Abstract
Childhood cerebellar ataxias, and particularly congenital ataxias, are heterogeneous disorders and several remain undefined. We performed a muscle biopsy in patients with congenital ataxia and children with later onset undefined ataxia having neuroimaging evidence of cerebellar atrophy. Significant reduced levels of Coenzyme Q10 (COQ10) were found in the skeletal muscle of 9 out of 34 patients that were consecutively screened. A mutation in the ADCK3/Coq8 gene (R347X) was identified in a female patient with ataxia, seizures and markedly reduced COQ10 levels. In a 2.5-years-old male patient with non syndromic congenital ataxia and autophagic vacuoles in the muscle biopsy we identified a homozygous nonsense mutation R111X mutation in SIL1 gene, leading to early diagnosis of Marinesco-Sjogren syndrome. We think that muscle biopsy is a valuable procedure to improve diagnostic assesement in children with congenital ataxia or other undefined forms of later onset childhood ataxia associated to cerebellar atrophy at MRI.
Keywords: Inherited cerebellar ataxias, Marinesco-Sjogren syndrome, Coenzyme Q10 deficiency
1. Introduction
Inherited cerebellar ataxias (ICA) in children are an extremely heterogeneous group of disorders. According to inheritance, inherited cerebellar ataxias can be classified into autosomal recessive, autosomal dominant, X-linked and maternally inherited forms.1,2
Autosomal recessive (AR) ataxias are the most frequent group of inherited ataxias with onset in childhood, particularly Friedreich ataxia and Ataxia-telangectasia. Different criteria have been used to classify these forms.2–4 Palau and Espinos (2006), in a pathogenic and clinically-oriented classification, established five subgroups of autosomal recessive ataxia including childhood and adult onset forms and in this classification they incorporated the metabolic ataxias, a growing group of genetically defined disorders.5 Clinical criteria based on age at onset can distinguish inherited cerebellar ataxias (ICA) with onset in childhood as following: a)congenital ataxias (CA), characterized by neonatal hypotonia, developmental delay and early-onset ataxia, and b) later onset childhood ataxias (CHA). Moreover all these conditions can be progressive or non progressive forms, and syndromic or non syndromic ataxias. Syndromic ataxias have associated symptoms such as dysmorphia, oculomotor apraxia, peripheral neuropathy, deafness, optic atrophy, congenital cataracts, pigmented retinopathy, Lebers’ amaurosis, microcephaly, recurrent infections, immunodeficiency, that besides are helpful key signs to define the various conditions and address diagnosis.
Because ICA are neurological disorders resulting from degeneration or abnormal development of the cerebellum, brain MRI is of pivotal importance for the subclassification of cerebellar abnormalities (dysgenesis, hypoplasia and/or atrophy) adding the potential association with supratentorial abnormalities.6 In cases of cerebellar atrophy, only few main pathogenetic categories have been defined7,8: metabolic, DNA repair defects and neurodegenerative, often responsible for congenital or later onset childhood ataxias.
In a series of patients affected by non syndromic congenital or later–onset childhood ataxia with neuroradiological evidence of cerebellar atrophy in whom known forms of childhood onset ataxia were ruled out by extensive metabolic, neurophysiological and laboratory examinations, we performed systematically a muscle biopsy in a selected cohort of 34 consecutive patients in order to analyze muscle morphology, and the mitochondrial respiratory chain enzyme activities together with the determination of CoQ10 levels in muscle. Our studies led to a definitive diagnosis in two patients: Marinesco-Sjogren syndrome in one sporadic patient with apparently non syndromic congenital ataxia, and another sporadic patient with a childhood onset non syndromic ataxia with a primary defect of CoQ10 biogenesis.
2. Materials and methods
2.1. Patients
We have evaluated 68 consecutive children with ataxic syndromes that were referred to our centre from 1998 to 2008 and we selected a cohort of 34 unrelated patients with undetermined cause who showed MRI evidence of cerebellar atrophy. A group of patients (14 patients, 41%) were classified as affected by a congenital non syndromic ataxia (CA) whereas most of them (20 patients, 59%) had undetermined ataxia with later onset in childhood (CHA). In all patients, family history was unremarkable for ataxia or other relevant genetic disorders. The brain MRI showed isolated cerebellar atrophy and extensive laboratory investigations including metabolic screening tests (isoelectric focusing of serum transferrin, serum and urine aminoacid chromatography, urinary organic acid chromatography MS, serum lactate, alpha-fetoprotein), and echocardiography were negative. Neurophysiologic examinations excluded a peripheral sensory motor neuropathy and other cranial nerve involvement. Patients were followed up for at least 5 years. All patients were submitted to a skeletal muscle biopsy for measuring mitochondrial respiratory chain enzymes and coenzyme Q10 levels. The procedure of the muscle biopsy was approved by or local Ethics committee. In addition the neurological examination ruled out other relevant associated symptoms and confirmed that most (85%) patients with a congenital onset had early-onset strabismus. Thirthy three patients of our series have been clinically followed up in a range of 4–12 years and have not shown any substantial progression of the disease. Only one patient followed for 3 years and diagnosed with a Marinesco-Sjogren syndrome has a slowly progressive ataxia.
