Abstract
Imaging procedures are a mainstream tool in the daily ENT workflow. Cochlear Implant patients are representing a special population with specific demands for imaging. There are different imaging techniques available for pre-operative evaluation, surgery and postoperative controls with different indications and consequences. High-resolution computed tomography and magnetic resonance imaging are mainly used in the evaluation process. New procedures, as digital volume tomography, are increasingly used intra- and postoperatively. Especially the intracochlear positioning in malformations of the inner ear, eventually added with radiological assisted navigation, can be considered a standard of modern cochlear implant surgery. In addition, digital volume tomography may serve as a quality control tool focusing on the evaluation of the intracochlear electrode position. The range of applications, indications and current results are illustrated.
Keywords: cochlear implant, computed tomography, magnetic resonance imaging, digital volume tomography, malformation, navigation
1. Introduction
Electrical stimulation of the hearing nerve for auditory rehabilitation by cochlear implants is the standard therapy in congenital and acquired severe to profound deafness. Current developments are characterized by expanding indications for cochlear implant surgery, e. g. in residual hearing, single sided deafness [1], surgery in very young children following the successful introduction of newborn hearing screening, and geriatric patients due to demographic changes. According to the experience of the last 25 years, imaging procedures are of utmost importance. Computed tomography (CT) and magnet resonance imaging (MRI) reveal malformations of the inner ear in up to 20% of childhood patients [2], [3]. The improvement of surgical therapy by navigation and, in future, by robotics is based on the use of imaging procedures. This survey is supposed to give an overview on currently available and necessary imaging procedures for cochlear implant patients.
2. Basic considerations on imaging
Today radiologic and magnet resonance imaging procedures are available in the daily clinical use. Procedures are selected according to the goal of the examination and can be divided into preoperative, intraoperative, and postoperative imaging procedures.
3. Preoperative imaging
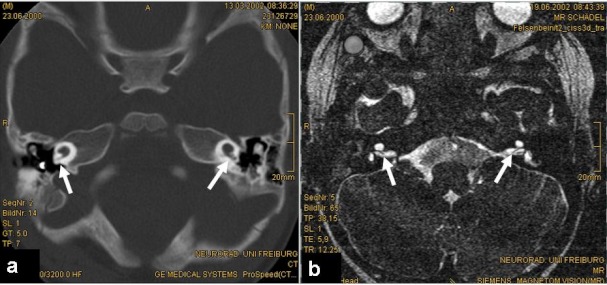
High resolution computed tomography (HRCT) and magnetic resonance imaging are regularly used for cochlear implant preoperative evaluation for the evaluation of inner ear malformations, surgical planning, and especially the imaging of the VIIIth nerve [2], [3], [4]. In children, these imaging procedures are especially important due to the high incidence of inner ear malformations. Evaluations at the University Clinic in Freiburg revealed malformations in up to 15% of computed tomographies in childhood cochlear implant candidates [3]. Malformations of the inner ear can be characterized by slight changes of the morphology (e. g. dysplasia of the vestibular organ, slight shortening of the cochlea) up to complex malformations of the complete temporal bone, e. g. CHARGE syndrome [5]. The features of surgical planning in complex malformations are discussed in the chapter Navigation. High resolution computed tomography is able to evaluate especially bony structures. An accurate analysis of the cochlear labyrinth is important for a precise surgical planning. The manufacturers of cochlear implant devices provide various electrodes (like short, long, preformed, straight, perimodiolar). After analyzing the malformation, the proper electrode has to be chosen by the surgeon. For example: In case that the analysis of the computed tomography reveals a hypoplastic cochlea (Figure 1 (Fig. 1)) with a total outer wall length of <20 mm, the surgeon has to choose the appropriate electrode.
Figure 1. HRCT of bilateral cochlear hypoplasia with a short small basal turn and cystic apex (a); total outer diameter is <20 mm; the VIIIth nerve is visible in MRI (arrows).
The use of high resolution computed tomography of the temporal bone in cochlear implant preoperative evaluation is currently discussed, as the identification of the VIIIth nerve by magnetic resonance imaging might be sufficient for the indication for surgery. However, imaging of the facial nerve with MRI is not sufficient today, and therefore the estimation of surgical risks or the accurate course of the facial nerve is difficult. Without a doubt, implant surgery should be performed by an experienced surgeon, but anyway, high resolution computed tomography is still the gold standard of preoperative diagnostics.
