Graphical abstract
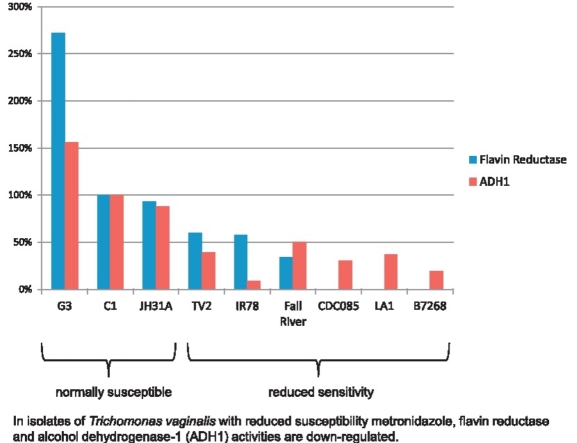
In isolates of Trichomonas vaginalis with reduced susceptibility metronidazole, flavin reductase and alcohol dehydrogenase-1 (ADH1) activities are down-regulated.
Highlights
► In clinical isolates of Trichomonas vaginalis with reduced metronidazole susceptibility flavin reductase is down-regulated. ► In clinical isolates of T. vaginalis with reduced metronidazole susceptibility alcohol dehydrogenase-1 (ADH1) is down-regulated. ► Thioredoxin reductase levels are not changed in metronidazole-resistant T. vaginalis clinical isolates.
Keywords: Trichomonosis, Metronidazole resistance, Thiordoxin reductase, Flavin reductase, Alcohol dehydrogenase 1
Abstract
The microaerophilic parasite Trichomonas vaginalis is a causative agent of painful vaginitis or urethritis, termed trichomoniasis, and can also cause preterm delivery or stillbirth. Treatment of trichomoniasis is almost exclusively based on the nitroimidazole drugs metronidazole and tinidazole. Metronidazole resistance in T. vaginalis does occur and is often associated with treatment failure. In most cases, metronidazole-resistant isolates remain susceptible to tinidazole, but cross resistance between the two closely related drugs can be a problem.
In this study we measured activities of thioredoxin reductase and flavin reductase in four metronidazole-susceptible and five metronidazole-resistant isolates. These enzyme activities had been previously found to be downregulated in T. vaginalis with high-level metronidazole resistance induced in the laboratory. Further, we aimed at identifying factors causing metronidazole resistance and compared the protein expression profiles of all nine isolates by application of two-dimensional gel electrophoresis (2DE).
Thioredoxin reductase activity was nearly equal in all strains assayed but flavin reductase activity was clearly down-regulated, or even absent, in metronidazole-resistant strains. Since flavin reductase has been shown to reduce oxygen to hydrogen peroxide, its down-regulation could significantly contribute to the impairment of oxygen scavenging as reported by others for metronidazole-resistant strains. Analysis by 2DE revealed down-regulation of alcohol dehydrogenase 1 (ADH1) in strains with reduced sensitivity to metronidazole, an enzyme that could be involved in detoxification of intracellular acetaldehyde.
1. Introduction
The microaerophilic protist parasite Trichomonas vaginalis is a causative agent of vaginits and urethritis, and ranks as an important human pathogen because of its very high occurrence [1–3] and possible complications, such as preterm-deliveries and stillbirth [reviewed in 3]. Treatment of T. vaginalis is practically exclusively based on metronidazole and other 5-nitroimidazole drugs such as tinidazole [reviewed in 3]. This group of drugs is highly effective against most microorganisms with microaerophilic or anaerobic metabolism and highly reliable. In case of T. vaginalis, however, the first strains refractory to metronidazole treatment were isolated very soon [4] after introduction of the drug in 1959 [5]. At first, it was not possible to demonstrate a correlation between treatment failure and resistance because resistance in clinical isolates only becomes manifest in the presence of oxygen [6]. A clear, although imperfect, correlation between in vitro susceptibility to metronidazole and treatment outcome, was demonstrated several years later [7]. The occurrence of metronidazole-resistant T. vaginalis varies strongly in different parts of the world, with only 2–5% of treated cases in the United States [8] and as much as 17% in Papua New Guinea [9]. Fortunately, most infections with metronidazole-resistant T. vaginalis can be successfully treated with tinidazole [8,10] but cross-resistance remains a concern [11].
Clinical resistance to metronidazole in T. vaginalis, also termed aerobic resistance, is fundamentally different from high-level metronidazole resistance induced in the laboratory. Laboratory-induced resistance is also termed anaerobic resistance, because it manifests itself also in the absence of oxygen and is the result of a loss of drug activating pathways which reduce the prodrug metronidazole to toxic intermediates [reviewed in 12]. Our recent results [13,14] suggest that a severe impairment of flavin-linked pathways, i.e. loss of thioredoxin reductase and flavin reductase activities, and depletion of intracellular free flavin concentrations might cause anaerobic resistance. Aerobic metronidazole resistance, however, seems to be caused by elevated intracellular oxygen concentrations due to a lowered oxygen scavenging capacity [15,16]. Oxygen interferes with activation of nitroimidazoles by either inhibiting drug activating pathways [as hypothesized in 12] or by re-oxidizing a critical toxic intermediate, the nitroradical anion [16]. This leads to a strongly reduced uptake of metronidazole in resistant isolates [17]. Interestingly, aerobic resistance can also be induced in the laboratory and has even been suggested to be an intermediate stage in the development of anaerobic resistance [18,19]. In contrast, anaerobic resistance does not practically occur in clinical isolates, with only one exceptional isolate known, BRIS/92/STDL/B7268 [20]. As compared to the very high levels of resistance in strains with laboratory induced resistance (300 μg/ml metronidazole and more), however, this strain displays only modest resistance (around 10 μg/ml).
