Abstract
We mimicked the effect of sphingomyelinase activity on lipid mixtures of palmitoyl-oleoyl-phosphatidylcholine, sphingomyelin, ceramide, and 10 mol % cholesterol. Using x-ray diffraction experiments in combination with osmotic stress we found, in agreement with previous studies, that ceramide induces a coexistence of Lα and Lβ domains. A detailed structural analysis of the coexisting domains demonstrated an increase of lipid packing density and membrane thickness in the Lα domains upon increasing overall ceramide levels. This provides evidence for a ceramide-driven accumulation of cholesterol in the Lα domains, in support of previous reports. We further determined the bending rigidities of the coexisting domains and found that the accumulation of cholesterol in the Lα domains stabilizes their bending rigidity, which experiences a dramatic drop in the absence of cholesterol. Deriving experimental estimates for the spontaneous curvature and Gaussian modulus of curvature, we show, using a simple geometric model for ion channels, that in this way changes in the conformational equilibrium of membrane proteins can be kept small.
Introduction
The organization of membranes into functional platforms (rafts), enabling the assembly of signaling proteins or transbilayer transport (1,2), is of particular interest in membrane research. Regarding lipid architecture, membrane rafts are generally considered to be rich in cholesterol and sphingolipids such as sphingomyelin (SM) (3). Under certain circumstances in the life of a cell (e.g., apoptosis), SM is hydrolyzed to ceramide (Cer) by sphingomyelinease (4,5). This significantly impacts lateral membrane structure (6–8), and it has been speculated that Cer stabilizes membrane rafts (9). In a previous report, performed on mixtures of palmitoyl-oleoyl phosphatidylcholine (POPC), SM, and Cer, we showed that Cer may induce macroscopic phase separation into stable lamellar fluid (Lα) and gel (Lβ) domains over a certain range of temperatures and Cer concentrations (10). In that study, we further showed that the phase separation is due to preferential interactions of Cer with SM, such as hydrogen bonding, leading to an enrichment of SM and Cer in the Lβ domains and an enrichment of POPC in the coexisting Lα domains. The depletion of SM from the Lα domains causes a fourfold drop in the bending rigidity of the fluid phase (11). This change of membrane elasticity can be coupled to transmembrane protein activity (12,13), and we estimated significant changes in the conformational equilibrium of simple geometric protein models (11).
In this report, we focus on the competition of Cer with cholesterol (Chol) in the context of sphingomyelinase activity, which is mimicked by gradually replacing SM with Cer in mixtures of POPC, SM, and Chol. In particular, we are interested in effects regarding membrane lateral structure and elasticity and their putative consequences for transmembrane protein activity. Cholesterol is abundant in plasma membranes (14) and interacts preferentially with saturated acyl chains as compared to unsaturated hydrocarbon chains (15,16). Therefore, it contends with Cer for SM (17–24). It is also well known that Chol condenses fluid membranes (see, e.g., (25–28)). Several ternary lipid mixtures of Chol mimicking the outer plasma membrane exhibit phase coexistence of liquid ordered (Lo), liquid disordered (Ld), or gel phases (29). Such phase coexistence has been reported also for POPC/SM/Chol, although the form of the phase coexistence regimes and direction of tie lines remain controversial (30,31). Although Chol levels are on the order of 30–50 mol % in plasma membranes (14), we chose an intermediate Chol concentration of 10 mol %. According to Veatch and Keller (31), this system offers the advantage of being just outside a Lo/Ld phase-coexistence regime, and we will thus be able to learn about the competition between Cer and Chol under simplified conditions, i.e., starting from a homogenous mixture. In this concentration range, Cer has been reported to displace Chol from Lo domains (17,19–21,23). In turn, an increase of Chol concentration was found to gradually dissolve Cer-rich gel domains (18,22–24).
In this report, we seek to understand 1), how Chol at intermediate concentrations affects the properties of the Cer-enriched Lβ phase and the coexisting Lα phase; and 2), how these properties are coupled to membrane protein activity. For this purpose, we compared our x-ray diffraction results to those of previous studies in the absence of Chol (10,11,32). Osmotic stress experiments showed that the bending rigidity of the Lα phase remains unaffected by Lβ-domain formation, which is attributed to the accumulation of Chol in Lα domains. We further estimated the spontaneous curvatures and Gaussian moduli of curvature of the coexisting phases, allowing us to address possible consequences of membrane restructuring for protein function in the case of a simple ion channel. Our calculations showed that Chol minimizes membrane-mediated activity changes of proteins located in the Lα domains that would take place in its absence (11).
Materials and Methods
Liposome preparation
POPC, egg-SM, and C16:0-Cer were purchased from Avanti Polar Lipids (Birmingham, AL) and used without further purification. Chol, polyethylene glycol (PEG, molecular mass 8000 g/mol), and all other chemicals (solvents in pro analysi grade) were from Sigma-Aldrich (Castle Hill, Australia).
Lipid stock solutions were prepared by dissolving weighted amounts of dry lipid powder from freshly opened sample containers from the suppliers in chloroform/methanol (2:1, v/v). The appropriate volumes of the stock solutions were mixed and the organic solvent was evaporated at room temperature under a gentle stream of nitrogen. The samples were then placed under vacuum for at least 12 h to form a thin lipid film on the bottoms of glass vials. Multilamellar vesicles (MLVs) were prepared by first suspending the dry lipid films in 18 MΩ/cm water (UHQ PS, USF Elga, Wycombe, UK) at a lipid concentration of 50 mg/ml and then incubating the dispersions for 4 h in the fluid phase of the system with intermittent vigorous vortex mixing. At least eight freeze-thaw circles were performed. For the osmotically stressed samples, the fully hydrated MLVs were prepared at a concentration of 200 mg/ml, as detailed above, and then diluted to 50 mg/ml by a PEG solution of a given concentration whose osmotic pressure was determined using a Knaur (Berlin, Germany) vapor-pressure osmometer. The sample was equilibrated at a given osmotic pressure for at least 2 days.
