Abstract
In this study, the effect of ordered rod-like FA coatings of metal discs on adipose-derived stem cell (ASC)’s growth, differentiation and mineralization was studied in vitro; and their mineral inductive effects in vivo. After 3 and 7 days, the cell number on the metal surfaces was significantly higher than those on the ordered and disordered FA surfaces. However, after 4 weeks much greater amounts of mineral formation was induced on the two FA surfaces with and even without osteogenesis induction. The osteogenic profiles showed the up regulation of a set of pro-osteogenic transcripts and bone mineralization phenotypic markers when the ASCs were grown on FA surfaces compared to metal surfaces at 7 and 21 days. In addition to BMP and TGFβ signaling pathways, EGF and FGF pathways also appeared to be involved in ASC differentiation and mineralization. In vivo studies showed accelerated and enhanced mineralized tissue formation integrated within ordered FA coatings. After 5 weeks, over 80 % of the ordered FA coating was integrated with a mineralized tissue layer covering the implants. Both the intrinsic properties of the FA crystals and the topography of the FA coating appeared to dominate the cell differentiation and mineralization process.
Keywords: Fluorapatite, differentiation, mineralization, gene profile, stem cells
1. Introduction
The use of biomedical implants for correction of skeletal defects caused by trauma, disease or genetic disorders has increased exponentially around the world. They have proved to be successful with failure rates of approximately 8 % in the maxilla, 5 % in the mandible [1] and 5 % for hip replacement [2]. Although implants are successful, it takes about 4–6 months for the healing and integration of dental implants with the existing bone to occur before functional loading of these implants can take place. Thus the real challenge in bone regeneration and repair lies in accelerating healing, reducing the time before loading and decreasing the recuperation time. In the last decades, coatings of hydroxyapatite (HA) have been introduced as a means to improve the osseointegration and fixation of implants. However the HA coating has been shown to have a potential risk of debonding from the implant causing them to fail [3]. Also, the limited stability of HA and the subsequent formation of heterogeneous calcium phosphates and its thermal decomposition products in the body has stimulated interest in bioactive materials with increased resorption resistance [4]. FA has been shown to have an increased stability and lower solubility than that of HA [3] and to have favored improved osseointegration [5]. Similarly, in our previous results [6], after cellular growth for 7 weeks, the ordered FA apatite surfaces showed higher binding strength with the metal surfaces than the ordered FA coatings without cellular involvement, which suggests that delamination of the FA coatings will not be a problem.
For tissue engineering purposes, the capability of the implant’s surface to induce or stimulate the osteogenic differentiation of mesenchymal stem cells is critical for the successful osseointegration between an implant and its surrounding tissue [7, 8]. In our previous studies, we have shown that the well aligned, ordered FA structures support the initial adhesion, long term growth of dental pulp stem cell (DPSC) and MG-63 osteoblast-like cells, and stimulated the differentiation and in vitro mineralization of the MG-63 cells [6, 9].
Recently, the adipose-derived stem cells (ASCs) have been of increasing interest regarding their application in the field of tissue engineering [10, 11]. It has been shown that ASCs resemble bone marrow stem cells (BMSCs) in their intrinsic characteristics including multilineage differentiation, immunophenotype, morphology and transcriptome profiles. More importantly, the easy accessibility, abundance and lower donor morbidity make ASCs more attractive and a better alternative to BMSC in bone regeneration studies [12–15]. Stem cell and biomaterial interactions provide valuable information of the relationship of material characteristics with cell phenotypic behavior (adherence, proliferation and differentiation etc.) which are fundamental to the future application of these biomedical materials in the orthopedic and dental fields [6]. It is thus essential to understand the mechanisms underlying the cell-material interaction, cell differentiation and mineralization, and osseointegration process. The ultimate goal is to manipulate these molecular events to obtain faster healing and better osseointegration of implants and/or apatite scaffolds. In this study, we use adipose-derived stem cells to study the effect of: fluorapatite crystal surfaces on cell differentiation; to compare the osteogenesis profiles of the ASCs grown on the metal and fluorapatite surfaces at different time points; and to study in vivo the newly induced mineral integration with the fluorapatite surfaces.
2. Materials and Methods
We have grown the FA apatite films on etched stainless steel (SS) and Ti surfaces and the crystal composition, alignment, size, shape and structure are exactly the same. We have therefore chosen to use the SS discs on which to grow the FA films rather than Ti discs because of reduced costs. However, as a “gold standard” for implants, etched Ti was used in the in vivo studies.
2.1. Synthesis of the ordered FA apatite surfaces
The synthesis of the ordered FA apatite surfaces has been previously described [6, 9]. Before the FA synthesis, the metal discs will be treated overnight with an etching solution containing 50% H2SO4 and 50% H2O2. For a typical synthesis of FA crystals, 9.36 g ethylenedeiaminetetraacetic acid calcium disodium salt (EDTA-Ca-Na2) and 2.07 g NaH2PO4.H2O were mixed with about 90 mL distilled water. The suspension was stirred continuously until the powder dissolved. The pH was adjusted to 6.0 using NaOH. Prior to mixing 0.21 g NaF in 90 mL of the EDTA-Ca-Na2 and NaH2PO4 solution, it was dissolved in 10 mL water (pH 7.0) and stirred continuously. The FA crystal growth on the substrates (15 mm 316 stainless steel discs) was achieved by adding the plates to 100 mL of newly prepared EDT-Ca-Na2/NaH2PO4 / NaF mixture and then autoclaving at 121 °C at pressure of 2.4 × 105 Pa for 10 hours. Ordered and disordered films were produced individually on the undersurfaces and upper surfaces of the stainless steel discs respectively.
2.2. Cell culture and cell attachment assay
The StemPro® Human Adipose-Derived Stem Cell (ASC) Kit (Invitrogen, NY, USA) was purchased, which contains cryopreserved normal human ASCs and MesenPRO RS™ Medium. The ASCs were then subcultured in reduced-serum (2 %) MesenPRO RS™ medium under standard culture conditions at 37 °C in a humidified atmosphere containing 5 % CO2 and 95 % air. The cells were grown on stainless steel (SS), etched stainless steel (SSE) and the SSE coated with ordered or disordered fluorapatite (FA). Before cell seeding, the experimental surfaces were equilibrated with 10 % FBS culture media for 2 hours. Briefly, the ASCs were seeded onto the above surfaces at a density of 2 × 104 cells/mL and cultured for 3 and 7 days. At the end of culture period, cells were detached with trypsin/EDTA, stained with trypan blue and counted using a haemocytometer. After osteogenic induction (OI) the osteogenic differentiation capability of ASCs was routinely monitored throughout the experimental period by the Alizarin red staining method.
2.3. SEM observation
After 4 weeks of culture, either with or without the osteogenic induction (OI) medium, the cells grown on the above experimental surfaces were rinsed and fixed in 2.5 % glutaraldehyde in distilled water, serially dehydrated and critical point dried. SEM analysis was conducted on a Phillips XL30FEG Scanning Electron Microscope (SEM) FEI company, Hillsboro, OR, USA) operated at 10 kV (Resolution: 2.0 nm at 30 kV, 5.0 nm at 1 kV).
2.4. RNA isolation and Reverse Transcription
Total cellular RNA was isolated from ASC cells grown on the experimental surfaces at day 7 and day 21 using the RNeasy Mini kit (Qiagen, CA, USA) according to the manufacturer’s instructions. The RNA was treated with the RNase-free DNase Set (Qiagen, CA, USA) during RNA isolation. The cDNA samples were prepared from the isolated RNA using the RT first strand kit (Cat. No. C-03, Qiagen, CA, USA) according to the manufacturer’s protocols. An average of 6–8 replicates of each surface on which the cells were grown has been used for the total cellular RNA isolation and cDNA sample preparation.
2.5. RT2 profiler PCR array analysis
Specimens were analyzed using the human pathway-focused osteogenesis PCR array, which combines the PCR sensitivity and the multi-gene profiling capability of a microarray. Briefly, the cDNA samples of the specimens from the experimental surfaces at day 7 and day 21 were added to the RT2 qPCR master mix containing SYBR Green and reference dye. The above mixture was then aliquoted across the PCR array templates which contain 84 human osteogenesis pathway-specific genes plus controls. The real-time PCR analysis was carried out using an ABI 7700 sequence detector (Applied Biosystems, Foster, USA). Relative gene expression values were analyzed using the Superarray web-based software package performing all ΔΔCt based fold-change calculations, which sets change of at least twofold as the “cutoff” value for the transcripts being differentially expressed in the experimental groups. Duplicate 96-well plates were used in this PCR array analyses. As an average of 6–8 replicates was used for the total RNA isolation and cDNA sample preparation, the expression change of at least 2 fold was set as the “cutoff” value for the transcripts being differentially expressed on the two crystal surfaces. In our previous studies, this “cutoff” value for the detection of the regulation of individual genes has been further confirmed by the single real-time PCR amplifications [16]. Similar methodology has been adopted in our previous publication[6].
2.6. Western Blots
After osteogenic induction for 4 weeks, cells grown on the experimental surfaces were washed with cold 1 × PBS, removed with a rubber policeman and lysed in 1 % Nonidet P-40 (NP-40) lysis buffer (50 mM Tris-HCL, PH 7.4, 200 mM NaCl, 2 mM MgCl2 and 10 % glycerol) containing protease inhibitors. Cell lysates were loaded in 15% SDS-PAGE gel. Membranes were incubated with mouse antihuman osteocalcin antibody at 1 to 1000 dilution (Santa Cruz Biotechnology, Santa Cruz, CA) and mouse anti-GAPDH at 1 to 106 dilution (Chemicon, Billerca, MA) overnight at 4 °C. Affinity-purified second antibodies conjugated with horseradish peroxidase (Jackson ImmunoResearch Laboratories) were used, and immunoreactive proteins were observed by SuperSignal West Pico chemiluminescent substrate (Thermo Scientific) and exposed to X-ray film. Three separate cell lysates from the experimental substrate surfaces were used for the experiment.