Physiologically, metronidazole-resistant clinical isolates differ from normal T. vaginalis strains in several aspects. They display strongly increased glucose consumption rates [21], produce higher amounts of lactate but smaller amounts of ethanol [21], and have diminished thiol reductase activity [22]. In addition, these strains are more susceptible to oxygen [23]. In contrast to anaerobically resistant strains, however, metronidazole-resistant clinical isolates have normally shaped hydrogenosomes [24] and fully active hydrogenosomal enzymatic pathways [17,21] although expression of ferredoxin has been reported to be down-regulated [25].
Despite a large body of data regarding clinical metronidazole resistance in T. vaginalis, its molecular background has remained so far elusive. It is also unknown, why some metronidazole-resistant isolates display cross resistance to tinidazole whereas others do not. Here, we conducted a study in which we compared thioredoxin reductase and flavin reductase activities in four susceptible and five resistant isolates as these two enzyme activities were identified to be minimal or absent in an anaerobically metronidazole-resistant cell line [13]. Flavin reductase had been originally described as “NADPH oxidase” and was found to be capable of reducing oxygen to hydrogen peroxide using FMN [26]. Therefore, we hypothesized that this enzyme is a possible candidate enzyme for being involved in clinical metronidazole resistance. Indeed, previous results by others [21] suggested this enzyme activity to be down-regulated in metronidazole-resistant clinical isolates. We also compared protein expression in all nine isolates by two-dimensional gel electrophoresis (2DE), in order to identify factors relevant not only for metronidazole resistance as such, but also for the variably pronounced cross-resistance to tinidazole.
2. Materials and methods
2.1. Strains
The T. vaginalis strains used in this study were C1:NIH (ATCC 30001), G3 (PRA-98), JH31A#4 (ATCC 30236), IR78 (ATCC 50138), CDC085 (ATCC 50143), Fall River, LA/03/CDC/1, BRIS/92/STDL/B7268, and TV2. Strains G3, JH31A#4, and CDC085 were purchased from LGC standards. Strains LA/03/CDC/1 and BRIS/92/STDL/B7268 were a gift from Melissa Conrad and Jane Carlton (New York University Langone Medical Center, USA), strains TV2 and IR78 were a gift from Julia Walochnik (Medical University of Vienna, Austria), and strain Fall River was a gift from Jan Tachezy (Charles University, Prague, Czech Republic). Strain C1:NIH had been in our possession before start of the study [13,14]. For the sake of simplicity, the strains C1:NIH, LA/03/CDC/1 and BRIS/92/STDL/B7268 are referred to as C1, LA1, and B7268, respectively, throughout the text. Further details on the strains are given in Table 1.
Table 1.
List of T. vaginalis strains used in this study and minimum lethal concentrations of metronidazole and tinidazole under aerobic conditions. The short designations are indicated in bold.
2.2. Culture
Trichomonas vaginalis was grown in trypticase, yeast extract, maltose (TYM) medium [27]. Ascorbate was always omitted but the medium was supplemented with 25 μg/ml ammonium iron (III) citrate if not indicated otherwise. Cultures were routinely grown in 40 ml culture polystyrene culture flasks (BD Biosciences). Trypticase was purchased from BD Biosciences, and yeast extract and ammonium iron (III) citrate were purchased from Merck chemicals.
2.3. Determination of sensitivities of strains to metronidazole and tinidazole
Sensitivity assays were performed in 96-well microtiter plates (TPP) under aerobic conditions. Parasites were exposed to 1, 2, 4, 16, 32, 64, 128, and 256 μg/ml of metronidazole or tinidazole. After placement of drug (solubilized in DMSO) into the wells, 300 μl/well of culture at a starting concentration of 100,000 cells/ml was added. The plates were sealed with Whatman paper in order to minimize evaporation of water and incubated at 37 °C for 48 h. After incubation, cell viability was evaluated by microscopy. The cells that had lost their motility were rated as non-viable. The minimal concentration of drug at which no viable parasites could be observed was defined as minimal lethal concentration (MLC). Assays were carried out in triplicates and repeated at least once. Untreated culture of the respective strain, incubated in the same plate, was used as a control.
2.4. Preparation of cell lysates for two-dimensional gel electrophoresis (2DE), 2DE, and image analysis
The preparation of T. vaginalis cell lysates for two-dimensional gel electrophoresis (2DE), 2DE (Bio-Rad), and image analysis with Melanie™ 2D-gel analysis software (GeneBio) was done as described before [28]. All gels in this study had a pI range of 5–8 and a density of 12.5% polyacrylamide.
2.5. Mass spectrometry
Spots of interest were cut from the 2DE gel, destained and digested with trypsin as described previously [29]. After extraction of tryptic peptides from the gel pieces the extract solution was dried in a vacuum centrifuge and taken up in 10 μl of 0.1% formic acid. Capillary Reversed Phase Liquid chromatography (LC–MS) was performed on a Dionex Ultimate 3000 (Dionex, Germany), using a ProteCol™ Capillary Column (SGE, Ringwood, Vic, Australia; 300 μm ID, 10 cm length) as described elsewhere [30]. Data Analysis software version 4 and Protein Scape 3.0 (Bruker Daltonics) were used for data processing, proteins were identified using Mascot server 2.3 searching against all eukaryotic proteins deposited on the NCBI database. Search parameters were as follows: protease: trypsin; allowed missed cleavages: 2; fixed modifications: carbamidomethylcystein; variable modifications: deamidation (NQ) and oxidation (M); MS and MS/MS tolerance: ±0.3 Da. Protein hits with a Mascot Score greater than 40 were considered for further evaluation, with a minimum individual peptide score greater than 20. At least two peptides had to be identified per protein and MS/MS spectra were furthermore re-evaluated manually to ensure identification.