Small and wide-angle x-ray diffraction
Synchrotron small- and wide-angle x-ray diffraction experiments were performed at the Austrian SAXS beamline at Elettra (Trieste, Italy) (33,34) using a photon energy of 8 keV. SAXD patterns were recorded in a q-range of 2.67 × 10−4 – 0.95 Å−1 (q = 4π/λ sinθ) using a mar300-image-plate detector (Marresearch, Norderstedt, Germany). Integration of scattered intensity and primary data reduction were performed using the program Fit2D. WAXD patterns (q-range = 0.77 – 2.79 Å−1), were recorded simultaneously using a Gabriel-type PSD. Samples were contained in 1-mm quartz-glass capillaries at a distance of 1.121 m to the image-plate detector. Before measurements, samples were equilibrated at 37°C for at least 10 min, using a circulating water bath (Unistat CC, Huber, Offenburg, Germany). Typical exposure times were 300 s. The instrumental resolution was determined to have a full width at half-maximum of δq = 2.23 × 10−3 Å−1.
Lamellar repeat distances, d, were derived from a Lorentzian fit to the first-order Bragg peaks. From the peaks, we derived the bilayer electron density profiles using standard techniques as reported previously (11). Further, we used the same definition for the membrane thickness dB = dHH + 10 Å (dHH is the distance between the two maxima of the electron-density profile).
Hydrocarbon chain positional correlation peaks occur in the WAXD regime. Peaks of the coexisting phases were fitted with two Lorentzians (Fig. S1 in the Supporting Material). For Cer = 30 mol % and 35 mol %, additional sharp peaks appeared that required additional Lorentz peak functions in the analysis. The Lβ phase is characterized by a single sharp peak occurring at q11 ∼ 1.51 Å−1. From the peak position, we calculated, as previously, the lateral area/lipid . The chain correlations of the Lα phase exhibit a broad peak at q0 ∼1.40 Å−1. Unlike the long-range order present in the Lβ phase, hydrocarbons in the Lα phase are fluid and exhibit short-range order. It has been shown (35), that the average chain separation can then be calculated using a = 9π/4q0. The decay constant of the short-range positional correlations was derived from ξ = 2/Δq0corr (35), using the corrected full width at half-maximum of the chain correlation peak.
Osmotic stress experiments and analysis
Osmotic pressure experiments combined with SAXD were performed as described previously using PEG 8000 (11). In the analysis of the isotherms, we considered van der Waals attraction, hydration repulsion, and repulsion due to bilayer bending fluctuations. The osmotic pressure, Π, is equated with the sum of disjoining pressures that results from these interactions, i.e., Π = PvdW + Phyd + Pflug using the same forms for the van der Waals pressure, PvdW, hydration pressure, Phyd, and fluctuation pressure, Pfluc, as reported previously (11). Parameters of PvdW are the Hamaker constant, H, and the membrane thickness, dB. The latter parameter is determined from SAXD (see above). For the Hamaker constant, we choose the theoretical value H = 4.3 × 10−21 J (36), as applied in previous studies from our laboratory (11,37). Parameters of hydration interactions are the empirical scaling constant, Ph, and the decay constant, λh. Fluctuation interactions were only considered for fluid phases. The presently used form of Pfl contains Ph, λh, and, in particular, the bending rigidity, KC, as adjustable parameters. As described previously (11,37), the isotherms were fitted iteratively, starting with the analysis of data with Π > 1 atm and a constrained estimate for KC. KC was subsequently adjusted in a fit of the full isotherm, returning with this new value of KC to Step 1 of the analysis, until the fit to the osmotic pressure data could not be improved any further.
Calculation of effects on transmembrane proteins
Bulk elastic properties of membrane lipids may affect the conformational equilibrium of membrane proteins (12,13,38). The basic idea is that conformational changes of proteins that go hand in hand with changes in the cross-sectional area, ΔA, need to take place against a distribution of repulsive and attractive intramembrane pressures, p, known as the lateral-pressure profile. Take, for example, the opening of an ion channel, which involves the opening of a passage for ions, i.e., the transition from a closed (r) to an open state (t). The thermodynamic equilibrium can be described by a distribution of closed to open channels K0 = [r0]/[t0]. Changes in the distribution of lateral pressures, e.g., upon changes in lipid composition due to sphingomyelinase activity, will also change the conformational equilibrium (39), which is then given by K = [r]/[t]. It can be shown that the two equilibria are related by K = K0 exp(α), with being the change of work associated with the opening of the channel in the changed lateral-pressure field (40).
For simple geometric shapes of proteins, α, and hence K/K0, can be calculated analytically (40). In the case of hourglass-shaped proteins, consisting of an n-mer of bent helices, we recently showed that (11,41), where rmax is the outer radius of the protein at the position of the lipid headgroups, φ the opening angle of the pore, and ΔP1 and ΔP2 the changes in the first and second integral moments of the lateral-pressure profile imposed in this case upon the addition of Cer. The two integral moments are related to membrane elasticity by (42) P1 = κmc0 and P2 = κG, where κm is the monolayer bending rigidity, c0 the spontaneous curvature, and κG the Gaussian modulus of curvature. These parameters can be determined from experiment. In particular, κm = KC/2 is derived from osmotic pressure experiments described above. We further derived c0 by measuring lipids in the inverted hexagonal phase (43) (see Supporting Material). κG was estimated using κG ∼ −0.8 κm (13).
