Abstract
The growth of airway and vascular smooth muscle cells occurs in various lung diseases including asthma, chronic obstructive pulmonary disease, bronchopulmonary dysplasia, lymphangioleiomyomatosis, and pulmonary hypertension. While inflammatory responses are critical in these diseases, the relationship between smooth muscle cell growth and inflammatory mediators is poorly understood. The present study demonstrates that PDGF promotes the expression of IL-13 in lung smooth muscle cells through an oxidant signaling mechanism. Treatment of cultured human airway or pulmonary vascular smooth muscle cells with PDGF promotes IL-13 mRNA and protein expression. IL-13 expression is also detected in smooth muscle of airways and pulmonary vessels in allergen-stimulated mice. PDGF activates the proximal 980 bp region of the IL-13 promoter. PDGF-induced IL-13 expression is suppressed by the inhibition of reactive oxygen species signaling such as by NAD(P)H oxidase inhibition, reactive oxygen species scavenging, and metal-chelation. Treatment of cells with hydrogen peroxide as low as 1 μM also promotes IL-13 gene expression. PDGF-induced cell growth is suppressed by the neutralizing antibody against IL-13 as well as by reactive oxygen inhibitors, and recombinant IL-13 promotes the growth of airway smooth muscle cells. These results demonstrate that oxidant signaling activates IL-13 gene transcription in lung smooth muscle cells and that this signaling mechanism regulates cell growth.
Keywords: Cell growth, Interleukin-13, Lung, Reactive oxygen species, Smooth muscle
Introduction
The growth of smooth muscle cells (SMCs) plays an important role in various lung diseases, including pulmonary hypertension, asthma, chronic obstructive pulmonary disease, bronchopulmonary dysplasia, and lymphangioleiomyomatosis. An increased pulmonary artery SMC mass contributes to the development of pulmonary vascular remodeling, which is a critical component of pulmonary hypertension (1). The growth of airway SMCs is also a major pathogenic feature of asthma, and it is thought to contribute to increased airway resistance (2). There is a definite role of inflammation in the pathogenesis of asthma (3), and accumulating evidence suggests the importance of inflammation in pulmonary hypertension (4). The relationships between lung SMC growth and inflammation are, however, poorly understood.
Platelet-derived growth factor (PDGF) exists as a hetero- or homo-dimer and stimulates the proliferation of SMCs, fibroblasts, and endothelial cells. PDGF is produced by various cell types including SMCs, monocytes/macrophages, endothelial cells, fibroblasts, epithelial cells, and eosinophils (5–8). PDGF mediates the development of airway smooth muscle thickening in asthmatics (8–11). Hirota et al. (12) recently reported that PDGF-BB is sufficient to induce airway SMC hyperplasia and change lung mechanics in mice. PDGF also plays an important role in lung remodeling observed in idiopathic pulmonary fibrosis (13) and pulmonary hypertension (14). Tyrosine kinase inhibitors such as imatinib, which inhibit PDGF signaling, have been shown to reverse pulmonary vascular remodeling and pulmonary hypertension (15,16).
Interleukin-13 (IL-13) is a cytokine produced by T-helper-2 (Th2) cells and is an important mediator of allergic inflammation. IL-13 acts through the IL-13Rα1/IL-4Rα complex to directly transduce signals in hematopoietic as well as non-hematopoietic cells including B cells, monocytes, eosinophils, mast cells, endothelial cells, SMCs, fibroblasts, and epithelial cells (17). IL-13 is implicated in airway hyperresponsiveness, lung eosinophilia, mucus generation and fibrosis in asthma (18). Genetic polymorphisms in IL-13, its receptor components, and its essential signaling element STAT6 have all been associated with an increased risk of asthma (19–23).
More recent studies suggest that IL-13 is also a regulator of tissue remodeling. IL-13 has been shown to promote airway SMC growth (24,25). There is also evidence linking an antigen-driven Th2 immune response and vascular remodeling in pulmonary hypertension. Daley et al. (26) showed that depleting IL-13 significantly ameliorated antigen-driven pulmonary arterial muscularization in mice, implicating IL-13 in disease development. Graham et al. (27) demonstrated that schistosomiasis-induced pulmonary hypertension is caused by upregulated IL-13 signaling. Hecker et al. (28), by contrast, showed the increased expression of the IL-13 decoy receptor, IL-13Rα2, in idiopathic pulmonary arterial hypertension patients, indicating that IL-13 is a negative regulator of pulmonary vascular remodeling.
The present study reports that PDGF promotes the gene transcription of IL-13 in airway SMCs and pulmonary vascular SMCs. The mechanism of PDGF signaling for IL-13 gene transcription involves reactive oxygen species (ROS)-mediated events.
Methods
Cell culture
Human airway (bronchial) SMCs from ScienCell Research Laboratories (Carlsbad, CA, USA) and human lung vascular (pulmonary artery) SMCs from Cell Applications, Inc. (San Diego, CA) were cultured in accordance with the manufacturers’ instructions in 5% CO2 at 37°C. Cells in passages 3–7 were used. Cells were growth-arrested for 2 days in media containing 0.01% FBS for proliferation assays or serum starved overnight for RNA and promoter studies. Cells were treated with human recombinant PDGF-BB (Invitrogen, Carlsbad, CA, USA) or recombinant human IL-13 (PeproTech, Inc., Rocky Hill, NJ, USA). For some experiments, cells were pretreated with the IL-13 neutralizing antibody or matching isotype control (BD Biosciences, San Jose, CA). To knock down, p22phox and STAT3, cells were transfected with siRNA Transfection Reagent and gene silencing siRNAs along with control with scrambled sequence from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Animal studies
Balb/c mice were sensitized by intraperitoneal injection of 10 mg/kg ovalbumin on day 0 and 1 mg/kg on day 10. Mice were then challenged with nebulization of 1% ovalbumin solution on days 21, 22 and 23 and with 10% solution on day 25. Lungs were harvested and fixed in buffered formalin 48 h after the final challenge. Control mice were challenged with saline. Georgetown University Animal Care and Use Committee approved all animal experiments, and the investigation conforms to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Four micron sections of formalin-fixed, paraffin-embedded lungs were stained with hematoxylin and eosin (H & E) and examined under a microscope at 400x magnification. Immunohistochemical (IHC) staining of IL-13 was performed using goat anti-mouse IL-13 antibody (R&D Systems, Inc., Minneapolis, MN).
