Abstract
Background
Mild cognitive impairment (MCI) is considered to represent a transitional stage between ageing and Alzheimer's disease (AD). To aim at identifying neuroimaging measures associated with cognitive changes in healthy elderly and MCI patients, longitudinal multicentre studies are ongoing in several countries. The patient profiles of each study are based on unique inclusion criteria.
Objectives
The purpose of the study is to clarify differences in baseline profiles of MCI patients between Studies on Diagnosis of Early Alzheimer's Disease—Japan (SEAD-J) and Alzheimer's Disease Neuroimaging Initiative (ADNI) and to examine the association between baseline profiles and risk of early conversion to AD.
Design
Prospective cohort study.
Setting and participants
SEAD-J recruited 114 patients from nine facilities in Japan. A total of 200 patients in ADNI with fluorodeoxyglucose–positron emission tomography (FDG-PET) were enrolled from the USA.
Methods
Baseline profiles were statistically analysed. For FDG-PET at a time of inclusion, associations between each profile and cerebral metabolic rate for glucose (CMRgl) were examined using SPM5 software. In each study, the ratio of conversion to AD within the 1-year and 2-year period after inclusion was investigated and differences in baseline profiles between AD converters and non-converters were analysed.
Results
SEAD-J included MCI patients with more severe verbal memory deficits and extracted patients with higher depressive tendencies. These differences were likely to be associated with criteria. SEAD-J exhibited a higher rate of conversion within 1 year compared with ADNI (24.5% vs 13.5%). In FDG-PET analyses of SEAD-J, AD converters within 1 year showed more severe decrease of FDG uptake in bilateral inferior parietal regions compared with non-converters.
Conclusions
Different inclusion criteria provided differences in baseline profiles. The severity of memory deficit might cause increase of the AD conversion within 1 year. Clinical outcomes of multicentre studies for early diagnosis of AD should be interpreted carefully considering profiles of patients.
Article summary
Article focus
To aim at identifying neuroimaging measures associated with cognitive changes in healthy elderly and MCI patients, longitudinal multicentre studies are ongoing in several countries.
The differences in baseline profiles of MCI patients between Studies on Diagnosis of Early Alzheimer's Disease—Japan (SEAD-J) and Alzheimer's Disease Neuroimaging Initiative (ADNI) multicentre studies are clarified.
Key messages
In association with criteria, SEAD-J recruited more patients with pre-dementia AD who had severe verbal memory deficits compared with ADNI.
In SEAD-J, AD converters within 1 year showed more severe decrease of FDG uptake in bilateral inferior parietal regions compared with non-converters. SEAD-J exhibited a higher rate of conversion within 1 year.
These results suggested that MCI patients with severe memory loss at the time of inclusion had an increased risk of early transition to AD.
Strengths and limitations of this study
This study reinforces that the results of multicentre studies should be interpreted carefully considering the impact of baseline profiles.
The present results were based on the analysis of data at the time of inclusion.
Introduction
The increasing prevalence of patients with dementia is a growing social problem. In particular, Alzheimer's disease (AD) is a common disease that causes progressive dementia. Mild cognitive impairment (MCI) is considered to represent a transitional stage between ageing and AD,1 and patients with MCI tend to progress to AD at a rate of approximately 10%–15% per year.2 3 In this context, early diagnosis of patients who show an increased risk of future conversion to AD represents an important step towards preventing progression of AD pathology when disease-modifying therapies for AD are finally developed.
Although the clinical evidence is not yet well established, fluorodeoxyglucose–positron emission tomography (FDG-PET) has recently been reported to provide useful findings of the cerebral metabolic rate for glucose (CMRgl) in both patients with AD4 5 and MCI patients.6 A pattern of CMRgl reduction in the posterior cingulate cortex and precuneus has been reported in MCI patients,7 and hypometabolism in these regions might contribute to prediction of clinical AD conversion.8 Furthermore, AD converters from among pre-MCI patients have shown correlations between CMRgl and future memory decline.9 Likewise, FDG-PET appears potentially useful for distinguishing MCI patients with increased risk of progressive dementia from patients with lower risk of future AD conversion.
Alzheimer's Disease Neuroimaging Initiative (ADNI) is a multicentre study aimed at identifying neuroimaging measures and biomarkers associated with cognitive and functional changes in healthy elderly, MCI and AD subjects.10 ADNI was launched in 2003 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies and non-profit organisations, as a $60 million 5-year public–private partnership. ADNI is the results of efforts by many co-investigators from a broad range of academic institutions and private corporations, and subjects have been recruited from over 50 sites across the USA and Canada (for additional information about ADNI, see http://www.adni-info.org). Studies on Diagnosis of Early Alzheimer's Disease—Japan (SEAD-J) was launched in 2005 by the National Center for Geriatrics and Gerontology. SEAD-J represents an ongoing follow-up of MCI patients, with the aim of achieving early prediction of AD conversion. Both studies have been investigating changes of serial neuroimaging findings and neuropsychological assessments, based on different patient samples enrolled with unique inclusion criteria to extract patients at increased risk of AD. Such differences in criteria appear likely to affect AD conversion.11 However, the impact of difference in baseline profiles of MCI patients for AD conversion has not been studied yet. The purpose of the study was to clarify this, comparing the results of statistical and imaging analyses of different multicentre studies: SEAD-J and ADNI. We investigated baseline profiles and AD conversion ratio within the 1-year and 2-year period after inclusion and then statistically analysed differences in baseline profiles between AD converters and non-converters.
