Abstract
Partial or whole-brain irradiation is often required to treat both primary and metastatic brain cancer. Radiation-induced normal tissue injury, including progressive cognitive impairment, however, can significantly affect the well-being of the approximately 200,000 patients who receive these treatments each year in the US. Although the exact mechanisms underlying radiation-induced late effects remain unclear, oxidative stress and inflammation are thought to play a critical role. Microglia are key mediators of neuroinflammation. Peroxisomal proliferator-activated receptor (PPAR)δ has been shown to be a potent regulator of anti-inflammatory responses. Thus, we hypothesized that PPARδ activation would modulate the radiation-induced inflammatory response in microglia. Incubating BV-2 murine microglial cells with the PPARδ agonist, L-165041, prevented the radiation-induced increase in: i) intracellular reactive oxygen species generation, ii) Cox-2 and MCP-1 expression, and iii) IL-1β and TNF-α message levels. This occured, in part, through PPARδ-mediated modulation of stress activated kinases and proinflammatory transcription factors. PPARδ inhibited NF-κB via transrepression by physically interacting with the p65 subunit, and prevented activation of the PKCα/MEK1/2/ERK1/2/AP-1 pathway by inhibiting the radiation-induced increase in intracellular reactive oxygen species generation. These data support the hypothesis that PPARδ activation can modulate radiation-induced oxidative stress and inflammatory responses in microglia.
Keywords: Ionizing radiation, PPARδ, radiation-induced brain injury, microglia, inflammation, NF-κB, PKCα/MEK1/2/ERK1/2/AP-1 pathway
Each year, approximately 200,000 patients will receive fractionated partial or whole-brain irradiation (fWBI) as treatment for primary or metastatic brain cancer [1]. Unfortunately, the radiation dose that can be delivered to the tumor is limited by the risk of toxicity to the surrounding normal brain tissue. Radiation-induced brain injury can lead to cognitive deficits several months to years after irradiation that affect patients' quality of life (QOL) [2–4]. This diminished QOL has become an important concern for these long-term survivors of brain irradiation, and is recognized as one of the most important measurements of brain tumor therapy outcomes in clinical trials, second only to survival [5, 6]. Although clinical trials have demonstrated that short-term interventions can modulate cognitive impairment [7], there are no proven long-term treatments for radiation-induced cognitive deficits; therefore, it is important to investigate new therapeutic approaches [8].
Although it was once believed that the brain was not susceptible to inflammation, studies have now demonstrated that inflammatory responses do occur and may contribute to radiation-induced brain injury. In vivo studies indicate that there is an increase in proinflammatory mediators within hours of irradiating the rodent brain [9–11]. Microglia are considered to be one of the key mediators of neuroinflammation [12–15]. In an uninjured brain, ramified microglia actively monitor the microenvironment to ensure that the brain is maintaining homeostasis [12]. Following injury, microglia become activated, a process characterized by rounding of the cell body, retraction of cell processes, proliferation, and an increased production of cytokines, chemokines, and reactive oxygen species (ROS) [12–15]. Although microglial activation plays an important role in phagocytosis of dead cells, sustained activation is thought to contribute to a chronic proinflammatory state in the brain [13, 15]. In vivo studies in rodents indicate that radiation leads to an increase in microglial activation [16, 17]. Irradiating microglia cells in vitro leads to an increase in proinflammatory mediators, such as the cytokines TNF-α and IL-1β, and the chemokines MCP-1 and ICAM-1 [18–20].
Radiation-induced chronic oxidative stress and inflammatory responses produced by microglia may: i) lead to a decrease in neurogenesis in the hippocampus, a critical region for learning and memory; and/or ii) alter the environment of the neurogenic niche and, in turn, the functions of the pre-existing neurons [15, 16, 21]. Studies in rodents demonstrate that the administration of anti-inflammatory drugs can decrease radiation-induced microglial activation. This decrease has been associated with an improvement in hippocampal neurogenesis [16, 17]. Moreover, administration of eicosapentaenoic acid, a polyunsaturated fatty acid with anti-inflammatory properties, restored the altered long-term potentiation (LTP) of hippocampal slices following irradiation of the rat brain [21]. LTP is thought to underlie memory and cognitive function; it is a measure of signal transmission between two neurons [22]. These findings provide a strong rationale for investigating anti-inflammatory therapies to mitigate radiation-induced brain injury.
PPARδ is a member of the nuclear hormone receptor superfamily of ligand-activated transcription factors and one of three subtypes (α, δ, and γ) that make up the PPAR family [23, 24]. PPARs regulate transcription by heterodimerizing with the retinoid × receptor (RXR) and binding to PPAR response elements (PPREs). PPREs, located in the enhancer regions of genes, consist of an AGGTCA hexameric direct repeat separated by one or two nucleotides [25]. PPAR subtypes are encoded by different genes, have unique tissue distributions, and exhibit overlapping and differential functions. PPARδ is thought to be expressed ubiquitously; it is the predominant PPAR subtype in the CNS [26]. Several studies have demonstrated that PPARδ activation can modulate oxidative stress and inflammatory processes [27–29]. PPARδ mediates many of its anti-inflammatory effects by preventing activation of stress-activated kinases and proinflammatory transcription factors [30, 31]. In addition, PPARδ has been shown to regulate oxidative stress by activating transcription of antioxidant genes, such as catalase and superoxide dismutase [32, 33].
Recent studies suggest that PPARδ agonists may ameliorate the severity of various acute and chronic CNS pathologies, including stroke, multiple sclerosis, and Alzheimer's disease, in large part, by modulating the oxidative stress and proinflammatory responses associated with these diseases [34–36]. The role of PPARδ in the modulation of radiation-induced brain injury is unknown. We hypothesized that activation of PPARδ would inhibit the radiation-induced oxidative stress and proinflammatory responses in microglia. Here, we report that PPARδ activation does indeed prevent the radiation-induced increase in: i) intracellular ROS generation, ii) Cox-2 and MCP-1 protein, and iii) IL-1β and TNF-α message levels. This occurred, in part, by transrepression of NF-κB and inhibition of the PKCα/MEK1/2/ERK1/2/AP-1 pathway.
Materials and methods
Materials
The PPARδ agonist, L-165041, was purchased from Calbiochem (San Diego, CA). The MEK inhibitor, U0126, the ERK1/2 inhibitor, FR180204, and the PKCα/β inhibitor, G06976, were purchased from EMD millipore (La Jolla, CA), Santa-Cruz Biotechnologies (Santa-Cruz, CA), and EMD millipore, respectively. All drugs were dissolved in Me2SO4 (DMSO). Goat anti-Cox-2, rabbit anti-MEK1/2, rabbit anti-p-MEK1/2, mouse anti-ERK1/2, mouse-anti-p-ERK1/2, goat anti-p-c-jun, and mouse anti-p-IκBα were purchased from Santa-Cruz Biotechnologies. Rabbit anti-p65, rabbit anti-PKCα, and rabbit anti-MCP-1 were purchased from Cell Signaling (Danvers, MA). Rabbit anti-p-PKCα was purchased from Epitomics (Burlingame, CA), and mouse anti-β-actin was purchased from Sigma-Aldrich (St. Louis, MO).
Cell culture and Irradiation
BV-2 cells, immortalized murine microglial cells, were cultured in DMEM high glucose media (Invitrogen, Carlsbad, CA) supplied with 5% fetal bovine serum (Sigma-Aldrich), 100 IU/mL penicillin, and 100 mg/mL streptomycin (Sigma-Aldrich) and were maintained at 37°C in 10% CO2 and 90% air. Twenty-four hours prior to irradiation, the culture medium was replaced with serum-free media. Cells were irradiated with a single dose of 10 Gy using a 137Cs irradiator (J.L. Shepherd and Associates, San Fernando, CA) at a dose rate of 3.56 Gy/min. Irradiations were conducted at room temperature and control cells received sham-irradiation. Following irradiation, culture dishes were returned to the incubator and maintained at 37°C in 10% CO2 and 90% air.
Short hairpin RNA targeting
Short hairpin RNA (shRNA) were generated as described by Sui et al. [37]. The PPARδ target site is: GGACCAGAACACACGCTTCCTT. BV-2 cells were infected with ecotropic virus and infected cells were selected by puromycin selection (Sigma-Aldrich). Single-cell clones were generated, and the expression of PPARδ was evaluated by western blot analysis.
Overexpression
BV-2 cells were transfected with the pcDNA3.1.PPARδ (Invitrogen) or the pcDNA3.1 empty vector control using Lipofectamine 2000 (Invitrogen), according to the manufacturer's protocol.
