Abstract
Objective
To investigate whether elevated urinary levels of vascular endothelial growth factor (VEGF), carbonic anhydrase 9 (CA9) and angiogenin are associated with BCa.
Methods
This is a case-control study in which voided urines from 127 patients: control subjects (n = 63) and tumor bearing subjects (n = 64) were analyzed. The urinary concentrations of VEGF, CA9, angiogenin and BTA were assessed by enzyme-linked immunosorbent assay (ELISA). We used the area under the curve (AUC) of receiver operating characteristic curves to determine the ability of VEGF, CA9, and angiogenin to detect BCa in voided urine samples. Data were also compared to a commercial ELISA-based BCa detection assay (BTA-Trak©). Sensitivity, specificity, positive and negative predictive values were calculated.
Results
Urinary concentrations of VEGF, CA9, angiogenin and BTA were significantly elevated in BCa. VEGF was the most accurate urinary biomarker (AUC: 0.886; 95% confidence interval [CI]: 0.8301–0.9418). Furthermore, multivariate regression analysis highlighted VEGF (OR: 5.90; 95% CI: 2.60–13.40, p < 0.0001) as an independent variable. The sensitivities and specificities for VEGF (sensitivity, 83% and specificity, 87%) outperformed BTA (sensitivity, 80% and specificity, 84%).
Conclusions
VEGF may be a valuable addition to voided urine sample analysis for the detection of BCa. Larger, prospective studies are needed to determine the clinical utility of urinary VEGF and angiogenin as biomarkers in the non-invasive evaluation of BCa patients.
Keywords: angiogenin, bladder cancer, biomarkers, diagnosis, VEGF
Introduction
Cystoscopy is the gold standard for evaluation of individuals in whom bladder cancer (BCa) is suspected, and for surveillance in patients with a history of BCa. The procedure can be uncomfortable and can lead to problems with compliance, and it does have a false-negative rate of 10–40% [1]. For these reasons, multiple tools to aid in the diagnosis of BCa, including urine-based assays are desirable. Voided urinary cytology (VUC) has been widely accepted as an adjunct to cystoscopy. While VUC has a specificity of >93%, its sensitivity is disappointing at 25–40%, especially for low-grade and low-stage tumors [2]. Cytology may be complemented with a voided urine biomarker with better performance characteristics for low-grade tumors, but the identification of accurate urinary biomarkers for the detection of BCa remains a challenge. To look for molecular markers with clinical utility we have performed genomic profiling [3] and proteomic profiling [4] of voided urine sample components and have identified diagnostic signatures that achieved high levels of accuracy in independent validation cohorts. We are the first group to then integrate our extensive genomic’s and proteomic’s data to generate a diagnostic signature comprised of 14 candidate biomarkers. In this study, we tested the potential clinical utility of three candidate biomarkers, vascular endothelial growth factor (VEGF), carbonic anhydrase 9 (CA9) and angiogenin, by measuring target protein levels in naturally voided urine samples obtained from a cohort of 127 subjects.
Materials and Methods
Specimen and Data Collection
Under Institutional Review Board approval and informed consent (IRB# 560–2006), voided urine samples, and associated clinical information were prospectively collected from 63 consecutive individuals without BCa and 64 consecutive individuals with newly diagnosed BCa. Median follow-up of our cohort was 11.5 months. This cohort of 127 subjects served as our phase II (validation) study (proteomic’s discovery [4] and genomic’s discover (manuscript under review) served as our feasibility phase I study) according to the International Consensus Panel on Bladder Tumor Markers [5] and data was reported based on the REMARK criteria [6].
All subjects were evaluated in the outpatient Urology clinic. In our cancer group, axial imaging of the abdomen and pelvis and cystoscopy were performed, and urothelial carcinoma was confirmed by histological examination of excised tissue. Fifty milliliters of urine was sent to the clinical laboratory for urinary cytology. In our control group, the patients presented with voiding symptoms (65%) or microscopic hematuria (35%). (None of the controls had a history of any type of malignancy.) All controls underwent cystoscopy and subjects with microscopic hematuria also underwent axial imaging of the abdomen and pelvis. All of the evaluations of the control group was negative. Pertinent information on clinical presentation, histologic grading, staging and outcome were recorded (Table 1). Clinical staging was reported in accordance with the 2003 TNM classification [7] and assigned a grade according to the World Health Organization classification [8].