Materials and methods
SEAD-J participants
Data set of SEAD-J was obtained from nine facilities in Japan. All data were checked and quality controlled at National Center for Geriatrics and Gerontology. A total of 114 patients with MCI (mean age (±SD), 70.8±7.5 years; 50 men, 64 women) were enrolled. A total of 56 normal age-matched subjects (20 men and 36 women) without evidence of neuropsychiatric impairment based on interviews were included to construct a normative imaging database. All participants provided informed consent in accordance with the trust ethics committee of National Center for Geriatrics and Gerontology. All data sets of clinical and FDG-PET findings over a follow-up period of 2 years have acquired.
Diagnosis of MCI was based on an interview with neurologists that contained evidence of reduced cognitive capacity, normal activities of daily living and absence of dementia.12 All patients were free of significant underlying medical, neurological or psychiatric illness. Patients were initially accessed using a neuropsychological test battery, including Mini-Mental Status Examination (MMSE), Clinical Dementia Rating (CDR),13 Geriatric Depression Scale (GDS)14 15 and Logical Memory subset of the Wechsler Memory Scale Revised (WMS-R LM).16 In accordance with the inclusion criteria, MCI patients were between 50 and 80 years old, with an MMSE score ≥24, a GDS score ≤10, a WMS-R LM I score ≤13, an LM II parts A and part B score (maximum, 50) ≤8 and a CDR memory box score restricted to 0.5. Patients with an educational level, defined as the number of completed years of formal education, <6 years were excluded.
ADNI participants
Data used in the preparation of this article were obtained from the ADNI Database (http://www.loni.ucla.edu/ADNI). Data sets of clinical and baseline FDG-PET recruited from a total of 200 MCI patients (mean age, 75.2±7.1 years; 134 men, 66 women) were downloaded from the ADNI public website (http://www.loni.ucla.edu/ADNI/). Data sets of baseline FDG-PET from 102 normal subjects were used as reference data to perform group comparisons of FDG-PET between these studies. MCI patients were without any other neuropsychological disease or symptoms and between 55 and 90 years old, with an MMSE score ≥24, verbal memory deficit as measured by WMS-R LM II part A score (maximum, 25) and a CDR memory box score 0.5 or 1. LM II part A score was used to select patients with verbal memory deficit measured by education-adjusted scores ≤8/25 (for ≥16 years of education, n=133), ≤4/25 (for 8–15 years of education, n=66) or ≤2/25 (for ≤7 years of education, n=1). In addition, patients who had experienced major depression or bipolar disorder within the past year were excluded, and patients with a Hamilton Depression Rating Scale17 score ≤12 (from a total of 17 items) were recruited.
Neuropsychological test batteries
The neuropsychological test batteries used in each study had three differences, regarding MMSE, WMS-R LM II and GDS scores. In different subscores of MMSE, patients in SEAD-J were scored using serial subtraction of 7 from 100 (5 points), while patients in ADNI were scored by reverse repetition of the word ‘earth’ (5 points). To adjust for this difference, modified MMSE score (maximum, 25) was calculated without the subscores from these 5-point subsets.
WMS-R LM II score contains parts A and B and reflects verbal memory deficits. The total score is 50 points. In SEAD-J, the cut-off score of WMS-R LM II for inclusion was ≤8/50. In ADNI, it was determined using the algorithm described above. For comparison of both profiles, only part A score (25 points) was used for analysis, and the normalised cut-off score for inclusion were calculated using a following calculation that took into account each weighting for the educational level: ∑ (cut-off score × patient number of each category)/total patient number. Using this measurement, the normalised cut-off score for ADNI was estimated as ≤6.65/25, while that for SEAD-J was ≤4/25. The difference also indicated that SEAD-J used more severe criteria to include patients with memory deficits.
To evaluate depressive tendencies, ADNI used the Hamilton Depression Rating Scale and GDS, while SEAD-J used a 15-item questionnaire (GDS-15). A higher GDS score (≥11) reflects depressive tendencies and represents a reliable instrument to diagnose depressive disorder.14 15 GDS-15 was considered a suitable short-form test for an elderly population.18 A higher GDS-15 score (≥6) was evaluated as having >90% sensitivity and specificity for depression in elderly individuals.19
FDG-PET and analyses
In SEAD-J, FDG-PET data at the time of inclusion were consolidated onto local servers. Scans were performed in a resting state in a dark room, 40–60 min after venous injection of FDG. Scans of MCI patients were compared with a normative reference database, controlling for global activity using iSSP software (http://MediPhysics.com) and then Z scores of FDG uptake were calculated voxel by voxel.
