Abstract
Objective
Abnormally elevated exercise blood pressure is associated with increased risk of cardiovascular disease. Aerobic exercise training has been shown to reduce exercise blood pressure. However, it is unknown if these improvements occur in a dose dependent manner. The purpose of the present study is to determine the effect of different doses of aerobic exercise training on exercise blood pressure in obese postmenopausal women.
Methods
Participants (n=404) were randomized to one of 4 groups: 4, 8, or 12 kilocalories per kilogram of energy expenditure per week (kcal/kg/week) or the non-exercise control group for 6 months. Exercise blood pressure was obtained during the 50 watts stage of a cycle ergometer maximal exercise test.
Results
There was a significant reduction in systolic blood pressure at 50 watts in the 4 kcal/kg/week (−10.9 mmHg, p< 0.001), 8 kcal/kg/week (−9.9 mmHg, p= 0.022), and 12 kcal/kg/week (−13.7 mmHg, p<0.001) compared to control (−4.2 mmHg). Only the highest exercise training dose significantly reduced diastolic blood pressure (−4.3 mmHg, p= 0.033) compared to control. Additionally, resting blood pressure was not altered following exercise training (p>0.05) compared to control, and was not associated with changes in exercise systolic (r=0.09, p=0.09) or diastolic (r=0.10, p=0.08) blood pressure.
Conclusions
Aerobic exercise training reduces exercise blood pressure and may be more modifiable than changes in resting blood pressure. A high dose of aerobic exercise is recommended to successfully reduce both exercise systolic and diastolic blood pressure, and therefore may attenuate the CVD risk associated with abnormally elevated exercise blood pressure.
Keywords: Postmenopausal, Exercise Training, Exercise Blood Pressure, Dose Response, Hypertension
Introduction
Abnormally elevated blood pressure during sub-maximal exercise is associated with future risk of cardiovascular disease (CVD)1–5. It has been suggested that exercise blood pressure may have prognostic value above and beyond resting blood pressure1, 2. This may be due to an inability to reduce peripheral arterial resistance appropriately in response to increased cardiac output during aerobic exercise 3, 5, increased vascular stiffness 5, and an exaggerated increase in sympathetic nervous system activity to a corresponding submaximal exercise intensity 3, 5. Thus, elevated exercise blood pressure may represent a more advanced stage of atherosclerosis or CVD risk profile 2, and has been associated with other CVD risk factors such as left ventricle hypertrophy 3, diabetes mellitus 5, hypertension 5 and dyslipidemia 5. This may in turn result in greater strain on the heart during submaximal activities of daily life. Thus, reducing exercise blood pressure may have clinical importance in sedentary postmenopausal women with elevated blood pressure who present with elevated CVD risk 6
Participation in sport 3 and aerobic exercise training 7, 8 has been shown to decrease exercise systolic (SBP) 7–12 and diastolic (DBP) 9, 10, 12 in a variety of populations including postmenopausal women. 9 This reduction of exercise blood pressure may be due to a reduction in catecholamines 13, increased fitness 10 and exercise tolerance 9 at the same absolute exercise intensity, and decreased peripheral resistance 10. However, it is unknown if improvements in SBP and DBP as a result of exercise training occur in a dose dependent manner. Considering the relationships between exercise blood pressure and CVD risk, it is important to determine if a larger amount (higher dose) of exercise training confers to a greater reduction in exercise blood pressure, as a low dose of exercise may be more attainable. Thus, the purpose of the present investigation is to evaluate the effect of three different doses of aerobic training on exercise blood pressure in postmenopausal women with elevated resting blood pressure from the Dose Response to Exercise in Women (DREW) trial.
Methods
Study Design and Participants
The full design and methodology for the DREW trial (www.clinicaltrials.gov, NCT00011193) have been published previously 14. In brief, the study was a randomized controlled trial evaluating the effects of aerobic exercise training at increasingly higher doses of energy expenditure in 464 sedentary, overweight or obese, postmenopausal women aged 45–75 years with elevated blood pressure. From this cohort, a subset of 404 participants had exercise blood pressure data at 50 W available at baseline and follow-up from their maximal exertion cycle ergometer tests. Written informed consent was obtained from all participants prior to enrollment. The research protocol was reviewed and approved annually by the Cooper Institute institutional review board, and subsequently approved by Pennington Biomedical Research Center for continued analysis.
Women recruited for this study were postmenopausal, overweight or obese (Body Mass Index (BMI) 25.0–43.0 kg/m2); were sedentary (no participation in greater than 20 minutes of exercise 3 times a week); had elevated resting SBP (120–159.0 mmHg); and were physically capable of participating in an exercise program. Exclusion criteria included cardiovascular disease, significant medical conditions, elevated LDL (≥ 3.36 mmol/L), or weight loss of 9.1 or more kg or more in the previous year 14.
