with antioxidant properties for the development of potential therapeutic agents [38–39]. Among several of the newly tested molecules, DPTVP has been characterized as the most potent antioxidant, as well as a hepato and neuroprotective compound [4, 27, 40–41].
Previous studies have indicated that mice and rats show distinct responses to organochalcogens, with rats appearing more sensitive to organotellurium compounds [1]. Notably, diphenyl ditelluride afforded protection in adult mice (but not rats) against 4-aminopyridine-induced neurotoxicity and oxidative stress [42]. Our studies suggest that analogous to mice, C. elegans exhibited relatively low sensitivity to the toxic effects of organotellurium compounds (Figure 1). Furthermore, diphenyl diselenide was more toxic to C. elegans than its tellurium analog. The rationale for the differential species sensitivity to diphenyl diselenide has yet to be determined; however, differences in the rate of metabolism and formation of toxic metabolites likely underlines this differential sensitivity [1].
In worms surviving the acute high-dose exposure to ebselen and (PhSe)2, the toxic effect were short-lasting (Figures 3A and 3B), with lifespan in treated and untreated worms revealing no significant differences, even when ebselen and (PhSe)2 were applied at doses as high as 100 μM. The same trend was also observed for the DPTVP curve (Figure 3C). Given these observations and the lack of chronic sequlae, we opted to study the efficacy of these compounds in attenuating Mn-induced toxicity in C. elegans.
Mn is an essential metal and important for brain development and the functioning of multiple enzymes, such as Mn-SOD and glutamine synthase [43–44]. Nevertheless, exposure to high levels of this metal leads to its preferential accumulation in basal ganglia [43–44], resulting in a parkinsonian-like symptoms [44]. Mn can oxidize dopamine and lead to mitochondrial dysfunction, causing excessive ROS generation [45]. Accordingly, oxidative stress is one of the main features of Mn-induced toxicity. The C. elegans model replicates features inherent to mammalian Mn exposure [16–17, 46], including specificity to DAergic neurons, increased generation of oxidative stress, as well as reduced lifespan and morphological alterations in dopamine-containing neurons [16–17, 46–47].
In the present study, treatments with DPTVP or ebselen failed to extend the worms’ longevity per se, although both protected against Mn-induced decrease in lifespan (Figure 5). This finding is in agreement with Zhang et al. (2009) who demonstrated that the antioxidant epigallocathechin gallate failed to alter lifespan in N2 (wild type) worms under normal conditions (20°C), yet it increased the redox potential and survival rate in response to environmental stress [48]. Notably, DPTVP and ebselen also decreased the Mn-induced mortality (observed after 24 h exposure, Figure 4), providing additional evidence for the ability of these compounds to confer protection against Mn-induced toxicity.
DPTVP and ebselen afford this protection by decreasing oxidative stress in response to Mn exposure. ROS generation, assayed by means of DCF-DA dye fluorescence, was significantly decreased when worms were pre-treated with these compounds, both in the presence or absence of Mn (Figure 6). In agreement, protein oxidation levels were restored to control levels in worms treated with DPTVP (Figure 7). This reduction in protein oxidation levels and in ROS production suggests that DPTVP may act as a scavenging agent, as other antioxidants do in worms [48–49].
In addition, DPTVP may also act by modulating intracellular processes that modulate antioxidant defenses. Accordingly, we further investigated whether DPTVP caused the translocation of the transcription factor DAF-16 into the nucleus. DAF-16 is the worm homologue of human Foxo (forkhead box O proteins) belonging to the insulin/IGF-like signaling pathway, where it is the central determinant of the endocrine control of stress response, aging, fat metabolism, fertility and diapause [18, 50]. Upstream of DAF-16 is the surface receptor DAF-2; its activity (upon ligand binding) triggers a phosphorylation cascade. AKT is downstream of DAF-2 and when phosphorylated, AKT, in turn, phosphorylates DAF-16, preventing its nuclear translocation. When the DAF-2 receptor is inactivated or deleted, DAF-16 remains non-phosphorylated and therefore translocates into the nucleus, where it activates the transcription of pro-longevity genes [18, 50]. In addition, other pathways interfere with proteins upstream of DAF-16, thus affecting the translocation of this transcription factor [51]. Herein, we demonstrated for the first time that an organotellurium compound caused DAF-16 translocation from the cytosol to the nucleus. This finding indicates that organotellurium can modulate this pathway, likely by inducing the production of enzymatic antioxidants that are dependent upon DAF-16, such as superoxide dismutase (SOD) and glutathione peroxidase (GPX) [18]. In turn, these antioxidants can neutralize ROS generated in response to Mn, thus attenuating protein oxidation.