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated using TRIzol Reagent (Invitrogen). cDNA made from 1 μg RNA via RT was amplified in two sets of PCR using primers for IL-13 (Santa Cruz Biotechnology), and nested PCR products were visualized on agarose gels with ethidium bromide staining. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as a housekeeping control. IL-13 expression values obtained from the densitometric analysis of gels were normalized with the corresponding GAPDH values.
Enzyme-linked immunosorbent assay (ELISA)
IL-13 protein levels in the culture media were monitored using the IL-13 Human ELISA Kit (Abcam, Cambridge, MA).
Promoter studies
The luciferase reporter plasmid containing the 980 bp human IL-13 promoter (29), that was submitted by Prof. Anjana Rao (Department of Pathology, Harvard Medical School, Boston, MA, USA), was purchased from Addgene Inc. (Cambridge, MA, USA). Cells on 6-well plates were transfected with 4 μg of the plasmid using Lipofectamine 2000 (Invitrogen). The co-transfection of a plasmid expressing renilla luciferase that is controlled by the thymidine kinase promoter (Promega Corporation, Madison, WI) was used to normalize assays. Then, 48 h after transfection, cells were serum starved and treated with PDGF overnight. A luciferase assay was performed using the Dual-Luciferase Reporter Assay System (Promega), according to the manufacturer’s instructions.
Immunoblotting
Cell lysates were prepared and immunoblotting was performed as previously described (30). Carbonyl content was monitored in 2,4-dinitrophenylhydrazine (DNPH)-derivatized proteins using an OxyBlot Protein Oxidation Detection Kit (Millipore, Billerica, MA, USA). To monitor the activation of STAT3, a phospho-STAT3 (Tyr705) antibody from Cell Signaling Technology (Danvers, MA, USA) was used. Electrophoresis through a reducing SDS-polyacrylamide gel was followed by electroblotting onto a nitrocellulose membrane. Enhanced Chemiluminescence System (Amersham Biosciences, Piscataway, NJ, USA) was used for detection.
Cell proliferation assay
Cell growth was assessed using a tetrazolium salt-based XTT assay (Trevigen, Inc., Gaithersburg, MD, USA) or by counting cells with a hemocytometer.
Statistical analysis
Means ± SEM were calculated, comparisons between 2 groups were analyzed by a two-tailed Student’s t test, and comparisons between 3 or more groups were analyzed by ANOVA with a Student-Newman-Keuls post-hoc test. P < 0.05 was considered to be significant.
Results
PDGF promotes IL-13 gene expression in airway and lung vascular SMCs
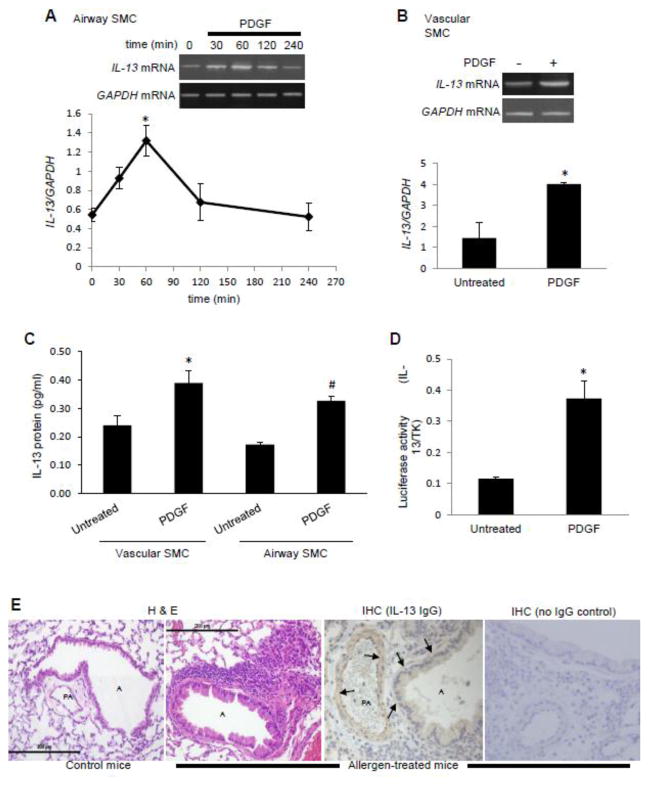
IL-13 mRNA was found to be expressed in both airway SMCs and lung vascular SMCs. The treatment of human bronchial SMCs with PDGF resulted in increased IL-13 mRNA with a peak at 60 min (Fig. 1A). Similarly, the treatment of human pulmonary artery SMCs with PDGF for 60 min increased IL-13 mRNA expression (Fig. 1B). ELISA measurements of IL-13 protein levels in the media demonstrated that PDGF caused IL-13 protein release from airway and vascular SMCs (Fig. 1C). PDGF also promoted the gene transcription of the luciferase reporter that is controlled by the proximal 980 bp region of the IL-13 promoter (Fig. 1D). IL-13 protein expression was also detected as brown IHC staining in smooth muscle layer of airways and pulmonary vessels in allergen-stimulated mice (Fig. 1E). These experiments demonstrated that lung SMCs express IL-13 in cultured cells and in intact animals, and that its gene transcriptional activity can be increased by PDGF.
Fig. 1. PDGF promotes IL-13 gene expression in lung SMCs.