Magnetic resonance imaging allows the identification of the structures of the internal auditory canal (for example if aplasia of the VIIIth nerve is suspected; [4]) but also the imaging of central structures. With an increasing number of older patients receiving cochlear implants, we can expect an increasing number of patients suffering from central morbidities, for example acoustic neuroma [6], [7]. Consequently, therapeutic decisions have to be considered but also the necessity of further controls ideally performed with magnetic resonance imaging. In addition, the choice of the device has to be critically discussed as further MRI controls should be able to be performed without additional surgery [8].
4. Intraoperative radiological procedures
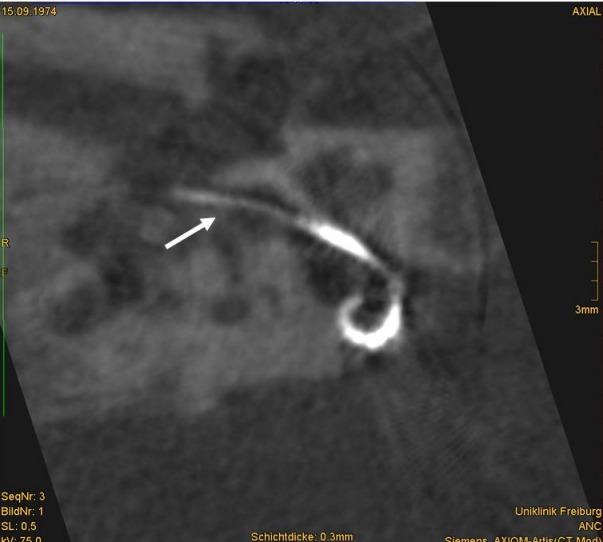
Until recently intraoperative imaging was limited to classical X-ray techniques (fluoros-copy). With expanding indications like CI in inner ear malformations, the intraoperative control of electrode positioning is required to exclude a misplaced insertion, for example into the internal auditory canal (Figure 2 (Fig. 2)), or to confirm correct intracochlear electrode placement [9]. High resolution CT and the newly developed digital volume tomography (DVT) are available for the intraoperative evaluation within the operation theatre. Mobile DVT or CT are cost effective and avoids additional surgical procedures as well as additional transport of the patient in general anaesthesia and as such reduce time and costs [10]. These imaging procedures are suitable especially for surgical centres with sufficient numbers of surgeries, thus the experience to perform such surgeries and evaluations.
Figure 2. Identification of the basal turn of the cochlea in postoperative DVT, cochleostomy (arrow) and insertion of the Contour Advance electrode array into the internal auditory meatus due to missing bony separation between cochlea and internal auditory meatus.
5. Navigation
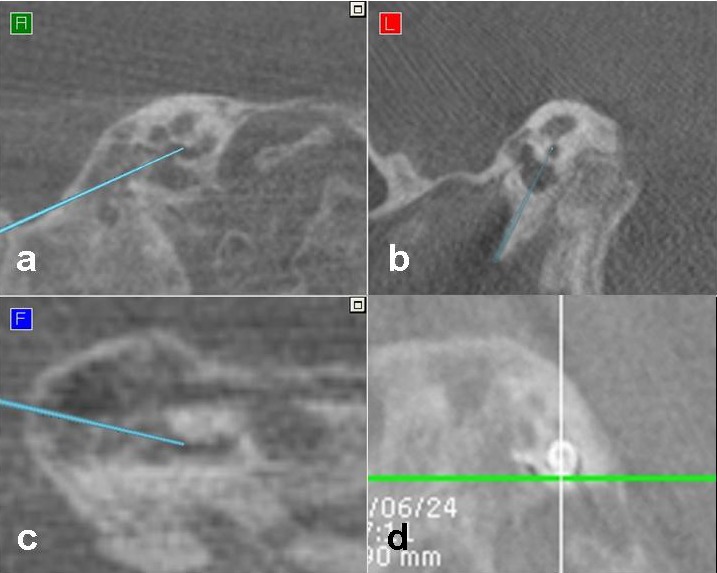
Neuronavigation in cochlear implant surgery is based on the use of imaging procedures. Typically HRCT (and MRI) are the basis for navigation. A new development is the combination of intraoperative imaging via DVT and navigation in the operation room [9]. Non-critical steps of the surgery (for example mastoidectomy) can be performed before using DVT to reduce the delay between the set-up of navigation and data acquisition. Critical surgical steps like the navigated cochleostomy in inner ear malformations (Figure 3 (Fig. 3)) are performed directly following the set-up of navigation. This is important as former evaluations revealed a correlation between quality of navigation and delay between set-up and data acquisition [11], [12]. By using mobile DVT, the insertion of the electrode array can then be performed under direct fluoroscopic control, or the result may be controlled by DVT or CT directly after insertion (Figure 3 (Fig. 3)). The use of robotics is currently under evaluation for atraumatic, minimal invasive cochlear implant surgery [13], [14], [15].