Table 1.
Demographic, clinicopathologic characteristics and concentration of urinary proteins in the study cohort
| Non-cancer (%) N=63 |
cancer (%) N=64 |
||||
|---|---|---|---|---|---|
| Median Age (range, y) | 60 | (30–81) | 69.5 | (22–90) | |
| Male : Female ratio | 55 : 8 | 55 : 9 | |||
| Race | |||||
| White | 41 | (65) | 58 | (91) | |
| African American | 8 | (13) | 0 | (0) | |
| Other | 14 | (22) | 6 | (9) | |
| Tobacco use | 25 | (40) | 54 | (84) | |
| Gross hematuria | 1 | (2) | 47 | (73) | |
| Suspicious/positive cytology | 1 | (2) | 18 | (28) | |
| Median follow-up (months) | 11.5 | 12.0 | |||
| Clinical stage | |||||
| Tis^ | n/a | 6 | (9) | ||
| Ta | n/a | 15 | (23) | ||
| T1 | n/a | 9 | (14) | ||
| T2 | n/a | 31 | (48) | ||
| T3 | n/a | 4 | (6) | ||
| T4 | n/a | 2 | (3) | ||
| N+ ~ | n/a | 3 | (5) | ||
| Grade | |||||
| Low | n/a | 9 | (14) | ||
| High | n/a | 55 | (86) | ||
| Urinary Proteins | Median (range) | Median (range) | |||
| VEGF | (pg/ml) | 0 | (0 – 904.76) | 335.34 | (0 – 9841.4) |
| Angiogenin | (pg/ml) | 44.58 | (20.48–696.18) | 410.98 | (3.28 – 17944) |
| CA9 | (pg/ml) | 0 | (0 – 28.28) | 10.36 | (0 – 4132.9) |
| BTA | (U/ml) | 12.55 | (0.5 – 36.87) | 179.34 | (0 – 24865.4) |
| Hemoglobin | (ng/ml) | 0 | (0 – 125.92) | 8.73 | (0 – 130367.5) |
4 subjects with concomitant cis had T1 (n=2) and T2 (n=2) disease
Subjects with T2 (n=1), T3 (n=1) and T4 (n=1) disease and node positive
Specimen processing and analysis
Prior to any type of therapeutic intervention, 50 mL of voided urine was obtained from each subject and assigned a unique identifying number before immediate delivery and laboratory processing. Each urine sample was centrifuged at 600 × g 4°C for 5 min. The supernatant was decanted and aliquoted, and the urinary pellet was snap frozen. Urine supernatant protein concentration was determined using Pierce 660-nm Protein Assay Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA).
Enzyme-linked Immunosorbent Assays for VEGF, CA9, Angiogenin, Hemoglobin and Bladder Tumor Antigen (BTA)
The levels of human VEGF (Cat # 100663 Abcam, Cambridge, MA, USA), human CA9 (Cat# DCA900 R&D Systems Inc., Minneapolis, MN, USA), human Angiogenin (Cat# CK400 CellSciences, Canton, MA, USA), hemoglobin (Cat#E88-135 Bethyl Laboratories Inc., Montgomery, TX, USA), and BTA (BTA-Trak© Ca# 662150 Polymedco Inc. Cortlandt Manor, NY, USA) were monitored in urine samples using enzyme-linked immunosorbent assays. The assays were conducted according to the manufacturer’s instructions. Calibration curves were prepared using purified standards for each protein assessed. Curve fitting was accomplished by either linear or four-parameter logistic regression following manufacturer’s instructions.
Creatinine Assay
The relative constant production of creatinine, a non-enzymatically metabolite of creatine, makes urinary creatinine a useful tool for normalizing the levels of other molecules found in urine [9]. The concentration of urinary creatinine was measured using a commercially available enzymatic assay (Cat# KGE005 R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions. Briefly, urine supernatants were treated with alkaline picrate solution, which when creatinine is present, turns an orange-red color. Intensity of the color at 490 nm corresponds to the concentration of creatinine in the sample. Creatinine concentrations of unknown samples were calculated by comparison to a standard curve. Due to the unavoidable variability of voided urine with respect to total volume and time within the bladder, each biomarker was normalized to urinary creatinine.