(A) Human bronchial SMCs were treated with 10 ng/ml of PDGF for the durations indicated. RNA was isolated and IL-13 and GAPDH mRNA levels were monitored. The values in the graph represent the means ± SEM of the ratio of IL-13 to GAPDH bands. (B) Human pulmonary artery SMCs were treated with 10 ng/ml PDGF for 60 min to monitor IL-13 mRNA levels. (C) Cells were treated with 10 ng/ml PDGF. Four days after the treatment, IL-13 protein levels were monitored in culture media by ELISA. (D) Human bronchial SMCs were transfected with an IL-13 promoter reporter plasmid. After 48 h, cells were serum starved for 8 h and treated with 10 ng/ml PDGF. A luciferase assay was performed the following day. Values were normalized with a co-transfected renilla luciferase activity that is controlled by the thymidine kinase (TK) promoter. Values in the graphs represent means ± SEM (n = 3 – 4). * and # denote values significantly different from the untreated control value at P < 0.05. (E) Formalin-fixed lung sections from control and allergen (ovalbumin)-treated mice were stained with H & E. Size marker = 200 μm. IHC staining shows IL-13 expression (brown staining) in smooth muscle layer (arrows) of airways, A, and pulmonary artery, PA, in allergen-stimulated mice.
Role of ROS in the regulation of PDGF-induced IL-13 gene expression
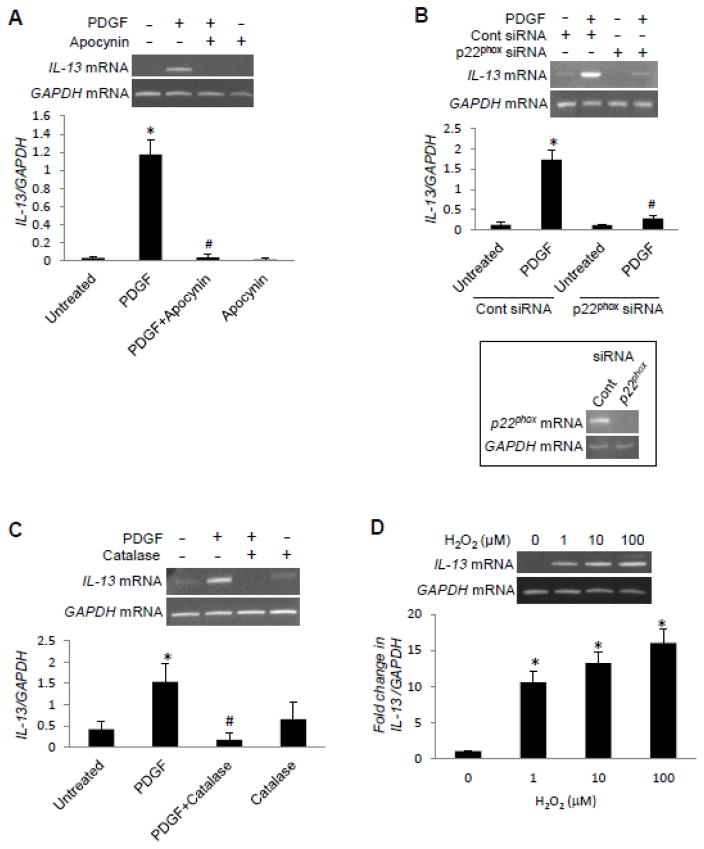
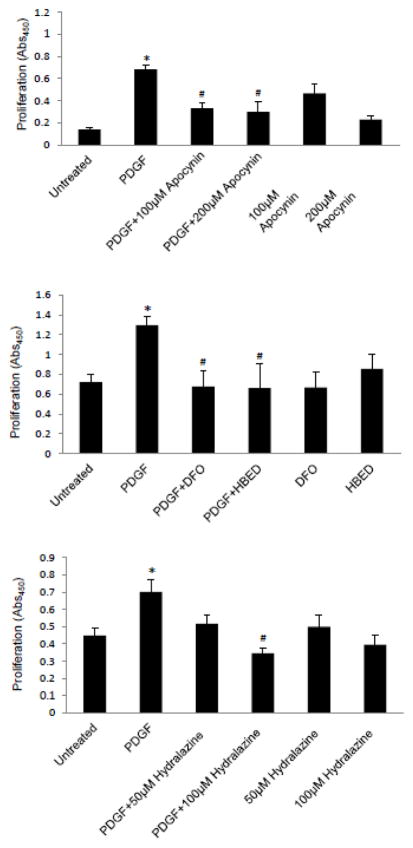
H2O2 that is produced through the activation of NAD(P)H oxidase has been shown to serve as a second messenger in response to various ligands, including PDGF (31–34). Thus, we tested the role of this pathway in PDGF signaling for IL-13 gene expression. An NAD(P)H oxidase inhibitor, apocynin (Fig. 2A), as well as the siRNA knock-down of p22phox NAD(P)H oxidase subunit (Fig. 2B) inhibited the PDGF-induced increase in IL-13 mRNA expression. An H2O2 scavenger, catalase, also inhibited the PDGF-induced IL-13 expression (Fig. 2C). Consistent with the previous report (33), PDGF increased dichlorofluorescein fluorescence in airway SMCs, indicating the production of ROS (data not shown). The treatment of cells with H2O2 (1–100 μM) increased IL-13 expression (Fig. 2D).
Fig. 2. PDGF-induced IL-13 gene expression is regulated by ROS.
Human bronchial SMCs were serum starved overnight and (A) treated with 100 μM apocynin for 30 min, (B) subjected to siRNA knock-down of p22phox NAD(P)H oxidase subunit, and (C) treated with 1,000 units/ml catalase for 30 min, followed by 10 ng/ml of PDGF for 1 h. (D) Human bronchial SMCs were treated with different concentrations of H2O2 for 1 h. RNA was isolated, and IL-13 and GAPDH mRNA levels were monitored. The values in the graph represent the means ± SEM of the ratio of IL-13 to GAPDH bands (n = 3 – 4). Values denoted with * are significantly different from the untreated control value and those with # are different from the PDGF value at P < 0.05.