2.2. Muscle biopsy
After obtaining an informed written consent, open muscle biopsies were performed in all patients.
Frozen muscle sections were stained using standard histochemical and histoenzymatic methods, and when appropriate were selected for ultrastructural examination.
Mitochondrial respiratory chain enzymes were analyzed spectrophotometrically in all muscle biopsies. Spectrophotometric measurements of mitochondrial respiratory chain enzyme activities were carried out as reported with modifications.9 Briefly, approximately 50 mg of muscle biopsy were homogenized in Tris HCl/KCl (pH 7.4), centrifuged at 800× g for 10 min and the enzyme activities assayed on the supernatants in a UV double-beam spectrophotometer. The rotenone–sensitive Complex I activity was measured by following the rate of NADH oxidation at 340 nm for 1 min. Complex III specific activity was measured by monitoring the reduction of cytochrome c at 550 nm and the reaction started by adding reduced DB. Complex IV was assayed by following the oxidation of reduced cytochrome c at 550 nm. SDH was assessed by following the reduction of 2,6-dichlorophenolindophenol at 600 nm for 1 min in the presence of succinate. Complex II activity was measured in the same reaction mixture by adding 50 μM DB and monitoring the enzyme kinetic for 3 min. The coupled Complex II + III assay was also determined by starting the reaction with succinate and measuring the reduction of cytochrome c at 550 nm. Complex II + III was performed only in patients who received a muscle biopsy after 2001.In all muscle extracts the levels of CoQ10 were measured using the method developed in our laboratory.10 Summarizing approximately 2 mg of −80 °C frozen fragments of muscle biopsy specimens were homogenized with 250 μL methanol and 500 μL hexane (containing 50 μL of 100 nmol/L CoQ9 as internal standard) in a Potter-Elvehjem type homogenizer and a 5 μL aliquot of hexane extract was immediately injected into a 150 × 4.6 mm Hypersil-ODS column. Reduced and oxidized CoQ10 was isocratic eluted at a flow rate of 1 mL/min and the retention times for each analyte was calculated using external standards at five different concentrations.
CoQ10 levels were measured with a HPLC-system by an Agilent 1100 Series Liquid Chromatograph with a coulometric electrochemical detector (Coulochem® II) equipped with a Model 5020 Guard Cell (−600 mV) and a Model 5011 Analytical Cell (first electrode operating at −150 mV; second electrode operating at +600 mV). Data obtained are analyzed by the ChemStation for LC program of Agilent Technologies.
Results were considered as significantly low CoQ10 levels when they were below 20 μmol/g tissue (se Results for criteria used to define significantly abnormal low CoQ10 concentrations).
2.3. Mutation analysis
In patient CA096, the 9 coding exons of SIL1 and their flanking intronic sequences, were amplified by PCR from genomic DNA isolated from blood lymphocyte of the patients, according to standard procedures. PCR products where directly sequenced on both strands using BigDye 3.1 chemistry (Applied Biosystems, Foster City CA, USA) on an ABI3130xl automatic sequencer (Applied Biosystems, Foster City CA, USA).
In all the 9 patients with significant reduction of Coenzyme Q10 in muscle we systematically performed molecular analysis of the human genes involved in ubiquinone synthesis. Based on published data we sequenced eleven known human genes (PDSS1, PDSS2, COQ2, COQ3, COQ4, COQ5, COQ6, COQ7, ADCK3, COQ9, ADCK2) encoding COQ10 biosynthetic proteins, and prioritizing genes typically involved in cerebellar sub-phenotype (ADCK3). Exons and flanking intronic regions of 50nt was PCR amplified using intronic primers. PCR products were directly sequenced with the BigDye v 3.1 sequencing Kit (Applied Biosystems, Foster City CA, USA) on an ABI3130xl automatic sequencer (Applied Biosystems, Foster City CA, USA).
3. Results
Muscle morphology showed no relevant abnormalities in all patients except one with a CA (patient CA096) who demonstrated numerous rimmed vacuoles (see detailed report below in the Case reports and Fig. 1). We found a significant reduction of Coenzyme Q10 in the muscle biopsy of 9 patients (Table 2). In this group, 5 patients had the clinical presentation of a congenital ataxia while the remaining 4 started showing ataxic symptoms after the 3rd year of life and so far in the first decade of life (childhood onset ataxia). We defined as significative reduction of Coenzyme Q10 those values that were related to the arbitrary range of Coenzyme Q10 levels (0.79–12.5) obtained from our past experience in 3 patients with confirmed mutations in genes involved in Coenzyme Q10 biogenesis, adding a fourth mutated patient CHA987 in this series who was mutated in the ADCK3 gene. Three of these patients were mutated in COQ2, two of which have been published11; details of patient CHA987 are reported in the case reports below. Thus significative reduced values that allegedly predicted a primary Coenzyme Q10 deficiency were considered those under 20 μg/g.