2.7. Mineralization induction and Alizarin Red Staining
ASCs grown on the experimental surfaces were further cultured in OI medium for 4 weeks, with medium changes twice per week. The STEMPRO Osteogenesis Differentiation Kit (A10072-01, Invitrogen) was used as the OI medium for the ASCs. After OI, the seeded cells from different experimental surfaces were used for Alizarin Red staining following the standard protocol as described in the Osteogenesis Assay Kit (ECM815, Millipore).
2.8. In vivo ectopic transplantation
A total of 14 BALB/c57bl mice (approximate weight 20–25 g) were utilized in this study. All animal procedures were approved by the University of Michigan University Committee on Use and Care of Animals and were in accordance with the National Institutes of Health guidelines. Briefly, under anesthesia with Ketamine (50 mg/kg) and Xylazine (10 mg/kg), midsagittal incisions were made on the dorsa of BALB/c57bl mice, the Ti (4.0 mm diameter, 0.7 mm thickness, GoodFellow, PA, USA) and coated FA samples were inserted into surgical pockets, and the incisions were stapled closed. Tetracycline, a bone fluorochrome was administered 1 day after surgery and repeated every week (im injection of tetracycline, 25 mg/kg) to label the newly formed mineralized tissue formation. At 2 and 5 weeks, the animals were sacrificed by CO2 euthanasia, and then six biopsy specimens from each group were harvested, immediately fixed and stored in 10% formalin. The specimens were subsequently dehydrated in step gradients of alcohol and infiltrated and embedded in methyl methacrylate by routine histological methods with each biopsy block containing one implant. Serial sections of approximately 150 µm in thickness were made by using a diamond saw, which were then attached to plastic slides (Wasatch Histo Consultants, Inc., Winnemucca, NV, USA). Individual sample was then ground to approximately 10 µm using a microgrinding machine system (D-2000; Exakt-Apparatebau, Norderstedt, Germany), and polished to an optical finish. The unstained sections were observed and imaged under fluorescence microcopy and further stained using basic fuschin/toluidine blue for histological observation. After merging with corresponding toluidine blue staining pictures, the area of fluorescence labeling over the total area was analyzed by Image J software (NIH).
2.8. Statistical analysis
The cell counting and the Image J area value results were analyzed statistically using GraphPad Prism 5 for one-way ANOVA (and post-hoc pairwise comparisons) of an average of three to five replicates and significance was considered at p < 0.05. Data are expressed as means ± standard deviation.
3. Results
3.1. Hydrothermal synthesis of ordered and disordered FA crystal surfaces
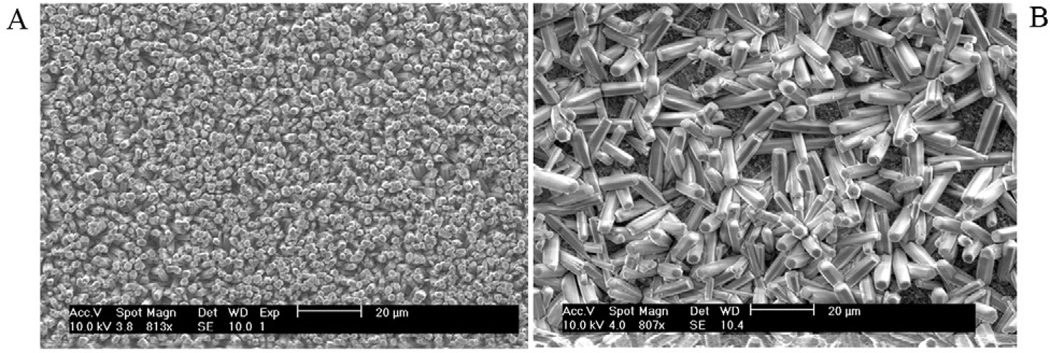
The ordered rod-like FA crystals were very well aligned along the c-axis after being grown on the undersurfaces of the 316 stainless steel discs. The densely packed growth mode along the c-axis forced the rod-like crystals to align parallel to each other (Fig. 1A). On the upper surfaces of the discs the FA crystals are randomly arranged without forming the well-aligned structure (Fig. 1B).
Fig. 1.
Scanning Electron Micrograph of synthesized ordered (A) and disordered (B) FA crystal surfaces. Ordered and disordered crystal layers grew on the undersurfaces (A) and upper surfaces (B) of the stainless steel discs respectively.
3.2. Cell culture and cell attachment assay
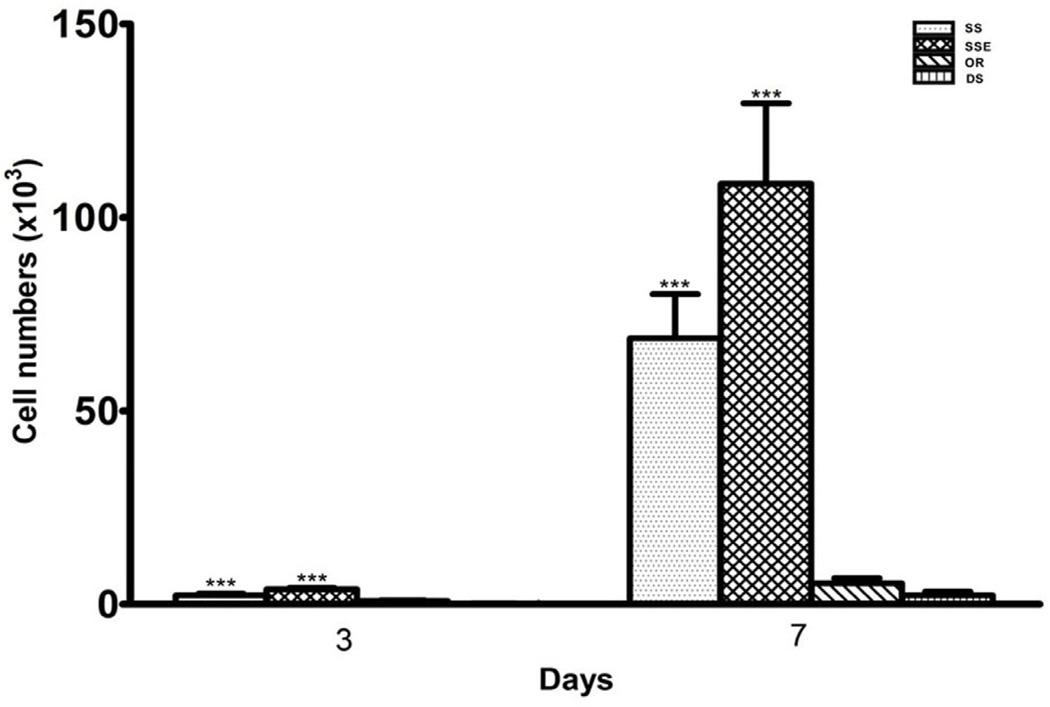
After 3 and 7 days of growth, the attached cell numbers on the metal surfaces were significantly higher than those on the disordered and ordered FA surfaces (One-way ANOVA, P<0.0001) (Fig. 2).
Fig. 2.
Cell density on the stainless steel (SS), etched stainless steel (SSE), disordered (DS) and ordered (OR) FA surfaces at 3 and 7 days. Data show that, the attached cell numbers on the metal surfaces were significantly higher than those on the disordered and ordered FA surfaces. Each bar represents the mean ± standard deviation. One-way ANOVA, ***P<0.0001.
3.3. SEM observation
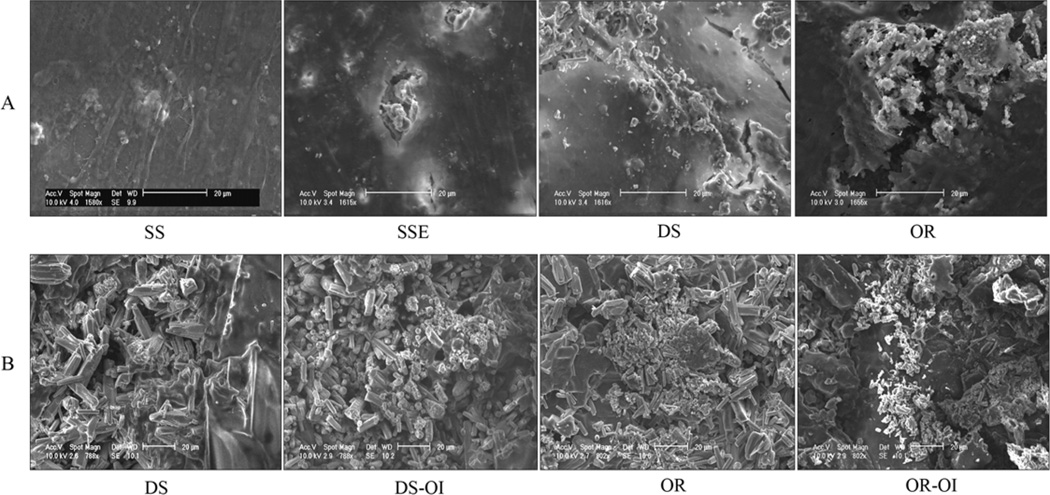
After 4 weeks of osteogenic induction, mineral deposition was seen beneath the densely formed cell-matrix layers on SSE, disordered and ordered FA surfaces (Fig. 3A). After removal of the ASCs, more densely deposited amorphous mineral nodules were observed on the ordered FA crystal surfaces with and even without the OI (Fig. 3B). No SEM pictures were taken of the samples of metal surfaces either with or without OI, since the surface layers were easily removed after the cells had been detached.
