Abstract
Objectives
Reasons for choosing active surveillance for the treatment of small renal masses are poorly understood. We aim to better delineate which factors influence the decision to undergo active surveillance.
Methods
We identified 204 consecutive patients at our institution with clinical T1 renal masses from June 2009 through June 2010. A variety of demographic and clinical characteristics were measured. Based on our prior work, “ideal” criteria for active surveillance included: tumor size ≤ 4cm, Charlson Comorbidity Index (CI) ≥ 2, Eastern Cooperative Oncology Group Performance Status (ECOG PS) ≥ 2, and estimated glomerular filtration rate (eGFR) < 60ml/min. We performed sensitivity analyses to identify characteristics associated with choice of active surveillance and compared these to our “ideal” criteria.
Results
Seventy-three (36%) and 131 (64%) patients underwent active surveillance and treatment, respectively. Patients undergoing active surveillance versus treatment differed with respect to distance from hospital > 60 miles (p=0.04), ECOG PS ≥ 2 (p<0.01), tumor size (p<0.01), multifocality (p=0.03), and endophytic nature of lesion (p=0.04) as well as whether the patient’s surgeon generally uses a robotic, laparoscopic, or open approach (p=0.01). Neither baseline eGFR (p=0.91) nor CI (p=0.69) were significant factors. The combination of tumor size < 3cm, ECOG PS ≥ 2, and an endophytic lesion were most predictive of active surveillance.
Conclusions
Patient, tumor, and surgeon characteristics all influence active surveillance. Based on sensitivity analyses, active surveillance was driven by a tumor size <3cm, poor performance status (i.e., ECOG PS ≥ 2), and an endophytic lesion.
Keywords: renal cell carcinoma, active surveillance, treatment, small renal mass, comorbidity
INTRODUCTION
The incidence of renal cell carcinoma has been steadily rising, with an estimated 58,240 new cases and 13,040 deaths in 2010.1 The increased incidence largely stems from the expanded use of cross-sectional abdominal imaging, which has increased the detection of small, incidental lesions.2,3 Despite the earlier detection of lesions, mortality rates for kidney cancer have continued to rise, suggesting that the immediate removal of small renal masses may not be beneficial in all cases.4 Traditionally, radical nephrectomy has been the standard of care for the treatment of these lesions. More recently, partial nephrectomy has demonstrated equivalent oncological outcomes as well as improved feasibility and has become the preferred approach for many clinical T1 lesions.5-8
While both approaches provide good oncologic control, radical and partial nephrectomy is associated with surgical morbidity, which can be substantial. Radical nephrectomy is associated with chronic renal insufficiency, which increases the risk of cardiovascular events and mortality.9 Partial nephrectomy spares nephrons, but has a higher rate of peri-operative complications than radical nephrectomy.5,10 In contrast to these invasive approaches, active surveillance is an alternative management that obviates the risks of surgery. Clinical T1 lesions frequently have an indolent course, and patients choosing active surveillance rarely have lesions that progress to metastatic disease.11 Further, the risk of progression is not jeopardized by delaying treatment.12 Given these findings, deciding the best treatment option for patients with clinical T1 lesions can be difficult. Clinicians must weigh the benefits of definitive treatment versus the immediate peri-operative risks associated with surgery and the potential long-term consequences of decreased renal function. In this context, active surveillance is an attractive option for certain patients.
To better delineate which factors influence the decision to pursue active surveillance, we analyzed our small renal mass database to compare patient, tumor, and surgeon characteristics between those undergoing active surveillance and those receiving treatment. Based on our prior work,13 we expected significant patient comorbidity, poor renal function, and lesions < 4cm to be significant predictors of active surveillance.