Results
Structural properties of coexisting fluid and gel domains
Fig. 1 shows the SAXD and WAXD patterns of fully hydrated MLVs at 37°C. Bilayers were initially composed of POPC/SM/Chol (45:45:10); subsequently, SM was gradually substituted by Cer in the concentration range from 0 to 35 mol %. SAXD patterns up to 23 mol % Cer were characteristic for a single lamellar phase. However, the sharp peak on top of a diffuse peak observed in the WAXD regime in the presence of Cer clearly demonstrates the coexistence of Lα and Lβ domains. The observation of a single d-value for coexisting phases may have several reasons, some of which are detailed in Mills et al. (44). For example, like domains may not be in registry, or they may happen to have similar d-values, i.e., the smaller dB of Lα and larger dB of Lβ phases, is compensated by a larger bilayer separation, dW = d − dB for Lα and smaller dW for Lβ. Phase coexistence in the SAXD regime will be revealed upon the application of osmotic pressure (see below). However, to differentiate between different domain alignments in MLVs is not within the scope of this work. For Cer concentrations ≥25 mol %, SAXD patterns showed an additional peak with d = 41.9 Å, which became increasingly prominent with Cer concentration. The d-value is comparable to that reported previously for pure Cer phases (10,32,45) and signifies the formation of Cer crystallites, which are insoluble in the lipid membrane. Precipitation of Cer crystallites was also observed as a decrease of the Lβ chain correlation peak intensity and the formation of higher q satellite peaks (Fig. 1 B). Concentrations of 25 mol % Cer correspond to a Cer/SM molar ratio of 1.25. Thus, as soon as the Cer level increases over that of SM, any additional Cer cannot be integrated into the lipid bilayer but forms a crystalline-like precipitate. In the absence of Chol, Cer precipitates at significantly higher concentrations (35 mol %) (10,32).
Figure 1.
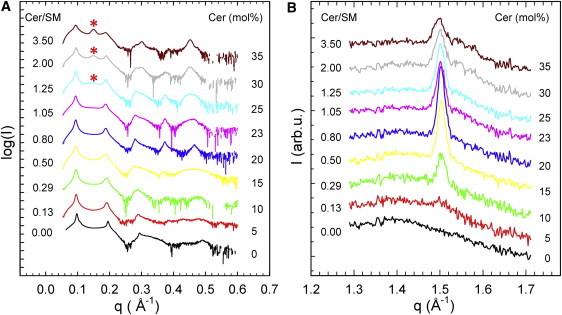
Changes in scattering patterns (panel A, SAXD; panel B, WAXD) of MLVs composed of POPC/SM/Chol upon replacing SM gradually with Cer. Asterisks in A indicate the position of the first diffraction order of the Cer phase.
As a next step, we performed a detailed analysis of the WAXD data, in comparison to previously published data that did not contain Chol (10). This is facilitated by plotting all structural parameters as a function of the Cer/SM molar ratio. We first focus on the Lβ domains using the data analysis described in the Materials and Methods section. The area/lipid, A, of the gel domains exhibited a value of 40.3 Å2 at the lowest Cer concentration, decreased slightly at Cer/SM = 0.3, and then increased linearly by ∼0.3 Å2 toward higher Cer/SM ratios (Fig. 2). The increase of A for Cer/SM ≥ 0.29 is most likely due to the splay induced by the spontaneous curvature of Cer (see Supporting Material). In the absence of Chol, A increased over the entire range of Cer concentrations studied. Differences in absolute A-values in the absence and presence of Chol show that lipid compositions of the domains differ. Most likely, this is due to Chol partitioning into the Lβ domains. In general, the overall extent of area variation is much more pronounced in the absence of Chol. Thus, the difference between the area/lipid of Chol-containing and that of Chol-free bilayers decreases with Cer content, which could indicate a displacement of Chol from the gel domains, as described in reports of previous studies that used different experimental techniques (17,19–21,23).
Figure 2.
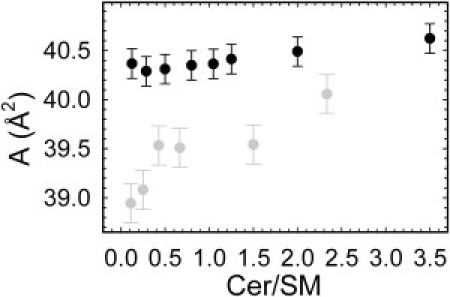
Comparison of lateral area/lipid of Lβ domains in the presence (black circles) and absence (gray circles) of Chol (10) as a function of the Cer/SM molar ratio.
A consequence of this scenario would be that Chol accumulates in the Lα domains. Since Chol is well known for its membrane-condensing effect (see e.g., Hodzic et al. (27)), we expect to observe changes of the corresponding broad WAXD peak. Indeed, the average distance between the acyl chains in the Lα domains provides clear evidence for such a scenario (Fig. 3). In the Cer/SM range 0–1.25, the distance a decreased quasilinearly and then remained constant toward higher Cer concentrations. The change in the slope of a coincided with the solubility limit of Cer in this system. Thus, Chol accumulates in the Lα phase, as long as Cer is able to form an Lβ phase with SM (Cer/SM ≤ 1). This leads to an increase of packing density in Lα because of the significantly smaller lateral area/molecule of Chol (46) as compared to SM (47) and due to the umbrella effect of POPC (26). At the same time, the decay of the positional correlations did not show significant changes, with an average value of ξ = 5.4 Å. This is a typical value for fluid hydrocarbons (35) and demonstrates that Chol accumulation in the Lα domains does not affect the short-range order of the acyl chains.
Figure 3.
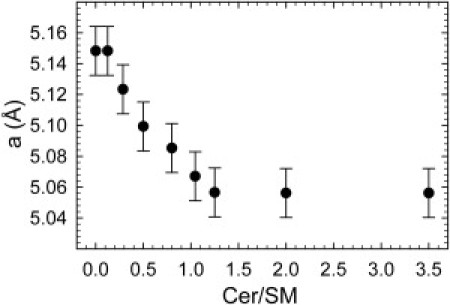
Average distance between acyl chains in the Lα domains as a function of Cer concentration.