Luciferase assay
Cells were plated on 24-well plates; 24 h later, cells were cotransfected with i) 0.2 μg of PPRE-ACOX (PPRE consensus sequence for the rat acyl-CoA oxidase gene, a kind gift from Dr. Thomas McIntyre, Univ. of Utah) or pGL3 control vector (Promega, Madison, WI), and ii) 0.02 μg pRL-SV40 renilla vector (a kind gift from Dr. Lee Yong Woo, Univ. of Virginia). Lipofectamine 2000 (Invitrogen) was used to transfect cells according to the manufacturer's protocol. Transfected cells were treated with vehicle control or L-165041. Twenty-four hours later, the Dual Luciferase Assay (Promega) was conducted according to the manufacturer's protocol, and the Terner Designs Reporter Microplate Luminometer (Promega) was used to measure luciferase activity. Fold change in luminescence, a measure of luciferase activity, was calculated by the relative luminescence units (RLU) of firefly/RLU renilla luciferase.
Measurement of intracellular ROS generation
Intracellular ROS generation was measured using 2'7'-dichlorofluorescein (DCFH-DA) as previously described [38]. DCFH-DA is permeable to cell membranes; once in cells, it is cleaved by cellular esterases and becomes impermeable. When oxidized by ROS, the probe becomes fluorescent. Briefly, cells were washed with PBS+ (1× PBS supplemented with 0.14 g/L CaCl2 and 0.1 g/L of MgCl2), incubated with 10 μM DCFH-DA (Invitrogen, CA/Molecular Probes, Eugene, OR) for 45 min, washed with PBS+ to remove excess probe, and then incubated with 5 μM L-165041. Three hours after L-165041 treatment, cells were irradiated with a single dose of 10 Gy of 137Cs γ rays. One hour post-irradiation, ROS generation was measured using a FACS BDCalibur (Becton Dickinson, Bedford, MA), and BD CellQuest™ Pro 6.0 software was used to analyze the data.
RNA isolation and qRT-PCR Syber Green
RNA was harvested using Trizol reagent (Invitrogen) according to the manufacturer's protocol. DNA contamination was removed by acid-phenol chloroform extraction (pH 4.6, 125:24:1, Ambion Inc., Austin, TX). Real-time PCR amplifications were conducted in a 25 μL reaction volume containing 1 μL cDNA, 12.5 μL SYBR Green PCR Master Mix (Roche, Indianapolis, IN), 0.1 μM upstream and downstream primers, and 10.5 μL nuclease-free water. Real-time PCR was carried out in an ABI Prism® 7000 at 50°C for 2 min, 95°C for 2 min, and 45 cycles of 95°C for 15 min, 55°C for 30 sec, and 72°C for 30 sec. The fold changes in MCP-1, IL-1β, and TNF-α gene expression were calculated using the comparative Ct (cross threshold) method. In brief, the Ct of the housekeeping gene GAPDH was subtracted from the Ct of MCP-1, IL-1β, or TNF-α to get ΔCt. The ΔCt of the sham-irradiated group was then subtracted from the ΔCt for each of the other treatment groups to get ΔΔCt. Fold changes compared to the sham-irradiated group were determined by calculating 2−ΔΔCt. Data represent the mean ± S.E.M of three independent experiments.
Immunoblotting
Total cellular protein was harvested using M-PER lysis buffer (Pierce Biotechnology, Inc., Rockford, IL) supplemented with 1 mg/mL aprotinin (Sigma-Aldrich), 1 mg/mL leupetin (Sigma-Aldrich), 10 mg/mL phenylmethylsulfonyl fluoride (PMSF), 1 mM Na3VO4 (Sigma-Aldrich), and 150 mM of NaCl. Lysates were centrifuged at 12,500 rpm for 10 min, and the supernatant was collected. Protein concentrations were measured using the Bradford assay (BioRad, Hercules, CA) at absorbance 595 nm. Five to 50 μg of protein were separated by SDS-PAGE. Protein was transferred to polyvinylidene fluoride (PVDF) membrane for 1.5–3 h at 80 V, blocked in 2.5% BSA in TBST (0.02 M Tris, 0.015 M NaCl, 0.05% Tween 20, pH 7.5), and incubated overnight with primary antibody. Membranes were washed, incubated with the appropriate HRP-conjugated secondary antibody, developed using the ECL detection system (GE Healthcare, NJ), and processed using a Kodak Processing System. Films were scanned and densitometry was conducted to quantify the signal intensity using Adobe Photoshop Elements 6.0.
Electromobility shift assay (EMSA)
Cells were lysed on ice with Buffer A (10 mM Hepes, pH 7.9, 1.5 mM MgCl2,10 mM KCl, 0.5 mM DTT); lysates were homogenized using a dounce homogenizer, which was followed by centrifugation at 12,000 rpm for 10 min. To extract nuclear protein, the nuclear pellets were lysed with Buffer C (5 mM Hepes, pH 7.9, 1.5 mM MgCl2, 25% v/v glycerol, 400 mM NaCl, 1 mM EDTA 0.5 mM DTT, 0.5 mM PMSF, 2 mg/mL aprotinin, 2 mg/mL leupeptin, and 1 mM Na3VO4). This was followed by centrifugation at 12,000 rpm for 10 min. Protein concentrations were measured using the Bradford assay (BioRad) at absorbance 595 nm. The EMSA procedure was performed using the Promega Gel-Shift Core Assay following the manufacturer's protocol. In brief, 10 μg of nuclear protein were incubated with 2 μL Binding Buffer (Promega) for 10 min. Consensus binding sequences of NF-κB (5'-AGTTGAGGGGACTTTCCCAGGC-3' and 3'TCAACTCCCCTGAAAGGGTCCG-5') and AP-1 (5'-CGCTTGATGAGTCAGCCGGAA-3' and 3'-GCGAACTACTCAGTCGGCCTT-5') were labeled with 10 μCi γ-P32 (GE Healthcare, Piscataway, NJ) and T4 polynucleotide kinase (Promega). The consensus sequences were then incubated with the nuclear protein for 20 min and electrophoresed on a 4% non-denaturing polyacrylamide gel. An X-ray film was then placed on top of the gel and developed overnight at −80°C. The X-ray film was processed using a Kodak Processing System. Films were scanned and densitometry was performed.
Co-Immunoprecipitation
Cell lysates were collected using RIPA buffer (50mM Tris, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 1% SDS, 1 mg/mL aprotinin, 1 mg/mL leupetin, 10 mg/mL PMSF, and 1 mM Na3VO4). Lysates were incubated with 2 μg anti-p65 at 4°C for 3 h, and immunocomplexes were captured by incubating lysates with Protein A/G PLUS agarose beads (Santa Cruz) at 4°C overnight. Agarose beads were centrifuged and washed 8 times with RIPA buffer. After centrifugation, the pellet was diluted with 80 μl 4× SDS-PAGE sample buffer (0.25M Tris-HCL, pH 6.8, 8% SDS, 30% Glycerol, 0.02% Bromophenol Blue, and 10% B-ME), and boiled for 5 min. The samples were then subjected to electrophoresis, and immunoblotted with an anti-PPARδ antibody.
Statistical Analysis
Each experiment was repeated a minimum of three independent times. All analyses were carried out using SAS software (SAS Inc, Cary, NC). Although the sample size within each treatment group is not large, we believe that the outcome measure distribution is normally distributed in the population. To determine statistical significance between two treatment groups, when appropriate, either a one- (when compared to sham) or two-sample t test was used. If more than two treatment groups were compared, analysis of variance (ANOVA) was used to explore the overall association. Bonferroni and Tukey's studentized range tests were used for pairwise comparisons. Levene's test was used to examine the homogeneity of variance, an assumption of variance. When this assumption was not valid, the Kruskal-Wallis test was used.
Results
BV-2 cells express a functional PPARδ
To demonstrate that BV-2 cells contain a functional PPARδ and were appropriate for our studies, we performed a luciferase reporter assay. Cells were co-transfected with: i) a PPRE-driven luciferase vector or a pGL3 control vector, and ii) a renilla vector. They were then incubated with the PPARδ agonist, L-165041, or vehicle control for 24 h, and luciferase activity was measured. Incubating BV-2 cells with 5 μM of L-165041 led to a 2-fold increase in luciferase activity, suggesting that these cells do express a functional PPARδ (SFig. 1). Since 5 μM of L-165041 led to a significant increase in luciferase fluorescence, we chose to use this concentration of the PPARδ agonist for the remainder of our studies.