A previous study in rats exposed to Mn for 4 months and thereafter co-treated with DPTVP for 2 weeks revealed a decrease in Mn levels in the striatum of these animals compared with controls [4]. Analogous to the present findings in C. elegans, there was decreased oxidative stress in rats treated with DPTVP. Accordingly, we investigated whether Mn levels would be decreased in worms in response to DPTVP pre-treatment. The results showed no change upon such treatment, with levels indistinguishable from Mn-treated wild type worms. Accordingly, tellurium, the metal inherent to DPTVP, does not seem to compete with Mn for the same transporters (Figure 9). In fact, Wetli et al. demonstrated that ebselen decreased iron uptake in HEK293 cells; however this decrease was not mediated through DMT-1, which also transports Mn [52]. Thus, this study and ours indicate that uptake of Se and Te ions is not mediated via the DMT1.
Taken together, our study showed that in addition to a direct antioxidant effect [4, 41], pre-treatment with DPTVP caused DAF-16 nuclear translocation, protecting the worms from Mn-induced oxidative stress. Since aging is modulated by oxidative stress and the DAF-16 pathway [50], we posit that DPTVP possesses antioxidant and anti-aging properties, thus modulating the insulin/IGF-like signaling in C. elegans. Our findings illustrate the utility of the worm model in elucidating protective and toxic mechanisms as well as for the identification of pharmacological targets for antioxidant drug development. Future studies could be profitably directed at identifying additional targets for the organochalcogens and potential pathways that reduce oxidative damage and delay the aging process.
Highlights.
Ebselen and DPTVP showed antioxidant efficacy in C. elegans.
Manganese (Mn) led to increased protein oxidation and reduction in life span of worms after chronic exposure.
Ebselen and DPTVP increased life span in Mn-exposed worms.
DPTVP protected against Mn-induced toxicity by reducing protein oxidation.
DPTVP increased the translocation of the transcription factor DAF-16 into the nucleus.
Acknowledgments
Authors would like to acknowledge the financial support provided by grants from the NIEHS R01 ES 07731 and R01 ES 10563.
Footnotes
Conflict of interest
There was no conflict of interest in the preparation of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nogueira CW, Zeni G, Rocha JB. Organoselenium and organotellurium compounds: toxicology and pharmacology. Chem Rev. 2004;104:6255–6285. doi: 10.1021/cr0406559. [DOI] [PubMed] [Google Scholar]
- 2.Ba LA, Doring M, Jamier V, Jacob C. Tellurium: an element with great biological potency and potential. Organic & biomolecular chemistry. 8:4203–4216. doi: 10.1039/c0Ob00086h. [DOI] [PubMed] [Google Scholar]
- 3.Zeni G, Ludtke DS, Panatieri RB, Braga AL. Vinylic tellurides: from preparation to their applicability in organic synthesis. Chem Rev. 2006;106:1032–1076. doi: 10.1021/cr0505730. [DOI] [PubMed] [Google Scholar]
- 4.Avila DS, Colle D, Gubert P, Palma AS, Puntel G, Manarin F, Noremberg S, Nascimento PC, Aschner M, Rocha JB, Soares FA. A possible neuroprotective action of a vinylic telluride against Mn-induced neurotoxicity. Toxicol Sci. 2010;115:194–201. doi: 10.1093/toxsci/kfq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imai H, Graham DI, Masayasu H, Macrae IM. Antioxidant ebselen reduces oxidative damage in focal cerebral ischemia. Free Radic Biol Med. 2003;34:56–63. doi: 10.1016/s0891-5849(02)01180-2. [DOI] [PubMed] [Google Scholar]
- 6.Ineu RP, Pereira ME, Aschner M, Nogueira CW, Zeni G, Rocha JB. Diphenyl diselenide reverses gastric lesions in rats: Involvement of oxidative stress. Food Chem Toxicol. 2008;46:3023–3029. doi: 10.1016/j.fct.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Jesse CR, Savegnago L, Nogueira CW. Mechanisms involved in the antinociceptive and anti-inflammatory effects of bis selenide in mice. J Pharm Pharmacol. 2009;61:623–630. doi: 10.1211/jpp/61.05.0011. [DOI] [PubMed] [Google Scholar]