2.6. Enzymatic assays with hydrogenosome-free cell homogenates of T. vaginalis
Fully grown cultures (40 ml) were harvested by centrifugation at 800 × g for 5 min. Pellets were washed once in 20 ml 1× PBS followed by another round of centrifugation at 800 × g for 5 min. For measurements of thioredoxin reductase and flavin reductase activities, pellets were resuspended in 500 μl of 100 mM Tris/Cl, pH 7.5. Cells were lysed with 25 strokes in a Dounce homogenizer. Cell debris and large organelles were removed by centrifugation at 20,000 × g for 10 min. Protein concentrations of lysates were measured using the Bradford assay (Bio-Rad). Thioredoxin and flavin reductase activities were exactly measured as described before [13]. When preparing homogenates for measurements of ADH1 activity, cell pellets were resuspended in 500 μl of 100 mM potassium phosphate buffer, pH 6.25. Otherwise, the same protocol was applied as described above for thioredoxin reductase and flavin reductase. ADH1 activity of the cell homogenate was measured as the NADPH-dependent reduction of acetaldehyde. The reaction in a buffer containing 1 mM acetaldehyde, 100 mM potassium phosphate, pH 6.25, was started by adding cell homogenate (10 μg protein/ml assay buffer) and NADPH to 200 μM final concentration. The decrease of NADPH was measured photometrically at λ = 340 nm (Δɛ340 = 6.2 mM−1 cm−1). NADPH oxidation measured in the absence of acetaldehyde was interpreted as unspecific background oxidation of NADPH and subtracted from values measured with substrate.
3. Results
3.1. Choice of strains and sensitivity testing to metronidazole and tinidazole
In total, nine T. vaginalis strains (Table 1) were selected for a comparative analysis. Of these, one (C1) had already been shown to be susceptible to metronidazole, another three had been isolated from patients successfully treated with metronidazole (G3, JH31#4, TV2), and five were known to be metronidazole-resistant and had been isolated from patients refractory to metronidazole treatment (IR78, Fall River, CDC085, LA1, and B7268). The sensitivities of all strains against metronidazole and tinidazole were determined under aerobic conditions. The concentration at which no more motile parasites could be observed after 48 h was defined as the minimum lethal concentration (MLC). In case of metronidazole, we could confirm previous results of others with strains C1, IR78, Fall River, CDC085, LA1, and B7268 (Table 1). Interestingly, IR78 and Fall River were much more affected by higher concentrations of the drug than the other three metronidazole-resistant isolates, with only a few intact and motile cells remaining. However, due to our definition of the MLC, i.e. the concentration of drug at which no motile cells can be detected, both strains have nominally high levels of resistance. Of the three strains tested for the first time, at least to the best of our knowledge, G3 and JH31A#4 proved to be highly susceptible to metronidazole, whereas TV2 displayed borderline resistance which lies near serum concentrations of metronidazole in treated patients [31].
Tinidazole was far more effective than metronidazole with only two metronidazole-resistant strains (CDC085, LA1) giving an equally high MLC for tinidazole as for metronidazole (Table 1). In case of LA1, this had been already demonstrated before [11]. In case of CDC085, however, our measured MLC for tinidazole was clearly higher than previously determined [11]. B7268 and IR78 were roughly one order of magnitude more susceptible to tinidazole as compared to metronidazole, and Fall River was hardly resistant to tinidazole at all. Also the mildly metronidazole-resistant isolate TV2 perished at the lowest tinidazole concentration used (1 μg/ml).
3.2. Reduced sensitivity to metronidazole in clinical isolates is associated with diminished flavin reductase activity but not with diminished thioredoxin reductase activity
Previously, we had identified thioredoxin reductase and flavin reductase activities to be strongly diminished or even absent in a C1 cell line with laboratory-induced high level resistance to metronidazole [13]. Thioredoxin reductase was shown to be normally expressed but almost inactive due to the lack of the FAD cofactor. Since thioredoxin reductase is capable of reducing nitroimidazoles to toxic intermediates [13] we argued that an impairment of the flavin-linked metabolism, and thereby loss of thioredoxin and flavin reductase activities, is the cause of anaerobic metronidazole resistance. In order to evaluate whether similar changes are associated with clinical metronidazole resistance, thioredoxin and flavin reductase activities were measured in all nine isolates.
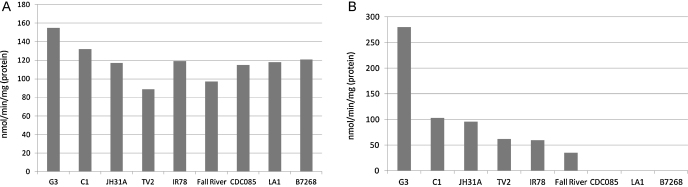
Thioredoxin reductase activity was similar in all nine isolates and no tendency in metronidazole-susceptible isolates towards stronger thioredoxin reductase activity could be observed (Fig. 1A). In contrast, flavin reductase activity was clearly decreased in all isolates with lowered sensitivity to metronidazole (Fig. 1B), and even absent in the three strains which display the highest level of metronidazole resistance, i.e. CDC085, LA1, and B7268 (Table 1). Flavin reductase activity in IR78 and CDC085 had already been found by others to be lower than in a metronidazole-susceptible isolate [21]. The measured values in the previous publication were congruent, although higher, than those measured here, with CDC085 retaining remnant flavin reductase activity (about 10% of the susceptible isolate). This can be probably ascribed to a much higher concentration of FMN applied in the assay (100 μM vs. 10 μM). The values of thioredoxin and flavin reductase activities in numbers and with standard error of the mean are given in Supplementary Table 1.
Fig. 1.