PPARδ activation prevents the radiation-induced increase in intracellular ROS generation and proinflammatory mediators in BV-2 cells
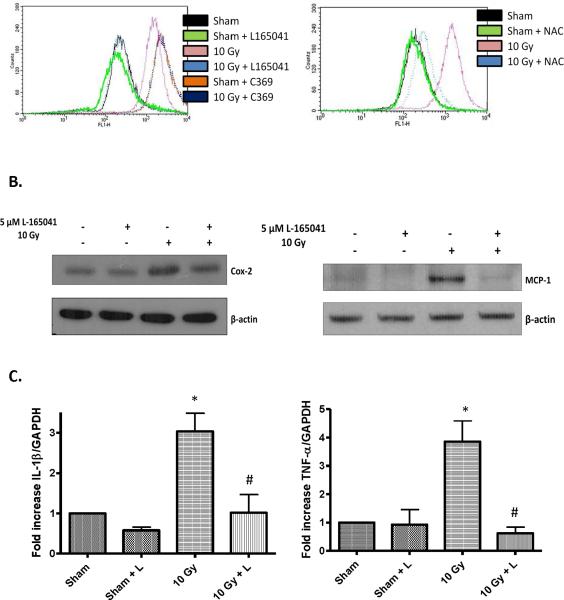
Radiation induces increased intracellular ROS generation in BV-2 cells [18]; we hypothesized that PPARδ activation could modulate this response. To test this hypothesis, we used the oxidation sensitive probe DCFH-DA to measure intracellular ROS generation in BV-2 cells, which were incubated with L-165041 prior to irradiation with a single dose of 10 Gy. As predicted, irradiating the cells resulted in increased ROS production 1 h post-irradiation, and this response was inhibited in cells treated with L-165041 (Fig. 1A). Incubating the cells with nacetyl-cysteine, an ROS scavenger, also inhibited the radiation-induced increase in DCF fluorescence (Fig. 1A). As expected, the non-oxidizable control probe, carboxy-DCF (C369) did not show any radiation-induced difference in fluorescence (Fig. 1A).
Fig. 1.
PPARδ activation prevents the radiation-induced increases in intracellular ROS generation, Cox-2 and MCP-1 protein, and IL-1β and TNF-α mRNA levels. A, BV-2 cells were incubated with 10 μM DCFH-DA or 10 μM C369 for 45 min, the probe was washed off using PBS+, and the cells were pretreated with 5 μM L-165041, NAC, or vehicle control for 3 h. The cells were then irradiated with a single dose of 10 Gy of 137Cs γ rays or sham irradiated, and intracellular ROS generation was measured 1 h post-irradiation as described in the Materials and methods. The results are presented as arbitrary fluoresence units. B & C, BV-2 cells were pretreated with 5 μM of L-165401 and irradiated with a single dose of 10 Gy. B, Protein was harvested 7 h post-irradiation and subjected to western blot analysis for Cox-2 or MCP-1; β-actin was used as a loading control. See SFig. 2 for densitometric analysis. C, RNA was harvested 24 h post-irradiation; the expression levels of IL-1β and TNF-α were analyzed using SYBER Green Real-time PCR and normalized with GAPDH expression levels. Changes in gene expression were calculated using the 2ΔΔCt methods (see Materials and methods). Mean ± S.E.M; *, p≤ 0.05 vs. sham-irradiated, #, p≤ 0.05 vs. 10 Gy; n=3.
Previous reports have demonstrated that irradiating BV-2 cells leads to an increase in Cox-2 protein and IL-1β and TNF-α message levels [18, 19]. We hypothesized that PPARδ activation would inhibit the radiation-induced inflammatory response in BV-2 cells. As shown in Fig. 1B (SFig. 2), L-165041 inhibited the increase in Cox-2 and MCP-1 protein levels observed 7 h post-irradiation. The increase in IL-1β and TNF-α message levels determined 24 h post-irradiation was also significantly inhibited when cells were pretreated with L-165041 (Fig. 1C).
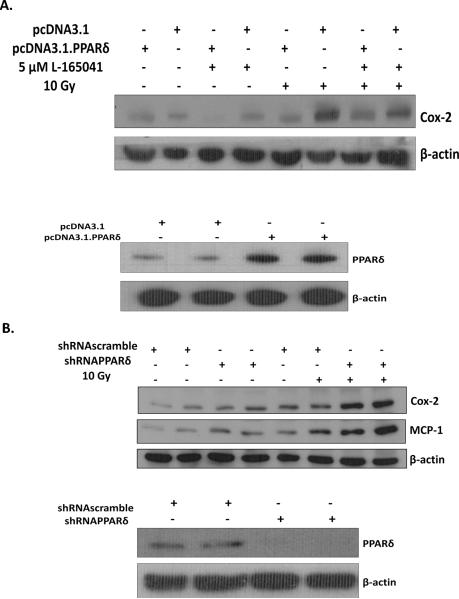
This effect appeared to be PPARδ-dependent; L-165041 failed to inhibit the radiation-induced increase in Cox-2 in the presence of the PPARδ antagonist GSK0660 (SFig. 3) [39]. Moreover, overexpressing PPARδ by transfecting cells with the pcDNA3.1.PPARδ vector inhibited the radiation-induced increase in Cox-2 expression (Fig. 2A). In contrast, shRNA-mediated knockdown of PPARδ (Fig. 2B) led to an increase in the radiation-induced Cox-2 and MCP-1 expression compared to cells infected with scrambled control shRNA. These results indicate that: i] overexpression of PPARδ prevents the radiation-induced increase in Cox-2 expression, and ii] loss of PPARδ enhances the radiation-induced increase in Cox-2 and MCP-1 expression. We also examined if PPARδ knockdown leads to an increase in IL-1β or TNF-α message levels, or intracellular ROS generation; however, we did not observe any differences in these endpoints between cells expressing scramble- or PPARδ-targeted shRNA (data not shown).
Fig. 2.
Overexpression or knockdown of PPARδ modulates Cox-2 and MCP-1 expression in BV-2 cells. A, BV-2 cells were transfected with 24 μg of the pcDNA3.1PPARδ vector or 24 μg of the empty pcDNA3.1 vector using Lipofectamine 2000 according to the manufacturer's protocol. Twenty-four h post-transfection, cells were serum starved for 24 h, treated for 3 h with 5 μM L-165041 or vehicle control, irradiated with a single dose of 10 Gy, and protein was harvested 7 h post-irradiation. Protein was subjected to western blot analysis for Cox-2; β-actin was used as a loading control. B, BV-2 cells were infected with scramble control or shRNA targeting PPARδ. Single knockdown clones were selected, irradiated with a single dose of 10 Gy, and protein was harvested 7 h post-irradiation. Protein was subjected to western blot analysis for Cox-2 or MCP-1. β-actin was used as a loading control.
PPARδ activation inhibits NF-κB activation via transrepression by physically interacting with the p65 subunit
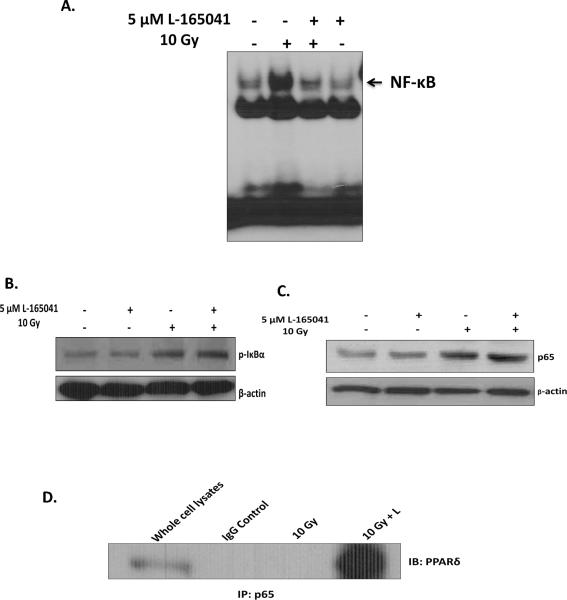
The radiation-induced proinflammatory response in BV-2 cells is regulated, in part, by NF-κB [18, 19]. Thus, we examined if PPARδ activation could modulate NF-κB activation in BV-2 cells following irradiation. As predicted, pre-incubating BV-2 cells with L-165041 prevented the radiation-induced increase in NF-κB DNA binding observed 30 min post-irradiation (Fig 3A; SFig 4A). To investigate the mechanism by which PPARδ modulates NF-κB activation, we examined the phosphorylation of Iκ-Bα and the nuclear translocation of p65. Thirty minutes post-irradiation, we observed the predicted increases in the phosphorylation of Iκ-Bα at Ser 32 and the nuclear translocation of p65; L-165041 did not modulate these responses (Figs. 3B & 3C; SFig 4B and SFig 4C). Next, we examined if PPARδ inhibited NF-κB activation via transrepression by binding to the p65 subunit. Indeed, when we immunoprecipitated cellular lysates for p65 and then immunoblotted for PPARδ, we observed that L-165041 led to an increase in the binding of PPARδ to p65 30 min post-irradiation (Fig. 3D). Mechanistically, these data suggest that PPARδ activation prevents the DNA binding activity of NF-κB via transrepression by physically interacting with the p65 subunit.