Three-dimensional stereotactic surface projections20 of Z scores were generated to visualise imaging differences for MCI patients compared with age-matched controls and AD converters compared with age-matched controls. In line with the same procedure mentioned above, we performed a comparison for scans of MCI patients in ADNI, using data sets restricted to participants <80 years old, to reduce differences in age for comparisons of results.
We also performed correlation analyses to investigate the impact of baseline patient profiles on CMRgl reduction using SPM5 software (http://www.fil.ion.ucl.ac.uk/spm/). Each image was deformed to the Montreal Neurological Imaging template and then normalised for variations in whole-brain measurements using proportionate scaling. Post-processed images were smoothed to a spatial resolution of 8 mm full width at half maximum. Analyses were conducted using MMSE score, WMS-R LM II score, GDS score and age as independent variables and CMRgl as the dependent variable. Statistical parametric maps for each of the contrasts and correlations were used in computations. The level of significance was set at p<0.01 (uncorrected).
Statistical analyses
SPSS V.17.0 was used for the analyses of baseline profiles. Independent sample t-tests were used to assess differences in clinical and cognitive variables. The χ2 test was used for the analysis of gender difference between studies and used to determine group differences in the ratio of AD conversion (AD converters vs non-converters; MCI stables) within the 1-year and 2-year period after inclusion.
Results
Differences in criteria and clinical profiles
The inclusion criteria of SEAD-J and ADNI and the differences in demographic characteristics of MCI patients are summarised in tables 1 and 2. In comparisons of neuropsychological test batteries at the time of inclusion, mean MMSE score was lower for SEAD-J patients (26.4±1.9) than for ADNI patients (27.2±1.7, p<0.001), and mean WMS-R LM score was lower for SEAD-J patients (1.8±1.8) than for ADNI patients (4.0±2.7, p<0.001). However, modified MMSE score did not differ significantly between studies, suggesting that there is little difference in global cognitive function compared with verbal memory deficits.
Table 1.
Differences in inclusion criteria for mild cognitive impairment
| SEAD-J | ADNI | |
| Age (yrs) | 50–80 | 55–90 |
| MMSE | 24–30 | 24–30 |
| CDR memory | 0.5 | 0.5 or 1 |
| WMS-R LM I | 0–13 | None |
| WMS-R LM II | 0–8 | * |
| GDS | 0–10 | None |
| HAM-D | None | 0–12 |
See the Materials and methods section.
ADNI, Alzheimer's Disease Neuroimaging Initiative; CDR memory, memory subscore for Clinical Dementia Rating; GDS, Geriatric Depression Scale; HAM-D, Hamilton Depression Rating Scale; MMSE, Mini-Mental Status Examination; SEAD-J, Studies on Diagnosis of Early Alzheimer's Disease—Japan; WMS-R LM II, Logical Memory part II subset of the Wechsler Memory Scale Revised; WMS-R LM I, Logical Memory part I subset of the Wechsler Memory Scale Revised.
Table 2.
Demographic characteristics of patients at the time of inclusion
| SEAD-J | ADNI | p Value | |
| Age (yrs) | 70.8±7.5 | 75.2±7.1 | <0.001 |
| Gender (M:F) | 50:64 | 134:66 | <0.001 |
| Education (yrs) | 11.5±3.0 | 15.8±2.9 | <0.001 |
| MMSE | 26.4±1.9 | 27.2±1.7 | <0.001 |
| Modified MMSE | 22.4±1.7 | 22.5±1.5 | 0.642 |
| WMS-R LM | 1.8±1.8 | 4.0±2.7 | <0.001 |
| GDS | 4.3±2.2 | 1.6±1.4 | <0.001 |
Values are mean±SD. The Modified MMSE represents the sum of total scores except for different subscores in both studies (maximum 25). WMS-R LM is taken as the score for the Logical Memory II part A (maximum 25).
ADNI, Alzheimer's Disease Neuroimaging Initiative; GDS, Geriatric Depression Scale; MMSE, Mini-Mental Status Examination; SEAD-J, Studies on Diagnosis of Early Alzheimer's Disease—Japan; WMS-R LM, Logical Memory subset of the Wechsler Memory Scale Revised.
MCI patients in SEAD-J showed a lower educational level (SEAD-J, 11.5±3.0 years; ADNI, 15.8±2.9 years, p<0.001). The percentage of patients with education level ≥16 years (corresponding to post-university) was 18.4% in SEAD-J and 66.5% in ADNI, indicating the inclusion of a larger proportion of patients with higher education in ADNI. A positive correlation between WMS-R LM score and education level was found in ADNI patients (r=0.30, p<0.001) but not in SEAD-J patients (r=0.04, p=0.67). No association with MMSE scores was found in either study.