- 8.Briviba K, Tamler R, Klotz LO, Engman L, Cotgreave IA, Sies H. Protection by organotellurium compounds against peroxynitrite-mediated oxidation and nitration reactions. Biochem Pharmacol. 1998;55:817–823. doi: 10.1016/s0006-2952(97)00542-x. [DOI] [PubMed] [Google Scholar]
- 9.Engman L, Kandra T, Gallegos A, Williams R, Powis G. Water-soluble organotellurium compounds inhibit thioredoxin reductase and the growth of human cancer cells. Anticancer Drug Des. 2000;15:323–330. [PubMed] [Google Scholar]
- 10.Scambia G, Panici PB, Battaglia F, Ferrandina G, Gaggini C, Mancuso S. Growth inhibitory effect of diheptyl diselenide on various human cancer cell lines. Anticancer Res. 1989;9:1697–1699. [PubMed] [Google Scholar]
- 11.Posser T, de Paula MT, Franco JL, Leal RB, da Rocha JB. Diphenyl diselenide induces apoptotic cell death and modulates ERK1/2 phosphorylation in human neuroblastoma SH-SY5Y cells. Arch Toxicol. doi: 10.1007/s00204-010-0602-0. [DOI] [PubMed] [Google Scholar]
- 12.Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 13.Gerstein MB, Lu ZJ, Van Nostrand EL, Cheng C, Arshinoff BI, Liu T, Yip KY, Robilotto R, Rechtsteiner A, Ikegami K, Alves P, Chateigner A, Perry M, Morris M, Auerbach RK, Feng X, Leng J, Vielle A, Niu W, Rhrissorrakrai K, Agarwal A, Alexander RP, Barber G, Brdlik CM, Brennan J, Brouillet JJ, Carr A, Cheung MS, Clawson H, Contrino S, Dannenberg LO, Dernburg AF, Desai A, Dick L, Dose AC, Du J, Egelhofer T, Ercan S, Euskirchen G, Ewing B, Feingold EA, Gassmann R, Good PJ, Green P, Gullier F, Gutwein M, Guyer MS, Habegger L, Han T, Henikoff JG, Henz SR, Hinrichs A, Holster H, Hyman T, Iniguez AL, Janette J, Jensen M, Kato M, Kent WJ, Kephart E, Khivansara V, Khurana E, Kim JK, Kolasinska-Zwierz P, Lai EC, Latorre I, Leahey A, Lewis S, Lloyd P, Lochovsky L, Lowdon RF, Lubling Y, Lyne R, MacCoss M, Mackowiak SD, Mangone M, McKay S, Mecenas D, Merrihew G, Miller DM, 3rd, Muroyama A, Murray JI, Ooi SL, Pham H, Phippen T, Preston EA, Rajewsky N, Ratsch G, Rosenbaum H, Rozowsky J, Rutherford K, Ruzanov P, Sarov M, Sasidharan R, Sboner A, Scheid P, Segal E, Shin H, Shou C, Slack FJ, Slightam C, Smith R, Spencer WC, Stinson EO, Taing S, Takasaki T, Vafeados D, Voronina K, Wang G, Washington NL, Whittle CM, Wu B, Yan KK, Zeller G, Zha Z, Zhong M, Zhou X, Ahringer J, Strome S, Gunsalus KC, Micklem G, Liu XS, Reinke V, Kim SK, Hillier LW, Henikoff S, Piano F, Snyder M, Stein L, Lieb JD, Waterston RH. Integrative analysis of the Caenorhabditis elegans genome by the modENCODE project. Science. 2010;330:1775–1787. doi: 10.1126/science.1196914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helmcke KJ, Avila DS, Aschner M. Utility of Caenorhabditis elegans in high throughput neurotoxicological research. Neurotoxicol Teratol. 2010;32:62–67. doi: 10.1016/j.ntt.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Peterson RT, Nass R, Boyd WA, Freedman JH, Dong K, Narahashi T. Use of non-mammalian alternative models for neurotoxicological study. Neurotoxicology. 2008;29:546–555. doi: 10.1016/j.neuro.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benedetto A, Au C, Avila DS, Milatovic D, Aschner M. Extracellular dopamine potentiates mn-induced oxidative stress, lifespan reduction, and dopaminergic neurodegeneration in a BLI-3-dependent manner in Caenorhabditis elegans. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Settivari R, Levora J, Nass R. The divalent metal transporter homologues SMF-1/2 mediate dopamine neuron sensitivity in caenorhabditis elegans models of manganism and parkinson disease. J Biol Chem. 2009;284:35758–35768. doi: 10.1074/jbc.M109.051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumeister R, Schaffitzel E, Hertweck M. Endocrine signaling in Caenorhabditis elegans controls stress response and longevity. J Endocrinol. 2006;190:191–202. doi: 10.1677/joe.1.06856. [DOI] [PubMed] [Google Scholar]