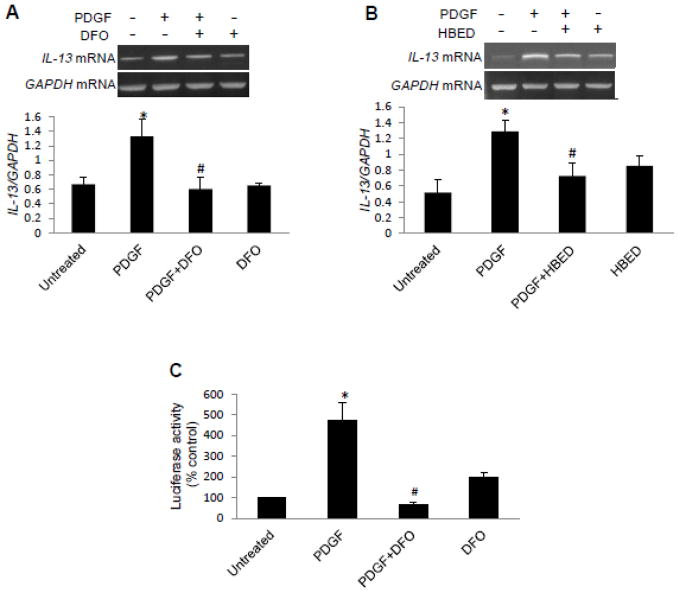
H2O2, in the presence of Fe2+ ions, forms the highly reactive hydroxyl radical in the Fenton reaction (35). Iron chelators such as deferoxamine (DFO) and N,N′-bis (2-hydroxybenzyl) ethylenediamine-N,N′-diacetic acid (HBED) limit the generation of hydroxyl radicals. The pre-treatment of cells with DFO (Fig. 3A) or HBED (Fig. 3B) significantly inhibited PDGF-induced IL-13 mRNA expression. DFO also inhibited the PDGF-induced activation of IL-13 promoter-dependent luciferase reporter activity (Fig 3C).
Fig. 3. The role of iron in PDGF-induced IL-13 gene expression.
Human bronchial SMCs were serum starved overnight and treated with (A) 50 μM DFO or (B) 50 μM HBED for 30 min, followed by 10 ng/ml of PDGF for 1 h. RNA was isolated, and IL-13 and GAPDH mRNA levels were monitored. (C) Human bronchial SMCs transfected with an IL-13 reporter plasmid were pre-treated with DFO for 30 min and then treated with PDGF overnight. The values in the graph represent the means ± SEM (n = 4). Values denoted with * are significantly different from the untreated control value and those with # are different from the PDGF value at P < 0.05.
Protein carbonylation and PDGF-induced IL-13 gene expression
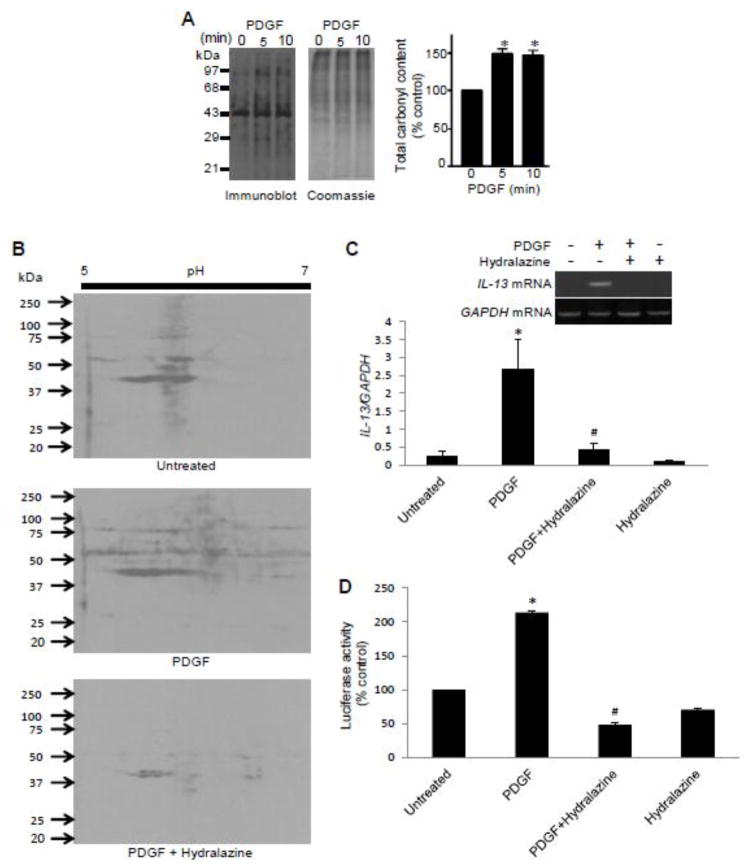
Our laboratory previously provided evidence that protein carbonylation may serve as a mechanism of ROS signaling in SMCs (30,36). Thus, we tested the role of protein carbonylation in PDGF-induced IL-13 gene expression. The treatment of cells with PDGF promoted total protein carbonyl content (Fig. 4A). A carbonyl inhibitor, hydralazine (37), inhibited PDGF-induced promotion of protein carbonylation (Fig. 4B), IL-13 mRNA expression (Fig. 4C), and the luciferase reporter activity controlled by the IL-13 gene promoter (Fig. 4D).
Fig. 4. Protein carbonylation is a mechanism of ROS signaling.
(A) Human bronchial SMCs were treated with 10 ng/ml PDGF for the durations indicated. Cells were lysed and derivatized with DNPH, and carbonylated proteins were detected by immunoblotting. Coomassie blue staining showed equal loading. (B) Cells were pre-treated with hydralazine for 30 min and then treated with PDGF for 10 min. DNPH-derivatized cell lysates were subjected to two-dimensional gel electrophoresis and immunoblotting. (C) Cells were pre-treated with 50 μM hydralazine for 30 min and then treated with PDGF for 1 h. RNA was isolated, and IL-13 and GAPDH mRNA levels were monitored. (D) Transfected cells were pre-treated with hydralazine for 30 min followed by PDGF overnight, and the IL-13 promoter activity was measured. The values represent the means ± SEM (n = 3 – 4). Values denoted with * are significantly different from the untreated control value and those with # are different from the PDGF value at P < 0.05.