Regarding depressive tendencies using GDS, mean score was higher in SEAD-J patients (4.3±2.2) than in ADNI patients (1.6±1.4, p<0.001). In SEAD-J, 18 patients (9%) were over the cut-off for GDS-15 (6/15 points), while in ADNI, no patients were over the cut-off (11/30 points). Thus, SEAD-J included more patients with higher depressive tendency compared with ADNI. The difference in GDS score might have been caused by the exclusive criteria using the Hamilton Depression Rating Scale. The mean age of patients was younger in SEAD-J (70.8±7.5 years) compared with ADNI (75.2±7.1 years, p<0.001), presumably due to the inclusion criteria for age.
Baseline FDG-PET: group comparisons and correlation analyses
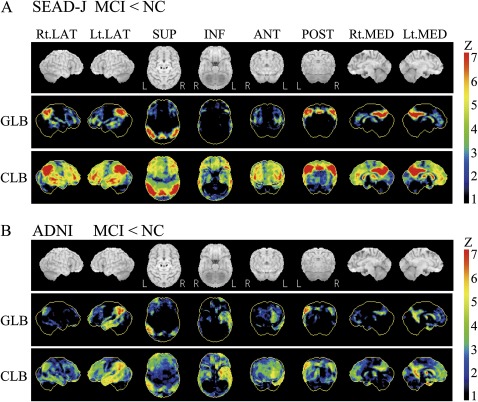
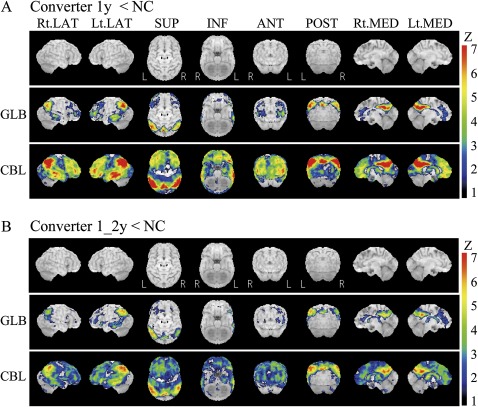
Compared with normal controls, MCI patients in SEAD-J showed considerably lower CMRgl in the regions preferentially affected by AD, including the precuneus, posterior cingulate and parietotemporal regions (AD-associated hypometabolism) (figure 1A). In ADNI, MCI patients exhibited similar patterns of reduced CMRgl in these regions. The CMRgl reduction was also found in medial temporal regions with left dominance (figure 1B). In both studies, MCI patients showed lower CMRgl in bilateral frontal regions compared with normal subjects. Furthermore, in SEAD-J, FDG-PET analysis revealed that the converters during 1 year after inclusion showed AD-associated hypometabolism compared with non-converters. The difference in hypometabolism was more severe in the converters within 1 year compared with the converters within the following 1 year (figure 2).
Figure 1.
3D-SSP analyses of baseline fluorodeoxyglucose–positron emission tomography in Studies on Diagnosis of Early Alzheimer's Disease—Japan (SEAD-J) (A) and Alzheimer's Disease Neuroimaging Initiative (ADNI) (B). These are the results of group comparison between MCI patients and normal controls (NC). MCI patients showed a significant decrease of the cerebral metabolic rate for glucose (CMRgl) not only in the regions preferentially affected by Alzheimer's disease (including the inferior parietal lobules and precuneus) but also in the frontal lobules. Colour bar indicates the mean Z score of CMRgl. LAT, lateral view; SUP, superior view; INF, inferior view; ANT, anterior view; POST, posterior view; MED, medial view; GLB, reference region in global brain; CLB, reference region in cerebellum.
Figure 2.
3D-SSP analyses of baseline fluorodeoxyglucose–positron emission tomography in Studies on Diagnosis of Early Alzheimer's Disease—Japan. These are the results of group comparisons between Alzheimer's disease (AD) converters and non-converters. AD converters show a greater reduction in glucose metabolism for AD-associated and frontal regions. This hypometabolism was more evident in the converters within 1 year after inclusion compared with the converters from 1 year to 2 years after inclusion. (A) AD converters within 1 year after inclusion and non-converters. (B) AD converters from 1 year to 2 years after inclusion and non-converters.