Activities thioredoxin reductase (A) and flavin reductase (B) in T. vaginalis homogenates. Values were determined in at least three independent experiments.
3.3. Comparative 2DE analysis of metronidazole-sensitive and–resistant strains
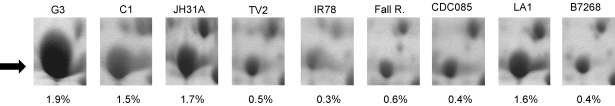
In the search for other factors contributing to clinical metronidazole resistance, we performed 2DE with all nine strains and searched for proteins which are consistently differentially expressed in metronidazole-resistant isolates as compared to normally sensitive isolates. Unfortunately, the obtained 2D profiles were rather divergent, thereby reflecting the genetic diversity within the species [32]. This circumstance made it difficult to identify relevant proteins which were differentially expressed in sensitive and resistant isolates. However, one protein was found to be clearly expressed more weakly in four metronidazole-resistant strains (IR78, CDC085, Fall River, B7268) and the mildly resistant strain TV2, than in the three highly metronidazole-susceptible isolates C1, G3, and JH31A#4 (Fig. 2). This protein spot was isolated from a 2D-gel of C1 cell extract and identified by mass spectrometric analysis (Supplementary Figure 1) as alcohol dehydrogenase-1 (ADH1). Although three homologous enzymes with this predicted function are encoded in the T. vaginalis genome (XP_001580601, XP_001314162, and XP_001316031), only one homologue located to the isolated protein spot (XP_001580601). This type of alcohol dehydrogenases is NADP-dependent and utilizes zinc as cofactor for the conversion of secondary alcohols and aldehydes or ketones. The homologous enzymes in Entamoeba histolytica [33] and Tritrichomonas foetus [34] have already been characterized and were shown to exert these activities. As observed before in E. histolytica [33,35], ADH1 is one of the most strongly expressed proteins in the cell, in case of metronidazole-susceptible T. vaginalis, 1.5–2% of the total protein content visualized by 2DE (Fig. 2).
Fig. 2.
Expression of ADH1 as visualized by 2DE. The position of ADH1 is indicated by the arrow. Intensities of the spots are given below the gel images as percentage of total protein visualized.
3.4. Reduced sensitivity to metronidazole correlates to diminished ADH1 activity
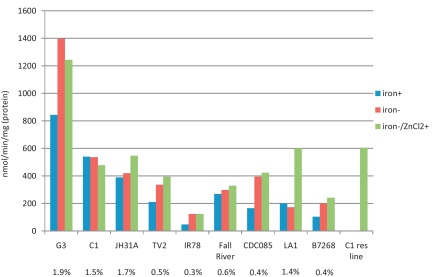
NADPH-dependent reduction of acetaldehyde was measured in all nine isolates in order to confirm that diminished expression of ADH1 also results in reduced enzyme activity (Fig. 3). Measurements were performed with homogenates from cells grown either in the presence or absence of supplemented iron in the growth medium. This was done because iron is known to substantially affect the activities of several metabolic enzymes in T. vaginalis [14,36,37]. In general, the measured rates of acetaldehyde reduction corresponded well to expression levels of ADH1 in the respective isolates (Fig. 3). A concentration of 1 mM acetaldehyde was used in the experiments which is close to the Km of approximately 700 μM, as determined with purified recombinant ADH1 (manuscript in preparation). An obvious exception was LA1 which displays a high expression level of ADH1 but, nevertheless, only slowly reduces acetaldehyde (Fig. 3). Omission of supplemented iron from the growth medium had a marked effect on acetaldehyde reduction only in four of the isolates tested, G3, TV2, CDC085, and B7268 (Fig. 3). In case of CDC085 and B7268, acetaldehyde reduction rates were approximately doubled. Densitometric analysis of 2D-gels from CDC085 cultures, grown with and without supplemented iron, revealed up-regulation of ADH1 in the absence of added iron; i.e. 1.2% of total protein visualized in the absence of supplemented iron (gel not shown) as compared to 0.4% in iron-supplemented medium (Fig. 2). However, this effect was not observed in B7268 (gel not shown).
Fig. 3.
ADH1 activity in T. vaginalis homogenates. The first bar (iron+) represents the acetaldehyde reduction rate in homogenates from cells grown in the presence of supplemented iron. The second and third bars represent the acetaldehyde production rate in cells grown without supplemented iron, either without (iron−) or with (iron−/ZnCl2+) 0.5 mM ZnCl2 added to the homogenates prior to the assay. Values were determined in at least two independent experiments. For comparison, the rate of expression of ADH1 is given as percentage of total protein visualized by 2DE (Fig. 2) below the bars.
It was puzzling that isolate LA1, in contrast to all other isolates, did not show any correlation between ADH1 expression level and acetaldehyde reduction rate (Fig. 3). We speculated that insufficient intracellular concentrations of zinc could result in low ADH1 enzyme activity despite normal expression levels of the enzyme. Indeed, when 0.5 mM ZnCl2 were added to LA1 homogenate prior to the acetaldehyde reduction assay, ADH1 activity increased to a level which was similar to that of C1 (Fig. 3). Tellingly, the expression level of ADH1 is practically equally high in C1 as in LA1 (Fig. 2). Addition of ZnCl2 to the homogenates of all other strains had a much smaller effect, if any (Fig. 3). However, when we performed the assay with cell homogenate from our highly metronidazole-resistant C1 cell line, displaying anaerobic, i.e. laboratory-induced resistance [13], we again observed a similar effect as with LA1 (Fig. 3). In the absence of ZnCl2, no reduction of acetaldehyde was measured. After addition of 0.5 mM ZnCl2, the rate of acetaldehyde reduction was very similar to that of the normally metronidazole-sensitive parent, C1 (Fig. 3). As observed in LA1, ADH1 remains normally expressed in the highly metronidazole-resistant C1 cell line (data not shown). The values of ADH1 activity in numbers and with standard error of the mean are given in Supplementary Table 2.