The involvement of STAT3 in PDGF-induced IL-13 expression
Because PDGF-induced STAT3 activation has been shown to involve ROS in airway SMCs (38), we tested if this pathway is involved in PDGF-induced IL-13 gene expression. Knocking-down STAT3 with siRNA inhibited PDGF-induced IL-13 mRNA expression, while control siRNA had no effects (Fig. 5A). PDGF induced the phosphorylation of STAT3 at Tyr705 that is known to activate this transcription factor. The phosphorylation is inhibited by hydralazine (Fig. 5B), indicating that the ROS-signaling mechanism for PDGF-induced IL13 expression resides upstream of STAT3.
Fig. 5. The role of STAT3 in PDGF-induced IL-13 expression.
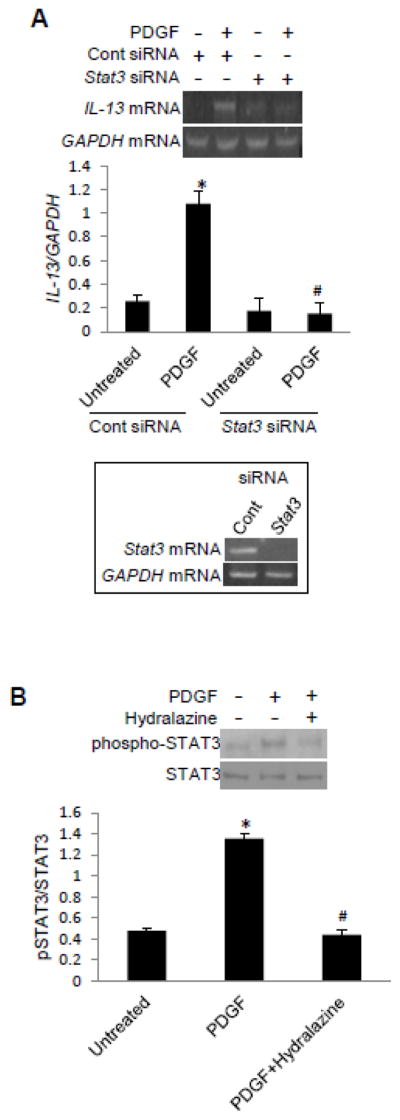
(A) Human bronchial SMCs were transfected with control or Stat3 siRNA and then treated with PDGF for 1 h. RNA was isolated, and IL-13 and GAPDH mRNA levels were monitored. (B) Cells were treated with hydralazine for 30 min and then treated with PDGF for 10 min. Cell lysates were subjected to western blotting to monitor phosphorylation of STAT3 at Tyr705. The values in the graph represent the means ± SEM (n = 3). Values denoted with * are significantly different from the untreated control value and those with # are different from the PDGF value at P < 0.05.
Role of ROS signaling in SMC growth
To test whether ROS signaling triggered by PDGF is also involved in cell growth, cells were treated with the ROS inhibitors before the addition of PDGF, and a cell proliferation assay was performed 4 days after the stimulation. Apocynin, DFO, HBED, and hydralazine all significantly inhibited PDGF-induced cell growth (Fig. 6).
Fig. 6. PDGF-induced SMC growth is mediated by ROS.
(A) Human bronchial SMCs were pre-treated with apocynin, 50 μM DFO, 50 μM HBED, or hydralazine for 30 min and then treated with 10 ng/ml PDGF. Proliferation was assessed after 4 days using an XTT assay. Each experiment was performed in triplicate. The values represent the means ± SEM of the three experiments for A and C and eight experiments for B. Values denoted with * are significantly different from the untreated control value and those with # are different from the PDGF value at P < 0.05.
Differential roles of IL-13 in the growth of airway and vascular SMCs
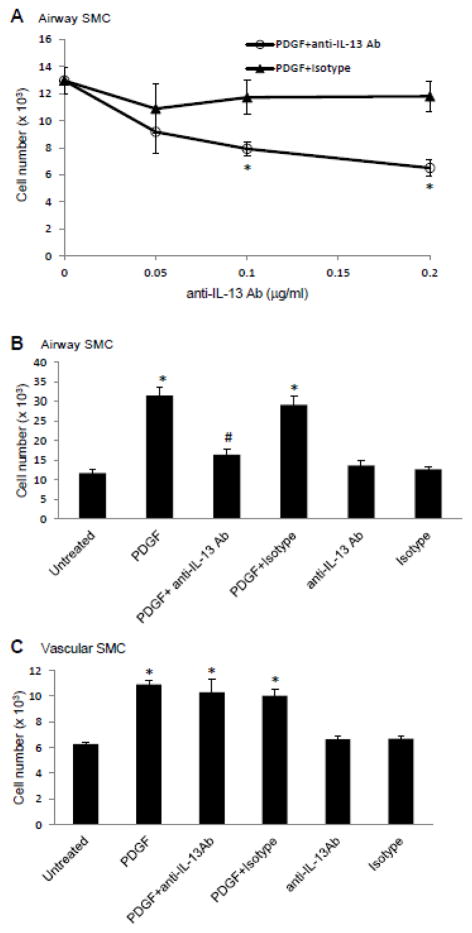
Since a potent growth factor of SMCs, PDGF, promotes IL-13 expression in human airway SMCs and in human lung vascular SMCs, we tested whether IL-13 plays a role in PDGF-induced cell growth. Cells were pre-treated with different concentrations of an IL-13 neutralizing antibody or isotype control for 1 h before PDGF stimulation, and cell growth was monitored after 4 days. The IL-13 neutralizing antibody inhibited the PDGF-induced growth of airway SMCs in a dose-dependent fashion, while the isotype control had no effect (Fig. 7A). Results using human bronchial SMCs from two different donors show that the IL-13 neutralizing antibody almost completely inhibited PDGF-induced cell growth (Fig. 7B). Neither the IL-13 neutralizing antibody nor the isotype affected cell growth in the absence of PDGF (Fig. 7B). By contrast, the IL-13 neutralizing antibody did not affect the PDGF-induced growth of human pulmonary artery SMCs (Fig. 7C).
Fig. 7. Effects of neutralizing antibody against IL-13 on PDGF-induced cell growth.