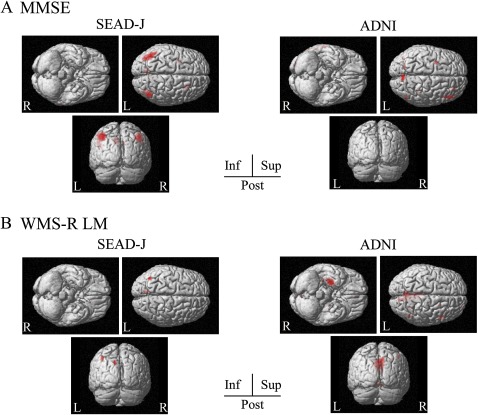
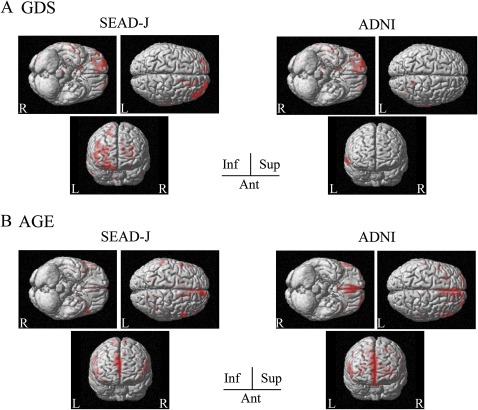
In correlation analyses for FDG-PET, the association between patient profiles and glucose metabolism are depicted in figures 3 and 4. In SEAD-J, bilateral inferior parietal regions correlated with MMSE score, whereas ADNI showed no specific regions (figure 3A). Both studies showed different patterns of correlation with WMS-R LM score. In SEAD-J, a correlation was found in the left inferior parietal region, while ADNI showed correlations in the precuneus and left medial temporal region (figure 3B). Furthermore, GDS score showed an inverse correlation in the frontal regions. In SEAD-J, regions with significant correlations showed a greater distribution over the lateral and inferior frontal regions (figure 4A). As for correlations with age, both studies showed an inverse correlation in bilateral medial frontal regions (figure 4B).
Figure 3.
Statistical parametric mapping of the brain regions correlated with baseline profiles in Studies on Diagnosis of Early Alzheimer's Disease—Japan (SEAD-J) and Alzheimer's Disease Neuroimaging Initiative (ADNI). The regions displayed in red indicate significant regional hypometabolism (p<0.05). (A) Correlation between lower Mini-Mental Status Examination (MMSE) scores and glucose metabolism. (B) Correlation between lower Logical Memory subset of the Wechsler Memory Scale Revised (WMS-R LM) scores and glucose metabolism.
Figure 4.
Statistical parametric mapping of the brain regions correlated with baseline profiles in Studies on Diagnosis of Early Alzheimer's Disease—Japan (SEAD-J) and Alzheimer's Disease Neuroimaging Initiative (ADNI). The regions displayed in red indicate significant regional hypometabolism (p<0.05). (A) Inverse correlation between Geriatric Depression Scale (GDS) scores and glucose metabolism. (B) Inverse correlation between age and glucose metabolism.
Differences between AD converters and non-converters
In comparisons with AD conversion within 2 years, we revealed the difference in profiles between converters and non-converters (table 3). Patients who had dropped out or returned to normal were excluded from statistical analysis. In terms of patients to follow-up and patients dropping out, the studies did not show any significant differences in clinical profiles. The conversion ratio during 1 year was higher in SEAD-J than in ADNI (24.5% vs 13.5%; χ2=5.33, p<0.05). Conversely, conversion ratio over 2 years showed no difference between studies (SEAD-J, 35.6%; ADNI, 33.3%; χ2=0.097, p=0.77). Comparing the baseline profiles associated with conversion during 1 year of follow-up, SEAD-J converters showed significantly lower MMSE and WMS-R LM scores than non-converters (p<0.01). In ADNI, WMS-R LM score was lower in converters (p<0.01), but no difference in MMSE score was evident. Regarding the profiles associated with conversion from 1 year to 2 years after inclusion, MMSE score was lower for SEAD-J converters than for non-converters (p<0.05). Among ADNI converters, no profiles showed significant differences.
Table 3.
Differences in baseline profiles between the converters to AD and non-converters
| SEAD-J |
ADNI |
|||
| Conv/non-conv | p Value | Conv/non-conv | p Value | |
| 1-year conversion | ||||
| MMSE | 25.3±1.3/26.6±1.9 | 0.002 | 26.8±1.8/27.2/1.7 | NS |
| Modified MMSE | 21.6±1.3/22.6±1.8 | 0.012 | 21.8±1.7/22.5±1.5 | NS |
| WMS-R LM | 0.7±1.3/1.9±1.8 | 0.003 | 2.5±2.3/4.2±2.7 | 0.004 |
| GDS | 4.3±2.0/4.2±2.4 | 0.003 | 1.3±1.4/1.7±1.4 | NS |
| Age (yrs) | 70.6±6.9/71.6±6.7 | NS | 75.5±6.1/75.7±7.3 | NS |
| Education (yrs) | 12.1±3.1/11.5±3.0 | NS | 15.8±2.8/15.9±2.9 | NS |
| 1–2-year conversion | ||||
| MMSE | 25.9±1.8/26.4±1.9 | 0.001 | 27.1±1.6/27.3±1.6 | NS |
| Modified MMSE | 22.1±1.5/22.5±2.0 | NS | 22.5±1.5/22.5±1.4 | NS |
| WMS-R LM | 1.6±1.9/1.9±1.9 | NS | 3.8±2.7/4.3±2.7 | NS |
| GDS | 4.9±2.6/3.9±2.1 | NS | 1.6±1.2/1.5±1.4 | NS |
| AGE (yrs) | 70.9±6.4/71.5±6.5 | NS | 73.7±7.6/75.9±6.8 | NS |
| Education (yrs) | 12.4±3.4/11.7±3.1 | NS | 16.6±2.5/15.8±2.9 | NS |
Values are mean±SD. 1-year conversion, AD conversion within 1 year after inclusion; 1–2-year conversion, AD conversion from 1 year to 2 years after inclusion.