4. Discussion
In this study we performed a comparative analysis with four metronidazole-susceptible and five metronidazole-resistant T. vaginalis isolates (Table 1) in order to identify factors involved in clinical metronidazole resistance, also termed aerobic resistance. Further, we aimed at elucidating the differences between metronidazole-resistant strains that display cross resistance to tinidazole and those which do not, or only imperfectly. The parameters studied, i.e. thioredoxin reductase and flavin reductase activities, and overall protein expression, allowed differentiation between metronidazole-sensitive and – resistant strains by activity of flavin reductase and by expression and activity of ADH1. Both activities were down-regulated in metronidazole-resistant isolates.
Our results show that thioredoxin reductase has no role in clinical metronidazole resistance, not even in the isolate which shows low level anaerobic resistance to metronidazole, B7268. Activity of the enzyme was similar in all nine strains tested which is consistent with the notion that clinical resistance is not caused by a loss of drug activating pathways, as observed in anaerobic resistance [reviewed in 12]. This is likely to apply also for B7268, as indicated by its low level of resistance to tinidazole, because the nitroimidazole activating pathways known in T. vaginalis, i.e. ferredoxin-coupled reduction and thioredoxin reductase, reduce tinidazole with similar efficiency as metronidazole [13,38]. Accordingly, anaerobically metronidazole-resistant T. vaginalis which lack both pathways, are also highly resistant to other nitroimidazoles, including tinidazole (own unpublished results). The observed down-regulation of flavin reductase activity in strains with reduced sensitivity to metronidazole, however, is likely to have an important role in the establishment of clinical metronidazole resistance. Importantly, flavin reductase activity was absent in those three strains (Fig. 1B) that displayed the most strongly pronounced resistance to metronidazole, CDC085, LA1, and B7268 (Table 1), and was clearly diminished in the two other resistant isolates, IR78 and Fall River (Fig. 1B). Flavin reductase had been originally designated as “NADPH oxidase” and was shown to reduce oxygen to hydrogen peroxide, using free FMN as a cofactor [26]. It is, therefore, plausible that diminished flavin reductase activity leads to impaired oxygen scavenging. Another oxygen scavenging enzyme, NADH oxidase [26,39], has also been described in T. vaginalis. However, NADH oxidase is normally expressed in metronidazole-resistant isolates but almost absent in the highly susceptible strain C1 [17]. A role of NADH oxidase in metronidazole resistance is, therefore, highly unlikely. In contrast, diminished or even absent flavin reductase activity has not only been observed with both types of metronidazole-resistance in T. vaginalis [13,21, this study], but also with laboratory-induced metronidazole resistance in G. lamblia [40,41]. Consequently, it seems justified to define down-regulation of flavin reductase activity as a hallmark event of metronidazole resistance. Arguably, this is an early event in the establishment of metronidazole resistance as already the mildly resistant strain TV2 displays lowered flavin reductase activity (Table 1B). It is even possible that down-regulation of flavin reductase is a prerequisite for the loss of thioredoxin reductase activity, as observed in our anaerobically metronidazole-resistant C1 line. NADPH-dependent consumption of oxygen, i.e. flavin reductase activity, was identified as a major source of hydrogen peroxide in T. vaginalis [42]. Since the thioredoxin-dependent redox system is crucial for the removal of hydrogen peroxide [43–45], loss of thioredoxin reductase activity would probably be lethal unless flavin reductase be down-regulated or even deactivated. However, it is also important to note that decrease of flavin reductase activity and the degree of metronidazole resistance are not fully proportional as the mildly resistant isolate TV2 and the highly resistant isolate IR78 have similar flavin reductase levels (Fig. 1B). This suggests the existence of other, yet unidentified, factors that contribute to aerobic metronidazole resistance.
The comparison of the protein expression profiles of the nine selected strains was far less informative than expected. Only the expression of one enzyme, ADH1, could be reliably identified as down-regulated in metronidazole-resistant isolates. Differentiation between metronidazole-resistant isolates that are cross-resistant to tinidazole, and such which are not, was not possible. Arguably, in the pursuit of further nitroimidazole-related factors in the proteome, the rather high divergence between the protein profiles of the strains has to be allowed for by studying larger numbers of strains. Of course, also methodological constraints of 2DE, i.e. poor representation of very large, of weakly expressed, and of hydrophobic proteins, probably added to the failure of identifying any further factors.
Nevertheless, the 2DE approach allowed the establishment of ADH1 as a factor correlated to metronidazole resistance (Figs. 2 and 3). In isolates with reduced metronidazole sensitivity lower expression rates of ADH1 were observed (Fig. 2). Congruently, acetaldehyde reduction rates were also lower in these isolates (Fig. 3). One resistant isolate, however, LA1, displayed normal expression levels of ADH1 but strongly decreased activity due to an obvious lack of intracellular zinc, a cofactor of ADH1. In four of the strains, most strongly pronounced in the metronidazole-resistant isolates CDC085 and B7268, omission of iron from the growth medium resulted in higher acetaldehyde reduction rates. A comparison of ADH1 expression levels in CDC085, grown with and without supplemented iron, suggested that low concentrations of iron could lead to increased ADH1 expression.