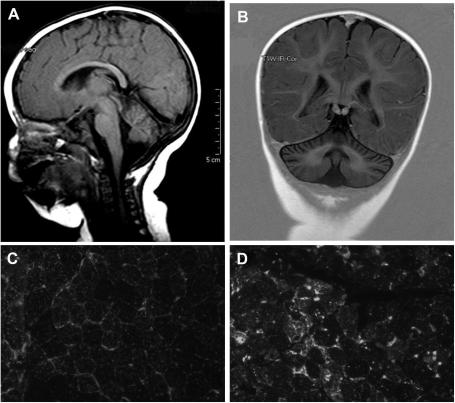
Fig. 1.
A–D. Fig A (T1 weighted, saggittal) and B (inversion recovery, coronal) are neuroimages of patient CA096 with a Marinesco-Sjogren s. and SIL1 mutations showing the presence of a mild cerebellar atrophy at the age of 5 months. Fig. 1D shows the morphology of the muscle biopsy performed at age 2 years showing increased immunofluorescence for LAMP-2 in relation to a control sample (Fig. 1C).
Table 2.
Summary of patients with either CA or CHA. Legends: CA: congenital ataxia; CHA: childhood onset ataxia; &: published in Diomedi-Camassei et al., J Am Soc Nephrol 2007, 18: 2773–2780; # the mean and SD value includes the value of patient CHA987. Only abnormal values of mitochondrial respiratory chain enzymes are reported; ND: not done; NL: normal; mt: mitochondrial.
| Patients |
Diagnosis |
Coenzyme Q10 levels in muscle (μmol/g tissue) |
Mitochondrial Respiratory chain enzymes (nmol/min/mg prot.) |
||
|---|---|---|---|---|---|
| Mean |
SD |
Range |
|||
| Total 24 | Normal controls | 37.4 | 18.5 | 20–79 | Normal ranges |
| Patients with CA (9) or CHA (16) ataxia (total 25) | Normal CoQ10 levels | 34.6 | 16 | 20–77.2 | Normal ranges |
| CA096 | CA; Marinesco-Sjogren S. | 40 | Normal ranges | ||
| Patients with CA (5) and CHA (4) ataxia and significatively reduced CoQ10 levels (total 9) | 13.1 | 2.6 | 9.24–17.25 | ||
| CA982 | CA | 10.2 | Complex II + III: ND | ||
| CHA995 | CHA | 14.38 | Complex II + III: 0.02 | ||
| CHA016 | CHA | 15.1 | Complex II + III: NL | ||
| CA028 | CA | 17.25 | Complex II + III: ND | ||
| CHA034 | CHA | 10 | Complex II + III: ND | ||
| CA056 | CA | 15 | Complex II + III: NL | ||
| CA073 | CA | 14.2 | Complex II + III: ND | ||
| CA978 | CA | 9.24 | Complex II + III: ND | ||
| CHA987 | CHA; ADK3 mutations | 3.69 | Complex II + III: 0.006 | ||
| Additional patients with confirmed mutations in genes of CoQ10 biogenesis | 7.345# | 6# | 0.79–12.5 | ||
| VA/07 | Leigh syndrome | 0.79 | Other myx enzymes NL | Complex II + III: 0.013 | |
| CB/09 | Myopathy + Encephalopathy | 12.4 | Other mtx enzymes NL | Complex II + III: 0.018 | |
| CV/06 | Myopathy + Encephalopathy | 12.5 | Other mtx enzymes NL | Complex II + III: 0.020 | |
In the 9 patients with significative reduction of Coenzyme Q10 we also found abnormalities of the mitochondrial respiratory chain enzymes in some, particularly Complex II + complex III activity was reduced in 2 patients and was normal in additional 2 patients (Table 2) but no mutations were found in 8 patients after sequencing 11 genes involved in ubiquinone biosynthesis. In patient CHA987 we found a homozygous c.1042C > T, p.R348X (see below for details on the clinical report). Notably, in this patient Coenzyme Q10 values and Complex II + III enzyme activity was the lowest of all the series. In the 8 patients in which we did not find any mutations in known genes of Coenzyme Q10 biogenesis, the supplementation of Coenzyme Q10 biogenesis at the dose of 5 mg/kg/day was delivered and in the follow-up from 4 to 12 years we did not observe neither improvement not worsening of the ataxic syndrome similarly to what we observed in the sub-group of patients with CA or CHA that had normal biochemical results. Patient with a defect in Coenzyme Q10 biogenesis and genetic confirmation (CHA987) will be described in detail below.