AD, Alzheimer's disease; ADNI, Alzheimer's Disease Neuroimaging Initiative; Conv, AD converters; GDS, Geriatric Depression Scale; MMSE, Mini-Mental Status Examination; non-conv, AD non-converters; NS, no significance; SEAD-J, Studies on Diagnosis of Early Alzheimer's Disease—Japan; WMS-R LM, Logical Memory subset of the Wechsler Memory Scale Revised.
Discussion
From analyses of baseline profiles, SEAD-J included patients with more severe verbal memory deficits and extracted patients with higher depressive tendencies compared with ADNI. These differences in profiles of MCI patients were likely to be associated with operating criteria. In FDG-PET, both studies showed considerably lower CMRgl in the regions preferentially affected by AD and the frontal cortices. The baseline profiles provided characteristic pattern of correlations between CMRgl on baseline FDG-PET and scores of neuropsychological tests.
Despite some studies have reported associations between lower MMSE score of AD patients and higher Z score in the regions preferentially affected by AD,21 22 such associations in MCI patients have not been demonstrated. In this study, MCI patients in SEAD-J had association between hypometabolism in bilateral inferior parietal regions and MMSE score. The modified MMSE score showed same pattern of correlation (data not shown). However, we could not find any association between MMSE score of patients in ADNI and CMRgl, as a result of previous report.23 In WMS-R LM score, SEAD-J showed a weak regional correlation in the part of right inferior parietal cortex, while ADNI showed correlations in the precuneus and right dominant medial–temporal cortices. These results might reflect difference in disease severity of the patient samples, that is, how close an individual is to a clinical transition to AD.
Concerning the hypometabolism in frontal cortices, it might be an additional finding associated with the conversion from MCI to AD.8 In patients with depressed mood disorders, an FDG-PET study has shown a lower CMRgl in bilateral frontal and temporal cortices, inferior parietal lobules and left cingulate cortex.24 In AD patients with depressive syndrome, a greater decrease of CMRgl has been found in right suprafrontal lobules than in non-depressive AD.25 In our analyses, CMRgl in the right dominant suprafrontal regions showed an inverse correlation with GDS scores. In particular, the SEAD-J, which included patients with higher depressive tendencies, showed wider regions with correlation compared with ADNI. Although the prevalence of patients with depressive tendencies was not as high in SEAD-J, the inclusion of patients with depressive tendencies might affect CMRgl. In addition, CMRgl in medial frontal regions showed an inverse correlation with age, indicating the ageing effect of glucose metabolism,26 or possibly containing a partial volume effect.27 These results reflected patient demographics of each study.
In baseline profiles, high educational level was another characteristic of patients in ADNI. The WMS-R LM score for ADNI patients correlated with educational level. This correlation was likely to be associated with categorical inclusion criteria for educational level. High education might mask expression of dementia symptoms. Several studies have supported the hypothesis that highly educated subjects tend to cope better with the onset of dementia.28–30 In FDG-PET studies, higher education has been documented as a proxy for brain functional reserve.31 32 The impact of educational level might complicate the interpretation of subtle changes in neuropsychological test results for patients with high education. A combination of neuropsychological testing with FDG-PET might thus help the accuracy for AD diagnosis in such cases. One study reported an association between higher education and lower CMRgl in the temporoparietal cortex and precuneus in AD and MCI converters.33 However, we did not find evidence that high education affected AD conversion in MCI patients. The impact of education remains controversial and might depend on the patient sample.34
We revealed that SEAD-J patients exhibited a significantly higher rate of conversion within 1 year after inclusion compared with ADNI. Deficits in verbal memory and psychomotor speed/executive function abilities might be associated with conversion to AD.35 Actually, in the present analyses, comparisons of baseline profiles between AD converters and non-converters revealed that SEAD-J converters had lower global cognitive and verbal memory compared with ADNI converters. Furthermore, in SEAD-J, AD converters during 1 year after inclusion showed more severe CMRgl reductions in bilateral inferior parietal regions compared with converters during the following year. Based on these results, the difference in AD conversion ratio might be dependent on the severity of pre-dementia AD, reflecting that MCI patients with severe baseline memory deficits rapidly converted to AD. It suggested that inclusion and diagnostic criteria were likely to be associated with the incidence of AD. However, there was no difference in conversion ratio seen within 2 years of follow-up period. Concerning the discrepancy due to follow-up period, it is likely that the difference in AD conversion ratio may not be limited by criteria only but be affected by another factor such as genotype in MCI population. The CMRgl reductions in AD-associated regions have been reported in cognitively normal people with the apolipoprotein E ɛ4 allele, a common AD susceptibility gene, many years before the onset of symptoms of cognitive disturbance.36 It suggests that FDG-PET findings may associate with pathogenesis of AD. Although our observation was too short to make clear the impact of criteria and baseline profiles on the risk of AD conversion, it is likely that the incidence of AD may not have greater difference in groups with greater susceptibility symptoms, if there are no operational criteria as for prevalence in genotype.