- 19.Lee SS, Kennedy S, Tolonen AC, Ruvkun G. DAF-16 target genes that control C. elegans life-span and metabolism. Science. 2003;300:644–647. doi: 10.1126/science.1083614. [DOI] [PubMed] [Google Scholar]
- 20.Murphy CT. The search for DAF-16/FOXO transcriptional targets: approaches and discoveries. Exp Gerontol. 2006;41:910–921. doi: 10.1016/j.exger.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 21.Braga AL, Alves EF, Silveira CC, Andrade LH. Stereoselective addition of sodium organyl chalcogenolates to alkynylphosphonates: synthesis of diethyl 2-(organyl)-2-(organochalcogenyl)vinylphosphonates. Tetrahedron Letters. 2000;41:161–163. [Google Scholar]
- 22.Haller WS, Irgolic KJ. Diaryl ditellurides from grignard-reagents and elemental tellurium. Journal of organonetallic chemistry. 1972;39:97–101. [Google Scholar]
- 23.Paulmier C. Selenoorganic functional groups. In: Paulmier C, editor. Selenium Reagents and Intermediates in Organic Synthesis. Oxford: Pergamom Press; 1986. [Google Scholar]
- 24.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stiernagle T. Maintenance of C. elegans. In: Hope IA, editor. C elegans: A Practical Approach. New York: Oxford University Press; 1999. [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.de Avila DS, Beque MC, Folmer V, Braga AL, Zeni G, Nogueira CW, Soares FA, Rocha JB. Diethyl 2-phenyl-2 tellurophenyl vinylphosphonate: an organotellurium compound with low toxicity. Toxicology. 2006;224:100–107. doi: 10.1016/j.tox.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 28.Schafer JC, Winkelbauer ME, Williams CL, Haycraft CJ, Desmond RA, Yoder BK. IFTA-2 is a conserved cilia protein involved in pathways regulating longevity and dauer formation in Caenorhabditis elegans. J Cell Sci. 2006;119:4088–4100. doi: 10.1242/jcs.03187. [DOI] [PubMed] [Google Scholar]
- 29.Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 30.Toda T, Nakamura M, Morisawa H, Hirota M, Nishigaki R, Yoshimi Y. Proteomic approaches to oxidative protein modifications implicated in the mechanism of aging. Geriatr Gerontol Int. 2010;10(Suppl 1):S25–31. doi: 10.1111/j.1447-0594.2010.00606.x. [DOI] [PubMed] [Google Scholar]
- 31.Simmons SO, Fan CY, Ramabhadran R. Cellular stress response pathway system as a sentinel ensemble in toxicological screening. Toxicol Sci. 2009;111:202–225. doi: 10.1093/toxsci/kfp140. [DOI] [PubMed] [Google Scholar]
- 32.Engman L, Al-Maharik N, McNaughton M, Birmingham A, Powis G. Thioredoxin reductase and cancer cell growth inhibition by organotellurium compounds that could be selectively incorporated into tumor cells. Bioorg Med Chem. 2003;11:5091–5100. doi: 10.1016/j.bmc.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 33.Parnham M, Sies H. Ebselen: prospective therapy for cerebral ischaemia. Expert Opin Investig Drugs. 2000;9:607–619. doi: 10.1517/13543784.9.3.607. [DOI] [PubMed] [Google Scholar]
- 34.Sies H, Arteel GE. Interaction of peroxynitrite with selenoproteins and glutathione peroxidase mimics. Free Radic Biol Med. 2000;28:1451–1455. doi: 10.1016/s0891-5849(00)00253-7. [DOI] [PubMed] [Google Scholar]
- 35.Sarma BK, Mugesh G. Antioxidant activity of the anti-inflammatory compound ebselen: a reversible cyclization pathway via selenenic and seleninic acid intermediates. Chemistry. 2008;14:10603–10614. doi: 10.1002/chem.200801258. [DOI] [PubMed] [Google Scholar]