Fig. 3.
PPARδ activation prevents radiation-induced NF-κB activation by physical interaction with the p65 subunit. BV-2 cells were pretreated with 5 μM of L-165401 or vehicle control and irradiated with a single dose of 10 Gy. A, Nuclear protein was collected 30 min post-irradiation. Gel-Shift analysis was carried out by incubating 10 μg of nuclear protein with γ-ATP P32 end- labeled NF-κB consensus oligo (see Materials and methods). The samples were run on a 4% non-denaturing acrylamide gel, stained with 7% Acetic acid, and exposed to X-ray film. B, Protein was harvested 30 min post-irradiation; whole cell lysates were subjected to western blot analysis for p-IκBα. C, Nuclear protein was harvested 30 min post-irradiation; nuclear protein was subjected to western blot analysis for nuclear p65. B & C, β-actin was used as a loading control. A–C, See SFig. 4 for densitometric analysis. D, Co-immunoprecipitation was carried out using anti-p65 and immunoblotting for PPARδ (see Materials and methods).
PPARδ activation prevents AP-1 activation by inhibiting c-jun phosphorylation and upstream activation of the stress-activated kinases MEK1/2 and ERK1/2
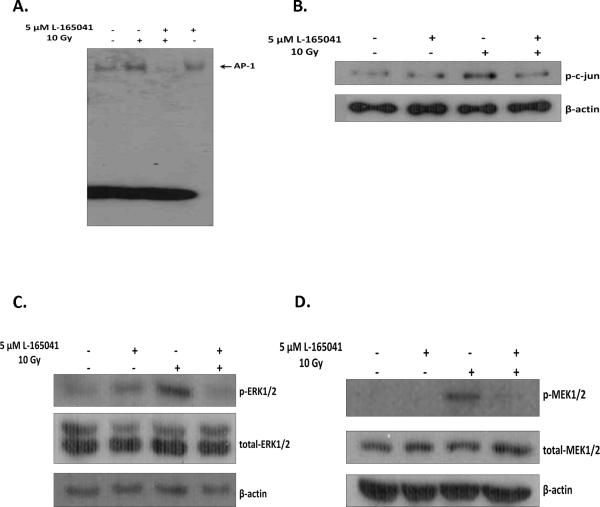
In addition to NF-κB activation, activation of AP-1 is thought to underlie the radiation-induced increase in proinflammatory mediators in BV-2 cells [18]. We therefore investigated if L-165041 could also modulate AP-1 activation. Pre-incubating BV-2 cells with L-165041 prevented the radiation-induced increase in AP-1 DNA binding activity observed 30 min post-irradiation (Fig. 4A; SFig. 5A). Activation of AP-1, a dimeric protein, is mediated, in part, by phosphorylation of the c-jun subunit. We observed that L-165041 inhibited the phosphorylation of nuclear c-jun at Ser73 following irradiation, suggesting that PPARδ activation inhibits AP-1 activation by preventing c-jun activation (Fig. 4B; SFig. 5B).
Fig. 4.
PPARδ activation prevents radiation-induced AP-1 activation and upstream activation of c-jun, MEK1/2, and ERK1/2. BV-2 cells were pretreated with 5 μM of L-165041 or vehicle control and irradiated with a single dose of 10 Gy. A, Nuclear protein was collected 30 min post-irradiation. Gel-Shift analysis was carried out by incubating 10 μg of nuclear protein with γ-ATP P32 end- labeled AP-1 consensus oligo (see Materials and methods). The samples were run on a 4% non-denaturing acrylamide gel, stained with 7% Acetic acid, and exposed to X-ray film. B, Nuclear protein was collected 30 min post-irradiation; nuclear protein was subjected to western blot analysis for p-c-jun; β-actin was used as a loading control. C & D, Protein was harvested 30 min post-irradiation and subjected to western blot analysis for C, p-MEK1/2 and total-MEK1/2 and D, p-ERK1/2 and total-ERK1/2; β-actin was used as a loading control. A–D, See SFig. 5 for densitometric analysis.
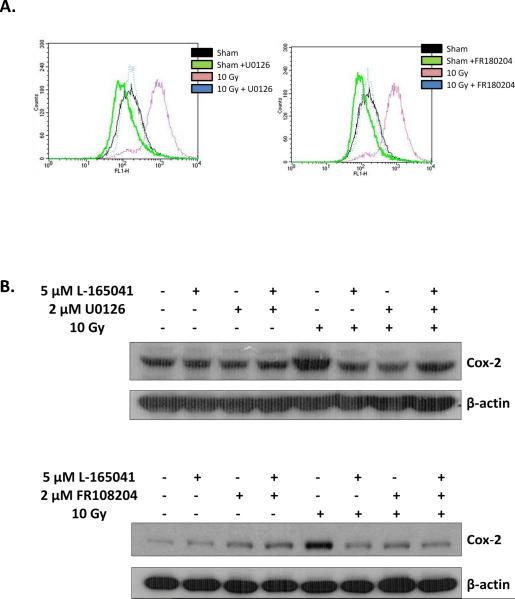
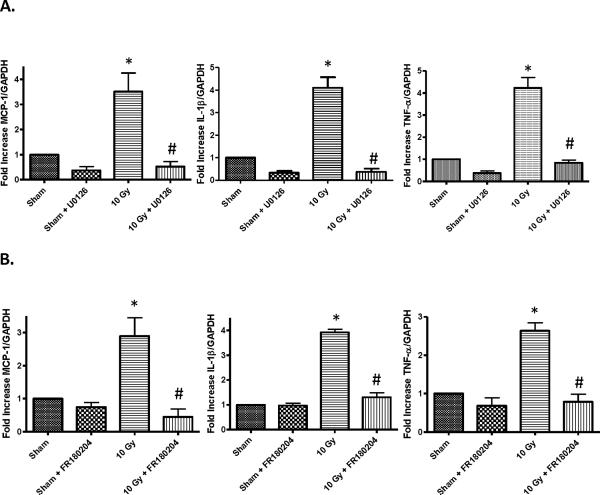
MEK1/2 and ERK1/2 activation regulates c-jun phosphorylation and AP-1 activation in BV-2 cells [Zhiyong Deng and Weiling Zhao, unpublished data]. Interestingly, studies have demonstrated that PPARδ can modulate activation of the MEK1/2/ERK1/2 pathway [31, 40]. Thus, we hypothesized that PPARδ modulates AP-1 activation via inhibition of MEK1/2 and ERK1/2 phosphorylation. Consistent with our hypothesis, pre-incubating BV-2 cells with L-165041 reduced the radiation-induced phosphorylation of MEK1/2 at Ser218 and Ser222, and that of ERK1/2 at Thr 202 and Tyr 204 (Figs. 4C & 4D; SFigs. 5C & 5D). Furthermore, pretreating BV-2 cells with 2 μM of the MEK1/2 inhibitor, U0126, or 2 μM of the ERK1/2 inhibitor, FR180204, inhibited the radiation-induced increase in intracellular ROS generation and Cox-2 expression (Figs. 5A & 5B; SFigs. 6). MEK1/2 or ERK1/2 inhibition also prevented the radiation-induced increases in MCP-1, IL-1β, and TNF-α gene expression (Figs. 6A & 6B).
Fig 5.
The MEK1/2 inhibitor, U0126, or the ERK1/2 inhibitor, FR180204, prevent the radiation-induced increase in intracellular ROS generation and Cox-2 expression. A, BV-2 cells were incubated with 10 μM DCFH-DA for 45 min, the probe was washed off using PBS+, and the cells were pretreated with 2 μM U0126, 2 μM FR180204, or vehicle control for 3 h. The cells were then irradiated with a single dose of 10 Gy of 137Cs γ rays or sham irradiated, and intracellular ROS generation was measured 1 h post-irradiation. B, BV-2 cells were pretreated with 2 μM U0126 or 2 μM FR180204, or vehicle control for 1 h prior to treatment with 5 μM L-165041 or vehicle control. Three hours post-L-165041 treatment, cells were irradiated with a single dose of 10 Gy and whole cell lysates were collected. Protein was subjected to western blot analysis for Cox-2. β-actin was used as a loading control. See SFig. 6 for densitometric analysis.
Fig. 6.