In our analyses, these comparisons of different multicenter studies have some limitations. Quality control protocols for data acquisition caused different pattern of CMRgl in comparison of FDG-PET between SEAD-J and ADNI. We carried out the analyses comparing the baseline FDG-PET between two studies. However, the result contaminated non-specific changes especially in the frontal and parietal regions. In this reason, we presented the difference in glucose metabolism between MCI patients and normal subjects, in each study. In addition, the present results were based on data sets at the time of inclusion. To clarify further association between each patient's profile and risk of AD conversion, multimodal analyses of data are needed for longer follow-up period.
In conclusion, our study revealed that the participants of each study showed some differences in baseline profiles because the two studies applied own original inclusion criteria to MCI patients. SEAD-J had more strict criteria to include patients with severe verbal memory deficits. The characteristics of baseline profiles are closely related to AD conversion ratio within 1 year after inclusion. Furthermore, we compared national differences between multicentre studies to show that inclusion criteria were associated with pattern of regional glucose metabolism. We suggest that severity of AD assessed by neuropsychological tests were a function of the recruitment criteria. To evaluate the value of neuroimaging measures in the early diagnosis of AD, the results of multicenter studies, even though focusing on amnestic MCI, should be compared carefully considering difference in characteristics of inclusion criteria and profiles.
Supplementary Material
Acknowledgments
The authors thank Ken Fujiwara and Yuki Okamoto for their technical assistance and data acquisition of SEAD-J.
Footnotes
To cite: Kawashima S, Ito K, Kato T, et al. Inclusion criteria provide heterogeneity in baseline profiles of patients with mild cognitive impairment: comparison of two prospective cohort studies. BMJ Open 2012;2:e000773. doi:10.1136/bmjopen-2011-000773
Contributors: The investigators within ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in the analysis or writing of this report. We had completely followed the review for ADNI Publications Policy. ADNI DPC approved it as acceptable for submission to a journal.
Funding: SEAD-J was supported by the Health Labour Sciences Research Grant from the Ministry of Health, Labour and Welfare of Japan (H17-Tyojyu-023) and the Research Funding for Longevity Sciences from National Center for Geriatrics and Gerontology, Japan.
Competing interests: None.
Ethics approval: SEAD-J was approved by the medical ethics committee of the Center for Development of Advanced Medicine for Dementia, National Center for Geriatrics and Gerontology.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
References
- 1.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–8 [DOI] [PubMed] [Google Scholar]
- 2.Bowen J, Teri L, Kukull W, et al. Progression to dementia in patients with isolated memory loss. Lancet 1997;349:763–5 [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Stevens JC, Ganguli M, et al. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:1133–42 [DOI] [PubMed] [Google Scholar]
- 4.Hoffman JM, Welsh-Bohmer KA, Hanson M, et al. FDG PET imaging in patients with pathologically verified dementia. J Nucl Med 2000;41:1920–8 [PubMed] [Google Scholar]
- 5.Silverman DH, Small GW, Chang CY, et al. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA 2001;286:2120–7 [DOI] [PubMed] [Google Scholar]
- 6.Drzezga A, Grimmer T, Riemenschneider M, et al. Prediction of individual clinical outcome in MCI by means of genetic assessment and (18)F-FDG PET. J Nucl Med 2005;46:1625–32 [PubMed] [Google Scholar]
- 7.Mosconi L, Tsui WH, Herholz K, et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer's disease, and other dementias. J Nucl Med 2008;49:390–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drzezga A, Lautenschlager N, Siebner H, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer's disease: a PET follow-up study. Eur J Nucl Med Mol Imaging 2003;30:1104–13 [DOI] [PubMed] [Google Scholar]
- 9.Caselli RJ, Chen K, Lee W, et al. Correlating cerebral hypometabolism with future memory decline in subsequent converters to amnestic pre-mild cognitive impairment. Arch Neurol 2008;65:1231–6 [DOI] [PubMed] [Google Scholar]
- 10.Mueller SG, Weiner MW, Thal LJ, et al. Ways toward an early diagnosis in Alzheimer's disease: he Alzheimer's Disease Neuroimaging Initiative (ADNI). Alzheimers Dement 2005;1:55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saxton J, Snitz BE, Lopez OL, et al. Functional and cognitive criteria produce different rates of mild cognitive impairment and conversion to dementia. J Neurol Neurosurg Psychiatry 2009;80:737–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001;58:1985–92 [DOI] [PubMed] [Google Scholar]