Figure 3. Intraoperative situation, (multiplanar reconstruction, a-c), performing navigated cochleostomy: the tip of the drill (arrow) is located within the cochlea and intraoperative control of electrode position (d).
6. Postoperative imaging procedures
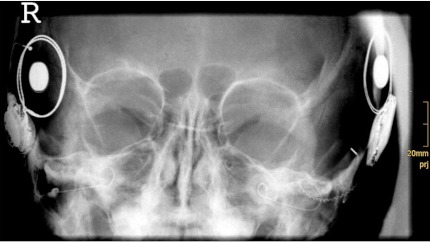
For postoperative evaluation HRCT, DVT, and MRI are available. The aim of postoperative evaluation is the documentation of the electrode placement, quality control of cochlear implant surgery, and the evaluation of the temporal bone in case of complications or additional central morbidities (e. g. acoustic neuroma, cerebral tumor, cerebral-vascular diseases, etc.). In children, a transorbital X-ray or cochlear view [16] is commonly used. Documentation of the electrode insertion by simple X-ray in the temporal bone (Figure 4 (Fig. 4)) allows later comparison of the electrode position even years after surgery. Due to the low radiation exposure by simple X-ray, the use of extensive postoperative radiological evaluation in childhood is not indicated and should only be used in case of malformations, additional surgeries or in complications.
Figure 4. Postoperative transorbital X-ray for documentation of inserted electrode following sequential bilateral implantation, right side: Nucleus Freedom with straight electrode, left side: Nucleus CI512 with Contour Advance electrode.
7. Basics of electrode evaluation with DVT
Evaluations of the electrode position in human temporal bones are essential for the use of DVT in human implantation [17], [18], [19], [20]. In these temporal bone trials, DVT after the insertion of the electrode array is followed by evaluation of the electrode position by DVT. Afterwards, the temporal bone is processed histologically using standard techniques [20]. DVT allows the reconstruction on any plane so that the histological slices can be matched to the identical plane in the DVT. Already in 1945, Wullstein [21] pointed out that any radiological technique will be able to demonstrate its quality when radiological and histological results are compared.
8. Comparison of HRCT and DVT in postoperative evaluation
The basis of DVT are isovolumetric data characterized by a very small metal artefact and a low radiation exposure [15], [22], [23], [24]. The weakness of DVT is its insufficient delineation of soft tissues. HRCT has probably a higher radiation exposure and a larger metal artefact. Human temporal bone trials could demonstrate the advantages of DVT especially due to the smaller metal artefact and voxel size (depending on manufacturer) of only 0.3 mm for evaluation of the intracochlear position of the electrode array.
In adults, the postoperative quality control can be performed with DVT [25], [26]. The interest of evaluation is the individual surgical result as well as the intracochlear electrode positioning. The surgical aim of an atraumatic insertion into the scala tympani is generally accepted today. On the one hand, the scala tympani is of larger size for the placement of electrodes, on the other hand, the preservation of residual hearing is only possible in scala tympani insertions. Own experience could demonstrate that the rate of scala vestibuli insertions is widely underestimated in cochleostomy approaches. Quality control of the electrode position in the human cochlea is supposed to reveal in which of the two scalae the electrode was inserted and also, if a dislocation from one scala to the other had occurred. These data are either underestimated or unknown, too. Moreover, quality control should answer the question if the electrode position has any influence on rehabilitation results following cochlear implant surgery. In our PORT study (postoperative rotation tomography) the postoperative control of the electrode position after adult cochlear implant surgery is performed routinely with DVT. DVT appears advantageous [17], [22], [24], [25], [26] and is available for all electrodes and manufacturers (Figure 5 (Fig. 5)). Lane et al. 2007 [27] evaluated the electrode position by comparing pre- and postoperative HRCT but were not able to perform an evaluation in up to 30%. Therefore this evaluation technique for postoperative control (with increased radiation exposure) does not seem appropriate for routine control. Similar results are available from Skinner et al. 2007 [28]. Transmodiolar reconstructions of HRCT pre- and postoperatively were compared – an extremely time-consuming procedure that will not become accepted in a daily routine.
Figure 5. Postoperative DVT following human implantation of different electrode arrays, right side: overview, left side: transmodiolar reconstruction, a: Hybrid L electrode, b: Contour Advance electrode, c: MedEl Flex Soft electrode, d: Advanced Bionics Helix electrode.