A link between down-regulation of ADH1 and metronidazole resistance is not obvious. A direct role in the activation of metronidazole can be ruled out due to the low levels of this enzyme in strain TV2 (Fig. 2) which is only mildly resistant to metronidazole (Table 1). In addition, all metronidazole-resistant clinical isolates, with the exception of B7268 [20], are normally susceptible to metronidazole under anaerobic conditions, indicating that drug activating pathways are intact. There is also no indication that a metabolic enzyme like ADH1 could be involved in oxygen scavenging. Interestingly, however, down-regulation of ADH1 could be responsible for the reduced production of ethanol in metronidazole-resistant isolates as compared to susceptible isolates [21]. Ethanol is only a minor end product of T. vaginalis metabolism and its source has been hitherto unknown. Based on the observations in this study, we propose that ADH1 acts as a detoxifying enzyme of intracellular acetaldehyde and that the ethanol produced by T. vaginalis is the reduction product of acetaldehyde and NADPH. The source of intracellular acetaldehyde could be pyruvate:ferredoxin oxidoreductase (PFOR). The homologous enzyme in Pyrococcus furiosus was shown to produce acetaldehyde by decarboxylation of pyruvate under anaerobic conditions as a side product, in addition to the normal oxidation product acetyl-CoA [46]. In the presence of oxygen, decarboxylation of pyruvate does not occur. A similar scenario in T. vaginalis would be consistent with the observation that ethanol is never formed in the presence of oxygen [21]. As metronidazole-resistant strains have impaired oxygen scavenging mechanisms [15,16], it is possible that acetaldehyde is only rarely formed, rendering a detoxifying enzyme, i.e. ADH1, redundant, or at least, less important. Therefore, it is possible that down-regulation of ADH1 is an adaptation to elevated intracellular oxygen levels and, therefore, not causative for but rather a result of metronidazole resistance. In this context, it is interesting to note that in metronidazole-resistant T. foetus activity of NADP-dependent alcohol dehydrogenase remains unaltered [47]. Still, rather in contrast with this notion is the observation that the highly metronidazole-resistant strain LA1 expresses normal levels of ADH1 but reduces acetaldehyde very slowly due to a lack of intracellular zinc (Fig. 3). A similar observation was made with our anaerobically resistant C1 cell line (Fig. 3). Since it is hardly conceivable that T. vaginalis reduces zinc uptake only to down-regulate a redundant enzyme, more investigations on ADH1 and its possible role in metronidazole resistance are warranted.
Acknowledgements
This study was supported by project P22546 of the Austrian Science Fund (FWF). Daniel Kolarich was supported by an Erwin Schrödinger Fellowship from the Austrian Science Fund (grant J2661) and Macquarie University.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.molbiopara.2012.03.003.
Appendix A. Supplementary data
Measured values of thioredoxin reductase and flavin reductase activities in the nine strains studied. All measurements were done in at least three independent experiments. All values are given with the standard error of the mean.
ADH1 activity in homogenates of all nine T. vaginalis assayed. Cells were grown either with supplementation of iron (+iron) or without (−iron). When indicated (−iron, +ZnCl2), 0.5 mM ZnCl2 were added to homogenates before start of the assay. All measurements were done in at least two independent experiments, with the exception of the C1 highly metronidazole-resistant cell line (C1 res line) which was only measured once. All values are given with the standard error of the mean. ND: not determined.
References
- 1.Wendel K.A., Workowski K.A. Trichomoniasis: challenges to appropriate management. Clin Infect Dis. 2007;44(Suppl 3):123–129. doi: 10.1086/511425. [DOI] [PubMed] [Google Scholar]
- 2.Petrin D., Delgaty K., Bhatt R., Garber G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300–317. doi: 10.1128/cmr.11.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nanda N., Ross G.M., Kurdgelashvili G., Wendel K.A. Trichomoniasis and its treatment. Expert Rev Anti Infect Ther. 2006;4:125–135. doi: 10.1586/14787210.4.1.125. [DOI] [PubMed] [Google Scholar]
- 4.Robinson S.C. Trichomonal vaginitis resistant to metronidazole. Can Med Assoc J. 1962;86:665. [PMC free article] [PubMed] [Google Scholar]
- 5.Watt L., Jennison R.F. Clinical evaluation of metronidazole. Br Med J. 1960;2:902–905. doi: 10.1136/bmj.2.5203.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meingassner J.G., Thurner J. Strain of Trichomonas vaginalis resistant to metronidazole and other 5-nitroimidazoles. Antimicrob Agents Chemother. 1979;15:254–257. doi: 10.1128/aac.15.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müller M., Lossick J., Gorrell T. In vitro susceptibility of Trichomonas vaginalis to metronidazole and treatment outcome in vaginal trichomoniasis. Sex Transm Dis. 1988;15:17–24. doi: 10.1097/00007435-198801000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Schwebke J.R., Barrientes F.J. Prevalence of Trichomonas vaginalis isolates with resistance to metronidazole and tinidazole. Antimicrob Agents Chemother. 2006;50:4209–4210. doi: 10.1128/AAC.00814-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Upcroft J.A., Dunn L., Wal T. Metronidazole resistance in Trichomonas vaginalis from highland women in Papua New Guinea. Sex Health. 2009;6:334–338. doi: 10.1071/SH09011. [DOI] [PubMed] [Google Scholar]