Data Analysis
The association between a biomarker and BCa was tested using Wilcoxon rank sum test. Spearman rank correlation coefficients were used to examine the correlation between urinary tumor biomarker concentrations and urinary hemoglobin concentration. Nonparametric receiver operating characteristic (ROC) curves were generated in which the value for sensitivity is plotted against false-positive rate (1-specificity). We defined a diagnostic test (positive vs. negative) for BCa using a cutoff threshold for each biomarker. The optimal cutoff (Youden index) was selected to maximize the sum of the sensitivity and specificity [10]. The overall accuracy of a biomarker to predict BCa is defined as the average of the sensitivity and the specificity. To assess the independent association between biomarkers and BCa, logistic regression analysis was performed with BCa status (yes vs. no) as the response variable, and age, gender, VEGF, CA9, angiogenin, and BTA concentrations as the explanatory variables. Statistical significance in this study was set at p < 0.05 and all reported p values were 2-sided. All analyses were performed with SAS software version 9.1.3.
Results
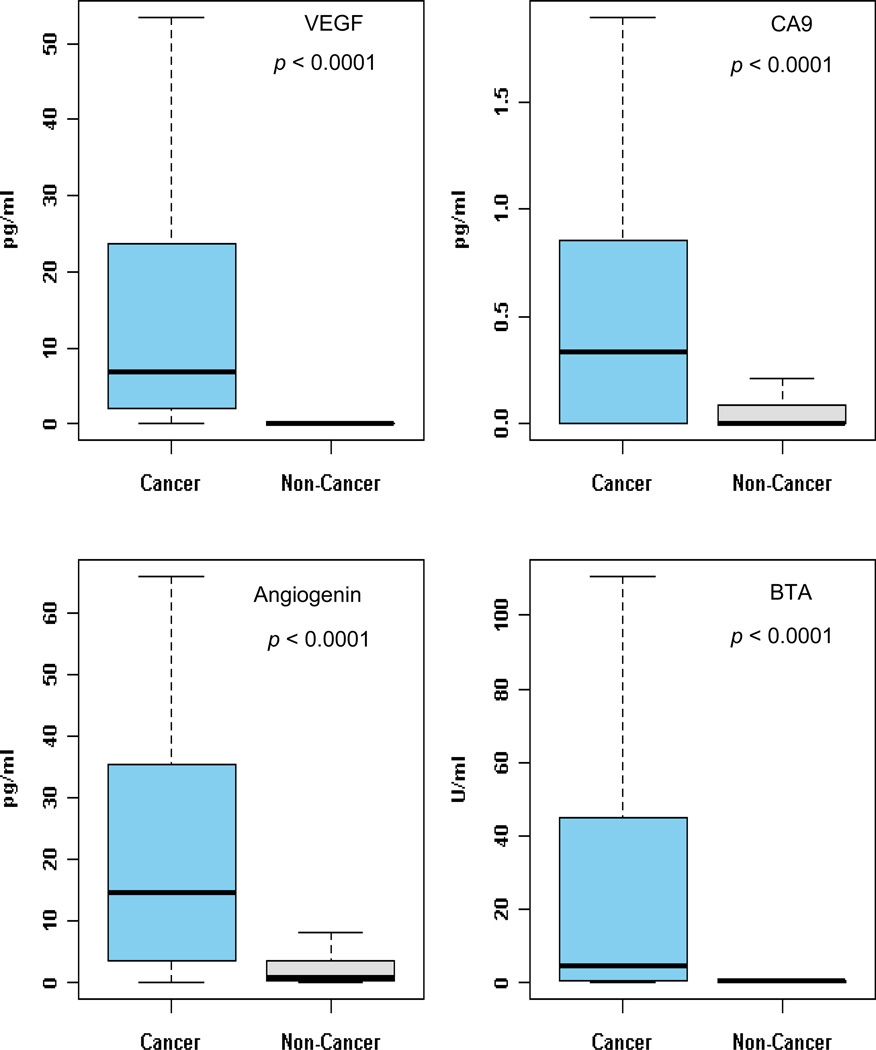
The cohort of 127 consisted of 64 subjects with active urinary BCa and 63 subjects without active BCa, history of BCa, gross hematuria, urolithiasis or urinary tract infection. Demographic, clinical and pathologic characteristics of both groups are illustrated in Table 1. Only 28% of the cancer cohort had a positive urinary cytology. Urinary VEGF, CA9 and hemoglobin levels were undetectable in the majority of subjects without BCa, whereas both angiogenin and BTA were detected in both groups. Median urinary levels were significantly higher in subjects with BCa compared to subjects without BCa for all biomarkers tested; VEGF (335.34 pg/ml vs. 0 pg/ml, p < 0.0001), CA9 (10.36 pg/ml vs. 0 pg/ml, p < 0.0001), angiogenin (410.98 pg/ml vs. 44.58 pg/ml, p < 0.0001) and BTA (179.34 U/ml vs. 12.55 U/ml, p < 0.0001). ELISA data are presented in a boxplot figure (Figure 1).