3.1. Case reports
3.1.1. Patient CA096
This boy was born from non consanguineous healthy parents both originating from South Italy. The baby had two healthy elder twin-brothers. Pregnancy had been complicated by transient polyhydramnios and poor fetal movements. Prenatal karyotype was normal. A caesarean section was programmed at 39 + 4 GW. Birth weight was 2890 g. During the neonatal period moderate axial hypotonia was noticed. Clinical examination at age 3 months revealed global hypotonia, psychomotor delay, convergent bilateral strabismus and cryptorchidism. At age 5 months brain MRI showed moderate global cerebellar atrophy (Fig. 1 A,B) and the child underwent several investigations including CK (93 IU/L), transferrin isofocusing and alpha-foetoprotein blood levels, and extensive metabolic screening in blood and urines. Ophtalmologic examination revealed epicanthus, oculomotor dyspraxia, intermittent nystagmus and strabismus excluding cataracts. Funduscopy, VEP-ERG and BAEP as well as nerve conduction studies were normal. At age 9 months Griffiths Mental Development Scale evidenced a GQ of 57 (below the expected level according to his age). Genetic analysis for Prader–Willi (methylation 15q11), FRAXA, OPHN1, PLP1 and CGH array were normal. At age 15 months a second MRI displayed marked global cerebellar atrophy (data not shown). By age 24 months the patient showed worsening of motor dysability with kyphosis, mild truncal ataxia and alternanting convergent strabismus; sitting posture without support was not achieved; growth parameters were in the standard range; social interactions and behavior seemed to be satisfactory. According to our diagnostic protocol we proceeded to a muscle biopsy. Histological examination showed myopathic changes characterized by variation in fiber size, mild increase of internal nuclei and the presence of numerous rimmed vacuoles which were positive to acid phosphatase and LAMP-2 antibody (Fig. 1C, D). Muscle mitochondrial respiratory chain studies and CoQ10 levels were in the normal range. The marked lysosomal proliferation in muscle prompeted us to screen for SIL1 gene although the child had no congenital cataracts. We identified a nonsense homozygous R111X mutation. The child is now 4 yeard old and the ophtalmological examination has excluded a bilateral cataracts.
3.1.2. Patient CHA987
This female was born after a normal pregnancy and delivery, from consanguineous italian parents (first cousins). Her sister was healthy. Psycomotor development was reportedly normal until the age of 6 years when she started to have partial seizures for which she was referred to our Hospital. Seizures were controlled by AEDs but she developed slowly progressive ataxic syndrome characterized by mild ataxic gait, intentional tremor, dysmetria and dysarthria. Brain MRI performed at age 7 years showed mild global isolated cerebellar atrophy (Fig. 2 A, B). Later a second MRI at age 13 years, after 5 years of CoQ10 supplementation, showed increased atrophy which was limited to the vermis and excluding other brain abnormalities (Fig. 2 C, D).Neuropsychological evaluation at age 8 years evidenced a mild cognitive delay. Laboratory tests (CK, vitamin E, alpha-foetoprotein, immunoglobulin electrophoresis) and metabolic investigations (transferrin isofocusing, serum lactate, serum and urine aminoacid chromatography, urine organic acid chromatography) were normal, as were neurophysiological examinations (BAEP, SEP, ERG, VEP). Ophthalmologic examination excluded a retinopathy. At the age of 8 years the child underwent a left quadriceps muscle biopsy that did not show any relevant changes, but mitochondrial respiratory chain enzymes in muscle extracts revealed decreased activities for complex II + III suggesting a CoQ10 defect. Indeed CoQ10 muscle levels were markedly reduced (2.9 μg/g) in this patient. We started CoQ10 supplementation (10 mg/kg/d) and within 6 months we observed clear improvement of cerebellar ataxia. A serial brain MRI at age 14 years did not reveal any progression of the cerebellar atrophy compared to the neuroimage performed one year before. Attempt to stop AEDs at age 11 failed, and now at the age of 17 the epileptic syndrome is well controlled by AEDs and CoQ10 supplementation, and the ataxic syndrome is persistently stable. She is able to walk independently and she is autonomous in daily life. The girl is attending school with some support.
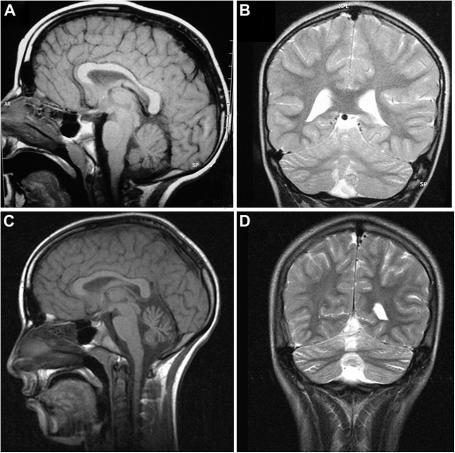
Fig. 2.
A–D. Brain MRI of patient CHA987 performed at age 7 (Fig. 2 A,B) and 13 years. In Fig. 2 A (saggittal, T1 weighted) and 2 B (coronal, T2 weighted) the atrophy is very mild while it is clearly evident and prominent at the vermis at a later age in Fig. 2C (saggital, T1 weighted) and 2 D (coronal, T2 weighted).
Clinical features of this patient, the reduced amounts of ubiquinone in its muscle and the very low activities of mitochondrial complex I + III and II + III, prompted us to analyze ADCK3/COQ8 gene with priority. We found a homozygous nonsense mutation (c.1042C > T, p.R348X) that was heterozygous in both healthy parents.