Resting Blood Pressure and Heart Rate
Resting blood pressure was evaluated in the supine position following heart rate variability measurements (we will not report on heart rate variability data as it has been previously published 15). We obtained a minimum of four blood pressure measurements, which were taken 2 min apart using a Colin STBP-780 automated BP unit (Colin Medical Instruments, Plainfield, NJ). Resting heart rate was obtained prior to blood pressure measurements.
Fitness Testing
Fitness testing was performed using a Lode Excalibur Sport rate-independent cycle ergometer (Groningen, the Netherlands). Participants cycled at 30 W for 2 minutes, 50 W for 4 minutes, followed by increases of 20 W every 2 minutes until they could no longer maintain a pedal cadence of 50 revolutions per minute. Respiratory gases were measured using a Parvomedics Truemax 2400 Metabolic Measurement Cart (Sandy, UT). Volume and gas calibrations were conducted before each test. Gas-exchange variables (VO2, VCO2 production, minute ventilation, and respiratory exchange ratio [RER]) were collected breath-by-breath and averaged into 15-second intervals. Heart rate was measured directly from the electrocardiographic monitoring system (Q-Stress, Quinton Instruments Co., Bothel WA). Two fitness tests were performed on separate days at both baseline and follow-up, and the mean of the VO2 max obtained from these two tests were defined as cardio-respiratory fitness.
Exercise Blood Pressure at 50 W
Exercise blood pressure measurements were performed during the 50 W stage of the maximal exercise test, using a Colin STBP-780 automated BP unit. Although, the unit was automated, the reported values for exercise blood pressure were based on the audible Korotkoff sounds determined by an experienced exercise physiologist. The Collins STBP unit allows for headphones to be inserted into the unit, which allows the Korotkoff sounds to be heard via a microphone inside the blood pressure cuff. The appropriate blood pressure cuff size was used for all participants. The SBP and DBP at 50 W were determined from the average of the two fitness tests performed at baseline and follow-up. The reproducibility of exercise SBP and DBP were similar at baseline and at follow-up with an intra-class correlation coefficient (ICC) of 0.72 and 0.79 respectively. Similarly, the reproducibility of exercise DBP was similar at baseline (ICC=0.64) at follow-up (ICC=0.71). If a participant did not complete both exercise tests, at baseline or follow-up, the exercise blood pressure measurements from the single exercise test were used in the analysis. Approximately, 6.6% and 3.2% of participants in the present study had performed only one of the two exercise tests at baseline and follow-up respectively.
Rate Pressure Product
Rate pressure product (RPP) was calculated at baseline and at follow-up by multiplying heart rate at 50 W by SBP at 50 W.
Randomization
Following baseline testing, participants were randomized as described previously to the 4, 8, or 12 kcal/kg/week exercise groups or to the non-exercise control group 14. There were a total of 404 participants in the analysis with 90 participants randomized to the control group, 140 randomized to the 4 kcal/kg/week group, 82 randomized to the 8 kcal/kg/week, and 93 randomized to the 12 kcal/kg/week group. The over-recruitment of the 4 kcal/kg/week group was done a priori in the parent study 16 in order to better detect changes associated with dose response.
Exercise Training
We calculated the exercise energy expenditure for women in the DREW age range associated with meeting the consensus public health recommendations. Details of these calculations are presented in the DREW design and methods report 14. Exercising women participated in 3 or 4 training sessions each week for 6 months with training intensity at the heart rate associated with 50% of each woman’s peak VO2. During the first week, each group expended 4kcal/kg. Those assigned to that level continued to expend 4kcal/kg per week for 6 months. All of the other groups increased their energy expenditure by 1 kcal/kg a week until they reached the level required for their group. All exercise sessions were performed under observation and supervision in an exercise laboratory with strict monitoring of the amount of exercise completed in each session. Women in the exercise groups alternated training sessions between semi-recumbent cycle ergometers and treadmills. Women in the non-exercise control group were asked to maintain their baseline level of activity during the 6-month study period. The total adherence for participants in the exercise groups was 98.0%.
Blinding
There were distinct and separate intervention and assessment teams, and assessment staff members were blinded to the randomization of study participants.
Statistical Procedure
All statistical analyses were conducted using SAS version 9.1 (Cary, NC), with the exception of reproducibility analyses, which were performed with SPSS version 18.0 (Somers, NY). Descriptive data of groups were tabulated as means (SD), and were tested for differences between groups using a one-way ANOVA for continuous variables and a chi-square for categorical variables. Pearson correlations were used to evaluate the relationship between change in exercise blood pressure and change in VO2max, exercise heart rate, and resting blood pressure. An analysis of covariance with a Tukey-Kramer adjustment for multiple comparisons was used to evaluate the effect of exercise training on exercise blood pressure. The primary outcome of the present study was change in exercise blood pressure following exercise training. Results are presented as adjusted least-square means with 95% confidence intervals and were adjusted for age, baseline value, presence of blood pressure medications, and ethnicity. In addition, we performed multiple linear regression to determine the trend between exercise dose and change in exercise blood pressure at 50 W, resting blood pressure, heart rate at 50 W, RPP, and fitness with adjustments for age ethnicity, blood pressure and baseline value. Exercise dose was recoded as a linear variable for the purpose of this analysis. For all analyses, a p value of less than 0.05 was used to reject the null hypothesis.