Figure 1.
Comparison of urine concentrations of Vascular Endothelial Growth Factor (VEGF), Carbonic Anhydrase 9 (CA9), Angiogenin and bladder tumor antigen (BTA) between the cancer and non-cancer groups. Data are normalized to urinary creatinine. Median levels are depicted by horizontal lines. Significance (p <0.05) was assessed by the Wilcoxon rank sum test.
The level of hematuria was assessed in all cases by performing a hemoglobin ELISA on all urine samples. The qualitative analyses revealed that 83% of BCa subjects were noted to have hemoglobin in their urine samples compared with only 23% of control subjects. The median urinary levels of hemoglobin in BCa subjects compared to control subjects were 8.73 ng/ml vs. <1.0 ng/ml, p = 0.005. Interestingly, of the four biomarkers assessed, BTA had the highest correlation coefficient with urinary hemoglobin (0.719).
The ability of the above biomarkers to predict the presence of BCa was analyzed using nonparametric ROC analyses, according to National Cancer Institute guidelines [11]. Based on the area under the ROC curve (AUROC) we determined Youden Index cutoff values to maximize the sum of sensitivity and specificity. Urinary VEGF was the most accurate biomarker to detect BCa in voided urine samples (area under the curve: 0.886; 95% CI: 0.8301–0.9418) (Figure 2). As would be expected, VEGF possessed the most favorable sensitivity, specificity, positive predictive value and negative predictive value. Using the Youden Index cutoff value (Figure 2), urinary VEGF provided a sensitivity of 83%, specificity of 87%, positive predictive value of 87% and negative predictive value of 83% (Figure 1). Urinary angiogenin was the next most accurate biomarker to detect BCa (area under the curve: 0.857; 95% CI: 0.7896–0.9252). Using the Youden Index cutoff value (Figure 2), urinary angiogenin analyses revealed a sensitivity of 67%, specificity of 97%, positive predictive value of 96% and negative predictive value of 74%. Of the three experimental biomarkers, urinary CA9 was the least favorable (area under the curve: 0.732; 95% CI: 0.6478–0.8165). Using the Youden Index cutoff value (Figure 2), urinary CA9 was found to have a sensitivity of 58%, specificity of 90%, positive predictive value of 86% and negative predictive value of 68%. Lastly, BTA served as our commercially available positive control test and was noted to have an AUROC of 0.818 (95% CI: 0.74–0.90). Using the Youden Index cutoff value, urinary BTA provided a sensitivity of 80%, specificity of 84%, positive predictive value of 84%, negative predictive value of 80%, and an overall accuracy of 82% (Figure 2).
Figure 2.
Receiver operating characteristic (ROC) curves for urinary VEGF, CA9, Angiogenin and BTA. Based on the area under the ROC curve (AUROC), Youden Index cutoff values that maximized the sum of sensitivity and specificity were determined for each biomarker (crossed square on curve). Table provides performance values for each biomarker. PPV, positive predictive value. NPV, negative predictive value.
In multivariate logistic regression analysis that adjusted for the effects of age and gender, only elevated urinary VEGF (OR: 5.90; 95% CI: 2.60–13.40; p < 0.0001) was determined to be an independent predictor of BCa. CA9, angiogenin and BTA levels were not significantly associated with BCa in the multivariate logistic regression analysis (Table 2). Analyses revealed VEGF as a promising urinary biomarker for BCa detection, outperforming BTA in both univariate and multivariate analyses.
Table 2.