Fig. 3.
Scanning Electron Micrograph of the mineral nodule formation of ASCs grown on stainless steel (SS), etched stainless steel (SSE), disordered (DS) and ordered (OR) FA surfaces after osteogenic induction (OI) for 4 weeks. The mineral deposition was beneath the densely formed cell-matrix layer (A). After removal of the cells, more densely deposited amorphous mineral nodules were observed on the ordered FA surfaces with and without the OI (B).
3.4. Human osteogenesis pathway-focused PCR array analysis
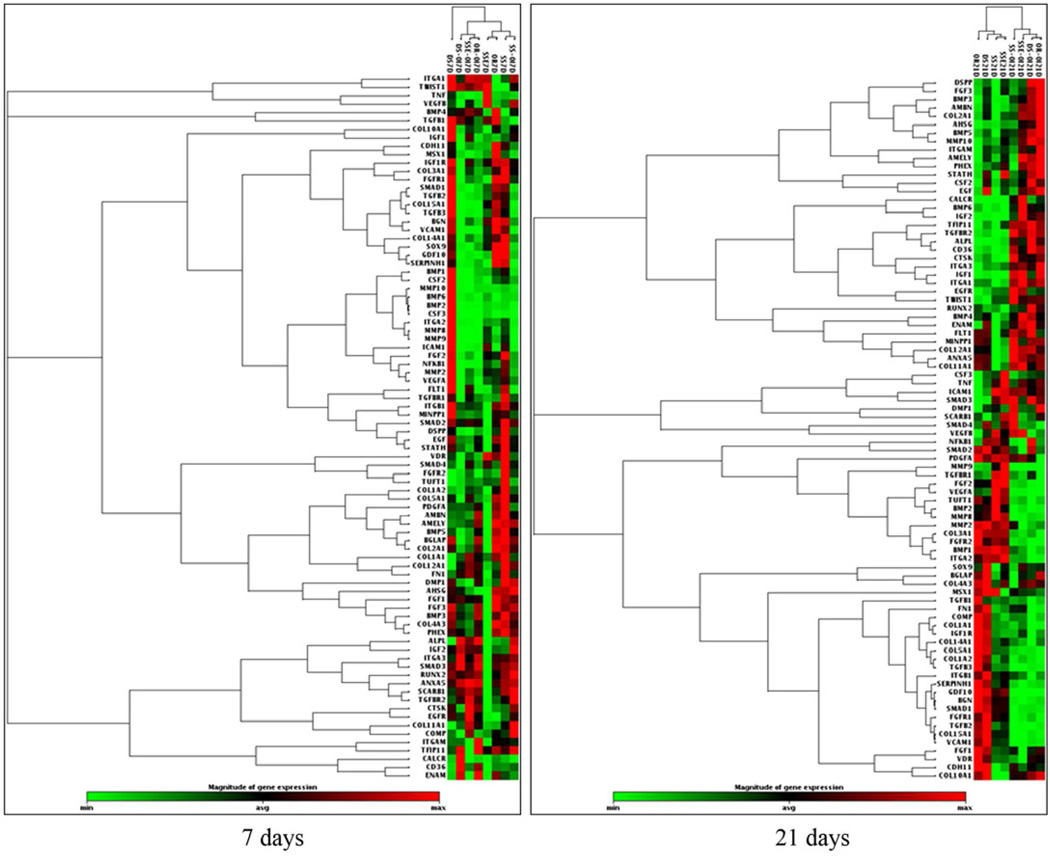
To simplify the multiple group osteogenesis profile analysis, we have chosen to compare the gene profiles of ASCs at two time points-7 (non-mineralizing stage) and 21 (mineralizing stage) days when grown on: SSE and ordered FA surfaces. All groups were either with or without OI (Fig. 4).
Fig. 4.
Cluster gram of the expression of osteogenesis-related molecules of ASCs grown on the stainless steel (SS), etched stainless steel (SSE), disordered (DS) and ordered (OR) FA surfaces, with or without OI at 7 and 21 days.
3.4.1. Osteogenesis profiles of ASCs grown on ordered FA crystal surfaces at 7 days
Without OI, a total of 40 genes were differentially regulated when the cells were grown on the ordered FA surfaces compared to the SSE surface, with 32 molecules up-regulated and 8 molecules down-regulated including the down-regulation of bone morphogenetic protein 6 (BMP6), colony stimulating factor 3 (CSF3), intercellular adhesion molecule 1 (ICAM1), matrix metallopeptidase 8 and 9 (MMP8 and 9), tumor necrosis factor (TNF) and twist homolog 1 (TWIST1). The ordered FA surfaces stimulated the expression of alkaline phosphatase (ALPL), bone morphogenic proteins including BMP3, 4 and 5, growth differentiation factor 10 (GDF10), Phosphate regulating endopeptidase homolog, X-linked (PHEX), Msh homeobox 1 (MSX1) and the expression of dentin sialophosphoprotein (DSPP). Most noticeably, the expression of type IV collagen, alpha 3 (COL4A3) was up-regulated by more than 500 times (Table 1A).
TABLE 1.
| A. Fold Change of the Human Pathway-focused Osteogenesis Molecules Expressed by the ASCs Grown on the Ordered FA Surface Relative to the SSE Surfaces at Day 7 (Without OI) | ||||
|---|---|---|---|---|
| Symbol | Unigene | Refseq | Description | Fold Change |
| AHSG | Hs.324746 | NM_001622 | Alpha-2-HS-glycoprotein | 7.32 |
| ALPL | Hs.75431 | NM_000478 | Alkaline phosphatase, liver/bone/kidney | 2.79 |
| AMBN | Hs.272396 | NM_016519 | Ameloblastin (enamel matrix protein) | 29.08 |
| AMELY | Hs.1238 | NM_001143 | Amelogenin, Y-linked | 17.29 |
| BGLAP | Hs.654541 | NM_199173 | Bone gamma-carboxyglutamate (gla) protein | 2.33 |
| BMP3 | Hs.387411 | NM_001201 | Bone morphogenetic protein 3 | 52.42 |
| BMP4 | Hs.68879 | NM_130851 | Bone morphogenetic protein 4 | 3.69 |
| BMP5 | Hs.296648 | NM_021073 | Bone morphogenetic protein 5 | 4.48 |
| CD36 | Hs.120949 | NM_000072 | CD36 molecule (thrombospondin receptor) | 2.45 |
| CDH11 | Hs.116471 | NM_001797 | Cadherin 11, type 2, OB-cadherin (osteoblast) | 2.72 |
| COL10A1 | Hs.520339 | NM_000493 | Collagen, type X, alpha 1 | 6.11 |
| COL2A1 | Hs.408182 | NM_001844 | Collagen, type II, alpha 1 | 32.49 |
| COL4A3 | Hs.570065 | NM_000091 | Collagen, type IV, alpha 3 (Goodpasture antigen) | 553.33 |
| COL5A1 | Hs.210283 | NM_000093 | Collagen, type V, alpha 1 | 2.13 |
| CSF2 | Hs.1349 | NM_000758 | Colony stimulating factor 2 (granulocyte-macrophage) | 2.15 |
| DSPP | Hs.678914 | NM_014208 | Dentin sialophosphoprotein | 2.59 |
| EGF | Hs.419815 | NM_001963 | Epidermal growth factor (beta-urogastrone) | 20.71 |
| ENAM | Hs.667018 | NM_031889 | Enamelin | 3.64 |
| FGF1 | Hs.483635 | NM_000800 | Fibroblast growth factor 1 (acidic) | 2.83 |
| FGF3 | Hs.37092 | NM_005247 | Fibroblast growth factor 3 (murine mammary tumor virus integration site (v-int-2) oncogene homolog) | 17.78 |
| FGFR1 | Hs.264887 | NM_015850 | Fibroblast growth factor receptor 1 | 2.18 |
| GDF10 | Hs.2171 | NM_004962 | Growth differentiation factor 10 | 7.53 |
| IGF2 | Hs.523414 | NM_000612 | Insulin-like growth factor 2 (somatomedin A) | 8.89 |
| ITGA3 | Hs.265829 | NM_002204 | Integrin, alpha 3 (antigen CD49C, alpha 3 subunit of VLA-3 receptor) | 8.29 |
| ITGAM | Hs.172631 | NM_000632 | Integrin, alpha M (complement component 3 receptor 3 subunit) | 2.72 |
| MSX1 | Hs.424414 | NM_002448 | Msh homeobox 1 | 2.32 |
| PHEX | Hs.495834 | NM_000444 | Phosphate regulating endopeptidase homolog, X-linked | 53.89 |
| SCARB1 | Hs.709216 | NM_005505 | Scavenger receptor class B, member 1 | 3.12 |
| SERPINH1 | Hs.596449 | NM_001235 | Serpin peptidase inhibitor, clade H (heat shock protein 47), member 1, (collagen binding protein 1) | 2.00 |
| STATH | Hs.654495 | NM_003154 | Statherin | 3.30 |
| TGFB3 | Hs.592317 | NM_003239 | Transforming growth factor, beta 3 | 2.37 |
| VCAM1 | Hs.109225 | NM_001078 | Vascular cell adhesion molecule 1 | 2.42 |
| BMP6 | Hs.285671 | NM_001718 | Bone morphogenetic protein 6 | −4.34 |
| CSF3 | Hs.2233 | NM_000759 | Colony stimulating factor 3 (granulocyte) | −18.48 |
| ICAM1 | Hs.643447 | NM_000201 | Intercellular adhesion molecule 1 | −6.95 |
| ITGA1 | Hs.644352 | NM_181501 | Integrin, alpha 1 | −2.17 |
| MMP8 | Hs.161839 | NM_002424 | Matrix metallopeptidase 8 (neutrophil collagenase) | −7.56 |
| MMP9 | Hs.297413 | NM_004994 | Matrix metallopeptidase 9 (gelatinase B, 92kDa gelatinase, 92kDa type IV collagenase) | −3.22 |
| TNF | Hs.241570 | NM_000594 | Tumor necrosis factor (TNF superfamily, member 2) | −6.63 |
| TWIST1 | Hs.66744 | NM_000474 | Twist homolog 1 (Drosophila) | −2.46 |
| B. Fold Change of the Human Pathway-focused Osteogenesis Molecules Expressed by the ASCs Grown on the Ordered FA Surface Relative to the SSE Surfaces at Day 7 (With OI) | ||||
|---|---|---|---|---|
| Symbol | Unigene | Refseq | Description | Fold Change |
| AMBN | Hs.272396 | NM_016519 | Ameloblastin (enamel matrix protein) | 4.14 |
| AMELY | Hs.1238 | NM_001143 | Amelogenin, Y-linked | 2.03 |
| BMP3 | Hs.387411 | NM_001201 | Bone morphogenetic protein 3 | 2.73 |