We performed post-hoc analyses to compare the effect of exercise training in participants with an abnormally elevated exercise SBP at baseline. In a separate analysis, we evaluated the same relationship in participants with abnormally elevated exercise diastolic blood pressure at baseline. These individuals are at elevated risk for CVD 1–5, and thus have the greatest need for improvement. We defined an abnormally elevated exercise blood pressure at baseline as greater than the median value (SBP>194 mmHg) and DBP (>96 mmHg). An ANCOVA was performed with adjustments for age, baseline value, presence of blood pressure medications, and ethnicity with a Tukey-Kramer adjustment for multiple comparisons to test the effect of exercise training in these sub-groups.
In order to determine the frequency at which individuals without hypertension have abnormally elevated blood pressure, we tabulated the percentage of individuals with an abnormally elevated exercise blood pressure in individuals with pre-hypertension (Resting SBP: 120–139 mmHg and/or DBP 80–89 mmHg) and hypertension (Resting SBP ≥ 140 mmHg and/or DBP ≥90 mmHg) at baseline 17.
Results
Demographic data for the participants are presented in Table I. There were no significant differences between groups. The participants had a mean (SD) age of 57.2 (6.3) yrs; a mean weight of 84.2 (11.8) kg; and a mean BMI of 31.9 (5.1) kg/m2. The study sample was approximately 64% White, 30% Black, 6% Hispanic, 1% Asian and 0.3% were classified as other. The mean resting SBP was 139.2 mmHg (12.8) and mean resting DBP was 80.7 mmHg (8.5). Baseline mean exercise SBP and DBP at 50 W was 192.6 (19.9) and 94.4 (11.1) mmHg respectively. There were 28.0% of participants on anti-hypertensive medications, of which 18.9% were on diuretics, 8.7% on ace-inhibitors, 6.7% on calcium channel blockers, 2.2% on beta-blockers, and 0.5% on alpha antagonists. The adherence in the exercise group was 97.9%, 98.2% and 98.1% in the 4, 8, and 12 kcal/kg/week groups respectively.
Table 1.
Baseline participant characteristics.1
| Control | 4 kcal/kg/week | 8 kcal/kg/week | 12 kcal/kg/week | |
|---|---|---|---|---|
| N | 89 | 140 | 82 | 93 |
| Age (yr) | 57.0 (5.8) | 57.2 (6.5) | 56.6 (6.3) | 56.8 (6.4) |
| White (%) (%) | 66.3 | 60.7 | 58.5 | 69.9 |
| Black (%) | 23.6 | 32.9 | 34.1 | 25.8 |
| Hispanic (%) | 9.0 | 5.7 | 7.3 | 2.2 |
| Asian (%) | 1.1 | 0.7 | 0.0 | 1.08 |
| Other (%) | 0.0 | 0.0 | 0.0 | 1.0 |
| Weight (kg) | 83.7 (11.4) | 84.7 (12.3) | 83.5 (11.4) | 83.3 (11.4) |
| Waist circumference (cm) | 102.2 (12.0) | 100.1 (11.3) | 101.5 (11.4) | 99.5 (12.0) |
| BMI (kg/m2) | 32.0 (4.0) | 32.2 (6.9) | 32.2 (4.0) | 31.2 (3.6) |
| VO2 max (ml/kg/min) | 15.7 (2.9) | 15.4 (3.0) | 15.0 (2.4) | 16.0 (2.9) |
| Resting heart rate (beats per min.) | 64.8 (8.6) | 65.0 (7.7) | 66.7 (9.0) | 65.1 (7.2) |
| Resting SBP (mmHg) | 141.0 (11.4) | 138.4 (12.9) | 140.1 (13.7) | 138.0 (12.9) |
| Resting DBP (mmHg) | 80.7 (7.9) | 80.4 (8.7) | 81.1 (8.6) | 80.9 (8.7) |
| Heart rate at 50 W (beats per min.) | 123.9 (14.7) | 125.2 (13.3) | 127.7 (15.8) | 123.7 (13.6) |