- 10.Sobel J.D., Nyiresy P., Brown W. Tinidazole therapy for metronidazole-resistant vaginal trichomonosis. Clin Infect Dis. 2001;33:1341–1346. doi: 10.1086/323034. [DOI] [PubMed] [Google Scholar]
- 11.Goldman L.M., Upcroft J.A., Workowski K., Rapkin A. Treatment of metronidazole-resistant Trichomonas vaginalis. Sex Health. 2009;6:345–347. doi: 10.1071/SH09064. [DOI] [PubMed] [Google Scholar]
- 12.Kulda J. Trichomonads, hydrogenosomes and drug resistance. Int J Parasitol. 1999;29:199–212. doi: 10.1016/s0020-7519(98)00155-6. [DOI] [PubMed] [Google Scholar]
- 13.Leitsch D., Kolarich D., Binder M., Stadlmann J., Altmann F., Duchêne M. Trichomonas vaginalis: metronidazole and other nitroimidazole drugs are reduced by the flavin enzyme thioredoxin reductase and disrupt the cellular redox system. Implications for nitroimidazole toxicity and resistance. Mol Microbiol. 2009;72:518–536. doi: 10.1111/j.1365-2958.2009.06675.x. [DOI] [PubMed] [Google Scholar]
- 14.Leitsch D., Kolarich D., Duchêne M. The flavin inhibitor diphenyleneiodonium renders Trichomonas vaginalis resistant to metronidazole, inhibits thioredoxin reductase and flavin reductase, and shuts off hydrogenosomal enzymatic pathways. Mol Biochem Parasitol. 2010;171:17–24. doi: 10.1016/j.molbiopara.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd D., Pedersen J.Z. Metronidazole radical anion generation in vivo in Trichomonas vaginalis: oxygen quenching is enhanced in a drug-resistant strain. J Gen Microbiol. 1985;131:87–92. doi: 10.1099/00221287-131-1-87. [DOI] [PubMed] [Google Scholar]
- 16.Yarlett N., Yarlett N.C., Lloyd D. Metronidazole-resistant clinical isolates of Trichomonas vaginalis have lowered oxygen affinities. Mol Biochem Parasitol. 1986;19:111–116. doi: 10.1016/0166-6851(86)90115-5. [DOI] [PubMed] [Google Scholar]
- 17.Müller M., Gorrell T.E. Metabolism and metronidazole uptake in Trichomonas vaginalis isolates with different metronidazole susceptibilities. Antimicrob Agents Chemother. 1983;24:667–673. doi: 10.1128/aac.24.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tachezy J., Kulda J., Tomková E. Aerobic resistance of Trichomonas vaginalis to metronidazole induced in vitro. Parasitology. 1993;106:31–37. doi: 10.1017/s0031182000074783. [DOI] [PubMed] [Google Scholar]
- 19.Rasoloson D., Vanacova S., Tomkova E. Mechanisms of in vitro development of resistance to metronidazole in Trichomonas vaginalis. Microbiology. 2002;48:2467–2477. doi: 10.1099/00221287-148-8-2467. [DOI] [PubMed] [Google Scholar]
- 20.Upcroft P., Upcroft J.A. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin Microbiol Rev. 2001;14:150–164. doi: 10.1128/CMR.14.1.150-164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis J.E., Cole D., Lloyd D. Influence of oxygen on the fermentative metabolism of metronidazole-sensitive and resistant strains of Trichomonas vaginalis. Mol Biochem Parasitol. 1992;56:79–88. doi: 10.1016/0166-6851(92)90156-e. [DOI] [PubMed] [Google Scholar]
- 22.Ellis J.E., Yarlett N., Cole D., Humphreys M.J., Lloyd D. Antioxidant defences in the microaerophilic protozoan Trichomonas vaginalis: comparison of metronidazole-resistant and sensitive strains. Microbiology. 1994;140:2489–2494. doi: 10.1099/13500872-140-9-2489. [DOI] [PubMed] [Google Scholar]
- 23.Rasoloson D., Tomková E., Cammack R., Kulda J., Tachezy J. Metronidazole-resistant strains of Trichomonas vaginalis display increased susceptibility to oxygen. Parasitology. 2001;123:45–56. doi: 10.1017/s0031182001008022. [DOI] [PubMed] [Google Scholar]
- 24.Wright J.M., Webb R.I., O’Donoghue P., Upcroft P., Upcroft J.A. Hydrogenosomes of laboratory-induced metronidazole-resistant Trichomonas vaginalis lines are downsized while those from clinical metronidazole-resistant isolates are not. J Eukaryot Microbiol. 2010;57:171–176. doi: 10.1111/j.1550-7408.2009.00455.x. [DOI] [PubMed] [Google Scholar]
- 25.Quon D.V., d’Oliveira C.E., Johnson P.J. Reduced transcription of the ferredoxin gene in metronidazole-resistant Trichomonas vaginalis. Proc Natl Acad Sci USA. 1992;89:4402–4406. doi: 10.1073/pnas.89.10.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linstead D.J., Bradley S. The purification and properties of two soluble reduced nicotinamide:acceptor oxidoreductases from Trichomonas vaginalis. Mol Biochem Parasitol. 1988;27:125–133. doi: 10.1016/0166-6851(88)90032-1. [DOI] [PubMed] [Google Scholar]
- 27.Diamond L.S. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957;3:488–490. [PubMed] [Google Scholar]
- 28.Leitsch D., Köhsler M., Marchetti-Deschmann M. Major role for cysteine proteases during the early phase of Acanthamoeba castellanii encystment. Eukaryot Cell. 2010;9:611–618. doi: 10.1128/EC.00300-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolarich D., Weber A., Turecek P.L., Schwarz H.P., Altmann F. Comprehensive glyco-proteomic analysis of human alpha1-antitrypsin and its charge isoforms. Proteomics. 2006;6:3369–3380. doi: 10.1002/pmic.200500751. [DOI] [PubMed] [Google Scholar]
- 30.Deshpande N., Jensen P.H., Packer N.H., Kolarich D. GlycoSpectrumScan: fishing lycopeptides from MS spectra of protease digests of human colostrum sIgA. J Proteome Res. 2010;9:1063–1075. doi: 10.1021/pr900956x. [DOI] [PubMed] [Google Scholar]