Logistic regression analysis of biomarkers in voided urine
| Variable | Coefficient | Odds Ratio |
95% C.I. | p-value |
|---|---|---|---|---|
| BTA-Trak | 0.83 | 2.30 | 0.78–6.79 | 0.13 |
| VEGF | 1.77 | 5.90 | 2.60–13.40 | < 0.0001 |
| CA9 | 0.42 | 1.52 | 0.12–19.61 | 0.75 |
| Angiogenin | 0.45 | 1.57 | 0.75–3.30 | 0.23 |
Discussion
The targets chosen for this study were highlighted in our previous biomarker discovery studies where we performed proteomic analysis [4, 12] or genomic analyses [3] in the search for BCa biomarkers that are amenable to urinalysis. From these studies we were able to derive a highly accurate 14-target transcriptomic diagnostic signature that achieved a specificity of 100% at 90% sensitivity, and which included VEGF, angiogenin and CA9. In this study, we leveraged the availability of commercial antibodies to these candidate biomarkers to investigate whether quantitative measurement of these proteins could facilitate the non-invasive detection of BCa.
CA9 is a hypoxia-inducible member of the carbonic anhydrase family that regulates intracellular pH and has been associated with cell proliferation, cell adhesion, and tumor progression [13]. Bladder tissue is not thought to express CA9, even at low levels, however, CA9 expression may increase in response to hypoxia, or as a result of genetic aberrations in tumor cells [14]. High CA9 expression has been associated with aggressive tumor phenotypes and a poor prognosis [15, 16]. However, there have been only a few reports of CA9 expression BCa, and they contain conflicting results. Turner et al. reported immunohistochemical staining results in a small cohort of patients. In patients with non-muscle invasive BCa, CA9 expression was not an adverse prognostic marker, however in a muscle invasive BCa cohort, CA9 expression was an adverse prognostic marker [17]. In another study, researchers were not able to demonstrate a link between elevated CA9 expression and a more aggressive BCa phenotype [18]. In the largest study to date, researchers from UCLA reported that normal urothelial tissue were negative for CA9 expression, whereas 71% of BCa tissue expressed CA9 [19]. In our study, urinary CA9 levels were significantly elevated in the urines from BCa subjects compared to control subjects 10.36 pg/ml vs. 0 pg/ml (p < 0.0001), but of the three biomarkers tested, CA9 was the least promising.
VEGF is pivotal to neovascular growth that is required to sustain solid tumor progression [20]. Elevated urinary levels of VEGF have been previously demonstrated in BCa specimens. For example, in 26 patients with BCa, Crews et al. were able to correlate bladder tumor VEGF protein levels with the VEGF protein level in matched urines, suggesting that the source of urinary VEGF is tumor tissue [21]. In a study of 219 patients, urinary supernatants were analyzed for VEGF by ELISA and Western blot analysis. Patients with BCa had elevated urinary levels of VEGF, and an assay comparison revealed an improved diagnostic sensitivity over VUC, 76% vs. 70%, respectively [22]. Similarly, Bian demonstrated improved sensitivity of detecting BCa with urinary VEGF compared to VUC 69% vs. 38%, respectively[23]. In another study, patients with elevated urinary VEGF levels had a higher risk of disease recurrence [24]. In this study, we demonstrated that VEGF was a highly accurate biomarker for voided urine diagnosis of BCa. Urinary VEGF assays predicted the presence of BCa with a sensitivity of 83%, and a specificity of 87%.
Human angiogenin, expressed in both normal and malignant cells, is a potent angiogenic factor that is believed to initiate cell migration and aid in cellular proliferation. Angiogenin binds to a cell-surface actin, and this complex results in plasmin generation, which directly degrades the extracellular matrix facilitating cell migration and invasion [25,26]. Elevated serum and plasma angiogenin levels have been found in patients with a variety of cancers including BCa [27–29]. Eissa et al. analyzed the supernatants of voided urine samples from a diverse cohort of 97 patients by immunoassay. The median urinary angiogenin levels in BCa, benign urological disorders and healthy volunteer groups were; 802.7, 425 and 33 pg/ml, respectively[30]. In the current study, we were able to confirm these results with median urinary angiogenin levels of 410.98 pg/ml vs. 44.58 pg/ml in cancer vs. benign subjects, respectively. Urinary angiogenin had a respectable diagnostic capability; sensitivity of 67%, specificity of 68%, positive predictive value of 96% and negative predictive value of 74%.