(A & B) Human bronchial SMCs and (C) human pulmonary artery SMCs were growth arrested and pre-treated with an IL-13 neutralizing antibody (anti-IL-13 Ab) or isotype control for 1 h and then treated with PDGF. Growth was assayed after 4 days by cell counting. For (B) and (C), 0.1 μg/ml of anti-IL-13 Ab or isotype control were used. The values represent the means ± SEM of the three experiments. Values denoted with * are significantly different from the untreated control value and those with # are different from the PDGF+Isotype value at P < 0.05.
Effects of IL-13 on cell growth
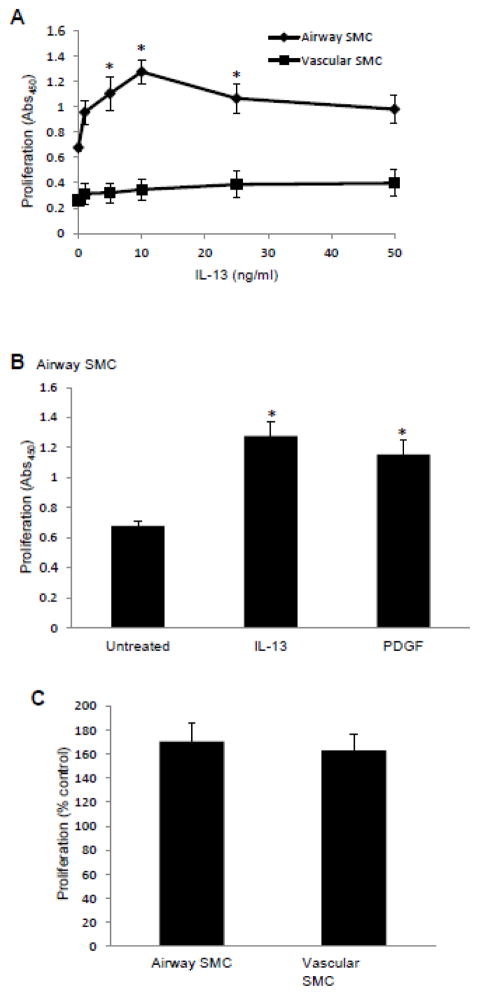
To test the hypothesis that the two lung SMC types we used in this study have different IL-13 signaling, cells were stimulated directly with recombinant human IL-13. IL-13 significantly promoted the growth of airway SMCs (Fig. 8A; closed diamonds). The growth of IL-13-induced airway SMCs was comparable to the growth induced by the same concentration of PDGF (Fig. 8B), whereas IL-13 did not promote the growth of lung vascular SMCs (Fig. 8A; filled squares). Despite the absence of growth promoting activity in vascular SMCs, IL-13 promoted phosphorylation of Stat6 in both airway and vascular SMCs (data not shown). By contrast, PDGF promoted the growth of both airway and lung vascular SMCs (Fig. 6C).
Fig. 8. IL-13 promotes the growth of airway SMCs, but not vascular SMCs.
(A) Human bronchial SMCs and human pulmonary artery SMCs were treated with different concentrations of recombinant human IL-13 as indicated. (B) Human bronchial SMCs were treated with 10 ng/ml IL-13 or 10 ng/ml PDGF. (C) Human bronchial SMCs and human pulmonary artery SMCs were treated with 10 ng/ml PDGF. Cell proliferation was assessed 2 days later using an XTT assay. Each experiment was performed in triplicate. The values represent the means ± SEM (n = 3 – 4). Values denoted with * are significantly different from the untreated control value at P < 0.05.
Discussion
The major finding of the present study is that SMCs in the lung (both airway and vascular SMC types) express IL-13, and PDGF increases its expression through a signal transduction mechanism, which involves ROS.
Grunstein et al. (39) first discovered that IL-13 mRNA is expressed in cultured human airway SMCs. In humans, Saha et al. (40) later showed increased IL-13 expression in airway smooth muscle bundles in the lungs of severe asthmatics compared with those from mild to moderate asthma patients. The present study demonstrated that SMCs do have the genetic and signal transduction machinery to activate IL-13 gene transcription. Our transfection experiments using a luciferase reporter revealed that an important transcriptional regulatory region in the IL-13 promoter that is activated by PDGF resides within 980 bp immediately proximal to the transcriptional start site. The present study identified the role of STAT3 in PDGF-signaling for IL-13 gene expression. The involvement of ROS in cell signaling for the activation of STAT3 has previously been demonstrated in airway SMCs (38).
Consistent with previous reports that PDGF activates NAD(P)H oxidase-dependent ROS signaling in SMCs (32–34), PDGF-induced IL-13 gene expression was blocked by the inhibitors of NAD(P)H oxidase and ROS metabolism. Further, H2O2 as low as 1 μM was found to significantly activate IL-13 gene expression. This sensitive ROS target may involve iron-catalyzed protein carbonylation, which was previously proposed to be involved in ROS signaling (30,36). Results of the present study, which shows that a carbonyl scavenger, hydralazine, inhibits PDGF-mediated events, further support the concept for the role of protein carbonylation in cell signaling. ROS appear to target upstream of STAT3, perhaps by either activating protein kinases or inhibiting protein phosphatases. Further work is needed to identify the exact target of oxidation in this signaling pathway.
The present study also provided evidence that IL-13 is involved in the mechanisms of growth factor-mediated growth of human bronchial SMCs. To our knowledge, this is the first demonstration for such a role of IL-13 or any Th2 cytokines in cell growth. We propose that IL-13 is released extracellularly and that it serves as an autocrine or paracrine signal to activate the IL-13 receptor. Consistent with previous findings (24,25), IL-13 can promote the growth of airway SMCs. Interestingly, however, these roles and actions of IL-13 only occur in airway SMCs, but not in lung vascular SMCs.
In summary, the present study revealed a novel ROS-dependent signaling event, in which PDGF increases IL-13 gene transcription in both airway and vascular SMC types. It is intriguing that this signaling pathway can be activated by low μM levels of H2O2. This suggests the existence of sensitive redox targets, which regulate gene transcription. More work is needed to determine the exact molecular mechanism of ROS signaling.
Highlights.
IL-13 is expressed in airway and lung vascular smooth muscle cells (SMCs).