- 31.Ralph E.D., Clarke J.T., Libke R.D., Luthy R.P., Kirby W.M. Pharmacokinetics of metronidazole as determined by bioassay. Antimicrob Agents Chemother. 1974;6:691–696. doi: 10.1128/aac.6.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conrad M., Zubacova Z., Dunn L.A. Microsatellite polymorphism in the sexually transmitted human pathogen Trichomonas vaginalis indicates a genetically diverse parasite. Mol Biochem Parasitol. 2011;175:30–38. doi: 10.1016/j.molbiopara.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar A., Shen P.S., Descoteaux S., Pohl J., Bailey G., Samuelson J. Cloning and expression of an NADP(+)-dependent alcohol dehydrogenase gene of Entamoeba histolytica. Proc Natl Acad Sci USA. 1992;89:10188–10192. doi: 10.1073/pnas.89.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleiner D.E., Johnston M. Purification and properties of a secondary alcohol dehydrogenase from the parasitic protozoan Tritrichomonas foetus. J Biol Chem. 1985;260:8038–8043. [PubMed] [Google Scholar]
- 35.Leitsch D., Radauer C., Paschinger K. Entamoeba histolytica: analysis of the trophozoite proteome by two-dimensional polyacrylamide gel electrophoresis. Exp Parasitol. 2005;110:191–195. doi: 10.1016/j.exppara.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Peterson K.M., Alderete J.F. Iron uptake and increased intracellular enzyme activity follow host lactoferrin binding by Trichomonas vaginalis receptors. J Exp Med. 1984;160:398–410. doi: 10.1084/jem.160.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorrell T.E. Effect of culture medium iron content on the biochemical composition and metabolism of Trichomonas vaginalis. J Bacteriol. 1985;161:1228–1230. doi: 10.1128/jb.161.3.1228-1230.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yarlett N., Gorell T.E., Marczak R., Müller M. Reduction of nitroimidazole derivatives by hydrogenosomal extracts of Trichomonas vaginalis. Mol Biochem Parasitol. 1985;14:29–40. doi: 10.1016/0166-6851(85)90103-3. [DOI] [PubMed] [Google Scholar]
- 39.Tanabe M. Trichomonas vaginalis: NADH oxidase activity. Exp Parasitol. 1979;48:143–145. doi: 10.1016/0014-4894(79)90063-8. [DOI] [PubMed] [Google Scholar]
- 40.Ellis J.E., Wingfield J.M., Cole D., Boreham P.F., Lloyd D. Oxygen affinities of metronidazole-resistant and -sensitive stocks of Giardia intestinalis. Int J Parasitol. 1993;23:35–39. doi: 10.1016/0020-7519(93)90095-g. [DOI] [PubMed] [Google Scholar]
- 41.Leitsch D., Burgess A.G., Dunn L.A. Pyruvate:ferredoxin oxidoreductase and thioredoxin reductase are involved in 5-nitroimidazole activation while flavin metabolism is linked to 5-nitroimidazole resistance in Giardia lamblia. J Antimicrob Chemother. 2011;66:1756–1766. doi: 10.1093/jac/dkr192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chapman A., Linstead D.J., Lloyd D. Hydrogen peroxide is a product of oxygen consumption by Trichomonas vaginalis. J Biosci. 1999;24:339–344. [Google Scholar]
- 43.Coombs G.H., Westrop G.D., Suchan P. The amitochondriate eukaryote Trichomonas vaginalis contains a divergent thioredoxin-linked peroxiredoxin antioxidant system. J Biol Chem. 2004;279:5249–5256. doi: 10.1074/jbc.M304359200. [DOI] [PubMed] [Google Scholar]
- 44.Stanley B.A., Sivakumaran V., Shi S. Thioredoxin reductase-2 is essential for keeping low levels of H(2)O(2) emission from isolated heart mitochondria. J Biol Chem. 2011;286:33669–33677. doi: 10.1074/jbc.M111.284612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lloyd D., Cortassa S., O’Rourke B., Aon M.A. What yeast and cardiomyocytes share: ultradian oscillatory redox mechanisms of cellular coherence and survival. Integr Biol. 2012;4:65–74. doi: 10.1039/c1ib00124h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma K., Hutchins A., Sung S.J., Adams M.W. Pyruvate ferredoxin oxidoreductase from the hyperthermophilic archaeon, Pyrococcus furiosus, functions as a CoA-dependent pyruvate decarboxylase. Proc Natl Acad Sci. 1997;94:9608–9613. doi: 10.1073/pnas.94.18.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cerkasovová A., Cerkasov J., Kulda J. Metabolic differences between metronidazole resistant and susceptible strains of Tritrichomonas foetus. Mol Biochem Parasitol. 1984;11:105–118. doi: 10.1016/0166-6851(84)90058-6. [DOI] [PubMed] [Google Scholar]
- 48.Müller M., Meingassner J., Miller W.A., Ledger W.J. Three metronidazole-resistant strains of Trichomonas vaginalis from the United States. Am J Obstet Gynecol. 1980;138:808–812. doi: 10.1016/s0002-9378(16)32741-7. [DOI] [PubMed] [Google Scholar]
- 49.Narcisi E.M., Secor W.E. In vitro effect of tinidazole and furazolidone on metronidazole-resistant Trichomonas vaginalis. Antimicrob Agents Chemother. 1996;40:1121–1125. doi: 10.1128/aac.40.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Measured values of thioredoxin reductase and flavin reductase activities in the nine strains studied. All measurements were done in at least three independent experiments. All values are given with the standard error of the mean.
ADH1 activity in homogenates of all nine T. vaginalis assayed. Cells were grown either with supplementation of iron (+iron) or without (−iron). When indicated (−iron, +ZnCl2), 0.5 mM ZnCl2 were added to homogenates before start of the assay. All measurements were done in at least two independent experiments, with the exception of the C1 highly metronidazole-resistant cell line (C1 res line) which was only measured once. All values are given with the standard error of the mean. ND: not determined.