Membrane thickness and domain elasticity from osmotic stress experiments
We performed SAXD experiments under osmotic stress, as described previously in detail (11). Fig. 4 A shows the SAXD patterns for Cer/SM = 0 and Cer/SM = 0.5 at mild osmotic pressure (Π = 1.31 atm). In the presence of Cer, this pressure was sufficient to reveal the two lamellar lattices of the Lα and Lβ phase. Only a single set of lamellar peaks, and hence no coexistence of Lo and Ld domains, was observed in the initial POPC/SM/Chol mixture. Also, application of higher osmotic pressures and lowering sample temperature to 10°C did not reveal the coexistence of macroscopically stable Lo and Ld domains (Fig. S3). Note that for the Chol concentration studied here, this is in agreement with a previous fluorescence microscopy study (31). There is a small, but distinct, difference from the compositional phase diagram based on fluorescence spectroscopy (30), where 10 mol % Chol would be just within a Lo/Ld coexistence regime. However, as discussed previously (10,29,48), this difference could be due to using egg-SM (here) instead of palmitoyl-SM (30), or different experimental windows of the applied techniques. Further, there are subtle differences between stable and unstable lipid domains, as well as macroscopic and nanoscopic domains, which can be discerned only upon the application of different experimental techniques (10,48). Note that we recently observed phase coexistence in the same system at higher Chol concentrations (to be published in a separate report). Thus, x-rays are sensitive to phase coexistence in POPC/SM/Chol.
Figure 4.
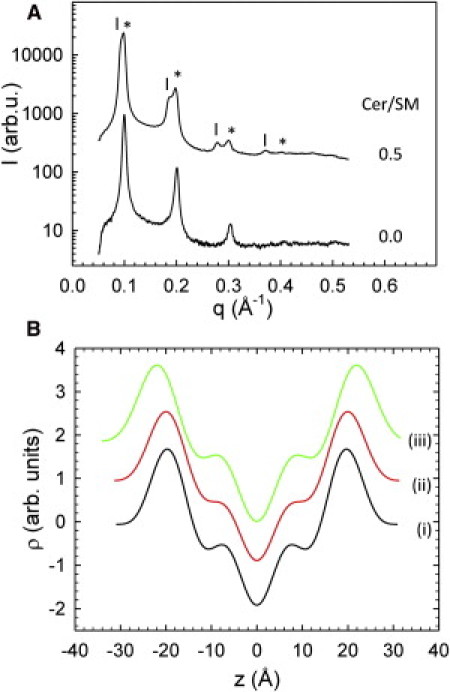
(A) SAXD patterns of osmotically stressed MLVs (Π = 1.31 atm) in the presence and absence of Cer. Two lamellar phases (Lα (∗) and Lβ (|)) are observed for Cer/SM = 0.5. (B) The corresponding electron-density profiles derived from the peak intensities. Profiles shown are for POPC/SM/Chol (i), the Lα phase of POPC/SM/Chol (ii), and the coexisting Lβ domains (iii).
From the SAXD patterns shown in Fig. 4 A, we were able to derive the electron-density profiles and the corresponding membrane thicknesses, dB, as described previously (11). Results are shown in Fig. 4 B and Table 1, which, to facilitate a comparison with other reports, also includes the headgroup-to-headgroup distances, dHH. In the absence of Cer, we found a membrane thickness of dB = 49.5 ± 0.2 Å, which is 1.1 Å larger than our previously reported value in the absence of Chol (11). The increase of dB goes hand in hand with the bilayer condensation by Chol, as reported previously for several lipid/ Chol mixtures (see, e.g., Hodzic et al. (27).). In the presence of Cer (Cer/SM = 0.5), we found dB = 50.2 ± 0.2 Å for the Lα domains and dB = 54.1 ± 0.2 Å for the coexisting Lβ domains. Naturally, the gel phase shows the largest membrane thickness due to the all-trans conformations of the hydrocarbon chains. Interestingly, also the dB of the coexisting Lα phase is larger than the membrane thickness of the non-phase-separated system (Cer/SM = 0). This supports the above-discussed scenario in which Cer leads to accumulation of Chol in the Lα phase, as additional Chol will also increase dB (see e.g., Hodzic et al. (27).). In the absence of Chol, the dBs of the Lα phases were found to be comparable (11).
Table 1.
Structural and interaction parameters
| Cer (mol %) | Phase | dB (Å) | dHH (Å) | Ph (atm) | λh (Å) | Kc (kBT) |
|---|---|---|---|---|---|---|
| 0 | Lα | 49.5 | 39.5 | 4555 | 1.51 | 78.9 |
| 10 | Lα | 50.2 | 40.2 | 4627 | 1.51 | 77.3 |
| 10 | Lβ | 54.1 | 44.1 | 1.38 × 104 | 1.51 | >100 |
Data were derived from osmotic isotherms of POPC/SM/Chol (45:45:10) and the coexisting Lα/Lβ domains of POPC/SM/Cer/Chol (45:30:15:10). KC was not measured for Lβ domains. We give a lower limit instead.
Accumulation of Chol in the Lα domains certainly has significant consequences for the elasticity of coexisting phases. This can be probed by variation of the osmotic pressure in combination with SAXD. Fig. 5 shows the osmotic pressure data in the presence of Chol. At the highest Π measured, hydration repulsion dominated the disjoining pressures. As Π was decreased, fluctuation repulsion, which has a larger decay constant, became more and more prominent. Finally, we observed a typical deviation from linearity in the semilogarithmic plot, which is when van der Waals attraction between the bilayers comes into play and the bilayers obtain their equilibrium separation. Visual inspection of the data presented in Fig. 5 shows that the equilibrium separation of the Lβ domains obtained at Π = 0 is significantly smaller than that of the Lα phases. This is characteristic for gel phases, where repulsion from bending fluctuations plays no role. Further, the two isotherms for the fluid phases are practically identical. Thus, we expect that all adjustable interaction parameters of the Lα phase of POPC/SM/Chol, including the bending rigidity, will not change significantly upon the addition of Cer. Indeed, all values for the interaction parameters Ph, λh, and KC obtained from fits to the osmotic pressure data agree within experimental error (Table 1). Compared to our previous study in the absence of Chol, the KC of the fluid domains is ∼34% larger (11). This effect is typical for the condensing abilities of Chol in fluid lipid bilayers (28,49).