| CD36 | Hs.120949 | NM_000072 | CD36 molecule (thrombospondin receptor) | 2.28 |
| COL2A1 | Hs.408182 | NM_001844 | Collagen, type II, alpha 1 | 2.22 |
| COL4A3 | Hs.570065 | NM_000091 | Collagen, type IV, alpha 3 (Goodpasture antigen) | 2.84 |
| CSF3 | Hs.2233 | NM_000759 | Colony stimulating factor 3 (granulocyte) | 3.07 |
| DMP1 | Hs.652366 | NM_004407 | Dentin matrix acidic phosphoprotein 1 | 2.04 |
| DSPP | Hs.678914 | NM_014208 | Dentin sialophosphoprotein | 1.80 |
| EGF | Hs.419815 | NM_001963 | Epidermal growth factor (beta-urogastrone) | 2.38 |
| ENAM | Hs.667018 | NM_031889 | Enamelin | 5.02 |
| FGF3 | Hs.37092 | NM_005247 | Fibroblast growth factor 3 (murine mammary tumor virus integration site (v-int-2) oncogene homolog) | 2.90 |
| ITGAM | Hs.172631 | NM_000632 | Integrin, alpha M (complement component 3 receptor 3 subunit) | 4.85 |
| MMP10 | Hs.2258 | NM_002425 | Matrix metallopeptidase 10 (stromelysin 2) | 2.75 |
| PHEX | Hs.495834 | NM_000444 | Phosphate regulating endopeptidase homolog, X-linked | 2.26 |
| STATH | Hs.654495 | NM_003154 | Statherin | 2.17 |
| COL14A1 | Hs.409662 | NM_021110 | Collagen, type XIV, alpha 1 | −2.04 |
| FGFR2 | Hs.533683 | NM_000141 | Fibroblast growth factor receptor 2 | −2.91 |
| IGF1 | Hs.160562 | NM_000618 | Insulin-like growth factor 1 (somatomedin C) | −5.36 |
| VCAM1 | Hs.109225 | NM_001078 | Vascular cell adhesion molecule 1 | −2.32 |
| VEGFA | Hs.73793 | NM_003376 | Vascular endothelial growth factor A | −2.81 |
After the real-time PCR amplification, relative gene expression values were analyzed using the Superarray web-based software package performing all ΔΔCt based fold-change calculations.
With OI, a total of 21 genes were differentially regulated when cells were grown on ordered FA compared to those grown on the SSE surface including collagen, type XIV, alpha 1 (COL14A1), dentin matrix protein 1 (DMP1), fibroblast growth factor receptor 2 (FGFR2), insulin-like growth factor 1 (IGF1) and vascular endothelia growth factor A (VEGFA), which were not detected in the OI untreated group. Two other molecules showed a reversal in their gene regulation, with vascular cell adhesion molecule 1 (VCAM1) being down-regulated and CSF 3 being up-regulated (Table 1B).
3.4.2. Osteogenesis profiles of ASCs grown on ordered FA crystal surfaces at 21 days
Without OI, a total of 15 osteogenesis related molecules were differentially regulated when the ASCs were grown on FA surfaces compared to the cells grown on SSE surfaces. Noticeably, the up-regulation of type X collagen, alpha 1 (COL10A1), cartilage oligomeric matrix protein (COMP), fibroblast growth factor 1 (FGF1) and transforming growth factor, beta 3 (TGFB3) was accompanied with the down-regulation of 11 other molecules including BMP5, DMP1, CSF2 and 3, and VEGFA etc (Table 2A).
TABLE 2.
| A. Fold Change of the Human Pathway-focused Osteogenesis Molecules Expressed by the ASCs Grown on the Ordered FA Surface Relative to the SSE Surfaces at Day 21 (Without OI) | ||||
|---|---|---|---|---|
| Symbol | Unigene | Refseq | Description Fold | Fold Change |
| COL10A1 | Hs.520339 | NM_000493 | Collagen, type X, alpha 1 | 4.14 |
| COMP | Hs.1584 | NM_000095 | Cartilage oligomeric matrix protein | 3.03 |
| FGF1 | Hs.483635 | NM_000800 | Fibroblast growth factor 1 (acidic) | 2.85 |
| TGFB3 | Hs.592317 | NM_003239 | Transforming growth factor, beta 3 | 3.41 |
| AMELY | Hs.1238 | NM_001143 | Amelogenin, Y-linked | −3.18 |
| BMP5 | Hs.296648 | NM_021073 | Bone morphogenetic protein 5 | −2.53 |
| CSF2 | Hs.1349 | NM_000758 | Colony stimulating factor 2 (granulocyte-macrophage) | −2.10 |
| CSF3 | Hs.2233 | NM_000759 | Colony stimulating factor 3 (granulocyte) | −8.63 |
| DMP1 | Hs.652366 | NM_004407 | Dentin matrix acidic phosphoprotein 1 | −2.13 |
| ICAM1 | Hs.643447 | NM_000201 | Intercellular adhesion molecule 1 | −3.48 |
| ITGAM | Hs.172631 | NM_000632 | Integrin, alpha M (complement component 3 receptor 3 subunit) | −2.83 |
| MMP9 | Hs.297413 | NM_004994 | Matrix metallopeptidase 9 (gelatinase B, 92kDa gelatinase, 92kDa type IV collagenase) | −12.55 |
| STATH | Hs.654495 | NM_003154 | Statherin | −2.75 |
| TNF | Hs.241570 | NM_000594 | Tumor necrosis factor (TNF superfamily, member 2) | −4.32 |
| VEGFA | Hs.73793 | NM_003376 | Vascular endothelial growth factor A | −2.35 |
| B. Fold Change of the Human Pathway-focused Osteogenesis Molecules Expressed by the ASCs Grown on the Ordered FA Surface Relative to the SSE Surfaces at Day 21 (With OI) | ||||
|---|---|---|---|---|
| Symbol | Unigene | Refseq | Description | Fold Change |
| DMP1 | Hs.652366 | NM_004407 | Dentin matrix acidic phosphoprotein 1 | 2.39 |
| DSPP | Hs.678914 | NM_014208 | Dentin sialophosphoprotein | 2.27 |
| FGF1 | Hs.483635 | NM_000800 | Fibroblast growth factor 1 (acidic) | 129.25 |
| BMP2 | Hs.73853 | NM_001200 | Bone morphogenetic protein 2 | −2.09 |
| CALCR | Hs.489127 | NM_001742 | CALCITONIN RECEPTOR | −2.34 |
| FGFR2 | Hs.533683 | NM_000141 | Fibroblast growth factor receptor 2 | −2.06 |
| PDGFA | Hs.535898 | NM_002607 | Platelet-derived growth factor alpha polypeptide | −6.71 |
| VEGFA | Hs.73793 | NM_003376 | Vascular endothelial growth factor A | −2.14 |
After the real-time PCR amplification, relative gene expression values were analyzed using the Superarray web-based software package performing all ΔΔCt based fold-change calculations.
With OI, a total of 8 genes were differentially regulated when cells were grown on ordered FA compared to those grown on the SSE surface. The expression of DMP1, DSPP and FGF1 was stimulated, whereas the expression of BMP2, calcitonin receptor (CALCR), fibroblast growth factor receptor 2 (FGFR2), platelet-derived growth factor alpha polypeptide (PDGFA) and VEGFA were inhibited (Table 2B).
3.4.3. Osteogenesis profiles of ASCs grown on ordered FA crystal surfaces at 7 and 21 days
Without OI, when obvious mineralization occurred at 21 days, 27 genes were differentially regulated compared to the 7 day results (no mineralization occurring) and these included the up regulation of BMP2, FGF1, TGFB3, TGFBR1 and the VEGF (Table 3A).