Though compelling, our study has several limitations. First, we are a tertiary care facility that is preferentially referred high grade, higher stage disease, which is reflected in our cohort. To confirm the robustness, subsequent studies will assess more urines from subjects with low-grade, low-stage disease. Second, the sensitivity of urinary cytology in our cohort of predominantly high grade disease was lower than would be expected. This calls to question the known inter-observer variability of urinary cytology. Subsequent studies we will utilize two cytopathologists to interpret these results. Third, processed, banked urines were analyzed in this study. It is feasible that freshly voided urine samples results may differ to some extent. Next, our sample size of 127 is small and the two groups that comprised the 127 patient cohort were relatively homogeneous, i.e. either active cancer, or control with no active cancer, no history of cancer, no urinary tract infection, no urolithiasis and no gross hematuria. Lastly, we believe our global biomarker discovery and validation schema along with the analysis of a panel of viable biomarkers may prove to be beneficial in screening for BCa, assessing the symptomatic patients (i.e., voiding symptoms or hematuria) for BCa, or surveillance after tumor resection. These groups will be assessed in subject studies to elucidate sensitivity and specificity for each group.
Conclusions
We have demonstrated that urinary levels of VEGF and angiogenin can be indicative of BCa with an accuracy at least as high as that achieved using the BTA-Trak© assay. Further prospective studies in diverse cohorts are required to validate our findings.
Acknowledgments
Support/Financial Disclosures
Financial disclosures: Charles J. Rosser, Steve Goodison and Virginia Urquidi are affiliated with NonaGen Bioscience Corp, Orlando, FL, USA.
Funding/Support and role of the sponsor: This work was supported by research grants from Flight Attendant Medical Research Institute (CJR), Florida Department of Health James and Esther King Team Science Award 10KT-01 (CJR), and National Cancer Institute RO1 CA116161 (SG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zaak D, Kriegmair M, Stepp H, et al. Endoscopic detection of transitional cell carcinoma with 5-aminolevulinic acid: results of 1012 fluorescence endoscopies. Urology. 2001;57:690–694. doi: 10.1016/s0090-4295(00)01053-0. [DOI] [PubMed] [Google Scholar]
- 2.Rife CC, Farrow GM, Utz DC. Urine cytology of transitional cell neoplasms. Urol Clin North Am. 1979;6:599–612. [PubMed] [Google Scholar]
- 3.Rosser CJ, Liu L, Sun Y, et al. Bladder cancer-associated gene expression signatures identified by profiling of exfoliated urothelia. Cancer Epidemiol Biomarkers Prev. 2009;18:444–453. doi: 10.1158/1055-9965.EPI-08-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang N, Feng S, Shedden K, et al. Urinary Glycoprotein Biomarker Discovery for Bladder Cancer Detection using LC-MS/MS and Label-free Quantification. Clin Cancer Res. 2011;17:3349–3359. doi: 10.1158/1078-0432.CCR-10-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lokeshwar VB, Habuchi T, Grossman HB, et al. Bladder tumor markers beyond cytology: International Consensus Panel on bladder tumor markers. Urology. 2005;66:35–63. doi: 10.1016/j.urology.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 6.McShane LM, Altman DG, Sauerbrei W, et al. Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics. REporting recommendations for tumor MARKer prognostic studies (REMARK) Nat Clin Pract Urol. 2005;2:416–422. [PubMed] [Google Scholar]
- 7.Greene FL. AJCC Cancer Staging Manual. 6th ed. New York: Springer-Verlag; 2002. American Joint Committee on Cancer, American Cancer Society. [Google Scholar]
- 8.Montironi R, Lopez-Beltran A. The 2004 WHO classification of bladder tumors: a summary and commentary. Int J Surg Pathol. 2005;13:143–153. doi: 10.1177/106689690501300203. [DOI] [PubMed] [Google Scholar]
- 9.Pu FS. Urinary excretion of creatinine in normal subjects. Chinese Pharmaceutical Journal. 1992;44:235–240. [Google Scholar]