| SBP at 50 W (mmHg) | 193.9 (19.2) | 193.1 (19.8) | 192.8 (21.3) | 190.2 (19.5) |
| DBP at 50 W (mmHg) | 93.4 (11.5) | 94.3 (10.0) | 94.7 (12.2) | 95.6 (9.7) |
| Rate pressure product at 50 W | 24,155.3 (4385.4) | 24,255.2 (4074.1) | 24,732 (4842.2) | 23,605.8 (4007.3) |
| Metabolic equivalents at 50 W | 3.09 (0.39) | 3.13 (0.36) | 3.08 (0.43) | 3.17 (0.38) |
Shown are the baseline characteristics of 404 postmenopausal women enrolled in the DREW trial. Continuous variables are presented as mean (SD). Categorical variables are expressed as (%). BMI: Body Mass Index, DBP: Diastolic blood
Change in Fitness
Participants had low fitness at baseline with a mean VO2 max of 15.6 (2.9) ml/kg/min. Similar to the findings of our main outcomes paper 16, there was a significant improvement in VO2 max following exercise training within the 4 (0.67 ml/kg/min, CI: 0.34, 0.93), 8 (1.32 ml/kg/min, CI: 0.93, 1.70), and 12 kcal/kg/week (1.67 ml/kg/min, CI: 1.30, 2.03) groups compared to control (−0.18, CI: −0.56, 0.18). In addition, there was a significant trend between change in VO2 max with exercise dose (p for trend< 0.001). Waist circumference was significantly reduced in the 4 (−2.7, CI: −4.1, −1.2) and 12 kcal/kg/week group (−2.7, CI: −4.4, −1.0). There were no significant reductions in weight following exercise training (p>0.05).
Change in Resting Blood Pressure Following Exercise Training
Following exercise training, there was no significant change in resting SBP in the 4 (0.6 mmHg, CI: −4.0, 0.78), 8 (−2.4 mmHg, CI: −1.29, 2.50), and 12 kcal/kg/week groups (−3.5, CI: −5.9, −1.16) compared to control (−1.59 mmHg, CI: −3.9, 0.79). Similarly, there was no significant change in resting DBP in the 4 (−0.83 mmHg, CI: −0.3, 2.0), 8 (−0.38 mmHG, CI: −1.86, 1.09) and 12 (−0.20, CI: −1.59, 1.19) kcal/kg/week groups following exercise training in compared to control (−0.48, CI: −1.89, 0.93). Analyses for trend between dose of exercise and resting blood pressure were not significant (p >0.05).
Effect of exercise training on Exercise Blood Pressure at 50 W
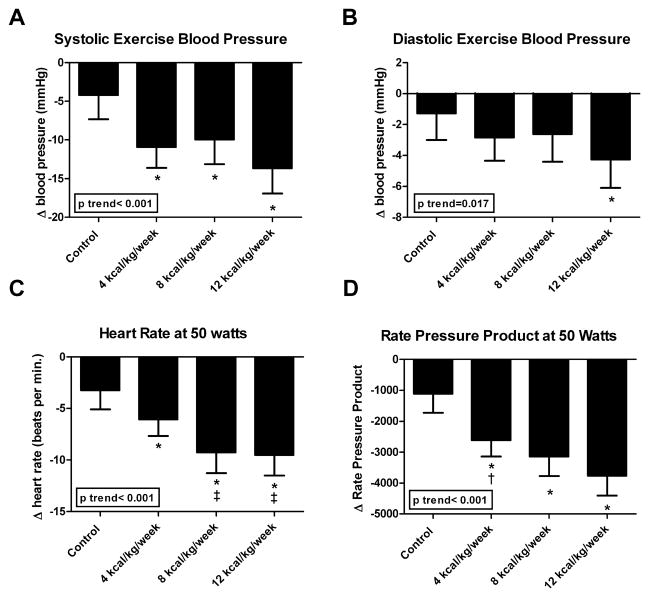
There was a significant reduction in exercise SBP (Figure 1A) at 50 W in the 4, 8 and 12 exercise groups compared with control (all p-values< 0.05). However, only the 12 kcal/kg/week significantly reduced DBP (Figure 1B) at 50 W (p= 0.026) compared with control. Analysis for trend revealed a significant improvement in SBP (p for trend <0.001) and DBP (p for trend=0.017) exercise blood pressure with increasing dose of exercise. The correlation between change in resting SBP and change in exercise SBP at 50 W was not significant (r=0.09, p=0.09). Similarly, the change in resting DBP was not significantly associated with change in exercise DBP pressure at 50 W (r=0.10, p=0.08). Change in exercise heart rate at 50 watts following exercise training was correlated with change in systolic (r=0.35, p<0.001) and diastolic (r=0.21, p<0.001) blood pressure at 50 watts. Change in VO2max was significantly correlated with change in systolic (r=−0.13, p=0.012), but not with diastolic blood pressure (r=−0.07, p=0.171) at 50 watts.
Figure 1.