PDGF promotes IL-13 gene transcription via NAD(P)H oxidase-generated H2O2.
This oxidant signaling mechanism may involve iron-dependent protein carbonylation.
This oxidant signaling mechanism occurs upstream of STAT3.
IL-13 is involved in PDGF-induced growth of airway SMCs, but not lung vascular SMCs.
Footnotes
This work was supported in part by grants from National Institutes of Health (R01HL72844 and R01HL97514) to YJS.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sakao S, Tatsumi K, Voelkel NF. Reversible or irreversible remodeling in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2010;43:629–634. doi: 10.1165/rcmb.2009-0389TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazaar AL, Panettieri RA., Jr Airway smooth muscle: a modulator of airway remodeling in asthma. J Allergy Clin Immunol. 2005;116:488–495. doi: 10.1016/j.jaci.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 3.Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38:872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 4.Hassoun PM, Mouthon L, Barberà JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54:S10–S19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Ross R. Platelet-derived growth factor. Lancet. 1989;1:1179–1182. doi: 10.1016/s0140-6736(89)92760-8. [DOI] [PubMed] [Google Scholar]
- 6.Ross R, Raines EW, Bowen-Pope DF. The biology of platelet-derived growth factor. Cell. 1986;46:155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- 7.Antoniades HN, Galanopoulos T, Neville-Golden J, O’Hara CJ. Malignant epithelial cells in primary human lung carcinomas coexpress in vivo platelet-derived growth factor (PDGF) and PDGF receptor mRNAs and their protein products. Proc Natl Acad Sci USA. 1992;89:3942–3946. doi: 10.1073/pnas.89.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohno I, Nitta Y, Yamauchi K, Hoshi H, Honma M, Woolley K, O’Byrne P, Dolovich J, Jordana M, Tamura G, et al. Eosinophils as a potential source of platelet-derived growth factor B-chain (PDGF-B) in nasal polyposis and bronchial asthma. Am J Respir Cell Mol Biol. 1995;13:639–647. doi: 10.1165/ajrcmb.13.6.7576701. [DOI] [PubMed] [Google Scholar]
- 9.Walker TR, Moore SM, Lawson MF, Panettieri RA, Jr, Chilvers ER. Platelet-derived growth factor-BB and thrombin activate phosphoinositide 3-kinase and protein kinase B: role in mediating airway smooth muscle proliferation. Mol Pharmacol. 1998;54:1007–1015. doi: 10.1124/mol.54.6.1007. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita N, Sekine K, Miyasaka T, Kawashima R, Nakajima Y, Nakano J, Yamamoto T, Horiuchi T, Hirai K, Ohta K. Platelet-derived growth factor is involved in the augmentation of airway responsiveness through remodeling of airways in diesel exhaust particulate-treated mice. J Allergy Clin Immunol. 2001;107:135–142. doi: 10.1067/mai.2001.111433. [DOI] [PubMed] [Google Scholar]
- 11.Humbert M, de Blay F, Garcia G, Prud’homme A, Leroyer C, Magnan A, Tunon-de-Lara JM, Pison C, Aubier M, Charpin D, Vachier I, Purohit A, Gineste P, Bader T, Moussy A, Hermine O, Chanez P. Masitinib, a c-kit/PDGF receptor tyrosine kinase inhibitor, improves disease control in severe corticosteroid-dependent asthmatics. Allergy. 2009;64:1194–1201. doi: 10.1111/j.1398-9995.2009.02122.x. [DOI] [PubMed] [Google Scholar]
- 12.Hirota JA, Ask K, Farkas L, Smith JA, Ellis R, Rodriguez-Lecompte JC, Kolb M, Inman MD. In Vivo Role of Platelet Derived Growth Factor-BB in Airway Smooth Muscle Proliferation in mouse lung. Am J Respir Cell Mol Biol. 2011;45:566–572. doi: 10.1165/rcmb.2010-0277OC. [DOI] [PubMed] [Google Scholar]
- 13.Antoniades HN, Bravo MA, Avila RE, Galanopoulos T, Neville-Golden J, Maxwell M, Selman M. Platelet-derived growth factor in idiopathic pulmonary fibrosis. J Clin Invest. 1990;86:1055–1064. doi: 10.1172/JCI114808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katayose D, Ohe M, Yamauchi K, Ogata M, Shirato K, Fujita H, Shibahara S, Takishima T. Increased expression of PDGF A and B-chain genes in rat lungs with hypoxic pulmonary hypertension. Am J Physiol. 1993;264:Ll00–L106. doi: 10.1152/ajplung.1993.264.2.L100. [DOI] [PubMed] [Google Scholar]
- 15.Hatano M, Yao A, Shiga T, Kinugawa K, Hirata Y, Nagai R. Imatinib mesylate has the potential to exert its efficacy by down-regulating the plasma concentration of platelet-derived growth factor in patients with pulmonary arterial hypertension. Int Heart J. 2010;51:272–276. doi: 10.1536/ihj.51.272. [DOI] [PubMed] [Google Scholar]
- 16.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, Grimminger F. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hershey GK. IL-13 receptors and signaling pathways: An evolving web. J Allergy Clin Immunol. 2003;111:677–690. doi: 10.1067/mai.2003.1333. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell J, Dimov V, Townley RG. IL-13 and the IL-13 receptor as therapeutic targets for asthma and allergic disease. Curr Opin Investig Drugs. 2010;11:527–534. [PubMed] [Google Scholar]
- 19.Vercelli D. Genetics of IL-13 and functional relevance of IL-13 variants. Curr Opin Allergy Clin Immunol. 2002;2:389–393. doi: 10.1097/00130832-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Schedel M, Carr D, Klopp N, Woitsch B, Illig T, Stachel D, Schmid I, Fritzsch C, Weiland SK, von Mutius E, Kabesch M. A signal transducer and activator of transcription 6 haplotype influences the regulation of serum IgE levels. J Allergy Clin Immunol. 2004;114:1100–1105. doi: 10.1016/j.jaci.2004.07.048. [DOI] [PubMed] [Google Scholar]