- 10.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47:458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 11.Pepe MS, Feng Z, Janes H, et al. Pivotal evaluation of the accuracy of a biomarker used for classification or prediction: standards for study design. J Natl Cancer Inst. 2008;100:1432–1438. doi: 10.1093/jnci/djn326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreunin P, Zhao J, Rosser C, et al. Bladder cancer associated glycoprotein signatures revealed by urinary proteomic profiling. J Proteome Res. 2007;6:2631–2639. doi: 10.1021/pr0700807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potter CP, Harris AL. Diagnostic, prognostic and therapeutic implications of carbonic anhydrases in cancer. Br J Cancer. 2003;89:2–7. doi: 10.1038/sj.bjc.6600936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanov S, Liao SY, Ivanova A, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trastour C, Benizri E, Ettore F, et al. HIF-1alpha and CA IX staining in invasive breast carcinomas: prognosis and treatment outcome. Int J Cancer. 2007;120:1451–1458. doi: 10.1002/ijc.22436. [DOI] [PubMed] [Google Scholar]
- 16.Loncaster JA, Harris AL, Davidson SE, et al. Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001;61:6394–6399. [PubMed] [Google Scholar]
- 17.Turner KJ, Crew JP, Wykoff CC, et al. The hypoxia-inducible genes VEGF and CA9 are differentially regulated in superficial vs invasive bladder cancer. Br J Cancer. 2002;86:1276–1282. doi: 10.1038/sj.bjc.6600215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussain SA, Palmer DH, Ganesan R, et al. Carbonic anhydrase IX, a marker of hypoxia: correlation with clinical outcome in transitional cell carcinoma of the bladder. Oncol Rep. 2004;11:1005–1010. [PubMed] [Google Scholar]
- 19.Klatte T, Seligson DB, Rao JY, et al. Carbonic anhydrase IX in bladder cancer: a diagnostic, prognostic, and therapeutic molecular marker. Cancer. 2009;115:1448–1458. doi: 10.1002/cncr.24163. [DOI] [PubMed] [Google Scholar]
- 20.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 21.Crew JP, O'Brien T, Bicknell R, et al. Urinary vascular endothelial growth factor and its correlation with bladder cancer recurrence rates. J Urol. 1999;161:799–804. [PubMed] [Google Scholar]
- 22.Eissa S, Salem AM, Zohny SF, et al. The diagnostic efficacy of urinary TGF-beta1 and VEGF in bladder cancer: comparison with voided urine cytology. Cancer Biomark. 2007;3:275–285. doi: 10.3233/cbm-2007-3601. [DOI] [PubMed] [Google Scholar]
- 23.Bian W, Xu Z. Combined assay of CYFRA21-1, telomerase and vascular endothelial growth factor in the detection of bladder transitional cell carcinoma. Int J Urol. 2007;14:108–111. doi: 10.1111/j.1442-2042.2007.01670.x. [DOI] [PubMed] [Google Scholar]
- 24.Jeon SH, Lee SJ, Chang SG. Clinical significance of urinary vascular endothelial growth factor in patients with superficial bladder tumors. Oncol Rep. 2001;8:1265–1267. doi: 10.3892/or.8.6.1265. [DOI] [PubMed] [Google Scholar]
- 25.Hu GF, Riordan JF. Angiogenin enhances actin acceleration of plasminogen activation. Biochem Biophys Res Commun. 1993;197:682–687. doi: 10.1006/bbrc.1993.2533. [DOI] [PubMed] [Google Scholar]
- 26.Badet J, Soncin F, Guitton JD, et al. Specific binding of angiogenin to calf pulmonary artery endothelial cells. Proc Natl Acad Sci U S A. 1989;86:8427–8431. doi: 10.1073/pnas.86.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chopra V, Dinh TV, Hannigan EV. Serum levels of interleukins, growth factors and angiogenin in patients with endometrial cancer. J Cancer Res Clin Oncol. 1997;123:167–172. doi: 10.1007/BF01214669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyake H, Hara I, Yamanaka K, et al. Increased angiogenin expression in the tumor tissue and serum of urothelial carcinoma patients is related to disease progression and recurrence. Cancer. 1999;86:316–324. [PubMed] [Google Scholar]
- 29.Zhao H, Grossman HB, Delclos GL, et al. Increased plasma levels of angiogenin and the risk of bladder carcinoma: from initiation ot recurrence. Cancer. 2005;104:30–35. doi: 10.1002/cncr.21136. [DOI] [PubMed] [Google Scholar]
- 30.Eissa S, Swellam M, Labib RA, et al. A panel of angiogenic factors for early bladder cancer detection: enzyme immunoassay and Western blot. J Urol. 2009;181:1353–1360. doi: 10.1016/j.juro.2008.10.102. [DOI] [PubMed] [Google Scholar]