TABLE 3.
| A. Fold Change of the Human Pathway-focused Osteogenesis Molecules Expressed by the ASCs Grown on the Ordered FA Surface at Day 21 Relative to Day 7 (Without OI) | ||||
|---|---|---|---|---|
| Symbol | Unigene | Refseq | Description | Fold Change |
| BMP2 | Hs.73853 | NM_001200 | Bone morphogenetic protein 2 | 2.27 |
| COL10A1 | Hs.520339 | NM_000493 | Collagen, type X, alpha 1 | 2.99 |
| COL11A1 | Hs.523446 | NM_080629 | Collagen, type XI, alpha 1 | 3.04 |
| COL12A1 | Hs.101302 | NM_004370 | Collagen, type XII, alpha 1 | 2.06 |
| COL14A1 | Hs.409662 | NM_021110 | Collagen, type XIV, alpha 1 | 3.08 |
| COL15A1 | Hs.409034 | NM_001855 | Collagen, type XV, alpha 1 | 2.09 |
| COMP | Hs.1584 | NM_000095 | Cartilage oligomeric matrix protein | 6.69 |
| CTSK | Hs.632466 | NM_000396 | Cathepsin K | 2.30 |
| FGF1 | Hs.483635 | NM_000800 | Fibroblast growth factor 1 (acidic) | 2.85 |
| FGFR2 | Hs.533683 | NM_000141 | Fibroblast growth factor receptor 2 | 9.08 |
| IGF1 | Hs.160562 | NM_000618 | Insulin-like growth factor 1 (somatomedin C) | 3.16 |
| MMP8 | Hs.161839 | NM_002424 | Matrix metallopeptidase 8 (neutrophil collagenase) | 3.84 |
| TGFB3 | Hs.592317 | NM_003239 | Transforming growth factor, beta 3 | 2.47 |
| TGFBR1 | Hs.494622 | NM_004612 | Transforming growth factor, beta receptor 1 | 2.33 |
| VEGFA | Hs.73793 | NM_003376 | Vascular endothelial growth factor A | 2.68 |
| AHSG | Hs.324746 | NM_001622 | Alpha-2-HS-glycoprotein | −2.03 |
| AMBN | Hs.272396 | NM_016519 | Ameloblastin (enamel matrix protein) | −2.05 |
| AMELY | Hs.1238 | NM_001143 | Amelogenin, Y-linked | −3.60 |
| BMP3 | Hs.387411 | NM_001201 | Bone morphogenetic protein 3 | −2.44 |
| BMP5 | Hs.296648 | NM_021073 | Bone morphogenetic protein 5 | −2.58 |
| CSF2 | Hs.1349 | NM_000758 | Colony stimulating factor 2 (granulocyte-macrophage) | −2.38 |
| CSF3 | Hs.2233 | NM_000759 | Colony stimulating factor 3 (granulocyte) | −2.60 |
| ENAM | Hs.667018 | NM_031889 | Enamelin | −3.60 |
| IGF2 | Hs.523414 | NM_000612 | Insulin-like growth factor 2 (somatomedin A) | −2.29 |
| ITGAM | Hs.172631 | NM_000632 | Integrin, alpha M (complement component 3 receptor 3 subunit) | −2.69 |
| MMP9 | Hs.297413 | NM_004994 | Matrix metallopeptidase 9 (gelatinase B, 92kDa gelatinase, 92kDa type IV collagenase) | −3.09 |
| TNF | Hs.241570 | NM_000594 | Tumor necrosis factor (TNF superfamily, member 2) | −2.39 |
| B. Fold Change of the Human Pathway-focused Osteogenesis Molecules Expressed by the ASCs Grown on the Ordered FA Surface at Day 21 Relative to Day 7 (With OI) | ||||
|---|---|---|---|---|
| Symbol | Unigene | Refseq | Description | Fold Change |
| AHSG | Hs.324746 | NM_001622 | Alpha-2-HS-glycoprotein | 15.05 |
| AMELY | Hs.1238 | NM_001143 | Amelogenin, Y-linked | 2.09 |
| BGN | Hs.821 | NM_001711 | Biglycan | 2.66 |
| BMP5 | Hs.296648 | NM_021073 | Bone morphogenetic protein 5 | 2.83 |
| CALCR | Hs.489127 | NM_001742 | CALCITONIN RECEPTOR | 11.89 |
| CDH11 | Hs.116471 | NM_001797 | Cadherin 11, type 2, OB-cadherin (osteoblast) | 2.68 |
| COL10A1 | Hs.520339 | NM_000493 | Collagen, type X, alpha 1 | 6.69 |
| COL14A1 | Hs.409662 | NM_021110 | Collagen, type XIV, alpha 1 | 6.69 |
| COL15A1 | Hs.409034 | NM_001855 | Collagen, type XV, alpha 1 | 6.03 |
| COMP | Hs.1584 | NM_000095 | Cartilage oligomeric matrix protein | 2.87 |
| CSF2 | Hs.1349 | NM_000758 | Colony stimulating factor 2 (granulocyte-macrophage) | 2.03 |
| CTSK | Hs.632466 | NM_000396 | Cathepsin K | 4.20 |
| DMP1 | Hs.652366 | NM_004407 | Dentin matrix acidic phosphoprotein 1 | 3.12 |
| DSPP | Hs.678914 | NM_014208 | Dentin sialophosphoprotein | 3.21 |
| EGF | Hs.419815 | NM_001963 | Epidermal growth factor (beta-urogastrone) | 2.74 |
| FGF1 | Hs.483635 | NM_000800 | Fibroblast growth factor 1 (acidic) | 2.45 |
| FGF3 | Hs.37092 | NM_005247 | Fibroblast growth factor 3 (murine mammary tumor virus integration site (v-int-2) oncogene homolog) | 2.18 |
| ICAM1 | Hs.643447 | NM_000201 | Intercellular adhesion molecule 1 | 3.51 |
| IGF1 | Hs.160562 | NM_000618 | Insulin-like growth factor 1 (somatomedin C) | 62.77 |
| IGF1R | Hs.643120 | NM_000875 | Insulin-like growth factor 1 receptor | 2.16 |
| IGF2 | Hs.523414 | NM_000612 | Insulin-like growth factor 2 (somatomedin A) | 12.40 |
| MMP2 | Hs.513617 | NM_004530 | Matrix metallopeptidase 2 (gelatinase A, 72kDa gelatinase, 72kDa type IV collagenase) | 2.87 |
| MMP8 | Hs.161839 | NM_002424 | Matrix metallopeptidase 8 (neutrophil collagenase) | 2.12 |
| TGFBR2 | Hs.604277 | NM_003242 | Transforming growth factor, beta receptor II (70/80kDa) | 2.27 |
| VCAM1 | Hs.109225 | NM_001078 | Vascular cell adhesion molecule 1 | 17.78 |
| ENAM | Hs.667018 | NM_031889 | Enamelin | −2.51 |
| GDF10 | Hs.2171 | NM_004962 | Growth differentiation factor 10 | −2.46 |
| MSX1 | Hs.424414 | NM_002448 | Msh homeobox 1 | −2.05 |
| PDGFA | Hs.535898 | NM_002607 | Platelet-derived growth factor alpha polypeptide | −6.49 |
After the real-time PCR amplification, relative gene expression values were analyzed using the Superarray web-based software package performing all ΔΔCt based fold-change calculations.
With OI, 29 molecules were differentially regulated at 21 days compared to 7 days. Twenty five of the genes, including DMP1, DSPP, FGF1 and 3, and TGFBR2 etc, were up-regulated; only 4 genes, i.e. ENAM, GDF10, MSX1 and PDGFA were down regulated (Table 3B).
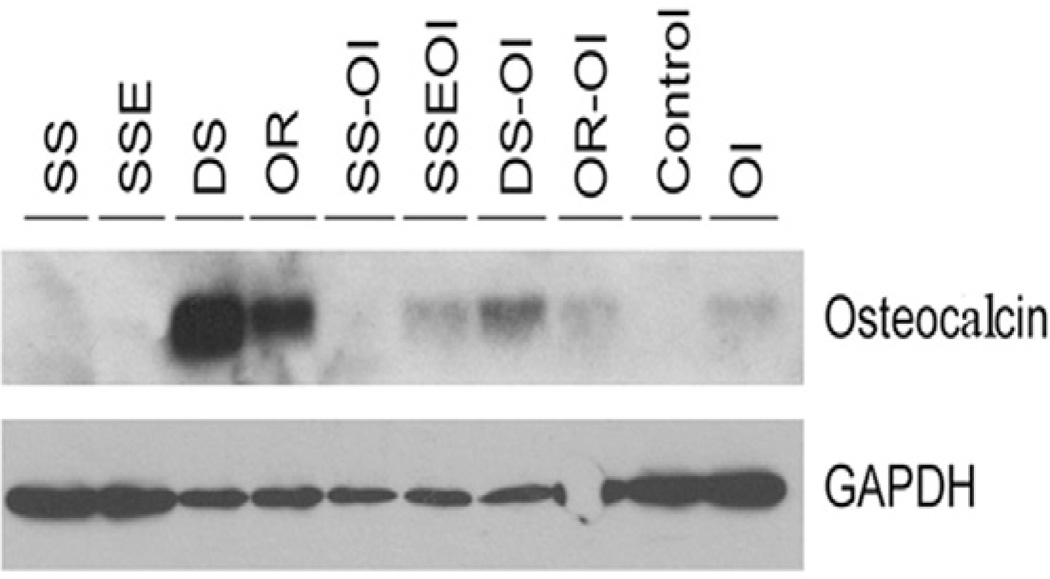
3.5. Western Blots
After 4 weeks, without OI, OCN expression was detected in cells grown on the ordered and disordered FA surfaces with the greatest intensity of OCN expression seen in cells grown on the disordered surfaces. With OI, much stronger OCN expression was detected in the cells grown on the ordered and disordered FA compared to the SSE surfaces. Without OI, no OCN was detected in cells grown on SS and SSE surfaces (Fig. 5).