MEK1/2 or ERK1/2 inhibition prevents the radiation-induced increase in MCP-1, IL-1β, and TNF-α gene expression. BV-2 cells were pretreated with A, 2 μM U0126, B, 2 μM FR180204, or vehicle control for 3 h. Three h post-treatment, cells were irradiated with a single dose of 10 Gy and RNA was harvested 24 h post-irradiation. The expression levels of MCP-1, IL-1β, and TNF-α were analyzed using SYBER Green Real-time PCR and normalized with GAPDH expression levels. Changes in gene expression were calculated using the 2−ΔΔCt methods (see Materials and methods). Mean ± S.E.M; *, p≤ 0.05 vs. sham-irradiated, #, p≤ 0.05 vs. 10 Gy; n=3.
PPARδ modulates MEK1/2 and ERK1/2 activation, in part, by inhibiting the radiation-induced phosphorylation and expression of PKCα
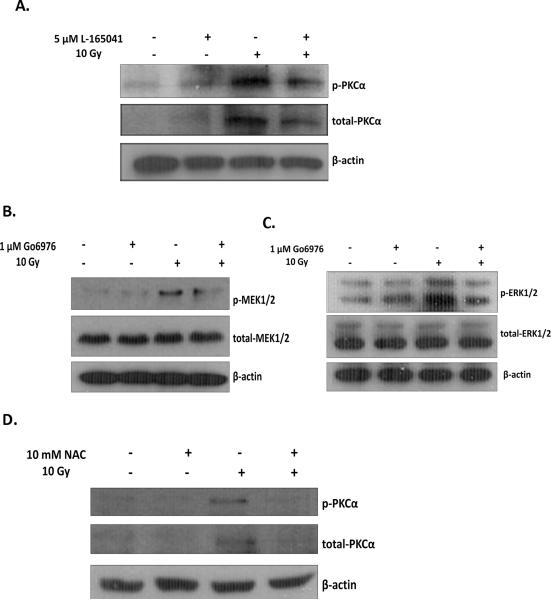
PKCα is an activator of the MEK1/2/ERK1/2 pathway [41, 42]. A radiation-induced activation of PKCα has not been previously reported in microglia. However, lipopolysaccaride (LPS), an inflammatory stimulus, has been shown to increase activation of PKCα in microglial cells [43]. Therefore, we next investigated if radiation induces PKCα activation in BV-2 cells, and further, if PPARδ activation can modulate this response. As shown in Fig. 7A (SFig. 7A), irradiating BV-2 cells with a single dose of 10 Gy led to an increase in the phosphorylation of PKCα at Thr638 and the expression of PKCα, and pre-treatment with L-165041 prevented these increases.
Fig. 7.
PPARδ modulates MEK1/2 and ERK1/2 activation, in part, by inhibiting upstream activation of PKCα. BV-2 cells were pretreated with A, 5 μM L-165041, B & C, 1 μM Go6976, D, 10 mM NAC, or vehicle control 3 h prior to a single dose of 10 Gy. Protein was harvested 30 min post-irradiation and subjected to western blot analysis for A & D, p-PKCα and total-PKCα, B, p-MEK1/2 and total-MEK1/2, or C, p-ERK1/2 and total-ERK1/2. A–D, β-actin was used as a loading control. A–D, See SFig. 7 for densitometric analysis.
To demonstrate that inhibition of PKCα prevents the radiation-induced activation of MEK1/2 and ERK1/2, we pre-treated BV-2 cells with 1 μM of the PKCα/β inhibitor, Go6976, and examined the phosphorylation of MEK1/2 and ERK1/2 30 min post-irradiation. As predicted, Go6976 prevented the radiation-induced phosphorylation of MEK1/2 and ERK1/2 (Figs. 7B & 7C; SFigs. 7B & 7C).
The radiation-induced phosphorylation of MEK1/2 and ERK1/2 is modulated by ROS production in BV-2 cells [Zhiyong Deng and Weiling Zhao, unpublished data]. Our results suggest that PKCα regulates MEK1/2 and ERK1/2 phosphorylation; thus, we examined whether PKCα phosphorylation is also modulated by ROS production. Indeed, pre-treating BV-2 cells with 10 mM of NAC inhibited the radiation-induced phosphorylation and expression of PKCα (Fig. 7D; SFig. 7D). Given that L-165041 inhibited the radiation-induced increase in intracellular ROS generation (Fig. 1A), these data suggest that PPARδ activation negatively regulates the PKCα/MEK1/2/ERK1/2 pathway by preventing ROS generation following irradiation.
Discussion
A growing body of evidence suggests that PPARδ activation can regulate oxidative stress and inflammatory responses following various cellular stresses. We therefore hypothesized that PPARδ activation would prevent the radiation-induced oxidative stress and proinflammatory responses in microglia. As predicted, pretreating BV-2 cells with L-165041 inhibited the radiation-induced increase in: i) intracellular ROS generation, ii) Cox-2 and MCP-1 protein expression, and iii) IL-1β and TNF-α message levels. This occurred, in part, by negatively regulating the DNA binding of NF-κB and AP-1. PPARδ activation: i) inhibited NF-κB via transrepression by physically interacting with the p65 subunit, and ii) prevented activation of the PKCα/MEK1/2/ERK1/2/AP-1 pathway by inhibiting the radiation-induced increase in intracellular ROS generation.
The radiation-induced proinflammatory response in BV-2 cells is regulated, in part, by NF-κB. There are several mechanisms by which PPARδ can modulate NF-κB, including: i) inhibiting the nuclear translocation of p65, ii) binding directly to p65, and iii) reducing acetylation of p65 [30, 44, 45]. In a rat model of reperfusion injury, the PPARδ agonist, GW0742, reduced myocardial infarct size, in part, by inhibiting nuclear translocation of p65 [44]. In cultured neonatal rat cardiomyocytes, L-165041 decreased LPS-stimulated NF-κB activation by increasing the physical interaction of PPARδ with the p65 subunit. This interaction is thought to interfere with the DNA binding of NF-κB [30]. The PPARδ agonist, GW501516, inhibited inflammation in human HaCaT keratinocytes by reducing TNF-α-induced p65 acetylation. Acetylation of p65 is regulated by the transcriptional co-activator p300; reduced acetylation of p65 decreases DNA binding and activation of NF-κB [45]. Our studies indicate that L-165041 increased the physical interaction of PPARδ to p65, suggesting that the receptor modulates NF-κB activation via transrepression.
In addition to NF-κB activation, the radiation-induced proinflammatory response in BV-2 cells is regulated, in part, by AP-1 activation. The transactivation function of the c-jun subunit of AP-1 depends on the phosphorylation of residues Ser63 and Ser73 [46]. Radiation induces phosphorylation of both residues in BV-2 cells [18]. We observed that PPARδ prevents the phosphorylation of nuclear c-jun at Ser73, and further, inhibits AP-1 DNA binding. Previous studies in BV-2 cells demonstrate that c-jun is phosphorylated by ERK1/2 [Zhiyong Deng and Weiling Zhao, unpublished data]. We observed that PPARδ activation prevents the radiation-induced activation of MEK1/2 and ERK1/2. In addition to AP-1, activation of the MEK1/2/ERK1/2 pathway has been shown to modulate NF-κB activation. In white adipose tissue, PPARδ activation prevented LPS-induced inflammation by inhibiting ERK1/2 activation and the downstream activation of NF-κB [40]. It is therefore possible that the PPARδ-mediated modulation of the MEK1/2/ERK1/2 pathway in our model also contributes to a reduction in radiation-induced proinflammatory responses, in part, by preventing NF-κB activation.
PKC is an upstream regulator of the MEK1/2/ERK1/2 pathway. In BV-2 cells, LPS-stimulated PKC activation led to ERK1/2 phosphorylation and increased Cox-2 protein levels [47]. Furthermore, in cultures of primary microglia, LPS-stimulated PKCα modulated TNF-α expression [43]. Studies have demonstrated that PPARδ can modulate PKCα. In PPARδ-null mice compared to wild-type mice, TPA induced significantly greater PKCα activity, enhancing MEK1/2 and ERK1/2 phosphorylation and Cox-2 expression [31]. Moreover, studies in platelets have demonstrated that PPARδ can physically interact with PKCα and suppress PKCα-mediated platelet activation [48]. Given the evidence that PKCα modulates both the MEK1/2/ERK1/2 pathway and inflammation, and further that PPARδ modulates PKCα activity, we examined if PKCα was an activator of MEK1/2 and ERK1/2 in our model. Indeed, we observed that the radiation-induced phosphorylation of PKCα activates the MEK1/2/ERK1/2 pathway, and PPARδ activation inhibits this response.