The effect of different doses of exercise training on (A) exercise systolic blood pressure, (B) exercise diastolic blood pressure, (C) exercise heart rate, and (D) rate pressure product at 50 W. – Shown are the effects of different doses of exercise on exercise blood pressure, heart rate and rate pressure product at 50 watts. Results from ANCOVA and p-trend analyses are displayed.* Denotes significant difference compared to control. † Significantly different from 4 kcal/kg/week/group. Results are presented as adjusted least-square means with 95% confidence intervals
Changes in heart rate at 50 W and RPP
Heart rate (Figure 1C) at 50 W and RPP (Figure 1D) was significantly reduced in all three exercise groups compared to control (all p-values < 0.05) following exercise training. There was a significant trend between dose of exercise and heart rate at 50 watt (p<0.001) and RPP (p<0.001).
Change in exercise blood pressure in participants with elevated exercise blood pressure
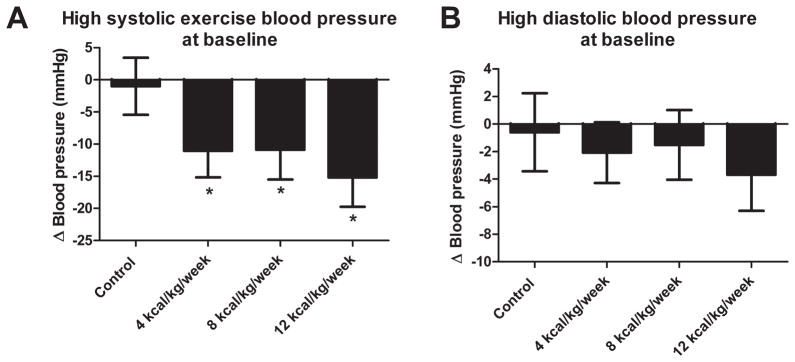
There were 199 participants with an abnormally elevated exercise SBP at baseline with a mean (SD) SBP at 50 W of 209.3 (10.7) mmHg and mean DBP of 97.4 mmHg. There were 93, 66, 42 and 46 participants in the 4, 8 12 kcal/kg/week and control groups respectively. As shown in figure 2A, we observed a significant reduction in exercise blood pressure in the 4 (−11.0 mmHg, CI: −15.16, −6.93) 8 (−10.9 mmHg, CI: −15.50, −6.29) and 12 kcal/kg/week (−15.2 mmHg, CI: −19.77, −10.65) compared to control participants with elevated SBP (−0.99, CI: −5.42, 3.44).
Figure 2.
(A) The effect of different doses of aerobic exercise training on exercise systolic blood pressure in individuals with systolic exercise blood pressure at baseline. (B) The effect of different doses of aerobic exercise training on exercise diastolic blood pressure in individuals with elevated baseline diastolic exercise blood pressure. * Denotes significant difference compared to control. Results are presented as adjusted least-square means with 95% confidence intervals
In participants with an abnormally elevated exercise DBP at baseline (n=178), the mean exercise DBP and SBP were 103.4 mmHg (5.0) and 198.1 mmHg (17.6) respectively. There were 32, 61, 44, and 41 participants in the 4, 8 12 kcal/kg/week and control group respectively. As shown in figure 2B, we observed no significant difference in the change in exercise DBP following exercise training compared to control participants with elevated DBP in the 4 (−2.08, CI: −4.28, 0.12), 8 (−1.5, CI: −4.04, −1.01) and 12 kcal/kg/week (−3.7, CI: −6.31, −1.04) compared to control participants with elevated exercise DBP (−0.60 mmHg, CI: −3.43, 2.24).
Elevated Exercise Blood Pressure in Pre-hypertensive and Hypertensive individuals
There were 204 participants with pre-hypertension, of which 40.2% had an abnormally elevated SBP (>194 mmHg) and 38.7% had an abnormally elevated DBP (>96 mmHg) at 50 W respectively. In hypertensive participants (n=200), 58.5% and 49.5% had an abnormally elevated exercise SBP and DBP respectively.
Discussion
The novel finding of the present study is that exercise dose is an important factor in the reduction of exercise blood pressure in obese postmenopausal women with elevated resting blood pressure. This may have important clinical implications as abnormally elevated SBP and DBP exercise blood pressure have been independently associated with a 20% and 35% increase in CVD events, respectively, after adjustments for resting blood pressure 1. Our results suggest that even a moderate increase in aerobic exercise training in previously sedentary postmenopausal women (50% below federal physical activity guidelines) can promote favorable reductions in systolic exercise blood pressure if higher doses of training are unattainable. However, a high dose of exercise should be recommended in order to reduce both exercise SBP and DBP exercise blood pressure.