Fig. 5.
Western blots of the osteocalcin expression of the ASCs grown on the stainless steel (SS), etched stainless steel (SSE), disordered (DS) and ordered (OR) FA surfaces with or without OI at 4 weeks.
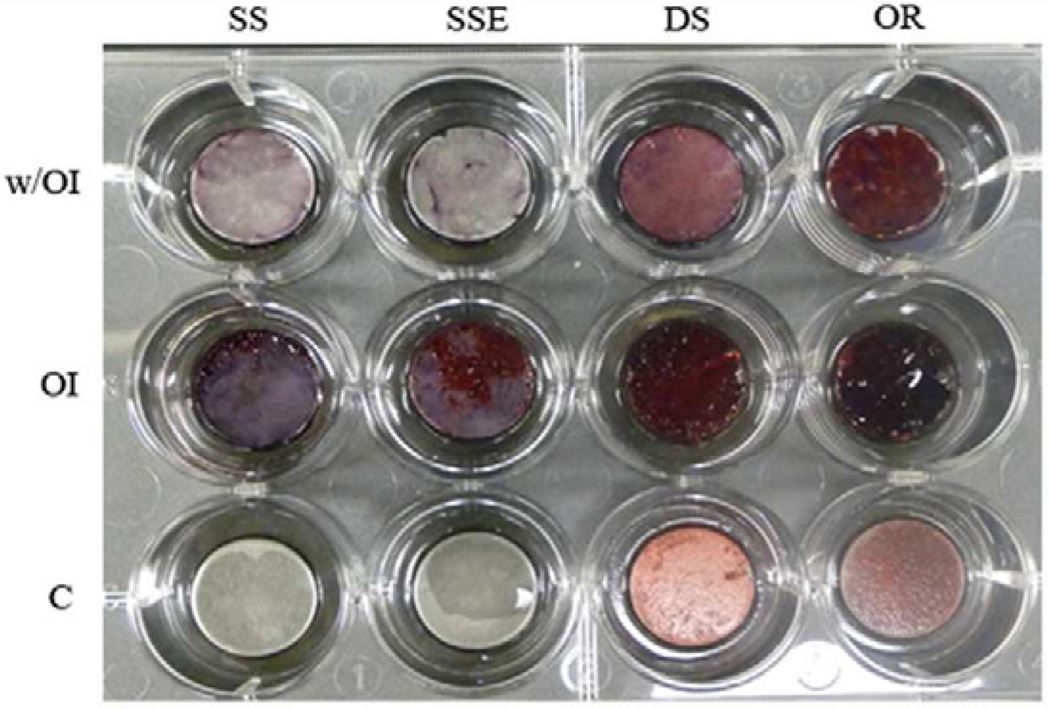
3.6. Mineralization induction and Alizarin Red Staining
Much stronger Alizarin Red staining was seen when the ASCs were grown on ordered and disordered FA surfaces than that seen in the cells grown on SSE and SS surfaces with OI. Interestingly, strong staining was also seen in the cells grown on both ordered and disordered FA crystal surfaces even without the OI. Without OI, no Alizarin Red staining was seen in cells grown on SS and SSE surfaces (Fig. 6).
Fig. 6.
Mineralization of the adipose derived stem cells (ASCs) on ordered and disordered FA either with or without OI as shown by Alizarin Red Staining.
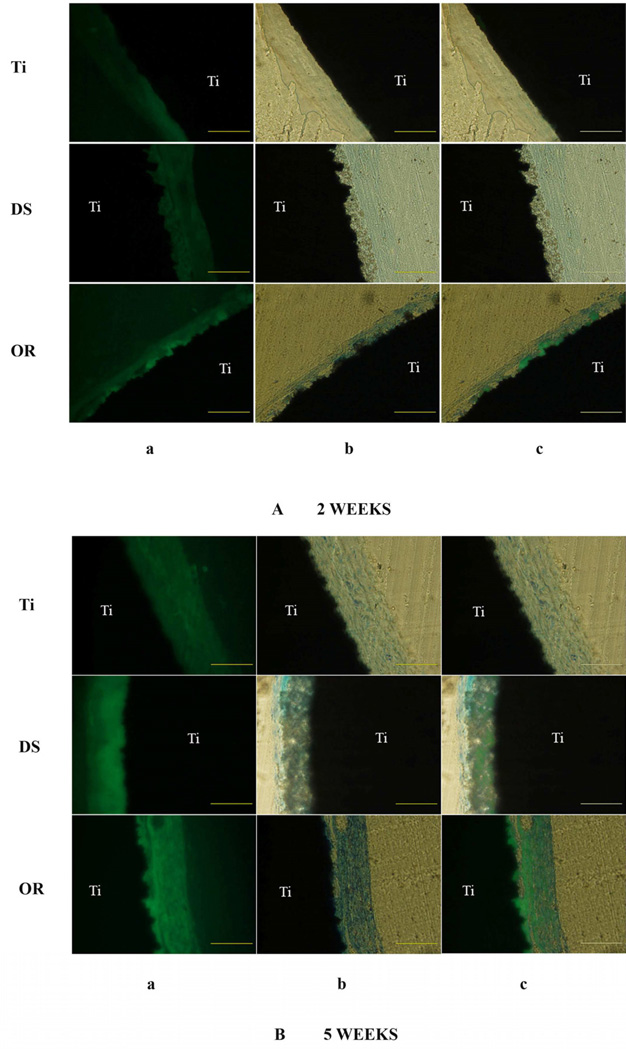
3.7. In vivo ectopic transplantation
After subcutaneous implantation of etched naked Ti and the FA coated Ti discs for 2 weeks, the ordered FA apatite surface stimulated scattered new mineral formation integrated within the ordered crystal layer (Fig. 7A). After 5 weeks, over 80 % of the ordered FA coating was integrated with the newly induced mineralized tissue compared to approximately 40 % in the disordered FA coating (One-way ANOVA, n=6, P<0.01) (Fig. 7B). The cellular involvement within the newly formed mineralized tissue was confirmed by the toluidine blue staining. The fluorescence intensity was also much stronger in the ordered FA crystal specimens compared to the disordered FA crystal samples. No new mineral formation was seen on the etched Ti surfaces (Fig. 7B).
Fig. 7.
Subcutaneous implantation of experimental discs on the dorsa of BALB/c mice for 2 weeks (A) and 5 weeks (B). a. Tetracycline, a bone fluorochrome was administered to label the newly formed bone-like mineralized tissue. b. The slides were stained with toluidine blue, which stains organic tissue components. c. Merged picture of micrograph a and b. After merging, the area of fluorescence labeling over the total area was analyzed by Image J software (NIH). After 5 weeks, over 80 % of the ordered FA coating was integrated with the newly induced mineralized tissue compared to approximately 40 % in the disordered FA coating (One-way ANOVA, P<0.01) (B).Scale bar: 200 µm. Ti, titanium. DS, disordered FA surface. OR, ordered FA surfaces.
4. Discussion
In our previous studies, the initial adhesion of the MG-63 osteoblast-like cells and the dental pulp stem cells (DPSCs) grown on the FA crystal surfaces was investigated and the results suggested that different cell types have different adhesion mechanisms during the initial cellular adhesion stage though grown on the same surfaces [6, 9]. In this study, we have used the ASCs which were seeded onto the FA crystal surfaces to study the effect of these surfaces on cell proliferation and growth compared to non-coated surfaces. After 3 and 7 days of growth, the attached cell numbers on the metal surfaces were significantly higher than those on the FA surfaces, however the osteocalcin expression and the mineral formation were most evident on the FA surfaces after 4 weeks with mineralization being greater on ordered FA surfaces than on the disordered with and without the OI. This would suggest that the topography of the FA crystal layer, in our case, the ordered structure of the FA crystals, stimulated the ASCs differentiation and mineralization process, which was further enhanced by the OI treatment. It would also indicate that the most favorable surfaces for the initial cellular adhesion might not necessarily result in favorable, or the expected cell fate selection. Thus we focused on exploring the mechanisms of cell differentiation and mineralization rather than initial cellular adhesion of the ASCs when they were grown on the FA crystal surfaces.
When the ASCs were grown for 7 days even without osteogenic induction, the ordered FA surfaces stimulated the expression of ALPL, bone morphogenic proteins including BMP3, 4 and 5, GDF10, EGF, PHEX, MSX1 and the expression of dentin sialophosphoprotein (DSPP). Bone morphogenetic proteins (BMPs) and growth differentiation factors (GDFs) belong to the transforming growth factor β (TGFβ) superfamily. Among the 15 known human BMPs, BMPs 2–7, 9 and 14 have been shown to induce undifferentiated mesenchymal stem cells to differentiate into chondroblasts or osteoblasts [17]. Similar to our data at 7 days, the BMP3, 4, 5 and BMP3b (GDF10) molecules were observed to be abundantly expressed at the initial murine bone fracture healing stage [18]. After 7 days, the expression of FGF1, FGF3 and FGFR1 was stimulated on the FA surfaces indicating the involvement FGF signaling pathway during this process. Noticeably, it has been previously shown that the application of a specific FGFR inhibitor completely blocked the osteogenic differentiation of the mesenchymal stem cell (MSC) and reduced chondrogenic differentiation of the MSCs [19]. Importantly, FGF1 has been show to have duel effects in favoring both angiogenesis and osteogenesis, which are crucial in the physiology of bone development and the repair process [20].