Figure 5.
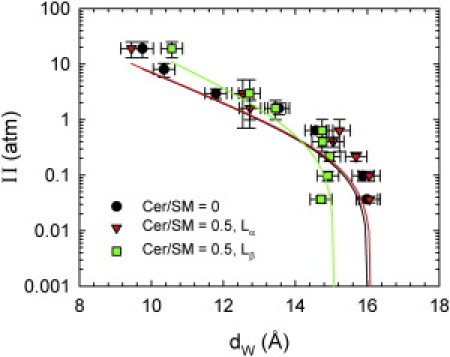
Equation of state for POPC/SM/Chol (45:45:10) (circles) and for the Lα domains (triangles) and Lβ domains (squares) of POPC/SM/Cer/Chol (45:30:15:10). Solid lines correspond to fits.
Discussion
Compared to our previous study (10), Cer precipitated at significantly lower concentrations (Fig. 1). Thus, the competition between Chol and Cer lowers the solubility of Cer in the bilayer. Our analysis of the WAXD data of the Lβ domains showed that the difference in lateral area/lipid between samples containing and those not containing Chol decreased with increasing Cer concentration (Fig. 2). Previously, we demonstrated by infrared spectroscopy preferential interactions between Cer and SM, leading to the formation of the gel phase (10). We assume that this is also the case in the presence of Chol, which is further supported by mass spectroscopy data (50). However, because of preferential interactions of Chol with saturated hydrocarbons (15,16), Chol will also partition into the Lβ domains, explaining the observed differences in A (Fig. 2). The decrease of this difference with increasing Cer content then suggests that Chol would be depleted from the Lβ domains and consequently accumulate in the Lα domains, in agreement with observations by others (17,19–21,23). This scenario is strongly supported by our WAXD analysis of the Lα domains, showing increased packing density as Cer levels are increased. Furthermore, the increase in dB of the Lα domains (Table 1) also agrees with increased Chol levels. In the absence of Chol, we observed previously an almost fourfold decrease of the bending rigidity of the Lα domains, which was explained by the depletion of SM from the fluid phase due to SM/Cer interactions (11). In this study, no significant changes in KC could be detected upon the addition of Cer (Table 1). Thus, Chol accumulation in the Lα domains recovers the drop in KC by depletion of SM from the fluid phase.
As a secondary effect, Chol accumulation in the Lα domains seems also to constrain POPC to the Lα domains. At Cer/SM = 1, all pairwise interactions between Cer and SM are saturated. Nonetheless, we were able to solubilize an additional amount of ∼5 mol % Cer in the bilayers in the absence of Chol (10). This was explained by the incorporation of some limited amounts of POPC in Lβ domains (32), which has a lower affinity for Cer than SM but still displays Lβ domains in binary mixtures with Cer (20). In the presence of Chol, we observed Cer crystallites as soon as Cer/SM > 1 (Fig. 1). This significantly lower solubility of Cer therefore suggests that POPC is not able to partition into the Lβ domains for Cer/SM > 1 but remains within the Lα domains, most likely due to preferred interactions with Chol as compared to Cer.
Potential effects on ion channels
It is instructive to ask how these changes in bulk membrane properties may affect the activity of membrane proteins. It is well known that changes in the distribution of lateral pressure are linked to protein function (12,13). In a previous study, based on the drop in KC in the Lα phase in the absence of Chol, we calculated possible consequences for the activity of channel proteins with hourglass-shaped geometry as a function of pore size (11). Since KC does not change significantly in this scenario, one could argue that protein activity will not be affected at all. However, in addition to KC, protein activity is also linked to the spontaneous curvature, c0, and the Gaussian modulus of curvature, κG (13).
It is not possible to measure the c0 for coexisting phases. However, c0 can be estimated from the molecular-mass sum of the c0s of the individual lipid components of a given phase (51). Of all the lipids used in this study, c0 has been reported only for Chol, where it was found to be between −0.036 Å−1 and −0.043 Å−1 (52). We thus decided to measure the spontaneous curvatures of all presently used lipids using a recently refined procedure (43). Details can be found in the Supporting Material. We arrive at c0 = −0.003 Å−1 for both SM and POPC, which is close to 0, as expected from their preference for forming lamellar phases. Further, c0 = −0.031 Å−1 for Cer, and c0 = −0.038 Å−1 for Chol. The latter value agrees well with previously published data (see above). To determine the c0s of the coexisting lipid phases, exact knowledge of the lipid composition of the individual domains is required. This is presently not available. However, for this discussion it is sufficient to consider the extreme cases in lipid sorting by Cer. Henceforth, we assume that in the absence of Chol, the Lα domains are composed of POPC only, whereas the coexisting Lβ domains contain an equimolar mixture of SM and Cer. In the presence of 10 mol % Chol, the Lα domains, in turn contain all POPC and all Chol, i.e., POPC/Chol = 0.815/0.185, whereas the Lβ phase is again given by SM/Cer = 1. Further, our calculation of c0 implicitly assumes random mixing of the lipids within a given domain, which should be fulfilled, neglecting boundary effects.
Table 2 shows the resulting spontaneous curvatures for these domain compositions. It is clear that the Lβ domains exhibit the largest negative value for spontaneous curvature due to the high concentration of Cer. Also, the presence of Chol in the Lα domains leads to a significant increase of c0. The monolayer bending rigidities were calculated from the measured KC values assuming that differences in lipid composition between experiment and those reported in Table 2 do not affect the κms significantly. Gaussian moduli of curvature were estimated as detailed in the previous section.
Table 2.