4. Discussion
Inherited cerebellar ataxias (ICA) in children are extremely heterogeneous disorders and ataxia is a frequent and a non-specific sign in many conditions.12 Most of the autosomal recessive conditions associated with ataxia are summarized in Table 1 and were excluded in our patients. Clinical criteria together with neuroimaging findings are crucial to establish a preliminary differential diagnosis of these conditions. Considering age of onset, ICA in children can be divided in 2 main groups: 1) congenital ataxia (CA), and 2) childhood onset ataxia (CHA). CA is characterized by neonatal hypotonia and developmental delay while patients with CHA show later onset ataxia. CA is frequently non progressive10 while CHA most frequently has a progressive course. In addition ICA can be distinguished in syndromic or non syndromic ataxias. Furthermore MRI is a valuable complementary tool for differential diagnosis of ICA and is capable of defining a possible brain involvement or simply of a cerebellar and brainstem malformation or a cerebellar atrophy.7
Table 1.
Summary of genetic conditions related to childhood onset autosomal recessive ataxias correlated with the presence or absence of cerebellar atrophy at neuroimaging.
| Autosomal Recessive Ataxia | Cerebellar atrophy | |
|---|---|---|
| Congenital ataxia | Cayman ataxia | + |
| Joubert syndrome | − | |
| Metabolic ataxia | Vitamin E deficiency | − |
| Abeta-lipoproteinemia | − | |
| Refsum disease | − | |
| Late-onset Tay-Sachs | + | |
| Niemann-Pick C | + | |
| CDG1a | + | |
| Cerebrotendinous xanthomatosis | + | |
| Neuronal ceroid lipofuscinoses | + | |
| 3-methylglutaconic aciduria | + | |
| Mevalonate kinase deficiency | + | |
| ADK3 mutaions and CoQ10 deficiency | + | |
| Leucodystrophy: L-2-hydroxyglutaric aciduria | +/− | |
| Menkes disease | +/− | |
| Autosomal recessive mitochondrial ataxias: | ||
| AR-CPEO, MIRAS, SANDO, SCAE, AHS, IOSCA, LBSL, Pyruvate decarboxylase deficiency, PDH deficiency | + | |
| Friedreich ataxia | − | |
| DNA repair defects | Ataxiatelangectasia (AT) | + |
| AT-like disorder | + | |
| AOA1 | + | |
| AOA2 | + | |
| Spinocerebellar ataxia with axonal neuropathy | + | |
| Xeroderma pigmentosum | + | |
| Cockayne syndrome | + | |
| Degenerative | Spastic ataxia of Charlevoix-Saguenay | + |
| Marinesco-Sjögren syndrome | + | |
| Infantile neuroaxonal dystrophy | + | |
| Leucodystrophy: CACH syndrome | − | |
| Hypomyelination: Salla disease, Pelizaeus-Merzbacher disease (PM), PM-like, leucoencephalopathy with ataxia, hypodontia and hypomyelination, | + | |
| Hypomyelination and atrophy of basal ganglia and cerebellum | + | |
Non syndromic CA or CHA associated to apparently “pure” cerebellar atrophy are clinical entities in which the genetic background has been defined in only very few known diseases.8,13 Currently a specific diagnosis is mostly available in syndromic ICA of childhood such as ataxia-telangectasia syndrome, autosomal dominant ataxia type 2, CDG syndrome for which some clinical and laboratory markers are known.
Following the participation of some of us to a preliminary collaborative study that measured the levels of Coenzyme Q10 in muscle in a series of patients affected by ICA with onset in childhood of unknown cause,14 after exclusion of known causes of ICA we decided to systematically carry out a muscle biopsy in patients with non syndromic CA or CHA associated to “pure” cerebellar atrophy in order to verify the impact of the muscle biopsy examination in the diagnosis of this group of disorders.
In our series of patients, muscle biopsy led to a definitive genetic diagnosis in two patients (5.5%) out of 34. One patient had clinical features of CA (patient A) while the second patient (patient B) was consistent with the diagnosis of non syndromic CHA.
In patient A with clinical featuires of CA the muscle biopsy was useful to formulate an early diagnosis of Marinesco-Sjogren syndrome (MSS). This sporadic patient had an unusual presentation of MSS lacking cardinal features of congenital or early-onset cataracts and normal CK levels in blood. Although cataracts is considered as a pathognomonic sign of MSS, it may seldom appear later in the course of the disease.15 Our patients is now 4-years-old and does not show any sign of bilateral cataracts. Muscular involvement has been reported since early descriptions of MSS16,17 and myopathic changes with proliferation of autophagic vacuoles at muscle biopsy is a constant feature of MSS carrying SIL1 mutations.18,19 In our patients typical muscle changes were detected as early as 2 years of age and were pivotal for addressing the diagnosis. The homozygous R111X change found in our patient has already been reported as recurrent mutation in the mediterranean population and southern Italy.20
Moreover, with the systematic application of a muscle biopsy to patients with undefined childhood ataxias with cerebellar atrophy we were also able to genetically detect a primary defect of COQ10 deficiency with a homozygous mutation in ADK3 in a sporadic CHA patient (CHA987). This homozygous nonsense mutation (c.1042C > T, p.R348X) has been recently detected in an informative Dutch family21 only after linkage analysis. The COQ10 levels in muscle were very low (3.69 μg/g) in this patient besides a normal appearance of light microscopy and ultrastructural morphology.