Noteworthily, the expression of EGF was up-regulated on the FA surfaces at 7 days since the effect of EGF on the MSC cell growth, differentiation and mineralization is still controversial. EGF has been found to play important roles in maintaining the undifferentiated osteoprogenitor cell populations, and in stimulating the proliferation of osteoblasts, while inhibiting the osteoblast differentiation and mineral nodule formation in various osteoblastic cell lines [21–23]. EGFR signaling has also been reported to negatively regulate bone cell differentiation [24]. However, in other studies, EGF and/or EGF-like ligands were shown to stimulate the osteoblast differentiation of human mesenchymal stem cells (HMSCs) through related EGFR phosphorylation [25], and synergistically enhanced the differentiation of MG-63 cells into osteoblasts [26]. The conflicting results of EGF signaling in the mediation of osteogenic differentiation were probably related to different species, different microenvironments of the tested cell/tissue models and the heterogenecity of the human MSC preparations [25, 27]. Phex (phosphate-regulating endopeptidase homolog, Xlinked) is a transmembrane metalloendoprotease predominantly localized on teeth and bone, which plays critical roles in the phosphate homeostasis and bone mineralization and is thought to be the candidate gene for the X-linked hypophosphatemic rickets (Hyp) [28]. Phex is thus thought to be one of the required genes for normal bone formation and biomineralization process [29]. The homeobox genes Msx1 and Msx2 regulate various cellular proliferation and differentiation during embryonic development [30, 31]. Msx genes are critical for the expression of Runx2 in the frontonasal neural crest cells and for the osteogenic lineage commitment [32]. It is thus very interesting to find that the up regulation of Msx 1 and especially Phex, at more than 50 times, in the ASCs stimulated by the ordered FA surfaces after their growth for 7 days.
In our study, the FA crystal surfaces also stimulated the expression of extracellular matrix related gene, i.e. type V, type X, type II, and most noticeable the type IV collagen, a more than 500 times up regulation, which may provide a favorable microenvironment for the differentiation of ASCs into osteoblast lineage cells. It is also surprising that not only ALPL, but the DSPP gene was up-regulated at 7 days, because in our previous studies enhanced DSPP gene expression was normally seen at a later stage growth of the dental pulp stem cells (DPSCs) when obvious mineralization occurred [16].
As we expected, when the ASCs were further treated with the OI for 7 days, a similar set of pro-osteogenic profile molecules including BMP3, EGF, FGF3, PHEX and DMP1 were stimulated when ASCs were grown on the ordered FA surfaces. DMP1, a non-collagenous matrix protein, has been shown to play essential roles in the maturation of odontoblast and osteoblasts and in the biomineralization process [33]. Mutations in DMP1 have been reported to cause autosomal recessive hypophosphatemic rickets [34]. Both DMP1 and Phex are essentially involved in the regulation of phosphate homeostasis and maintain appropriate serum phosphate levels required for normal mineralization of teeth and bone [29].
In our study even without OI, after 21 days when obvious mineralization was occurring, the FGF and TGF-beta signaling appear to be dominant with the up regulation of FGF1 and TGFB3 on the FA surfaces compared to the metal surfaces. When OI treated, most obviously, one of the important FGF signal molecules, FGF1 was up-regulated more than 100 times on the FA surfaces compared to the metal surfaces. At the same time point of 21 days, OI also enhanced the expression of the late mineralization markers, i.e. DMP1 and DSPP, on the FA surfaces, which is in accord with our mineralization data showing more mineral deposition compared to FA and metal surfaces without OI.
Comparing the gene profile of ASCs grown on ordered FA surfaces at 21 days to that of 7 days, it is not surprising to observe the up regulation of potent pro-osteogenic molecules, i.e. BMP2, FGF1, FGFR2 and the involvement of TGF beta signaling molecules. The up regulation of VEGF is fascinating since most recently it has been shown that locally applied VEGFA induces concurrent angiogenesis and osteogenesis by the human adipose derived stem cells [35]. Among the collagen members the type XI collagen seems to have a critical role in solid connective tissue development since mutation of this gene causes severe abnormalities of bone and tracheal cartilage normally resulting death of the animals at birth [36]. After OI, similar to the comparison between FA and metal surfaces at 21 days, non-collagenous matrix proteins, DSPP and DMP1 were consistently up-regulated on the ordered FA surfaces at 21 days compared to their expression at 7 days. Combined with our mineralization data, it would suggest that enhanced mineralization seems to correlate well with the stimulated expression of DSPP and DMP1 transcripts.
Among the two FA surfaces, without OI, after 7 days, it was noteworthy that ALPL gene, one of the early osteoblast differentiation and mineralization markers, was up-regulated on ordered versus disordered FA surfaces; whereas the OI treatment stimulated the expression of proosteogenic genes i.e. BMP2, BMP5 and GDF10 (Table 1_suppl). It seems likely that the ordered FA structure favors the recruitment of certain growth factors from the OI supplement thus creating an osteoinductive microenvironment which then stimulates the cells to produce potent inductive factors further enhancing this osteoinductive effect. After 21 days, there was no up regulation of pro-osteogenic genes when the cells were grown on ordered FA compared to disordered surfaces (Table 2_suppl). Combined with our 7 days data, we would assume that the ASC cell lineage commitment and differentiation was already pre-determined at 7 days, thus further stimulation of pro-osteogenic transcripts at 21 days may not be necessary for mineralization to occur.
For the use of biomedical implants in the treatment of skeletal defects caused by trauma, disease or genetic disorders, it would be ideal to make functional loading possible much earlier after surgery thus decreasing the recuperation time of patients and restoring tissue functions and cosmetics in a timely manner. In a previous study, the HA coating has been shown to shorten the implant healing period in a rabbit tibia model through enhanced bone implant interactions [37]. In our study, after subcutaneous implantation of Ti and FA discs into the surgical pockets for 2 weeks, it is encouraging to see that the ordered FA apatite surface induced scattered new mineral formation integrated within the coated ordered crystal layer. Naked titanium surfaces did not result in the formation of any mineralized tissue. The newly formed mineralized tissue was distinguished by the tetracycline labeling and toluidine blue staining, which indicated new mineral formation with a cellular component. According to our in vitro studies, in an in vivo situation the ordered FA surfaces may favor the recruitment of pro-osteogenic molecules and facilitate the migration of stem cells from the local tissue environment. These ordered FA surfaces then accelerated both the differentiation of the attracted stem cells into osteoblast-like cells and the subsequent mineralization process. Promisingly, after 5 weeks both the coverage and the intensity of the fluorescence of the integrated mineralized layer into the ordered FA samples were much more pronounced when compared to the disordered FA samples. This indicated a much greater amount and denser newly formed mineralized tissue in this layer. This is very strong evidence in support of the ordered FA coatings being able to accelerate and enhance the osseointegration of the implants.
Interestingly, one of our in vitro studies showed that after osteoblast-like cellular growth the binding of the ordered FA crystals to the metal surfaces was significantly stronger than that of the disordered crystals, which appeared to be closely related to the formation of greater amount of mineral nodules within the ordered FA crystal layers [6]. Therefore, we would assume that the ordered FA crystal structure not only accelerated and stimulated osseointegration, but the union between the implant and the coating was also further strengthened. This improvement is of obvious clinical significance because delamination of a coating material from an implant surface would cause implant failure, especially during the initial healing phase [38].
5. Conclusions
Therefore, within the limitation of this study, the ordered FA crystal surfaces stimulated the expression of a set of pro-osteogenic transcripts and bone mineralization phenotypic markers of ASCs compared to metal surfaces at 7 and 21 days. In addition to BMP and TGFβ signaling pathways, EGF and FGF pathways appeared to be involved in this ASCs differentiation and mineralization process. In vivo studies also showed accelerated and enhanced osseointegration of newly formed mineralized tissue on ordered FA samples. Both the properties and topography of the FA crystals appeared to dominate the cell differentiation and mineralization process. Thus, the ordered FA crystals show promise as an effective implant surface coating material.