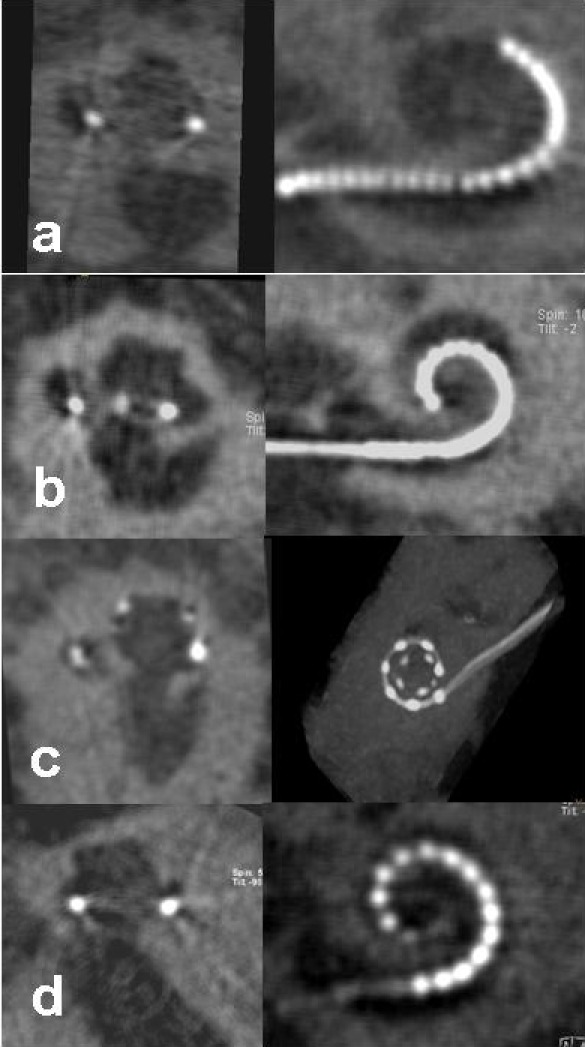
Evaluating the electrode position with the Contour electrode in a first group of patients [25] we found an unexpected amount of scala vestibuli insertions (62%). This can be assessed as poorer surgical quality at that time; in addition we found a dislocation rate from scala tympani to scala vestibuli of 71%; similarly this rate was not expected after the initial temporal bone trials [29], [30]. Following the optimization of the position of the cochleostomy (anterior and inferior of the round window) we observed a significant improvement of the surgical results [26] with an increase of the scala tympani insertion rate to 84%. Dislocations from scala tympani to scala vestibuli declined to 22%. The continuous radiological control and feed-back for the surgeons of insertion resulted in an improvement of the surgical procedure and also improved our understanding of the cochlear anatomy and the intraoperative behaviour of the electrode array. Only correct evaluation and apprehension of the cochlea anatomy with its rotation within the temporal bone and the course of the electrode insertion may explain the significant reduction of dislocations. In the following evaluations the Contour Advance electrode was inserted; improvement of the electrode tip may also have been critical for improvement of the dislocation rate. Also the control of the surgical results of individual surgeons could demonstrate that scala tympani insertion rates, learning curves, and dislocation rates are highly individual and improve over time.
For the first time a correlation between postoperative electrode position and results of rehabilitation could be identified [26]. Patients with scala tympani insertions and a short duration of deafness performed significantly better in typical German speech tests like Freiburg Numbers, Freiburg Monosyllables, and Oldenburg Sentence Test when compared to patients with scala vestibuli insertions and short duration of deafness. Finley et al. 2008 [31] were able to confirm our observations showing that results of rehabilitation are correlated to the number of electrode contacts within the scala tympani.