Supplementation of Coenzyme Q10 has improved and probably stabilized ataxia in this patient but from serial neuroimaging we did not observe any reversal of cerebellar atrophy. We have no explanation for this phenomenon at the moment also because pathogenesis of cerebellar atrophy in this disorder is currently not known. Additional serial MRIs are in program to monitor possible improvement of cerebellar atrophy in this patient. From our experience we can conclude that CHA due to mutations in ADK3 is a very rare condition because we have detected only one patient out of 20 with undetermined CHA. Once again a patient with a ADK3 mutation has the clinical pattern of a CHA rather that CA confirming the same clinical presentation that has been reported so far in this condition.22,23 Thus a clinical clue to suspect children with ataxia harboring mutations in the ADK3 gene is that ataxia does not have a congenital onset and conceivably occurs in the CHA category. Currently, patients with ICA and primary defect of COQ10 deficiency with mutations in ADK3 can be suspected by determining levels of CoQ10 in a muscle biopsy or fibroblasts or can be assumed by clinical and neuroimaging associated signs, because no other clues are avaliable. The determination of CoQ10 levels in the muscle biopsy and or in cultured fibroblasts is a rapid procedure rather that measuring CoQ10 levels of CoQ10 biogenesis in skin fibroblasts that warrants a highly skilled laboratory.24 A needle biopsy may also be sufficient to measure levels in Coenzyme Q10 in muscle reducing the more invasive open biopsy.
Moreover we detected 8 additional patients with a CA or CHA phenotype that showed significant reduction of COQ10 together with a reduction of complex II + III in the mitochondrial respiratory chain enzyme activity only in some patients. Nonetheless these patients did not show any mutations in the known disease genes (COQ2, ADCK3, PDSS1 and PDSS2, COQ9) and in additional 6 genes (COQ3, COQ4, COQ5, COQ6, COQ7, ADCK2) encoding for COQ10 biosynthetic proteins. The COQ10 muscle levels of these patients were clearly low, although most of them had values above 10 μg/g (Table 2) and complex II + III enzyme activity was within normal ranges in some. We found COQ10 muscle levels around 12 μg/g in two patients with genetically confirmed mutations in COQ225 so we cannot exclude that some of these patients may be affected by a primary COQ10 biosynthetic defect carrying mutations in genes that have not been characterized so far. These 8 patients had Coenzyme Q10 supplementation for several years and we did not observe any improvement of ataxia that has remained stable, similarly to the sub-group of our patients series that had normal levels of Coenzyme Q10 in muscle. Patients with CA or CHA and significative reduction of Coenzyme Q10 in muscle were also reported in the first description associating heterogeneous forms of ataxic syndromes with a COQ10 deficiency in muscle.14 This sub-group of patients with significative reduction of Coenzyme Q10 in muscle but without showing mutations in known genes of Coenzyme Q10 biogenesis mostly have clinical presentation of a CA rather than CHA. The muscle biopsy of all patients with significative reduction of Coenzyme Q10 showed no morphological clue abnormalities, including our patient CHA987 with ADK3 mutations, although proliferation of lysosomes and autophagic vacuoles have been reported in the fibroblasts of one patient.25 In contrast, muscle morphological abnormalities have been reported in some myopathic forms of secondary CoQ10 deficiency with ETFDH deficiency26 or other encephalomyopathic forms in which the genetic basis are unknown27–29 showing mitochondrial proliferation and lipid storage. Moreover we have described a reduction of SDH staining in the muscle biopsies of patients with CoQ10 defciency in muscle and mutations in COQ2.11
Finally, it has been described that some patients with ataxia may have low levels of COQ10 in muscle but this may not be related to a primary COQ10 biosynthetic defect as has been shown in conditions such as patients harboring aprataxin mutations.30
In conclusion in our series of patients with ICA, muscle biopsy led to genetic diagnosis in two patients (5.5%) and gave helpful indications for therapeutic advise in additional 8 patients that were treated with CoQ10 supplementation. Following these studies, we think that muscle biopsy is a valuable diagnostic approach and should be considered in the panel of investigations to enhance diagnostic chances in children with early-onset ataxia or genetically undiagnosed ataxia associated to cerebellar atrophy, after excluding other known conditions. ADK3 mutations should be suspected in ICA patients with a clinical presentation of CHA rather that CA. In these patients there is markedly reduced levels of CoQ10 in muscle (at least under 10 μg/g in our series) and in fibroblasts as already reported.31,32 In addition the finding of a relative reduction of COQ10 in muscle (levels between 10 and 20 μg/g) in patients which are negative for ADK3 mutations offers a possible clue for subgrouping conditions of CA and CHA of undetermined cause. However, increasing knowledge on the underlying genetic cause of these latter conditions is necessary to define whether supgrouping ICA patients with the finding of a relative reduction of CoQ10 in the muscle biopsy is a useful procedure for any preliminary diagnostic approach.