Our data suggest that PPARδ prevented activation of the PKCα/MEK1/2/ERK1/2/AP-1 pathway by inhibiting the radiation-induced increase in intracellular ROS generation. Of interest, MEK1/2 and ERK1/2 inhibition also prevented the radiation-induced increase in intracellular ROS generation, suggesting that the increase in intracellular ROS generation observed following irradiation reflects both direct and indirect effects, and that PPARδ activation can inhibit these responses. The antioxidant actions of PPARδ agonists have been observed in a variety of cell types [27–29]; we observed that PPARδ activation could inhibit intracellular ROS generation following irradiation of BV-2 cells. It should be noted that PPARδ can activate transcription of several antioxidant genes, including catalase, superoxide dismutase, glutathione peroxidase 1, heme oxygenase 1, and thioredoxin 1 [32, 33]. Previous studies in our lab have failed to detect changes in the expression levels of antioxidant genes in BV-2 cells; however, changes in the activity levels of antioxidant enzymes have not been examined [18]. Future studies should examine if radiation induces changes in the activity levels of antioxidant enzymes, and further, if PPARδ can modulate these changes. These studies will help elucidate the mechanisms by which PPARδ activation can modulate intracellular ROS.
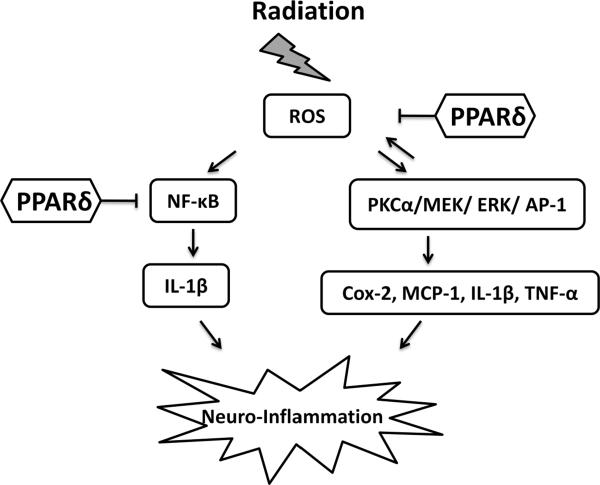
Based on the findings described above, we propose a model, outlined in Fig. 8, for the role of PPARδ in modulating radiation-induced proinflammatory responses in microglia. Following irradiation, increased intracellular ROS generation leads to activation of the stress-activated kinases, PKCα, MEK1/2, and ERK1/2, and the proinflammatory transcription factors, NF-κB and AP-1. Activation of these transcription factors increases the expression of Cox-2, MCP-1, IL-1β, and TNF-α; these proinflammatory mediators contribute to the inflammatory phenotype of microglia. PPARδ activation prevents the radiation-induced proinflammatory response, in part, by negatively regulating NF-κB and AP-1. Specifically, PPARδ physically interacts with p65 and inhibits the DNA binding of NF-κB. Futhermore, PPARδ inhibits the radiation-induced increase in intracellular ROS generation, which prevents PKCα, MEK1/2, ERK1/2, and c-jun phosphorylation and the DNA binding of AP-1. Taken together, our data indicate that PPARδ activation can prevent the acute radiation-induced oxidative stress and proinflammatory responses observed in microglia within hours of in vitro irradiation.
Fig. 8.
Proposed model outlining the role of PPARδ in the modulation of the radiation-induced proinflammatory response in BV-2 cells. Irradiation of BV-2 cells leads to an increase in intracellular ROS generation. This increases activation of the stress-activated kinases, PKCα, MEK1/2, and ERK1/2, and the proinflammatory transcription factors, NF-κB and AP-1. Activation of NF-κB and AP-1 enhance the expression of Cox-2, MCP-1, IL-1β, and TNF-α. PPARδ activation prevents radiation-induced neuroinflammation, in part, by transrepression of NF-κB by physical interaction with the p65 subunit. Additionally, PPARδ inhibits intracellular ROS generation, which prevents PKCα, MEK, and ERK1/2 phosphorylation and downstream activation of AP-1.
It is important to note that the microglia used in our in vitro studies were grown under 21% oxygen, a concentration much higher than would occur in vivo. Thus, the affect of “physiological” oxygen concentration on the radiation-induced inflammatory response of microglial cells remains to be determined. Furthermore, although our in vitro studies examined the radiation-induced inflammatory response in microglial cells, radiation-induced brain injury in vivo is a multicellular process. Indeed, we recognize that interpreting these data in terms of their relevance to the onset and progression of radiation-induced late effects in the brain is difficult and somewhat controversial. It is unlikely that the radiation-induced cognitive impairment observed 6 months or more after fWBI is a direct result of acute proinflammatory responses in the microglia alone. However, brain irradiation clearly leads to chronic, persistent increases in activated microglia [16, 17]; the resultant neuroinflammation has been associated with decreased neurogenesis and impaired neuronal function [15, 16, 21]. Thus, understanding the mechanisms by which radiation alters the microglia cell phenotype, and how PPARδ agonists might prevent/ameliorate these changes, offers the promise of identifying potential interventions that can be evaluated in vivo.
Moreover, since PPARδ activation has been shown to regulate inflammation in multiple cell types and promote the survival of neurons under stress conditions, it is possible that PPARδ can mitigate radiation-induced brain injury in multiple cell types [29, 49]. Additionally, animal studies have demonstrated that PPARδ agonists can cross the blood brain barrier and modulate oxidative stress and pro-inflammatory responses associated with acute and chronic CNS disorders [34–36]. Overall, our current in vitro findings indicate that PPARδ activation shows promise as a potential therapeutic strategy in the treatment and/or prevention of radiation-induced brain injury.
Supplementary Material
A functional PPARδ is expressed in BV-2 cells. BV-2 cells were cotransfected using Lipofectamine 2000 (Invitrogen, Carlsbad, California) with i) 0.2 μg of PPRE-ACOX (PPRE consensus rat acyl-CoA oxidase gene) or pGL3 (control vector) and ii) 0.02 μg of pRL-SV40 (a renilla plasmid). Twenty-four h post-transfection, cells were treated with 0, 1, or 5 μM of L-165041 or vehicle control. The Dual Luciferase Assay (Promega, Madison, Wisconsin) was used to measure luciferase activity according to the manufacturer's instructions. * = p≤ 0.05.
PPARδ activation prevents the radiation-induced increases in Cox-2 and MCP-1 protein expression. Densitometric analysis of Fig. 1B. Results are shown as fold changes compared to sham-irradiated controls. Mean ± S.E.M; *, p≤0.05 vs. sham; #, p≤0.05 vs. 10 Gy; n = 3.
The PPARδ antagonist, GSK0660, prevents the L-165041-mediated inhibition of the radiation-induced increase in Cox-2 protein. BV-2 cells were treated with 10 μM of the PPARδ antagonist GSK0660 or vehicle control 1 h prior to treatment with 5 μM L-165041 or vehicle control. Cells were then irradiated with a single dose of 10 Gy and protein was subjected to western blot analysis for Cox-2; β-actin was used as a loading control.
PPARδ activation prevents radiation-induced NF-κB activation. Densitometric analysis of Figs 3A–3C (A–C). Results are shown as fold changes compared to sham-irradiated controls.
PPARδ activation prevents radiation-induced AP-1 activation and upstream activation of c-jun, MEK1/2, and ERK1/2. Densitometric analysis of Figs 4A–4D (A–D). Results are shown as fold changes compared to sham-irradiated controls. Mean ± S.E.M; *, p≤0.05 vs. sham; #, p≤0.05 vs. 10 Gy; n = 3.
The MEK1/2 inhibitor, U0126, or the ERK1/2 inhibitor, FR180204, prevent the radiation-induced increase in Cox-2 expression. Densitometric analysis of Figs 5B. Results are shown as fold changes compared to sham-irradiated cells. Mean ± S.E.M; *, p≤0.05 vs. sham; n = 3.
PPARδ modulates MEK1/2/ERK1/2 activation, in part, by inhibiting upstream activation of PKCα. Densitometric analysis of Figs 7A–7D (A–D). Results are shown in fold changes compared to sham-irradiated controls. Mean ± S.E.M; *, p≤0.05 vs. sham; #, p≤0.05 vs. 10 Gy; n = 3.
Highlights
PPARδ activation prevents radiation-induced oxidative stress/inflammation
PPARδ inhibits NF-κB via transrepression by physically interacting with p65 subunit
PPARδ prevents activation of the PKCα/MEK1/2/ERK1/2/AP-1 pathway
PPARδ activation modulates radiation-induced microglial pro-inflammatory response.