In the present study, exercise at 50 W was associated with 3.1 metabolic equivalents (METS), which is similar to the level of METS (3.0) required for walking at 2.5 miles per hour with no grade 18. Since this level of exertion is very common during activities of daily living, abnormally elevated blood pressure in response to submaximal exertion may represent a considerable excess strain on the heart similar to hypertension at rest. The adoption of regular aerobic exercise program may significantly reduce blood pressure and cardiac workload during submaximal exercise as we saw a significant reduction in RPP, a measure of myocardial oxygen demand in all three exercise groups following exercise training. Additionally, changes in exercise blood pressure in response to exercise training may be more modifiable as we observed significant reductions in exercise blood pressure, without significant improvements in resting blood pressure, and both measures were not significantly correlated.
Our results are consistent with previous studies which have shown an improvement in exercise SBP 7–12 and DBP 9, 10, 12 with aerobic training. We also investigated the effect of exercise training in participants with an abnormally elevated exercise blood pressure (upper 50th percentile of our population) at baseline since they have a greater risk of CVD 1–5. We found a significant reduction in exercise SBP in these participants at every dose of exercise following exercise training. Regression to the mean was considered in this analysis, as the reduction in exercise blood pressure was significantly different from control participants with an abnormally elevated SBP at baseline. The same relationship was not found for participants with an abnormally elevated exercise DBP at baseline. Therefore, we can only conclude that high dose exercise training may generally reduce exercise DBP. However, this attenuation is of clinical importance as the American College of Sports Medicine guidelines 19 state that the normal physiologic response to aerobic exercise is a maintenance or slight decrease in DBP during exercise, which has been shown in older women 20. However, in our study and other previous reports in populations with elevated blood pressure 9, 12 a mean increase in DBP has been observed at sub-maximal intensities. Whether this increase in DBP is a hallmark of individuals with hypertension is beyond the scope of this paper, however it may warrant further study, as an increase in DBP with exercise has been associated with cardiovascular abnormalities in individuals with coronary artery disease 21, 22.
An interesting finding from the present study is that abnormally elevated exercise blood pressure can occur in the absence of hypertension. This supports the findings from previous data that suggest that exercise blood pressure may have independent prognostic value 1, 2. Although an elevated exercise blood pressure was more common in participants with hypertension, approximately 40% and 39% of participants with pre-hypertension at rest had an abnormally elevated exercise SBP and DBP respectively. Perhaps abnormalities in blood pressure control in this sub-group are not evident until the system is “stressed” with an acute bout of aerobic exercise. We could not evaluate individuals who were normotensive as the exclusion criteria for DREW excluded women with resting SBP lower than 120 mmHg 14. Thus, an abnormally elevated exercise blood pressure may place these individuals at increased risk for CVD, but high normal values at rest may mask this additional risk from their primary care physician. Since physicians may be more likely to recommend lifestyle changes to individuals with pre-hypertension 23, our study demonstrates that aerobic exercise training is an effective intervention strategy. This is an important finding as abnormally elevated exercise blood pressure has been associated with future hypertension in individuals with pre-hypertension 24.
It could be speculated that the improvements in exercise blood pressure seen in the present study only represents a reduction in heart rate at the same absolute intensity. Indeed the correlation between exercise blood pressure and change in heart rate was significant. However, when we entered the change in heart rate following exercise training into the statistical model of our main analyses to address this potential concern, there was still a significant reduction in systolic exercise blood pressure in the 4 and 12 kcal/kg/week group compared to control (p<0.05), and the 8 kcal/kg/week group was still significant within groups. When a similar analysis was performed with change in VO2 max, fitness level was not a significant covariate in the model (p=0.254) and did not affect the results. This suggests that other factors regulating blood pressure are also involved (ie: reductions in total peripheral resistance). Our data support that the reduction in exercise blood pressure is not simply due to a reduction in heart rate or an improvement in fitness level with exercise training although both those factors likely play a role in the response.
The strengths of the present investigation are that DREW is a dose-response, randomized controlled trial with a large study population. In addition, exercise blood pressure was measured twice at baseline and follow-up, with good reproducibility. Furthermore, each participant received a standardized supervised exercise intervention. Limitations of the present study are participants exercised at the heart rate associated with 50% of VO2 max, so it is possible that other exercise intensities may produce different results. Secondly, since resting blood pressure was evaluated in the supine position, it is possible that resting values would be lower if we used seated blood pressure 25 which is common in the clinical setting. Lu et al. found that in 40–59 year old hypertensive individuals, there was approximately a 2.3 mmHg elevation in SBP in supine compared to seated position without significant differences in DBP. This does not affect the results of our main outcomes, but may affect the classification of participants as being pre-hypertensive or hypertensive. Lastly, we did not specifically report on the change of blood pressure medications in the present study. However, our main outcomes paper 16 showed that there was no significant change in blood pressure medications in the control, 4 and 8 kcal/kg/week groups, with a trend for a reduction of medications (p= 0.07) in the 12 kcal/kg/week group. Therefore, an increase in medications is not responsible for the reductions in exercise blood pressure shown in our study.