Spontaneous curvature, monolayer bending rigidity, and Gaussian modulus of curvature for POPC/SM/Cer mixtures in the presence and absence of Chol
| Lipid system | Domain | Phase | Molar ratio | c0 (10−2 Å−1) | κm (kBT) | κG (kBT) |
|---|---|---|---|---|---|---|
| POPC/SM | — | Lα | 0.5:0.5 | −0.314 | 28.9 | −23.1 |
| POPC/SM/Cer | POPC | Lα | 1 | −0.326 | 7.9 | −6.3 |
| SM/Cer | Lβ | 0.5:0.5 | −3.41 | ∼50 | −40 | |
| POPC/SM/Chol | Lα | 0.45:0.45:0.1 | −0.662 | 39.5 | −31.6 | |
| POPC/SM/Cer/Chol | POPC/Chol | Lα | 0.815:0.185 | −0.967 | 38.7 | −30.9 |
| SM/Cer | Lβ | 0.5:0.5 | −3.41 | ∼50 | −40 |
With these data, we calculated the changes in first and second integral moments of the lateral pressure profile, ΔP1 and ΔP2, due to lipid sorting by Cer. The results reported in Table 3 show that both Lα domains exhibit positive changes in ΔP1 and ΔP2, regardless of the presence or absence of Chol. This signifies a shift of lateral pressure toward the lipid/water interface (11,12). The effect is more pronounced in the absence of Chol, in agreement with previous theoretical work on lipid mixtures showing that Chol shifts lateral pressure toward the membrane interior (39). In turn, ΔP1 and ΔP2 are negative for the Lβ domains, implying a redistribution of lateral pressure toward the bilayer center.
Table 3.
Effects on hourglass-shaped ion channels
| Phase | ΔP1 (kBT/Å) | ΔP2 (kBT) | α | K/K0 | ||
|---|---|---|---|---|---|---|
| POPC/SM/Cer | Lα | 0.062 | 16.8 | −7.7 | 4.5 × 10−4 | ↑ |
| Lβ | −0.31 | −16.9 | 18.3 | 9.3 × 107 | ↓ | |
| POPC/SM/Cer/Chol | Lα | 0.005 | 0.64 | −0.4 | 6.5 × 10−1 | ↑ |
| Lβ | −1.16 | −8.4 | 55.1 | 8.7 × 1023 | ↓ |
Changes of first and second integral moments, P1 and P2, of the lateral-pressure profiles and the consequences for conformational equilibria K/K0. Up and down arrows indicate shifts toward channel opening and channel closing, respectively.
Next, it is interesting to consider the effect of these changes on transmembrane proteins, e.g., the opening probability of ion channels. For these calculations, we chose an hourglass-shaped channel protein with the same geometry parameters listed previously (41). Table 3 reports the changes of work, α, to open the ion channel in the changed lateral-pressure field. Negative values of α indicate a shift of the conformational equilibrium toward open channels, and conversely, positive α values signify channel closing. Thus, ion channels in the Lα domains will have increased probability of being in an open state, whereas those located on Lβ domains will be inhibited. Inhibition of protein function in gel phases is a well-known fact. We therefore focus on the effects within the Lα domains and in particular on K/K0 (Table 3), which expresses the magnitude of the shifts of conformational equilibrium. Both Lα phases show a decrease of closed states. However, the effect is much less expressed in the presence of Chol, where the number of open states increases by a factor of ∼1.5, as compared to ∼2000 when no Chol is present.
Summary and Conclusions
We have performed a comprehensive structural analysis of POPC/SM/Chol/Cer mixtures with 10 mol % Chol and compared our results to structural data reported in the absence of Chol (10,11,32). Upon gradually substituting SM with Cer, we found, in particular, 1), a decrease in the difference in lateral area/lipid in the Lβ domains of Chol-free membranes; 2), tighter lipid packing in the Lα domains; and 3), an increase in the membrane thickness of the Lα domains. In support of previous studies (17,19–21,23), this provides evidence that competition between Cer and Chol leads to an accumulation of Chol in Lα domains. Theoretical calculations of the effects of ion channel activity showed that channel opening in the Lα domains occurs with a significantly higher probability in the absence of Chol. This highlights the important role of Chol in maintaining membrane stability and function. In the absence of Chol, lipid sorting by Cer induces strong shifts of the conformational equilibrium of ion channels in Lα domains toward open states, which could be detrimental to cell function and may interfere with the controlled disposal of cells during apoptosis. In the presence of Chol, these changes are much more moderate and can possibly be more easily handled.
Supporting Material
References
- 1.Lingwood D., Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 2.Simons K., Sampaio J.L. Membrane organization and lipid rafts. Cold Spring Harb. Perspect. Biol. 2011;3:a004697. doi: 10.1101/cshperspect.a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pike L.J. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J. Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.van Blitterswijk W.J., van der Luit A.H., Borst J. Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem. J. 2003;369:199–211. doi: 10.1042/BJ20021528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrieu-Abadie N., Levade T. Sphingomyelin hydrolysis during apoptosis. Biochim. Biophys. Acta. 2002;1585:126–134. doi: 10.1016/s1388-1981(02)00332-3. [DOI] [PubMed] [Google Scholar]
- 6.Goñi F.M., Alonso A. Biophysics of sphingolipids I. Membrane properties of sphingosine, ceramides and other simple sphingolipids. Biochim. Biophys. Acta. 2006;1758:1902–1921. doi: 10.1016/j.bbamem.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Cremesti A.E., Goni F.M., Kolesnick R. Role of sphingomyelinase and ceramide in modulating rafts: do biophysical properties determine biologic outcome? FEBS Lett. 2002;531:47–53. doi: 10.1016/s0014-5793(02)03489-0. [DOI] [PubMed] [Google Scholar]