Supplementary Material
Acknowledgements
This study was supported by NIH grants DE020983. We specially thanked Dr. David Kohn for the critical review of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moy PK, Medina D, Shetty V, Aghaloo TL. Dental implant failure rates and associated risk factors. Int J Oral Maxillofac Implants. 2005;20:569–577. [PubMed] [Google Scholar]
- 2.Makela K, Eskelinen A, Pulkkinen P, Paavolainen P, Remes V. Cemented total hip replacement for primary osteoarthritis in patients aged 55 years or older: results of the 12 most common cemented implants followed for 25 years in the Finnish Arthroplasty Register. J Bone Joint Surg Br. 2008;90:1562–1569. doi: 10.1302/0301-620X.90B12.21151. [DOI] [PubMed] [Google Scholar]
- 3.Dhert WJ, Klein CP, Wolke JG, van der Velde EA, de Groot K, Rozing PM. A mechanical investigation of fluorapatite, magnesiumwhitlockite, and hydroxylapatite plasma-sprayed coatings in goats. J Biomed Mater Res. 1991;25:1183–1200. doi: 10.1002/jbm.820251002. [DOI] [PubMed] [Google Scholar]
- 4.Fazan F, Marquis PM. Dissolution behavior of plasma-sprayed hydroxyapatite coatings. J Mater Sci Mater Med. 2000;11:787–792. doi: 10.1023/a:1008901512273. [DOI] [PubMed] [Google Scholar]
- 5.Bhadang KA, Gross KA. Influence of fluorapatite on the properties of thermally sprayed hydroxyapatite coatings. Biomaterials. 2004;25:4935–4945. doi: 10.1016/j.biomaterials.2004.02.043. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Jin T, Chang S, Czajka-Jakubowska A, Zhang Z, Nor JE, et al. The effect of novel fluorapatite surfaces on osteoblast-like cell adhesion, growth, and mineralization. Tissue Eng Part A. 2010;16:2977–2986. doi: 10.1089/ten.tea.2009.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavenus S, Louarn G, Layrolle P. Nanotechnology and dental implants. Int J Biomater. 2010;2010:915327. doi: 10.1155/2010/915327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmquist A, Omar OM, Esposito M, Lausmaa J, Thomsen P. Titanium oral implants: surface characteristics, interface biology and clinical outcome. J R Soc Interface. 2010;7(Suppl 5):S515–S527. doi: 10.1098/rsif.2010.0118.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Jin TC, Chang S, Czajka-Jakubowska A, Clarkson BH. Adhesion and growth of dental pulp stem cells on enamel-like fluorapatite surfaces. J Biomed Mater Res A. 2011;96:528–534. doi: 10.1002/jbm.a.33002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindroos B, Aho KL, Kuokkanen H, Raty S, Huhtala H, Lemponen R, et al. Differential gene expression in adipose stem cells cultured in allogeneic human serum versus fetal bovine serum. Tissue Eng Part A. 2010;16:2281–2294. doi: 10.1089/ten.tea.2009.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bieback K, Ha VA, Hecker A, Grassl M, Kinzebach S, Solz H, et al. Altered gene expression in human adipose stem cells cultured with fetal bovine serum compared to human supplements. Tissue Eng Part A. 2010;16:3467–3484. doi: 10.1089/ten.TEA.2009.0727. [DOI] [PubMed] [Google Scholar]
- 12.Haimi S, Suuriniemi N, Haaparanta AM, Ella V, Lindroos B, Huhtala H, et al. Growth and osteogenic differentiation of adipose stem cells on PLA/bioactive glass and PLA/beta-TCP scaffolds. Tissue Eng Part A. 2009;15:1473–1480. doi: 10.1089/ten.tea.2008.0241. [DOI] [PubMed] [Google Scholar]
- 13.Lu Z, Roohani-Esfahani SI, Kwok PC, Zreiqat H. Osteoblasts on Rod Shaped Hydroxyapatite Nanoparticles Incorporated PCL Film Provide an Optimal Osteogenic Niche for Stem Cell Differentiation. Tissue Eng Part A. 2011;17:1651–1661. doi: 10.1089/ten.TEA.2010.0567. [DOI] [PubMed] [Google Scholar]
- 14.Monaco E, Bionaz M, Hollister SJ, Wheeler MB. Strategies for regeneration of the bone using porcine adult adipose-derived mesenchymal stem cells. Theriogenology. 2011;75:1381–1399. doi: 10.1016/j.theriogenology.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Farre-Guasch E, Marti-Page C, Hernadez-Alfaro F, Klein-Nulend J, Casals N. Buccal fat pad, an oral access source of human adipose stem cells with potential for osteochondral tissue engineering: an in vitro study. Tissue Eng Part C Methods. 2010;16:1083–1094. doi: 10.1089/ten.TEC.2009.0487. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Jin T, Ritchie HH, Smith AJ, Clarkson BH. In vitro differentiation and mineralization of human dental pulp cells induced by dentin extract. In Vitro Cell Dev Biol Anim. 2005;41:232–238. doi: 10.1290/0502014.1. [DOI] [PubMed] [Google Scholar]
- 17.Marsell R, Einhorn TA. The role of endogenous bone morphogenetic proteins in normal skeletal repair. Injury. 2009;40(Suppl 3):S4–S7. doi: 10.1016/S0020-1383(09)70003-8. [DOI] [PubMed] [Google Scholar]
- 18.Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 19.Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, et al. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112:295–307. doi: 10.1182/blood-2007-07-103697. [DOI] [PubMed] [Google Scholar]
- 20.Kelpke SS, Zinn KR, Rue LW, Thompson JA. Site-specific delivery of acidic fibroblast growth factor stimulates angiogenic and osteogenic responses in vivo. J Biomed Mater Res A. 2004;71:316–325. doi: 10.1002/jbm.a.30163. [DOI] [PubMed] [Google Scholar]
- 21.Zhu J, Shimizu E, Zhang X, Partridge NC, Qin L. EGFR signaling suppresses osteoblast differentiation and inhibits expression of master osteoblastic transcription factors Runx2 and Osterix. J Cell Biochem. 2011;112:1749–1760. doi: 10.1002/jcb.23094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laflamme C, Curt S, Rouabhia M. Epidermal growth factor and bone morphogenetic proteins upregulate osteoblast proliferation and osteoblastic markers and inhibit bone nodule formation. Arch Oral Biol. 2010;55:689–701. doi: 10.1016/j.archoralbio.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Lu X, Wang Q, Hu G, Van Poznak C, Fleisher M, Reiss M, et al. ADAMTS1 and MMP1 proteolytically engage EGF-like ligands in an osteolytic signaling cascade for bone metastasis. Genes Dev. 2009;23:1882–1894. doi: 10.1101/gad.1824809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sibilia M, Wagner B, Hoebertz A, Elliott C, Marino S, Jochum W, et al. Mice humanised for the EGF receptor display hypomorphic phenotypes in skin, bone and heart. Development. 2003;130:4515–4525. doi: 10.1242/dev.00664. [DOI] [PubMed] [Google Scholar]
- 25.Kim SM, Jung JU, Ryu JS, Jin JW, Yang HJ, Ko K, et al. Effects of gangliosides on the differentiation of human mesenchymal stem cells into osteoblasts by modulating epidermal growth factor receptors. Biochem Biophys Res Commun. 2008;371:866–871. doi: 10.1016/j.bbrc.2008.04.162. [DOI] [PubMed] [Google Scholar]
- 26.Yarram SJ, Tasman C, Gidley J, Clare M, Sandy JR, Mansell JP. Epidermal growth factor and calcitriol synergistically induce osteoblast maturation. Mol Cell Endocrinol. 2004;220:9–20. doi: 10.1016/j.mce.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Tamama K, Fan VH, Griffith LG, Blair HC, Wells A. Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:686–695. doi: 10.1634/stemcells.2005-0176. [DOI] [PubMed] [Google Scholar]
- 28.Miao D, Bai X, Panda D, McKee M, Karaplis A, Goltzman D. Osteomalacia in hyp mice is associated with abnormal phex expression and with altered bone matrix protein expression and deposition. Endocrinology. 2001;142:926–939. doi: 10.1210/endo.142.2.7976. [DOI] [PubMed] [Google Scholar]
- 29.Gorski JP, Huffman NT, Chittur S, Midura RJ, Black C, Oxford J, et al. Inhibition of proprotein convertase SKI-1 blocks transcription of key extracellular matrix genes regulating osteoblastic mineralization. J Biol Chem. 2011;286:1836–1849. doi: 10.1074/jbc.M110.151647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roybal PG, Wu NL, Sun J, Ting MC, Schafer CA, Maxson RE. Inactivation of Msx1 and Msx2 in neural crest reveals an unexpected role in suppressing heterotopic bone formation in the head. Dev Biol. 2010;343:28–39. doi: 10.1016/j.ydbio.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung IH, Han J, Iwata J, Chai Y. Msx1 and Dlx5 function synergistically to regulate frontal bone development. Genesis. 2010;48:645–655. doi: 10.1002/dvg.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han J, Ishii M, Bringas P, Jr, Maas RL, Maxson RE, Jr, Chai Y. Concerted action of Msx1 and Msx2 in regulating cranial neural crest cell differentiation during frontal bone development. Mech Dev. 2007;124:729–745. doi: 10.1016/j.mod.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin C, D'Souza R, Feng JQ. Dentin matrix protein 1 (DMP1): new and important roles for biomineralization and phosphate homeostasis. J Dent Res. 2007;86:1134–1141. doi: 10.1177/154405910708601202. [DOI] [PubMed] [Google Scholar]
- 34.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Behr B, Tang C, Germann G, Longaker MT, Quarto N. Locally Applied VEGFA Increases the Osteogenic Healing Capacity of Human Adipose Derived Stem Cells by Promoting Osteogenic and Endothelial Differentiation. Stem Cells. 2011;29:286–296. doi: 10.1002/stem.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Lacerda DA, Warman ML, Beier DR, Yoshioka H, Ninomiya Y, et al. A fibrillar collagen gene, Col11a1, is essential for skeletal morphogenesis. Cell. 1995;80:423–430. doi: 10.1016/0092-8674(95)90492-1. [DOI] [PubMed] [Google Scholar]
- 37.Faeda RS, Tavares HS, Sartori R, Guastaldi AC, Marcantonio E., Jr. Biological performance of chemical hydroxyapatite coating associated with implant surface modification by laser beam: biomechanical study in rabbit tibias. J Oral Maxillofac Surg. 2009;67:1706–1715. doi: 10.1016/j.joms.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 38.DeLuca S, Habsha E, Zarb GA. The effect of smoking on osseointegrated dental implants. Part I: implant survival. Int J Prosthodont. 2006;19:491–498. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.