- 36.Sausen de Freitas A, de Souza Prestes A, Wagner C, Haigert Sudati J, Alves D, Oliveira Porciuncula L, Kade IJ, Teixeira Rocha JB. Reduction of diphenyl diselenide and analogs by mammalian thioredoxin reductase is independent of their gluthathione peroxidase-like activity: a possible novel pathway for their antioxidant activity. Molecules. 2010;15:7699–7714. doi: 10.3390/molecules15117699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, Yasuhara H. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke. 1998;29:12–17. doi: 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa A, Yoshimoto T, Kikuchi H, Sano K, Saito I, Yamaguchi T, Yasuhara H. Ebselen in acute middle cerebral artery occlusion: a placebo-controlled, double-blind clinical trial. Cerebrovasc Dis. 1999;9:112–118. doi: 10.1159/000015908. [DOI] [PubMed] [Google Scholar]
- 39.Saito I, Asano T, Sano K, Takakura K, Abe H, Yoshimoto T, Kikuchi H, Ohta T, Ishibashi S. Neuroprotective effect of an antioxidant, ebselen, in patients with delayed neurological deficits after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1998;42:269–277. doi: 10.1097/00006123-199802000-00038. discussion 277–268. [DOI] [PubMed] [Google Scholar]
- 40.Avila DS, Gubert P, Dalla Corte CL, Alves D, Nogueira CW, Rocha JB, Soares FA. A biochemical and toxicological study with diethyl 2-phenyl-2-tellurophenyl vinylphosphonate in a sub-chronic intraperitoneal treatment in mice. Life Sci. 2007;80:1865–1872. doi: 10.1016/j.lfs.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 41.Avila DS, Gubert P, Palma A, Colle D, Alves D, Nogueira CW, Rocha JB, Soares FA. An organotellurium compound with antioxidant activity against excitotoxic agents without neurotoxic effects in brain of rats. Brain Res Bull. 2008;76:114–123. doi: 10.1016/j.brainresbull.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 42.Brito VB, Rocha JB, Folmer V, Erthal F. Diphenyl diselenide and diphenyl ditelluride increase the latency for 4-aminopyridine-induced chemical seizure and prevent death in mice. Acta biochimica Polonica. 2009;56:125–134. [PubMed] [Google Scholar]
- 43.Aschner M, Erikson KM, Herrero Hernandez E, Tjalkens R. Manganese and its role in Parkinson’s disease: from transport to neuropathology. Neuromolecular Med. 2009;11:252–266. doi: 10.1007/s12017-009-8083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benedetto A, Au C, Aschner M. Manganese-induced dopaminergic neurodegeneration: insights into mechanisms and genetics shared with Parkinson’s disease. Chem Rev. 2009;109:4862–4884. doi: 10.1021/cr800536y. [DOI] [PubMed] [Google Scholar]
- 45.Graumann R, Paris I, Martinez-Alvarado P, Rumanque P, Perez-Pastene C, Cardenas SP, Marin P, Diaz-Grez F, Caviedes R, Caviedes P, Segura-Aguilar J. Oxidation of dopamine to aminochrome as a mechanism for neurodegeneration of dopaminergic systems in Parkinson’s disease. Possible neuroprotective role of DT-diaphorase. Pol J Pharmacol. 2002;54:573–579. [PubMed] [Google Scholar]
- 46.Au C, Benedetto A, Anderson J, Labrousse A, Erikson K, Ewbank JJ, Aschner M. SMF-1, SMF-2 and SMF-3 DMT1 orthologues regulate and are regulated differentially by manganese levels in C. elegans. PLoS One. 2009;4:e7792. doi: 10.1371/journal.pone.0007792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao J, Rui Q, Guo Y, Chang X, Wang D. Prolonged manganese exposure induces severe deficits in lifespan, development and reproduction possibly by altering oxidative stress response in Caenorhabditis elegans. J Environ Sci (China) 2009;21:842–848. doi: 10.1016/s1001-0742(08)62350-5. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, Jie G, Zhang J, Zhao B. Significant longevity-extending effects of EGCG on Caenorhabditis elegans under stress. Free Radic Biol Med. 2009;46:414–421. doi: 10.1016/j.freeradbiomed.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 49.Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 51.Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wetli HA, Buckett PD, Wessling-Resnick M. Small-molecule screening identifies the selanazal drug ebselen as a potent inhibitor of DMT1-mediated iron uptake. Chem Biol. 2006;13:965–972. doi: 10.1016/j.chembiol.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]