9. Postoperative MRI
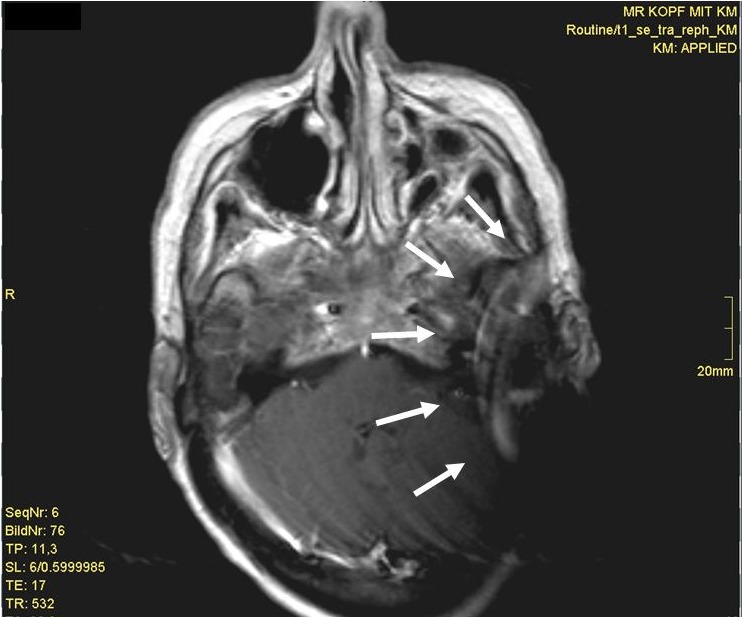
The use of postoperative MRI is a specific challenge. Additional surgical procedures may be necessary for the CI patient, e. g. for removement and replacement of the magnet, for additional revision surgery due to a dislocation of the internal magnet, or replacement of the internal magnet due to a loss of the magnet’s strength [8], [32]. Application of MRI depends on the implant type and can result in different sizes of the artefact (Figure 6 (Fig. 6)). In general, MRI evaluations are possible up to 1.5 tesla (with or without magnet, depending on recommendation of the manufacturer). In the future we expect an increasing number of postoperative MRI evaluations because of the demographic changes. The indication for performing a postoperative MRI could be complications or central morbidities. This will result in an increasing number of MRI evaluations with a working cochlear implant and in an increasing number of additional surgical procedures.
Figure 6. Postoperative MRI (T1 with contrast) and internal magnet in situ. The extensive artifact (arrow) obscures the internal auditory canal.
10. DVT for further diagnostic evaluations
Due to the CT-like quality with lower radiation exposure [22] DVT can be used as an alternative, for example if in later sequential cochlear implantation the initial CT scan is not available anymore. Further developments also indicate that DVT may be used for evaluation of the nasal sinuses and the middle ear as well as in maxillo-facial surgery [33], [34], [35]. Also the use of DVT in private practices is under discussion. Due to the improvement of quality DVT may be replace HRCT for diagnostics at least partially.
11. Imaging procedures outside the clinical routine
Highly specialized evaluation procedures such as SPECT, PET and fMRI are still not available within the clinical routine. The interest of these evaluations in cochlear implant patients is cortical plasticity [36], [37]. The activation of different parts of the central auditory pathway following electrostimulation may indicate the plasticity of different neural populations [38]. Giraud and Lee 2007 [39] used preoperative PET examinations and identified that specific central organisation structures might work as prognostic factors for results of rehabilitation in children. A critical point of PET and SPECT is the increased radiation exposure; thus the results are still limited to a small patient population.
12. Future perspectives
Imaging procedures in cochlear implant patients are an essential tool for pre-, intra- and postoperative diagnostics. Classic X-ray examinations, HRCT, MRI and DVT are available. DVT is characterized by its CT-like quality. We expect a broader use of this evaluation technique in the future. A fusion of DVT and MRI will be available and might improve the soft tissue information immensely.
Imaging procedures like PET, SPECT and fMRI for scientific evaluation will improve our knowledge of the central organisation and reorganisation of the auditory pathway. Navigation and robotic assistance at surgery will help to further improve surgical quality. Navigation and DVT assisted surgery will be performed mainly in complex malformations of the temporal bone.
Common goal of all imaging procedures in cochlear implant patients is the improvement of surgical planning and results, the control of surgical quality, and the reduction of complications.