- 8.Kolesnick R.N., Goñi F.M., Alonso A. Compartmentalization of ceramide signaling: physical foundations and biological effects. J. Cell. Physiol. 2000;184:285–300. doi: 10.1002/1097-4652(200009)184:3<285::AID-JCP2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Goñi F.M., Alonso A. Effects of ceramide and other simple sphingolipids on membrane lateral structure. Biochim. Biophys. Acta. 2009;1788:169–177. doi: 10.1016/j.bbamem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Boulgaropoulos B., Arsov Z., Pabst G. Stable and unstable lipid domains in ceramide-containing membranes. Biophys. J. 2011;100:2160–2168. doi: 10.1016/j.bpj.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pabst G., Boulgaropoulos B., Laggner P. Effect of ceramide on nonraft proteins. J. Membr. Biol. 2009;231:125–132. doi: 10.1007/s00232-009-9211-3. [DOI] [PubMed] [Google Scholar]
- 12.Cantor R.S. Lateral pressures in cell membranes: a mechanism for modulation of protein function. J. Phys. Chem. B. 1997;101:1723–1725. [Google Scholar]
- 13.Marsh D. Lateral pressure profile, spontaneous curvature frustration, and the incorporation and conformation of proteins in membranes. Biophys. J. 2007;93:3884–3899. doi: 10.1529/biophysj.107.107938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Meer G., Voelker D.R., Feigenson G.W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silvius J.R. Role of cholesterol in lipid raft formation: lessons from lipid model systems. Biochim. Biophys. Acta. 2003;1610:174–183. doi: 10.1016/s0005-2736(03)00016-6. [DOI] [PubMed] [Google Scholar]
- 16.Ramstedt B., Slotte J.P. Sphingolipids and the formation of sterol-enriched ordered membrane domains. Biochim. Biophys. Acta. 2006;1758:1945–1956. doi: 10.1016/j.bbamem.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Megha, London E. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J. Biol. Chem. 2004;279:9997–10004. doi: 10.1074/jbc.M309992200. [DOI] [PubMed] [Google Scholar]
- 18.Fidorra M., Duelund L., Bagatolli L.A. Absence of fluid-ordered/fluid-disordered phase coexistence in ceramide/POPC mixtures containing cholesterol. Biophys. J. 2006;90:4437–4451. doi: 10.1529/biophysj.105.077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiantia S., Kahya N., Schwille P. Effects of ceramide on liquid-ordered domains investigated by simultaneous AFM and FCS. Biophys. J. 2006;90:4500–4508. doi: 10.1529/biophysj.106.081026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva L.C., de Almeida R.F., Prieto M. Ceramide-domain formation and collapse in lipid rafts: membrane reorganization by an apoptotic lipid. Biophys. J. 2007;92:502–516. doi: 10.1529/biophysj.106.091876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sot J., Ibarguren M., Alonso A. Cholesterol displacement by ceramide in sphingomyelin-containing liquid-ordered domains, and generation of gel regions in giant lipidic vesicles. FEBS Lett. 2008;582:3230–3236. doi: 10.1016/j.febslet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Staneva G., Chachaty C., Quinn P.J. The role of sphingomyelin in regulating phase coexistence in complex lipid model membranes: competition between ceramide and cholesterol. Biochim. Biophys. Acta. 2008;1778:2727–2739. doi: 10.1016/j.bbamem.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 23.Castro B.M., Silva L.C., Prieto M. Cholesterol-rich fluid membranes solubilize ceramide domains: implications for the structure and dynamics of mammalian intracellular and plasma membranes. J. Biol. Chem. 2009;284:22978–22987. doi: 10.1074/jbc.M109.026567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staneva G., Momchilova A., Koumanov K. Membrane microdomains: role of ceramides in the maintenance of their structure and functions. Biochim. Biophys. Acta. 2009;1788:666–675. doi: 10.1016/j.bbamem.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Mouritsen O.G., Jørgensen K. Dynamical order and disorder in lipid bilayers. Chem. Phys. Lipids. 1994;73:3–25. doi: 10.1016/0009-3084(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 26.Huang J., Feigenson G.W. A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys. J. 1999;76:2142–2157. doi: 10.1016/S0006-3495(99)77369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodzic A., Rappolt M., Pabst G. Differential modulation of membrane structure and fluctuations by plant sterols and cholesterol. Biophys. J. 2008;94:3935–3944. doi: 10.1529/biophysj.107.123224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khelashvili G., Rappolt M., Harries D. Impact of sterol tilt on membrane bending rigidity in cholesterol and 7-DHC containing DMPC membranes. Soft Matter. 2011;7:10299–10312. doi: 10.1039/C1SM05937H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsh D. Cholesterol-induced fluid membrane domains: a compendium of lipid-raft ternary phase diagrams. Biochim. Biophys. Acta. 2009;1788:2114–2123. doi: 10.1016/j.bbamem.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 30.de Almeida R.F., Fedorov A., Prieto M. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts. Biophys. J. 2003;85:2406–2416. doi: 10.1016/s0006-3495(03)74664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veatch S.L., Keller S.L. Miscibility phase diagrams of giant vesicles containing sphingomyelin. Phys. Rev. Lett. 2005;94:148101. doi: 10.1103/PhysRevLett.94.148101. [DOI] [PubMed] [Google Scholar]
- 32.Boulgaropoulos B., Amenitsch H., Pabst G. Implication of sphingomyelin/ceramide molar ratio on the biological activity of sphingomyelinase. Biophys. J. 2010;99:499–506. doi: 10.1016/j.bpj.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernstorff S., Amenitsch H., Laggner P. High-throughput asymmetric double-crystal monochromator of the SAXS beamline at ELETTRA. J. Synchrotron Radiat. 1998;5:1215–1221. doi: 10.1107/S0909049597019110. [DOI] [PubMed] [Google Scholar]
- 34.Amenitsch H., Rappolt M., Bernstorff S. First performance assessment of the small-angle X-ray scattering beamline at ELETTRA. J. Synchrotron Radiat. 1998;5:506–508. doi: 10.1107/S090904959800137X. [DOI] [PubMed] [Google Scholar]