Acknowledgements
This work was supported by NIH grant CA112593 (MER). We thank Dr. Linda van Eldik, Northwestern University, USA, for generously providing the BV-2 cells, originally developed by Dr. V. Bocchini, University of Perugia, Italy. We thank Dr. Weiling Zhao (Department of Radiation Oncology, WFSM) for providing antibodies against phosphorylated c-jun and p65.
List of abbreviations
- AP-1
Activator protein-1
- CNS
Central nervous system
- Cox-2
Cyclooxygenase-2
- ERK1/2
Extracellular signal-related kinase 1/2
- Gy
Gray
- ICAM-1
Intracellular cellular adhesion molecule-1
- IL-1β
Interleukin-1 beta
- MCP-1
Monocyte chemoattractant protein-1
- MEK1/2
Mitogen-activated protein kinase kinase 1/2
- NF-κB
Nuclear factor kappa B
- PKCα
Protein Kinase C alpha
- PPARδ
Peroxisomal proliferator-activated receptor delta
- ROS
Reactive oxygen species
- TNF-α
Tumor necrosis factor-alpha
- WBI
Whole-brain Irradiation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J. Clin. Oncol. 2006;24:1295–304. doi: 10.1200/JCO.2005.04.6185. [DOI] [PubMed] [Google Scholar]
- [2].Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J. Clin. Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- [3].Johannesen TB, Lien HH, Hole KH, Lote K. Radiological and clinical assessment of long-term brain tumour survivors after radiotherapy. Radiother. Oncol. 2003;69:169–176. doi: 10.1016/s0167-8140(03)00192-0. [DOI] [PubMed] [Google Scholar]
- [4].Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J. Clin. Oncol. 2006;24:1305–1309. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- [5].Frost MH, Sloan JA. Quality of life measurements: a soft outcome--or is it? Am. J. Manag. Care. 2002;8:S574–9. [PubMed] [Google Scholar]
- [6].Liu R, Page M, Solheim K, Fox S, Chang SM. Quality of life measurements: A soft outcome – or is it? Am. J. Manag. Care. 2002;8:S330–339. [PubMed] [Google Scholar]
- [7].Shaw EG, Rosedahl R, D'Agostino RB, Lovato J, Naughton MJ, Robbins ME, Rapp SR. Phase II study of donepezil in irradiated brain tumor patients: Effect on cognitive function, mood, and quality of life. J. Clin. Oncol. 2006;24:1415–1420. doi: 10.1200/JCO.2005.03.3001. [DOI] [PubMed] [Google Scholar]
- [8].Shaw EG, Robbins ME. The management of radiation-induced brain injury. Cancer Treat. Res. 2006;128:7–22. doi: 10.1007/0-387-25354-8_2. [DOI] [PubMed] [Google Scholar]
- [9].Kyrkanides S, Moore AH, Olschowka JA, Daeschner JC, Williams JP, Hansen JT, Kerry O'Banion M. Cyclooxygenase-2 modulates brain inflammation-related gene expression in central nervous system radiation injury. Brain Res. Mol. Brain Res. 2002;104:159–69. doi: 10.1016/s0169-328x(02)00353-4. [DOI] [PubMed] [Google Scholar]
- [10].Chiang CS, Hong JH, Stalder A, Sun JR, Withers HR, McBride WH. Delayed molecular responses to brain irradiation. Int. J. Radiat. Biol. 1997;72:45–53. doi: 10.1080/095530097143527. [DOI] [PubMed] [Google Scholar]
- [11].Lee WH, Sonntag WE, Mitschelen M, Yan H, Lee YW. Irradiation induces regionally specific alterations in proinflammatory environments in rat brain. Int. J. Radiat. Biol. 2010;86:132–44. doi: 10.3109/09553000903419346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog. Neurobiol. 1999;58:233–47. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- [13].Gebicke-Haerter PJ. Microglia in neurodegeneration: molecular aspects. Microsc.. Res. Tech. 2001;54:47–58. doi: 10.1002/jemt.1120. [DOI] [PubMed] [Google Scholar]
- [14].Pocock JM, Liddle AC. Microglial signalling cascades in neurodegenerative disease. Prog. Brain. Res. 2001;32:555–65. doi: 10.1016/S0079-6123(01)32103-9. [DOI] [PubMed] [Google Scholar]
- [15].Kim SU, de Vellis J. Microglia in health and disease. J. Neurosci. Res. 2005;81:302–13. doi: 10.1002/jnr.20562. [DOI] [PubMed] [Google Scholar]
- [16].Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- [17].Ramanan S, Kooshki M, Zhao W, Hsu FC, Riddle DR, Robbins ME. The PPARalpha agonist fenofibrate preserves hippocampal neurogenesis and inhibits microglial activation after whole-brain irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2009;75:870–7. doi: 10.1016/j.ijrobp.2009.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ramanan S, Kooshki M, Zhao W, Hsu FC, Robbins ME. PPARalpha ligands inhibit radiation-induced microglial inflammatory responses by negatively regulating NF-kappaB and AP-1 pathways. Free Radic. Biol. Med. 2008;45:1695–704. doi: 10.1016/j.freeradbiomed.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dong X, Dong J, Zhang R, Fan L, Liu L, Wu G. Anti-inflammatory effects of tanshinone IIA on radiation-induced microglia BV-2 cells inflammatory response. Cancer Biother. Radiopharm. 2009;24:681–7. doi: 10.1089/cbr.2009.0640. [DOI] [PubMed] [Google Scholar]
- [20].Kyrkanides S, Olschowka JA, Williams JP, Hansen JT, O'Banion MK. TNFα and IL-1β mediate intercellular adhesion molecule-1 induction via microglia–astrocyte interaction in CNS radiation injury. J. Neuroimmunol. 1999;95:95–106. doi: 10.1016/s0165-5728(98)00270-7. [DOI] [PubMed] [Google Scholar]
- [21].Lonergan PE, Martin DS, Horrobin DF, Lynch MA. Neuroprotective effect of eicosapentaenoic acid in hippocampus of rats exposed to gamma-irradiation. J. Biol. Chem. 2002;277:20804–11. doi: 10.1074/jbc.M202387200. [DOI] [PubMed] [Google Scholar]
- [22].Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–7. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- [23].Blanquart C, Barbier O, Fruchart JC, Staels B, Glineur C. Peroxisome proliferator-activated receptors: regulation of transcriptional activities and roles in inflammation. J. Steroid Biochem. Mol. Biol. 2003;85:267–73. doi: 10.1016/s0960-0760(03)00214-0. [DOI] [PubMed] [Google Scholar]
- [24].Nuclear Receptors Nomenclature Committee A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97:161–163. doi: 10.1016/s0092-8674(00)80726-6. [DOI] [PubMed] [Google Scholar]
- [25].Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358:771–4. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Moreno S, Farioli-Vecchioli S, Cerù MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid × receptors in the adult rat CNS. Neuroscience. 2004;123:131–45. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- [27].Moraes LA, Piqueras L, Bishop-Bailey D. Peroxisome proliferator-activated receptors and inflammation. Pharmacol. Ther. 2006;110:371–85. doi: 10.1016/j.pharmthera.2005.08.007. [DOI] [PubMed] [Google Scholar]
- [28].Kostadinova R, Wahli W, Michalik L. PPARs in diseases: control mechanisms of inflammation. Curr. Med. Chem. 2005;12:2995–3009. doi: 10.2174/092986705774462905. [DOI] [PubMed] [Google Scholar]
- [29.Bishop-Bailey D, Bystrom J. Emerging roles of peroxisome proliferator-activated receptor-beta/delta in inflammation. Pharmacol. Ther. 2009;124:141–50. doi: 10.1016/j.pharmthera.2009.06.011. [DOI] [PubMed] [Google Scholar]
- [30].Planavila A, Rodríguez-Calvo R, Jové M, Michalik L, Wahli W, Laguna JC, Vázquez-Carrera M. Proliferator-activated receptor beta/delta activation inhibits hypertrophy in neonatal rat cardiomyocytes. Cardiovasc. Res. 2005;65:832–41. doi: 10.1016/j.cardiores.2004.11.011. [DOI] [PubMed] [Google Scholar]
- [31].Kim DJ, Murray IA, Burns AM, Gonzalez FJ, Perdew GH, Peters JM. Peroxisome proliferator-activated receptor-beta/delta inhibits epidermal cell proliferation by down-regulation of kinase activity. J. Biol. Chem. 2005;280:9519–27. doi: 10.1074/jbc.M413808200. [DOI] [PubMed] [Google Scholar]