References
- 1.Arndt S, Aschendorff A, Laszig R, Beck R, Schild C, Kröger S, Ihorst G, Wesarg T. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol Neurotol. 2011;32(1):39–47. doi: 10.1097/MAO.0b013e3181fcf271. Available from: http://dx.doi.org/10.1097/MAO.0b013e3181fcf271. [DOI] [PubMed] [Google Scholar]
- 2.Papsin BC. Cochlear implantation in children with anomalous cochleovestibular anatomy. Laryngoscope. 2005;115:1–26. doi: 10.1097/00005537-200501001-00001. Available from: http://dx.doi.org/10.1097/00005537-200501001-00001. [DOI] [PubMed] [Google Scholar]
- 3.Aschendorff A, Laszig R, Maier W, Beck R, Schild C, Birkenhäger R, Wesarg T, Kröger S, Arndt S. Kochlearimplantat bei Innenohrfehlbildungen. HNO. 2009;57:533–541. doi: 10.1007/s00106-009-1936-x. Available from: http://dx.doi.org/10.1007/s00106-009-1936-x. [DOI] [PubMed] [Google Scholar]
- 4.De Foer B, Kenis C, Van Melkebeke D, Vercruysse JP, Somers T, Pouillon M, Offeciers E, Casselman JW. Pathology of the vestibulocochlear nerve. Eur J Radiol. 2010;74:349–358. doi: 10.1016/j.ejrad.2009.06.033. Available from: http://dx.doi.org/10.1016/j.ejrad.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 5.Arndt S, Laszig R, Beck R, Schild C, Maier W, Birkenhäger R, Kroeger S, Wesarg T, Aschendorff A. Spectrum of hearing disorders and their management in children with CHARGE syndrome. Otol Neurotol. 2010;31:67–73. doi: 10.1097/MAO.0b013e3181c0e972. Available from: http://dx.doi.org/10.1097/MAO.0b013e3181c0e972. [DOI] [PubMed] [Google Scholar]
- 6.Trotter MI, Briggs RJ. Cochlear implantation in neurofibromatosis type 2 after radiation therapy. Otol Neurotol. 2010;31:216–219. doi: 10.1097/MAO.0b013e3181c348e7. Available from: http://dx.doi.org/10.1097/MAO.0b013e3181c348e7. [DOI] [PubMed] [Google Scholar]
- 7.Helbig S, Rader T, Bahmer A, Baumann U. A case of bilateral cochlear implantation in single-sided untreated acoustic neurinoma. Acta Otolaryngol. 2009;129:694–696. doi: 10.1080/00016480802527545. Available from: http://dx.doi.org/10.1080/00016480802527545. [DOI] [PubMed] [Google Scholar]
- 8.Crane BT, Gottschalk B, Kraut M, Aygun N, Niparko JK. Magnetic resonance imaging at 1.5 T after cochlear implantation. Otol Neurotol. 2010;31:1215–1220. doi: 10.1097/MAO.0b013e3181ec1d61. Available from: http://dx.doi.org/10.1097/MAO.0b013e3181ec1d61. [DOI] [PubMed] [Google Scholar]
- 9.Aschendorff A, Maier W, Jaekel K, Wesarg T, Arndt S, Laszig R, Voss P, Metzger M, Schulze D. Radiologically assisted navigation in cochlear implantation for X-linked deafness malformation. Cochlear Implants Int. 2009;10:14–18. doi: 10.1002/cii.379. Available from: http://dx.doi.org/10.1002/cii.379. [DOI] [PubMed] [Google Scholar]
- 10.Bischoff M, Hebecker A, Hartwig E, Gebhard F. Wirtschaftlichkeit der intraoperativen 3D-Bildgebung mit einem mobilen chirurgischen C-Bogen. Unfallchirurg. 2004;107:712–715. doi: 10.1007/s00113-004-0811-1. [DOI] [PubMed] [Google Scholar]
- 11.Schipper J, Aschendorff A, Arapakis I, Klenzner T, Teszler CB, Ridder GJ, Laszig R. Navigation as a quality management tool in cochlear implant surgery. J Laryngol Otol. 2004;118:764–770. doi: 10.1258/0022215042450643. Available from: http://dx.doi.org/10.1258/0022215042450643. [DOI] [PubMed] [Google Scholar]
- 12.Schipper J, Klenzner T, Aschendorff A, Arapakis I, Ridder GJ, Laszig R. Navigiert-kontrollierte Kochleostomie Ist eine Verbesserung der Ergebnisqualität in der Kochleaimplantatchirurgie möglich? HNO. 2004;52:329–335. doi: 10.1007/s00106-004-1057-5. Available from: http://dx.doi.org/10.1007/s00106-004-1057-5. [DOI] [PubMed] [Google Scholar]
- 13.Manrique MJ, Savall J, Cervera-Paz FJ, Rey J, Der C, Echeverria M, Ares M. Atraumatic surgical approach to the cochlea with a micromanipulator. Acta Otolaryngol. 2007;127:122–131. doi: 10.1080/00016480600827063. Available from: http://dx.doi.org/10.1080/00016480600827063. [DOI] [PubMed] [Google Scholar]