- 35.Spaar A., Salditt T. Short range order of hydrocarbon chains in fluid phospholipid bilayers studied by x-ray diffraction from highly oriented membranes. Biophys. J. 2003;85:1576–1584. doi: 10.1016/S0006-3495(03)74589-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Podgornik R., French R.H., Parsegian V.A. Nonadditivity in van der Waals interactions within multilayers. J. Chem. Phys. 2006;124:044709. doi: 10.1063/1.2150825. [DOI] [PubMed] [Google Scholar]
- 37.Pabst G., Danner S., Katsaras J. Entropy-driven softening of fluid lipid bilayers by alamethicin. Langmuir. 2007;23:11705–11711. doi: 10.1021/la701586c. [DOI] [PubMed] [Google Scholar]
- 38.Dan N., Safran S.A. Effect of lipid characteristics on the structure of transmembrane proteins. Biophys. J. 1998;75:1410–1414. doi: 10.1016/S0006-3495(98)74059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cantor R.S. Lipid composition and the lateral pressure profile in bilayers. Biophys. J. 1999;76:2625–2639. doi: 10.1016/S0006-3495(99)77415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cantor R.S. The influence of membrane lateral pressures on simple geometric models of protein conformational equilibria. Chem. Phys. Lipids. 1999;101:45–56. doi: 10.1016/s0009-3084(99)00054-7. [DOI] [PubMed] [Google Scholar]
- 41.Jerabek H., Pabst G., Stockner T. Membrane-mediated effect on ion channels induced by the anesthetic drug ketamine. J. Am. Chem. Soc. 2010;132:7990–7997. doi: 10.1021/ja910843d. [DOI] [PubMed] [Google Scholar]
- 42.Ben Shaul A. Molecular theory of chain packing, elasticity and lipid-protein interaction in lipid bilayers. In: Lipowsky R., Sackmann E., editors. Handbook of Biological Physics. Elsevier; Amterdam: 1995. pp. 359–401. [Google Scholar]
- 43.Alley S.H., Ces O., Templer R.H. X-ray diffraction measurement of the monolayer spontaneous curvature of dioleoylphosphatidylglycerol. Chem. Phys. Lipids. 2008;154:64–67. doi: 10.1016/j.chemphyslip.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Mills T.T., Tristram-Nagle S., Feigenson G.W. Liquid-liquid domains in bilayers detected by wide angle X-ray scattering. Biophys. J. 2008;95:682–690. doi: 10.1529/biophysj.107.127910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah J., Atienza J.M., Shipley G.G. Physical properties of ceramides: effect of fatty acid hydroxylation. J. Lipid Res. 1995;36:1945–1955. [PubMed] [Google Scholar]
- 46.Chiu S.W., Jakobsson E., Scott H.L. Cholesterol-induced modifications in lipid bilayers: a simulation study. Biophys. J. 2002;83:1842–1853. doi: 10.1016/S0006-3495(02)73949-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maulik P.R., Sripada P.K., Shipley G.G. Structure and thermotropic properties of hydrated N-stearoyl sphingomyelin bilayer membranes. Biochim. Biophys. Acta. 1991;1062:211–219. doi: 10.1016/0005-2736(91)90395-o. [DOI] [PubMed] [Google Scholar]
- 48.Heberle F.A., Wu J., Feigenson G.W. Comparison of three ternary lipid bilayer mixtures: FRET and ESR reveal nanodomains. Biophys. J. 2010;99:3309–3318. doi: 10.1016/j.bpj.2010.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan J., Tristram-Nagle S., Nagle J.F. Effect of cholesterol on structural and mechanical properties of membranes depends on lipid chain saturation. Phys. Rev. E. 2009;80:021931. doi: 10.1103/PhysRevE.80.021931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Popov J., Vobornik D., Johnston L.J. Chemical mapping of ceramide distribution in sphingomyelin-rich domains in monolayers. Langmuir. 2008;24:13502–13508. doi: 10.1021/la8007552. [DOI] [PubMed] [Google Scholar]
- 51.Siegel D.P. Determining the ratio of the Gaussian curvature and bending elastic moduli of phospholipids from Q(II) phase unit cell dimensions. Biophys. J. 2006;91:608–618. doi: 10.1529/biophysj.106.085225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Z., Rand R.P. The influence of cholesterol on phospholipid membrane curvature and bending elasticity. Biophys. J. 1997;73:267–276. doi: 10.1016/S0006-3495(97)78067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zimmerberg J., Kozlov M.M. How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell Biol. 2006;7:9–19. doi: 10.1038/nrm1784. [DOI] [PubMed] [Google Scholar]
- 54.Duesing P.M., Templer R.H., Seddon J.M. Quantifying packing frustration energy in inverse lyotropic mesophases. Langmuir. 1997;13:351–359. [Google Scholar]
- 55.Kozlov M.M., Winterhalter M. Elastic-moduli for strongly curved monolayers: position of the neutral surface. J. Phys. II. 1991;1:1077–1084. [Google Scholar]
- 56.Rappolt M. The biologically relevant lipid mesophases as “seen” by x-rays. In: Leitmannova-Liu A., editor. Vol. 5. Elsevier; Amsterdam: 2006. pp. 253–283. (Advances in Planar Lipid Bilayers and Liposomes). [Google Scholar]
- 57.Rand R.P., Fuller N.L., Parsegian V.A. Membrane curvature, lipid segregation, and structural transitions for phospholipids under dual-solvent stress. Biochemistry. 1990;29:76–87. doi: 10.1021/bi00453a010. [DOI] [PubMed] [Google Scholar]
- 58.Feigenson G.W. Phase diagrams and lipid domains in multicomponent lipid bilayer mixtures. Biochim. Biophys. Acta. 2009;1788:47–52. doi: 10.1016/j.bbamem.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolff J., Marques C.M., Thalmann F. Thermodynamic approach to phase coexistence in ternary phospholipid-cholesterol mixtures. Phys. Rev. Lett. 2011;106:128104. doi: 10.1103/PhysRevLett.106.128104. [DOI] [PubMed] [Google Scholar]
- 60.Honerkamp-Smith A.R., Veatch S.L., Keller S.L. An introduction to critical points for biophysicists; observations of compositional heterogeneity in lipid membranes. Biochim. Biophys. Acta. 2009;1788:53–63. doi: 10.1016/j.bbamem.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