Acknowledgments
This research was supported in part by grants from the Italian Ministry of Health, and the Telethon Foundation Onlus (Project GGP10225B on Autophagy to EB and GGP08145 on Joubert syndrome to EB, EMV and GZ).
References
- 1.Finsterer J. Ataxias with autosomal, X-chromosomal or maternal inheritance. Can J Neurol Sci. 2009;36:409–412. doi: 10.1017/s0317167100007733. [DOI] [PubMed] [Google Scholar]
- 2.Harding A.E. Clinical features and classification of inherited ataxias. In: Harding A.E., editor. Hereditary ataxias and related disorders. Churchill-Livingstone; Edinburgh: 1984. Adv Neurol. 1993; 61: 1–14. [Google Scholar]
- 3.Koenig M. Rare forms of autosomal recessive neurodegenerative ataxia. Semin Pediatr Neurol. 2003;10:183–192. doi: 10.1016/s1071-9091(03)00027-5. [DOI] [PubMed] [Google Scholar]
- 4.De Michele G., Coppola G., Cocozza S., Filla A. A pathogenetic classification of hereditary ataxias: is the time ripe? J Neurol. 2004;251:913–922. doi: 10.1007/s00415-004-0484-2. [DOI] [PubMed] [Google Scholar]
- 5.Palau F., Espinós Autosomal recessive cerebellar ataxias. Orphanet J Rare Dis. 2006;17(1):47. doi: 10.1186/1750-1172-1-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boddaert N., Desguerre I., Bahi-Buisson N., Romano S., Valayannopoulos V., Saillour Y., Seidenwurm D. Posterior fossa imaging in 158 children with ataxia. J Neuroradiol. 2010;37:220–230. doi: 10.1016/j.neurad.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Boltshauser E. Cerebellar hypoplasias. Handb Clin Neurol. 2007;87:115–127. doi: 10.1016/S0072-9752(07)87008-4. [DOI] [PubMed] [Google Scholar]
- 8.Poretti A., Wolf N.I., Boltshauser E. Differential diagnosis of cerebellar atrophy in childhood. Eur J Paediatr Neurol. 2008;12:155–167. doi: 10.1016/j.ejpn.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Zheng X.X., Shoffner J.M., Voljavec A.S., Wallace D.C. Evaluation of procedures for assaying oxidative phosphorylation enzyme activities in mitochondrial myopathy muscle biopsies. Review Biochim Biophys Acta. 1990;1019:1–10. doi: 10.1016/0005-2728(90)90118-n. [DOI] [PubMed] [Google Scholar]
- 10.Pastore A., Giovamberardino G.D., Bertini E., Tozzi G., Gaeta L.M., Federici G., Piemonte F. Simultaneous determination of ubiquinol and ubiquinone in skeletal muscle of pediatric patients. Anal Biochem. 2005;342:352–355. doi: 10.1016/j.ab.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Diomedi-Camassei F., Di Giandomenico S., Santorelli F.M., Caridi G., Piemonte F., Montini G. COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J Am Soc Nephrol. 2007;18:2773–2780. doi: 10.1681/ASN.2006080833. [DOI] [PubMed] [Google Scholar]
- 12.García-Cazorla A., Wolf N.I., Serrano M., Pérez-Dueñas B., Pineda M., Campistol J. Inborn errors of metabolism and motor disturbances in children. J Inherit Metab Dis. 2009;32:618–629. doi: 10.1007/s10545-009-1194-9. [DOI] [PubMed] [Google Scholar]
- 13.Zanni G., Bertini E., Bellcross C., Nedelec B., Froyen G., Neuhäuser G. X-linked congenital ataxia: a new locus maps to Xq25-q27.1. Am J Med Genet A. 2008;146A:593–600. doi: 10.1002/ajmg.a.32186. Institut Cochin, Université Paris Descartes, CNRS (UMR 8104) Paris, France. [DOI] [PubMed] [Google Scholar]
- 14.Lamperti C., Naini A., Hirano M., De Vivo D.C., Bertini E., Servidei S. Cerebellar ataxia and coenzyme Q10 deficiency. Neurology. 2003;60:1206–1208. doi: 10.1212/01.wnl.0000055089.39373.fc. [DOI] [PubMed] [Google Scholar]
- 15.Takahata T., Yamada K., Yamada Y., Ono S., Kinoshita A., Matsuzaka T. Novel mutations in the SIL1 gene in a Japanese pedigree with the Marinesco-Sjögren syndrome. J Hum Genet. 2010;55:142–146. doi: 10.1038/jhg.2009.141. [DOI] [PubMed] [Google Scholar]
- 16.Herva R., von Wendt L., von Wendt G., Saukkonen A.L., Leisti J., Dubowitz V. A syndrome with juvenile cataract, cerebellar atrophy, mental retardation and myopathy. Neuropediatrics. 1987;18:164–169. doi: 10.1055/s-2008-1052473. [DOI] [PubMed] [Google Scholar]