- 14.Klenzner T, Ngan CC, Knapp FB, Knoop H, Kromeier J, Aschendorff A, Papastathopoulos E, Raczkowsky J, Wörn H, Schipper J. New strategies for high precision surgery of the temporal bone using a robotic approach for cochlear implantation. Eur Arch Otorhinolaryngol. 2009;266:955–960. doi: 10.1007/s00405-008-0825-3. Available from: http://dx.doi.org/10.1007/s00405-008-0825-3. [DOI] [PubMed] [Google Scholar]
- 15.Majdani O, Rau TS, Baron S, Eilers H, Baier C, Heimann B, Ortmaier T, Bartling S, Lenarz T, Leinung M. A robot-guided minimally invasive approach for cochlear implant surgery: preliminary results of a temporal bone study. Int J Comput Assist Radiol Surg. 2009;4:475–486. doi: 10.1007/s11548-009-0360-8. Available from: http://dx.doi.org/10.1007/s11548-009-0360-8. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Xu SA, Cohen LT, Clark GM. Cochlear view: postoperative radiography for cochlear implantation. Am J Otol. 2000;21:49–56. [PubMed] [Google Scholar]
- 17.Husstedt HW, Aschendorff A, Richter B, Laszig R, Schumacher M. Nondestructive three-dimensional analysis of electrode to modiolus proximity. Otol Neurotol. 2002;23:49–52. doi: 10.1097/00129492-200201000-00012. Available from: http://dx.doi.org/10.1097/00129492-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Aschendorff A, Klenzner T, Richter B, Kubalek R, Nagursky H, Laszig R. Evaluation of the HiFocus electrode array with positioner in human temporal bones. J Laryngol Otol. 2003;117:527–531. doi: 10.1258/002221503322112932. Available from: http://dx.doi.org/10.1258/002221503322112932. [DOI] [PubMed] [Google Scholar]
- 19.Klenzner T, Richter B, Nagursky H, Schipper J, Laszig R, Aschendorff A. Evaluation des Insertionstraumas des Nucleus® Contour Advance™-Elektrodenträgers im humanen Felsenbeinmodell. Laryngo-Rhino-Otol. 2004;83:840–844. doi: 10.1055/s-2004-826067. Available from: http://dx.doi.org/10.1055/s-2004-826067. [DOI] [PubMed] [Google Scholar]
- 20.Richter B, Aschendorff A, Nagursky H, Schipper J, Laszig R, Klenzner T. Methodik der Evaluation perimodiolärer CI-Elektrodenträger im Felsenbeinmodell. Laryngo-Rhino-Otol. 2005;84:42–50. doi: 10.1055/s-2004-826002. Available from: http://dx.doi.org/10.1055/s-2004-826002. [DOI] [PubMed] [Google Scholar]
- 21.Wullstein H. Die Klinik der Labyrinthitis und Paralabyrinthitis auf Grund des Röntgenbefundes. Stuttgart: Georg Thieme Verlag; 1948. [Google Scholar]
- 22.Ruivo J, Mermuys K, Bacher K, Kuhweide R, Offeciers E, Casselman JW. Cone beam computed tomography, a low-dose imaging technique in the postoperative assessment of cochlear implantation. Otol Neurotol. 2009;30:299–303. doi: 10.1097/MAO.0b013e31819679f9. Available from: http://dx.doi.org/10.1097/MAO.0b013e31819679f9. [DOI] [PubMed] [Google Scholar]
- 23.Aschendorff A, Kubalek R, Hochmuth A, Bink A, Kurtz C, Lohnstein P, Klenzner T, Laszig R. Imaging procedures in cochlear implant patients – evaluation of different radiological techniques. Acta Otolaryngol. 2004;552:46–49. doi: 10.1080/03655230410017175. Available from: http://dx.doi.org/10.1080/03655230410017175. [DOI] [PubMed] [Google Scholar]
- 24.Kurzweg T, Dalchow CV, Bremke M, Majdani O, Kureck I, Knecht R, Werner JA, Teymoortash A. The value of digital volume tomography in assessing the position of cochlear implant arrays in temporal bone specimens. Ear Hear. 2010;31:413–419. doi: 10.1097/AUD.0b013e3181d3d6b6. Available from: http://dx.doi.org/10.1097/AUD.0b013e3181d3d6b6. [DOI] [PubMed] [Google Scholar]
- 25.Aschendorff A, Kubalek R, Turowski B, Zanella F, Hochmuth A, Schumacher M, Klenzner T, Laszig R. Quality control after cochlear implant surgery by means of rotational tomography. Otol Neurotol. 2005;26:34–37. doi: 10.1097/00129492-200501000-00007. Available from: http://dx.doi.org/10.1097/00129492-200501000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Aschendorff A, Kromeier J, Klenzner T, Laszig R. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear. 2007;28:75S–79S. doi: 10.1097/AUD.0b013e318031542e. Available from: http://dx.doi.org/10.1097/AUD.0b013e318031542e. [DOI] [PubMed] [Google Scholar]