Conclusions
The results of the present study found that aerobic exercise training significantly reduces both exercise SBP and DBP in obese postmenopausal women with elevated resting blood pressure. In addition, our results suggest that reductions in exercise blood pressure may be more modifiable than reductions in resting blood pressure. This has public health implications as many of the activities of daily living occur at sub-maximal intensities, and previous data suggest that exercise blood pressure may have independent prognostic value above resting blood pressure 1, 2. Clinicians should evaluate the exercise blood pressure response when available in at risk patients, and should recommend a high dose of exercise when attainable for patients with an abnormally elevated exercise blood pressure.
Table 2.
Resting and exercise blood pressure values in individuals with pre-hypertension and hypertension classified based on the presence of elevated systolic exercise blood pressure or diastolic exercise blood pressure. Results are presented as mean (SD).1
| Elevated exercise systolic blood pressure | ||
|---|---|---|
| Pre-hypertension | Mean resting systolic | Mean exercise systolic |
| Normal (n=122) | 127.3 (7.4) | 174.8 (12.1) |
| Elevated (n=82) | 132.3 (5.8) | 207.9 (10.4) |
| Hypertension | ||
| Normal (n=83) | 145.9 (5.0) | 178.6 (10.4) |
| Elevated (n=117) | 151.6 (9.6) | 210.2 (10.8) |
| Elevated exercise diastolic blood pressure | ||
|---|---|---|
| Pre-hypertension | Mean resting diastolic | Mean exercise diastolic |
| Normal (n=125) | 75.6 (6.6) | 87.2 (7.6) |
| Elevated (n=79) | 78.5 (6.9) | 102.4 (4.9) |
| Hypertension | ||
| Normal (n=101) | 82.4 (8.2) | 86.2 (8.6) |
| Elevated (n=99) | 87.2 (7.2) | 104.3 (4.9) |
Shown are the resting and exercise systolic blood pressure values in individuals with elevated systolic blood pressure in participants with pre-hypertension and hypertension. In a separate analysis, we tabulated resting and exercise diastolic blood pressure in individuals with elevated diastolic blood pressure in individuals with pre-hypertension and hypertension. Elevated exercise blood pressure was defined as greater than the median value (systolic blood pressure>194 mmHg) and diastolic blood pressure (>96 mmHg) for the study sample. Pre-hypertension was defined as a systolic blood pressure between 120–139 mmHg and/or diastolic blood pressure between80–89mmHg. Hypertension was defined as a resting systolic ≥140mmHgand/or diastolic ≥90mmHg blood pressure at baseline. Results are presented as mean (SD).
Acknowledgments
Funding: National Institute of Health [grant number HL66262] and unrestricted research support from Coca-Cola.
This work was performed at The Cooper Institute, and the staff is especially commended for their efforts. We thank The Cooper Institute Scientific Advisory Board and the DREW participants. In addition, we would like to thank the NIH T-32 postdoctoral fellowship (Obesity from Genes to man) awarded to Pennington Biomedical Research Center.
Footnotes
Conflicts of interest: None
References
- 1.Lewis GD, Gona P, Larson MG, et al. Exercise Blood Pressure and the Risk of Incident Cardiovascular Disease (from the Framingham Heart Study) Am J Cardiol. 2008;101:1614–20. doi: 10.1016/j.amjcard.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mundal R, Kjeldsen S, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Exercise blood pressure predicts cardiovascular mortality in middle- aged men. Hypertension. 1994;24:56–62. doi: 10.1161/01.hyp.24.1.56. [DOI] [PubMed] [Google Scholar]
- 3.Filipovsky J, Ducimetiere P, Safar M. Prognostic significance of exercise blood pressure and heart rate in middle-aged men. Hypertension. 1992;20:333–9. doi: 10.1161/01.hyp.20.3.333. [DOI] [PubMed] [Google Scholar]
- 4.Fagard RH, Pardaens K, Staessen JA, Thijs L. Prognostic Value of Invasive Hemodynamic Measurements at Rest and During Exercise in Hypertensive Men. Hypertension. 1996;28:31–6. doi: 10.1161/01.hyp.28.1.31. [DOI] [PubMed] [Google Scholar]