- [32].Pesant M, Sueur S, Dutartre P, Tallandier M, Grimaldi PA, Rochette L, Connat JL. Peroxisome proliferator-activated receptor delta (PPARdelta) activation protects H9c2 cardiomyoblasts from oxidative stress-induced apoptosis. Cardiovasc. Res. 2006;69:440–9. doi: 10.1016/j.cardiores.2005.10.019. [DOI] [PubMed] [Google Scholar]
- [33].Kim HJ, Ham SA, Paek KS, Hwang JS, Jung SY, Kim MY, Jin H, Kang ES, Woo IS, Kim HJ, Lee JH, Chang KC, Han CW, Seo HG. Transcriptional up-regulation of antioxidant genes by PPARδ inhibits angiotensin II-induced premature senescence in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2011;406:564–9. doi: 10.1016/j.bbrc.2011.02.091. [DOI] [PubMed] [Google Scholar]
- [34].Kalinin S, Richardson JC, Feinstein DL. A PPARdelta agonist reduces amyloid burden and brain inflammation in a transgenic mouse model of Alzheimer's disease. Curr. Alzheimer Res. 2009;6:431–7. doi: 10.2174/156720509789207949. [DOI] [PubMed] [Google Scholar]
- [35].Iwashita A, Muramatsu Y, Yamazaki T, Muramoto M, Kita Y, Yamazaki S, Mihara K, Moriguchi A, Matsuoka N. Neuroprotective efficacy of the peroxisome proliferator-activated receptor delta-selective agonists in vitro and in vivo. J. Pharmacol. Exp. Ther. 2007;320:1087–96. doi: 10.1124/jpet.106.115758. [DOI] [PubMed] [Google Scholar]
- [36].Kanakasabai S, Chearwae W, Walline CC, Iams W, Adams SM, Bright JJ. Peroxisome proliferator-activated receptor delta agonists inhibit T helper type 1 (Th1) and Th17 responses in experimental allergic encephalomyelitis. Immunology. 2010;13:572–88. doi: 10.1111/j.1365-2567.2010.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Su i G., Soohoo C, Affar el. B., Gay F, Shi Y, Forrester WC, Shi Y. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc. Natl. Acad. Sci. U S A. 2002;99:5515–20. doi: 10.1073/pnas.082117599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Smith PS, Zhao W, Spitz DR, Robbins ME. Inhibiting catalase activity sensitizes 36B10 rat glioma cells to oxidative stress. Free Radic. Biol. Med. 2007;42:787–797. doi: 10.1016/j.freeradbiomed.2006.11.032. [DOI] [PubMed] [Google Scholar]
- [39].Shearer BG, Steger DJ, Way JM, Stanley TB, Lobe DC, Grillot DA, Iannone MA, Lazar MA, Willson TM, Billin AN. Identification and characterization of a selective peroxisome proliferator-activated receptor beta/delta (NR1C2) antagonist. Mol. Endocrinol. 2008;22:523–9. doi: 10.1210/me.2007-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rodríguez-Calvo R, Serrano L, Coll T, Moullan N, Sánchez RM, Merlos M, Palomer X, Laguna JC, Michalik L, Wahli W, Vázquez-Carrera M. Activation of peroxisome proliferator-activated receptor beta/delta inhibits lipopolysaccharide-induced cytokine production in adipocytes by lowering nuclear factor-kappaB activity via extracellular signal-related kinase 1/2. Diabetes. 2008;57:2149–57. doi: 10.2337/db08-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schönwasser DC, Marais RM, Marshall CJ, Parker PJ. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol. Cell Biol. 1998;18:790–8. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marmé D, Rapp UR. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364:249–52. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- [43].Nakajima K, Tohyama Y, Kohsaka S, Kurihara T. Protein kinase C alpha requirement in the activation of p38 mitogen-activated protein kinase, which is linked to the induction of tumor necrosis factor alpha in lipopolysaccharide-stimulated microglia. Neurochem. Int. 2004;44:205–14. doi: 10.1016/s0197-0186(03)00163-3. [DOI] [PubMed] [Google Scholar]
- [44].Kapoor A, Collino M, Castiglia S, Fantozzi R, Thiemermann C. Activation of peroxisome proliferator-activated receptor-beta/delta attenuates myocardial ischemia/reperfusion injury in the rat. Shock. 2010;34:117–24. doi: 10.1097/SHK.0b013e3181cd86d6. [DOI] [PubMed] [Google Scholar]
- [45].Barroso E, Eyre E, Palomer X, Vázquez-Carrera M. The peroxisome proliferator-activated receptor β/δ (PPARβ/δ) agonist GW501516 prevents TNF-α-induced NF-κB activation in human HaCaT cells by reducing p65 acetylation through AMPK and SIRT1. Biochem. Pharmacol. 2011;81:534–43. doi: 10.1016/j.bcp.2010.12.004. [DOI] [PubMed] [Google Scholar]
- [46].Herdegen T, Waetzig V. AP-1 proteins in the adult brain: facts and fiction about effectors of neuroprotection and neurodegeneration. Oncogene. 2001;20:2424–37. doi: 10.1038/sj.onc.1204387. [DOI] [PubMed] [Google Scholar]
- [47].Egger T, Schuligoi R, Wintersperger A, Amann R, Malle E, Sattler W. Vitamin E (alpha-tocopherol) attenuates cyclo-oxygenase 2 transcription and synthesis in immortalized murine BV-2 microglia. Biochem. J. 2003;370:459–67. doi: 10.1042/BJ20021358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ali FY, Hall MG, Desvergne B, Warner TD, Mitchell JA. PPARbeta/delta agonists modulate platelet function via a mechanism involving PPAR receptors and specific association/repression of PKCalpha--brief report. Arterioscler. Thromb. Vasc. Biol. 2009;29:1871–3. doi: 10.1161/ATVBAHA.109.193367. [DOI] [PubMed] [Google Scholar]
- [49].Basu-Modak S, Braissant O, Escher P, Desvergne B, Honegger P, Wahli W, Smith SA, Monteith GR, Robinson JA, Venkata NG, May FJ, Roberts-Thomson SJ. Effect of the peroxisome proliferator-activated receptor beta activator GW0742 in rat cultured cerebellar granule neurons. J. Neurosci. Res. 2004;77:240–9. doi: 10.1002/jnr.20153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A functional PPARδ is expressed in BV-2 cells. BV-2 cells were cotransfected using Lipofectamine 2000 (Invitrogen, Carlsbad, California) with i) 0.2 μg of PPRE-ACOX (PPRE consensus rat acyl-CoA oxidase gene) or pGL3 (control vector) and ii) 0.02 μg of pRL-SV40 (a renilla plasmid). Twenty-four h post-transfection, cells were treated with 0, 1, or 5 μM of L-165041 or vehicle control. The Dual Luciferase Assay (Promega, Madison, Wisconsin) was used to measure luciferase activity according to the manufacturer's instructions. * = p≤ 0.05.
PPARδ activation prevents the radiation-induced increases in Cox-2 and MCP-1 protein expression. Densitometric analysis of Fig. 1B. Results are shown as fold changes compared to sham-irradiated controls. Mean ± S.E.M; *, p≤0.05 vs. sham; #, p≤0.05 vs. 10 Gy; n = 3.
The PPARδ antagonist, GSK0660, prevents the L-165041-mediated inhibition of the radiation-induced increase in Cox-2 protein. BV-2 cells were treated with 10 μM of the PPARδ antagonist GSK0660 or vehicle control 1 h prior to treatment with 5 μM L-165041 or vehicle control. Cells were then irradiated with a single dose of 10 Gy and protein was subjected to western blot analysis for Cox-2; β-actin was used as a loading control.
PPARδ activation prevents radiation-induced NF-κB activation. Densitometric analysis of Figs 3A–3C (A–C). Results are shown as fold changes compared to sham-irradiated controls.
PPARδ activation prevents radiation-induced AP-1 activation and upstream activation of c-jun, MEK1/2, and ERK1/2. Densitometric analysis of Figs 4A–4D (A–D). Results are shown as fold changes compared to sham-irradiated controls. Mean ± S.E.M; *, p≤0.05 vs. sham; #, p≤0.05 vs. 10 Gy; n = 3.
The MEK1/2 inhibitor, U0126, or the ERK1/2 inhibitor, FR180204, prevent the radiation-induced increase in Cox-2 expression. Densitometric analysis of Figs 5B. Results are shown as fold changes compared to sham-irradiated cells. Mean ± S.E.M; *, p≤0.05 vs. sham; n = 3.
PPARδ modulates MEK1/2/ERK1/2 activation, in part, by inhibiting upstream activation of PKCα. Densitometric analysis of Figs 7A–7D (A–D). Results are shown in fold changes compared to sham-irradiated controls. Mean ± S.E.M; *, p≤0.05 vs. sham; #, p≤0.05 vs. 10 Gy; n = 3.