- 27.Lane JI, Witte RJ, Driscoll CL, Shallop JK, Beatty CW, Primak AN. Evaluation Scalar localization of the electrode array after cochlear implantation: clinical experience using 64-slice multidetector computed tomography. Otol Neurotol. 2007;28:658–662. doi: 10.1097/MAO.0b013e3180686e26. Available from: http://dx.doi.org/10.1097/MAO.0b013e3180686e26. [DOI] [PubMed] [Google Scholar]
- 28.Skinner MW, Holden TA, Whiting BR, Voie AH, Brunsden B, Neely JG, Saxon EA, Hullar TE, Finley CC. In vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. Ann Otol Rhinol Laryngol. 2007;197:2–24. [PubMed] [Google Scholar]
- 29.Tykocinski M, Saunders E, Cohen LT, Treaba C, Briggs RJ, Gibson P, Clark GM, Cowan RS. The contour electrode array: safety study and initial patient trials of a new perimodiolar design. Otol Neurotol. 2001;22:33–41. doi: 10.1097/00129492-200101000-00007. Available from: http://dx.doi.org/10.1097/00129492-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Richter B, Aschendorff A, Lohnstein P, Husstedt H, Nagursky H, Laszig R. The Nucleus Contour electrode array: a radiological and histological study. Laryngoscope. 2001;111:508–514. doi: 10.1097/00005537-200103000-00023. Available from: http://dx.doi.org/10.1097/00005537-200103000-00023. [DOI] [PubMed] [Google Scholar]
- 31.Finley CC, Holden TA, Holden LK, Whiting BR, Chole RA, Neely GJ, Hullar TE, Skinner MW. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol. 2008;29:920–928. doi: 10.1097/MAO.0b013e318184f492. Available from: http://dx.doi.org/10.1097/MAO.0b013e318184f492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deneuve S, Loundon N, Leboulanger N, Rouillon I, Garabedian EN. Cochlear implant magnet displacement during magnetic resonance imaging. Otol Neurotol. 2008;29:789–790. doi: 10.1097/MAO.0b013e3181825695. Available from: http://dx.doi.org/10.1097/MAO.0b013e3181825695. [DOI] [PubMed] [Google Scholar]
- 33.Offergeld Ch, Kromeier J, Aschendorff A, Maier W, Klenzner T, Beleites T, Zahnert T, Schipper J, Laszig R. Rotational tomography of the normal and reconstructed middle ear in temporal bones: an experimental study. Eur Arch Otorhinolaryngol. 2007;264:345–351. doi: 10.1007/s00405-006-0180-1. Available from: http://dx.doi.org/10.1007/s00405-006-0180-1. [DOI] [PubMed] [Google Scholar]
- 34.Arndt S, Kromeier J, Berlis A, Maier W, Laszig R, Aschendorff A. Imaging procedures after bone-anchored hearing aid implantation. Laryngoscope. 2007;117:1815–1818. doi: 10.1097/MLG.0b013e3180f62b5e. Available from: http://dx.doi.org/10.1097/MLG.0b013e3180f62b5e. [DOI] [PubMed] [Google Scholar]
- 35.Bremke M, Sesterhenn AM, Murthum T, Hail AA, Kadah BA, Bien S, Werner JA. Digital volume tomography (DVT) as a diagnostic modality of the anterior skull base. Acta Otolaryngol. 2008;31:1–9. doi: 10.1080/00016480802620621. Available from: http://dx.doi.org/10.1080/00016480802620621. [DOI] [PubMed] [Google Scholar]
- 36.Limb CJ, Molloy AT, Jiradejvong P, Braun AR. Auditory cortical activity during cochlear implant-mediated perception of spoken language, melody, and rhythm. J Assoc Res Otolaryngol. 2010;11:133–143. doi: 10.1007/s10162-009-0184-9. Available from: http://dx.doi.org/10.1007/s10162-009-0184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coez A, Zilbovicius M, Ferrary E, Bouccara D, Mosnier I, Ambert-Dahan E, Bizaguet E, Syrota A, Samson Y, Sterkers O. Cochlear implant benefits in deafness rehabilitation: PET study of temporal voice activations. J Nucl Med. 2008;49:60–67. doi: 10.2967/jnumed.107.044545. Available from: http://dx.doi.org/10.2967/jnumed.107.044545. [DOI] [PubMed] [Google Scholar]
- 38.Tobey EA, Devous MD, Sr, Buckley K, Cooper WB, Harris TS, Ringe W, Roland PS. Functional brain imaging as an objective measure of speech perception performance in adult cochlear implant users. Int J Audiol. 2004;43:52–56. [PubMed] [Google Scholar]
- 39.Giraud AL, Lee HJ. Predicting cochlear implant outcome from brain organisation in the deaf. Restor Neurol Neurosci. 2007;25:381–390. [PubMed] [Google Scholar]