- 5.Weiss SA, Blumenthal RS, Sharrett AR, Redberg RF, Mora S. Exercise Blood Pressure and Future Cardiovascular Death in Asymptomatic Individuals. Circulation. 2010;121:2109–16. doi: 10.1161/CIRCULATIONAHA.109.895292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and Risk of Cardiovascular Disease. Ann Intern Med. 1976;85:447–52. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 7.Barone BB, Wang N-Y, Bacher AC, Stewart KJ. Decreased exercise blood pressure in older adults after exercise training: contributions of increased fitness and decreased fatness. Br J Sports Med. 2009;43:52–6. doi: 10.1136/bjsm.2008.050906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelissen VA, Verheyden B, Aubert AE, Fagard RH. Effects of aerobic training intensity on resting, exercise and post-exercise blood pressure, heart rate and heart-rate variability. J Hum Hypertens. 2010;24:175–82. doi: 10.1038/jhh.2009.51. [DOI] [PubMed] [Google Scholar]
- 9.Seals DR, Silverman HG, Reiling MJ, Davy KP. Effect of regular aerobic exercise on elevated blood pressure in postmenopausal women. Am J Cardiol. 1997;80:49–55. doi: 10.1016/s0002-9149(97)00282-8. [DOI] [PubMed] [Google Scholar]
- 10.Bond V, Millis RM, Adams RG, et al. Attenuation of exaggerated exercise blood pressure response in African-American women by regular aerobic physical activity. Ethn Dis. 2005;15:S10–S3. [PMC free article] [PubMed] [Google Scholar]
- 11.Blumenthal JA, Sherwood A, Gullette ECD, et al. Exercise and Weight Loss Reduce Blood Pressure in Men and Women With Mild Hypertension: Effects on Cardiovascular, Metabolic, and Hemodynamic Functioning. Arch Intern Med. 2000;160:1947–58. doi: 10.1001/archinte.160.13.1947. [DOI] [PubMed] [Google Scholar]
- 12.Ketelhut RG, Franz IW, Scholze J. Regular exercise as an effective approach in antihypertensive therapy. Med Sci Sports Exerc. 2004;36:4–8. doi: 10.1249/01.MSS.0000106173.81966.90. [DOI] [PubMed] [Google Scholar]
- 13.Peronnet F, Cleroux J, Perrault H, Cousineau D, Dechamplain J, Nadeau R. Plasma norepinephrine response to exercise before and after training in humans. J Appl Physiol. 1981;51:812–5. doi: 10.1152/jappl.1981.51.4.812. [DOI] [PubMed] [Google Scholar]
- 14.Morss GM, Jordan AN, Skinner JS, et al. Dose-Response to Exercise in Women Aged 45–75 yr (DREW): Design and Rationale. Med Sci Sports Exerc. 2004;36:336–44. doi: 10.1249/01.MSS.0000113738.06267.E5. [DOI] [PubMed] [Google Scholar]
- 15.Earnest CP, Lavie CJ, Blair SN, Church TS. Heart Rate Variability Characteristics in Sedentary Postmenopausal Women Following Six Months of Exercise Training: The DREW Study. PLoS ONE. 2008;3:e2288. doi: 10.1371/journal.pone.0002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of Different Doses of Physical Activity on Cardiorespiratory Fitness Among Sedentary, Overweight or Obese Postmenopausal Women With Elevated Blood Pressure. JAMA. 2007;297:2081–91. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 17.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 18.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of Physical Activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Whaley M, editor. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 8. Baltimore: Lippincott Williams& Williams; 2010. [Google Scholar]
- 20.Miyai N, Arita M, Miyashita K, Morioka I, Shiraishi T, Nishio I. Blood Pressure Response to Heart Rate During Exercise Test and Risk of Future Hypertension. Hypertension. 2002;39:761–6. doi: 10.1161/hy0302.105777. [DOI] [PubMed] [Google Scholar]
- 21.Akhras F, Upward J, Jackson G. Increased diastolic blood-pressure response to exercise testing when coronary-artery disease is suspected - an indication of severity. Br Heart J. 1985;53:598–602. doi: 10.1136/hrt.53.6.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paraskevaidis IA, Kremastinos DT, Kassimatis AS, et al. Increased response of diastolic blood pressure to exercise in patients with coronary artery disease: an index of latent ventricular dysfunction? Br Heart J. 1993;69:507–11. doi: 10.1136/hrt.69.6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svetkey LP. Management of Prehypertension. Hypertension. 2005;45:1056–61. doi: 10.1161/01.HYP.0000167152.98618.4b. [DOI] [PubMed] [Google Scholar]
- 24.Miyai N, Arita M, Morioka I, Miyashita K, Nishio I, Takeda S. Exercise BP response in subjects with high-normal BP: Exaggerated blood pressure response to exercise and risk of future hypertension in subjects with high-normal blood pressure. J Am Coll Cardiol. 2000;36:1626–31. doi: 10.1016/s0735-1097(00)00903-7. [DOI] [PubMed] [Google Scholar]
- 25.Lu LC, Wei TM, Li S, Ye XL, Zeng CL, Wang LX. Differences in blood pressure readings between supine and sitting positions in hypertensive patients. Acta Cardiol. 2008;63:707–11. doi: 10.2143/AC.63.6.2033387. [DOI] [PubMed] [Google Scholar]