- 17.Goto Y., Komiyama A., Tanabe Y., Katafuchi Y., Ohtaki E., Nonaka I. Myopathy in Marinesco-Sjögren syndrome: an ultrastructural study. Acta Neuropathol. 1990;80:123–128. doi: 10.1007/BF00308914. [DOI] [PubMed] [Google Scholar]
- 18.Senderek J., Krieger M., Stendel C., Bergmann C., Moser M., Breitbach-Faller N. Mutations in SIL1 cause Marinesco-Sjögren syndrome, a cerebellar ataxia with cataract and myopathy. Nat Genet. 2005;37:1312–1314. doi: 10.1038/ng1678. [DOI] [PubMed] [Google Scholar]
- 19.Mahjneh I., Anttonen A.K., Somer M., Paetau A., Lehesjoki A.E., Somer H. Myopathy is a prominent feature in Marinesco-Sjögren syndrome: a muscle computed tomography study. J Neurol. 2006;253:301–306. doi: 10.1007/s00415-005-0983-9. [DOI] [PubMed] [Google Scholar]
- 20.Annesi G., Aguglia U., Tarantino P., Annesi F., De Marco E.V., Civitelli D. SIL1 and SARA2 mutations in Marinesco-Sjögren and chylomicron retention diseases. Clin Genet. 2007;71:288–289. doi: 10.1111/j.1399-0004.2007.00759.x. [DOI] [PubMed] [Google Scholar]
- 21.Gerards M., van den Bosch B., Calis C., Schoonderwoerd K., van Engelen K. Nonsense mutations in CABC1/ADCK3 cause progressive cerebellar ataxia and atrophy. Mitochondrion. 2010;10:510–515. doi: 10.1016/j.mito.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Lagier-Tourenne C., Tazir M., López L.C., Quinzii C.M., Assoum M., Drouot N. ADCK3, an ancestral kinase, is mutated in a form of recessive ataxia associated with coenzyme Q10 deficiency. Am J Hum Genet. 2008;82:661–672. doi: 10.1016/j.ajhg.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mollet J., Delahodde A., Serre V., Chretien D., Schlemmer D., Lombes A. CABC1 gene mutations cause ubiquinone deficiency with cerebellar ataxia and seizures. Am J Hum Genet. 2008;82:623–630. doi: 10.1016/j.ajhg.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinzii C., Naini A., Salviati L., Trevisson E., Navas P., Dimauro S. A mutation in para-hydroxybenzoate-polyprenyl transferase (COQ2) causes primary coenzyme Q10 deficiency. Am J Hum Genet. 2006 Feb;78:345–349. doi: 10.1086/500092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodríguez-Hernández A., Cordero M.D., Salviati L., Artuch R., Pineda M., Briones P. Coenzyme Q deficiency triggers mitochondria degradation by mitophagy. Autophagy. 2009;5:19–32. doi: 10.4161/auto.5.1.7174. [DOI] [PubMed] [Google Scholar]
- 26.Gempel K., Topaloglu H., Talim B., Schneiderat P., Schoser B.G., Hans V.H. The myopathic form of coenzyme Q10 deficiency is caused by mutations in the electron-transferring-flavoprotein dehydrogenase (ETFDH) gene. Brain. 2007;130:2037–2044. doi: 10.1093/brain/awm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Giovanni S., Mirabella M., Spinazzola A., Crociani P., Silvestri G., Broccolini A. Coenzyme Q10 reverses pathological phenotype and reduces apoptosis in familial CoQ10 deficiency. Neurology. 2001;57:515–518. doi: 10.1212/wnl.57.3.515. [DOI] [PubMed] [Google Scholar]
- 28.Gironi M., Lamperti C., Nemni R., Moggio M., Comi G., Guerini F.R. Late-onset cerebellar ataxia with hypogonadism and muscle coenzyme Q10 deficiency. Neurology. 2004;62:818–820. doi: 10.1212/01.wnl.0000113719.67643.b7. [DOI] [PubMed] [Google Scholar]
- 29.Sobreira C., Hirano M., Shanske S., Keller R.K., Haller R.G., Davidson E. Mitochondrial encephalomyopathy with coenzyme Q10 deficiency. Neurology. 1997;48:1238–1243. doi: 10.1212/wnl.48.5.1238. [DOI] [PubMed] [Google Scholar]
- 30.Quinzii C.M., Kattah A.G., Naini A., Akman H.O., Mootha V.K., DiMauro S. Coenzyme Q deficiency and cerebellar ataxia associated with an aprataxin mutation. Neurology. 2005;64:539–541. doi: 10.1212/01.WNL.0000150588.75281.58. [DOI] [PubMed] [Google Scholar]
- 31.DiMauro S., Quinzii C.M., Hirano M. Mutations in coenzyme Q10 biosynthetic genes. J Clin Invest. 2007;117:587–589. doi: 10.1172/JCI31423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinzii C.M., López L.C., Naini A., DiMauro S., Hirano M. Human CoQ10 deficiencies. Biofactors. 2008;32:113–118. doi: 10.1002/biof.5520320113. [DOI] [PMC free article] [PubMed] [Google Scholar]