- 13.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–14 [DOI] [PubMed] [Google Scholar]
- 14.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982;17:37–49 [DOI] [PubMed] [Google Scholar]
- 15.Nyunt MS, Fones C, Niti M, et al. Criterion-based validity and reliability of the Geriatric Depression Screening Scale (GDS-15) in a large validation sample of community-living Asian older adults. Aging Ment Health 2009;13:376–82 [DOI] [PubMed] [Google Scholar]
- 16.Sullivan K. Estimates of interrater reliability for the Logical Memory subtest of the Wechsler Memory Scale-Revised. J Clin Exp Neuropsychol 1996;18:707–12 [DOI] [PubMed] [Google Scholar]
- 17.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Craen AJ, Heeren TJ, Gussekloo J. Accuracy of the 15-item geriatric depression scale (GDS-15) in a community sample of the oldest old. Int J Geriatr Psychiatry 2003;18:63–6 [DOI] [PubMed] [Google Scholar]
- 19.Fountoulakis KN, Tsolaki M, Iacovides A, et al. The validation of the short form of the geriatric depression scale (GDS) in Greece. Aging (Milano) 1999;11:367–72 [DOI] [PubMed] [Google Scholar]
- 20.Minoshima S, Frey KA, Koeppe RA, et al. A diagnostic approach in Alzheimer's disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med 1995;36:1238–48 [PubMed] [Google Scholar]
- 21.Hanyu H, Sato T, Hirao K, et al. The progression of cognitive deterioration and regional cerebral blood flow patterns in Alzheimer's disease: a longitudinal SPECT study. J Neurol Sci 2010;290:96–101 [DOI] [PubMed] [Google Scholar]
- 22.Chase TN, Foster NL, Fedio P, et al. Regional cortical dysfunction in Alzheimer's disease as determined by positron emission tomography. Ann Neurol 1984;(15 Suppl):S170–4 [DOI] [PubMed] [Google Scholar]
- 23.Langbaum JB, Chen K, Lee W, et al. Categorical and correlational analyses of baseline fluorodeoxyglucose positron emission tomography images from the Alzheimer's Disease Neuroimaging Initiative (ADNI). Neuroimage 2009;45:1107–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosokawa T, Momose T, Kasai K. Brain glucose metabolism difference between bipolar and unipolar mood disorders in depressed and euthymic states. Prog Neuropsychopharmacol Biol Psychiatry 2009;33:243–50 [DOI] [PubMed] [Google Scholar]
- 25.Lee DY, Choo IH, Jhoo JH, et al. Frontal dysfunction underlies depressive syndrome in Alzheimer disease: a FDG-PET study. Am J Geriatr Psychiatry 2006;14:625–8 [DOI] [PubMed] [Google Scholar]
- 26.Aston JA, Cunningham VJ, Asselin MC, et al. Positron emission tomography partial volume correction: estimation and algorithms. J Cereb Blood Flow Metab 2002;22:1019–34 [DOI] [PubMed] [Google Scholar]
- 27.Kantarci K, Senjem ML, Lowe VJ, et al. Effects of age on the glucose metabolic changes in mild cognitive impairment. AJNR Am J Neuroradiol 2010;31:1247–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson RS, Li Y, Aggarwal NT, et al. Education and the course of cognitive decline in Alzheimer disease. Neurology 2004;63:1198–202 [DOI] [PubMed] [Google Scholar]
- 29.Bennett DA, Wilson RS, Schneider JA, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology 2003;60:1909–15 [DOI] [PubMed] [Google Scholar]
- 30.Roe CM, Xiong C, Miller JP, et al. Education and Alzheimer disease without dementia: support for the cognitive reserve hypothesis. Neurology 2007;68:223–8 [DOI] [PubMed] [Google Scholar]
- 31.Stern Y, Alexander GE, Prohovnik I, et al. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer's disease. Ann Neurol 1992;32:371–5 [DOI] [PubMed] [Google Scholar]
- 32.Perneczky R, Drzezga A, Diehl-Schmid J, et al. Schooling mediates brain reserve in Alzheimer's disease: findings of fluoro-deoxy-glucose-positron emission tomography. J Neurol Neurosurg Psychiatry 2006;77:1060–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garibotto V, Borroni B, Kalbe E, et al. Education and occupation as proxies for reserve in a MCI converters and AD: FDG-PET evidence. Neurology 2008;71:1342–9 [DOI] [PubMed] [Google Scholar]
- 34.Landau SM, Harvey D, Madison CM, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology 2010;75:230–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabert MH, Manly JJ, Liu X, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry 2006;63:916–24 [DOI] [PubMed] [Google Scholar]
- 36.Reiman EM, Chen K, Alexander GE, et al. Correlations between apolipoprotein E ɛ4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci U S A 2005;102:8299–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.