- 21.Moissidis I, Chinoy B, Yanamandra K, Napper D, Thurmon T, Bocchini J, Jr, Bahna SL. Association of IL-13, RANTES, and leukotriene C4 synthase gene promoter polymorphisms with asthma and/or atopy in African Americans. Genet Med. 2005;7:406–410. doi: 10.1097/01.gim.0000170994.24960.48. [DOI] [PubMed] [Google Scholar]
- 22.Beghé B, Hall IP, Parker SG, Moffatt MF, Wardlaw A, Connolly MJ, Fabbri LM, Ruse C, Sayers I. Polymorphisms in IL13 pathway genes in asthma and chronic obstructive pulmonary disease. Allergy. 2010;65:474–481. doi: 10.1111/j.1398-9995.2009.02167.x. [DOI] [PubMed] [Google Scholar]
- 23.Bottema RW, Nolte IM, Howard TD, Koppelman GH, Dubois AE, de Meer G, Kerkhof M, Bleecker ER, Meyers DA, Postma DS. Interleukin 13 and interleukin 4 receptor-α polymorphisms in rhinitis and asthma. Int Arch Allergy Immunol. 2010;153:259–267. doi: 10.1159/000314366. [DOI] [PubMed] [Google Scholar]
- 24.Aravamudan B, Thompson M, Pabelick C, Prakash YS. Brain derived neurotrophic factor induces proliferation of human airway smooth muscle cells. J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2011.01356.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellner J, Gamarra F, Welsch U, Jörres RA, Huber RM, Bergner A. IL-13Ralpha2 reverses the effects of IL-13 and IL-4 on bronchial reactivity and acetylcholine-induced Ca2+ signaling. Int Arch Allergy Immunol. 2007;142:199–210. doi: 10.1159/000097022. [DOI] [PubMed] [Google Scholar]
- 26.Daley E, Emson C, Guignabert C, de Waal Malefyt R, Louten J, Kurup VP, Hogaboam C, Taraseviciene-Stewart L, Voelkel NF, Rabinovitch M, Grunig E, Grunig G. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med. 2008;205:361–372. doi: 10.1084/jem.20071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham BB, Mentink-Kane MM, El-Haddad H, Purnell S, Zhang L, Zaiman A, Redente EF, Riches DW, Hassoun PM, Bandeira A, Champion HC, Butrous G, Wynn TA, Tuder RM. Schistosomiasis-induced experimental pulmonary hypertension: role of interleukin-13 signaling. Am J Pathol. 2010;177:1549–1561. doi: 10.2353/ajpath.2010.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hecker M, Zaslona Z, Kwapiszewska G, Niess G, Zakrzewicz A, Hergenreider E, Wilhelm J, Marsh LM, Sedding D, Klepetko W, Lohmeyer J, Dimmeler S, Seeger W, Weissmann N, Schermuly RT, Kneidinger N, Eickelberg O, Morty RE. Dysregulation of the IL-13 receptor system: a novel pathomechanism in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182:805–818. doi: 10.1164/rccm.200909-1367OC. [DOI] [PubMed] [Google Scholar]
- 29.Macián F, García-Rodríguez C, Rao A. Gene expression elicited by NFAT in the presence or absence of cooperative recruitment of Fos and Jun. EMBO J. 2000;19:4783–4795. doi: 10.1093/emboj/19.17.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong CM, Cheema AK, Zhang L, Suzuki YJ. Protein carbonylation as a novel mechanism in redox signaling. Circ Res. 2008;102:310–318. doi: 10.1161/CIRCRESAHA.107.159814. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 32.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 33.Page K, Li J, Hodge JA, Liu PT, Vanden Hoek TL, Becker LB, Pestell RG, Rosner MR, Hershenson MB. Characterization of a Rac1 signaling pathway to cyclin D(1) expression in airway smooth muscle cells. J Biol Chem. 1999;274:22065–22071. doi: 10.1074/jbc.274.31.22065. [DOI] [PubMed] [Google Scholar]
- 34.Brar SS, Kennedy TP, Whorton AR, Murphy TM, Chitano P, Hoidal JR. Requirement for reactive oxygen species in serum-induced and platelet-derived growth factor-induced growth of airway smooth muscle. J Biol Chem. 1999;274:20017–20026. doi: 10.1074/jbc.274.28.20017. [DOI] [PubMed] [Google Scholar]
- 35.Freeman BA, Crapo JD. Biology of disease: free radicals and tissue injury. Lab Invest. 1982;47:412–426. [PubMed] [Google Scholar]
- 36.Wong CM, Marcocci L, Liu L, Suzuki YJ. Cell signaling by protein carbonylation and decarbonylation. Antioxid Redox Signal. 2010;12:393–404. doi: 10.1089/ars.2009.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galvani S, Coatrieux C, Elbaz M, Grazide MH, Thiers JC, Parini A, Uchida K, Kamar N, Rostaing L, Baltas M, Salvayre R, Nègre-Salvayre A. Carbonyl scavenger and antiatherogenic effects of hydrazine derivatives. Free Radic Biol Med. 2008;45:1457–1467. doi: 10.1016/j.freeradbiomed.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 38.Simon AR, Takahashi S, Severgnini M, Fanburg BL, Cochran BH. Role of the JAK-STAT pathway in PDGF-stimulated proliferation of human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1296–L1304. doi: 10.1152/ajplung.00315.2001. [DOI] [PubMed] [Google Scholar]
- 39.Grunstein MM, Hakonarson H, Leiter J, Chen M, Whelan R, Grunstein JS, Chuang S. IL-13-dependent autocrine signaling mediates altered responsiveness of IgE-sensitized airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2002;282:L520–L528. doi: 10.1152/ajplung.00343.2001. [DOI] [PubMed] [Google Scholar]
- 40.Saha SK, Berry MA, Parker D, Siddiqui S, Morgan A, May R, Monk P, Bradding P, Wardlaw AJ, Pavord ID, Brightling CE. Increased sputum and bronchial biopsy IL-13 expression in severe asthma. J Allergy Clin Immunol. 2008;121:685–691. doi: 10.1016/j.jaci.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]