MATERIALS AND METHODS
Study Population
We retrospectively analyzed 204 consecutive patients referred to our institution with a clinical T1 renal mass (i.e., ≤ 7cm) from June 2009 through June 2010. Data were collected prospectively and entered into our small renal mass database. Patients with metastatic disease were excluded. Patient demographics were recorded, including age, gender, race/ethnicity, socioeconomic status, and distance from the hospital. Patient zip codes were used to categorize socioeconomic status and calculate distance to our institution.14 Cancer-specific information obtained included clinical tumor stage, tumor anatomy, and type of treatment. R.E.N.A.L. nephrometry score provides a tool to quantify the anatomical characteristics of renal masses on cross-sectional imaging.15 Lesions were characterized as low (4-6), moderate (7-9), or high (10- 12) complexity lesions based on their nephrometry score. Specific components of the nephrometry score that were thought, a priori, to affect the treatment decision were evaluated individually. For each patient, comorbidities were documented, and both CI and ECOG PS were determined. We calculated eGFR based on the Modification of Diet in Renal Disease equation.
Outcomes
The primary outcome of interest was the use of active surveillance. Given the short follow-up time, the outcomes of active surveillance (i.e., delayed intervention, progression to metastatic disease, mortality, or continued surveillance) were not assessed. We evaluated an array of exposures (i.e., patient demographics, clinical factors, and tumor and surgeon characteristics) that may predict the utilization of active surveillance over treatment. Treatment included partial nephrectomy, radical nephrectomy, cryotherapy, and radiofrequency ablation. We then determined which combination of patient and tumor factors were most predictive of active surveillance in our study population. To do this, we initially examined characteristics felt to be the best candidates (i.e., tumor size ≤ 4cm, eGFR < 60ml/min, and CI ≥ 2) based on our prior work.13 Since the effectiveness of these criteria as predictors of active surveillance had not been previously validated, we then compared this group to other criteria. In deciding which characteristics to use in comparison, we selected factors that were either significantly associated with active surveillance in our analyses (e.g., small tumor size, poor performance status, and endophytic lesion) or previously associated with active surveillance in the literature (e.g., poor renal function).16
Statistical Analysis
Patient demographics as well as clinical and tumor characteristics were compared between patients receiving active surveillance versus treatment using parametric (Student t-test) and non-parametric (chi-squared or Fisher’s exact test) testing as appropriate. In addition, surgeons were characterized by their primary surgical approach (i.e., robotic, laparoscopic, or open surgery). Next, we fit a multivariable logistic regression model, accounting for age, gender, race/ethnicity, tumor size, patient comorbidities and renal function, distance from hospital, and surgical approach. Lastly, we performed sensitivity analyses to identify patient characteristics most predictive of active surveillance and examined how these compared to our initial selection criteria. All analyses were performed using STATA v11.0 (STATA Corp, College Station, Texas) at the 5% significance level. Our Institutional Review Board approved the study protocol.
RESULTS
Of the 204 patients in our small renal mass database, 73 (36%) underwent active surveillance and 131 (64%) underwent treatment. The two groups had similar demographic profiles except that patients undergoing treatment travelled farther distances (Table 1). In the treatment cohort, 68% underwent a partial nephrectomy, 19% underwent ablation (i.e., cryotherapy or radiofrequency ablation), and 13% underwent a radical nephrectomy.
Table 1.
Demographics – Active surveillance versus Treatment
| Characteristic | Active surveillance |
Treatment | P- value |
|---|---|---|---|
| Number of patients (%) | 73 (36) | 131 (64) | |
| Mean age ± standard deviation (years) |
63 ± 14 | 60 ± 12 | 0.12 |
| Socioeconomic Status (terciles) | |||
| Low (%) | 30 (43.5) | 39 (56.5) | 0.25 |
| Medium (%) | 21 (31) | 47 (69) | |
| High (%) | 22 (33) | 45 (67) | |
| Distance > 60 miles (%) | 27 (28) | 68 (72) | 0.04* |
| Male Gender (%) | 37 (33) | 75 (67) | 0.37 |
| Race | |||
| White (%) | 59 (34.5) | 112 (65.5) | 0.53 |
| Black (%) | 10 (45.5) | 12 (54.5) | |
| Other (%) | 4 (44) | 5 (56) | |
| Family history of kidney cancer (%) | 8 (57) | 6 (43) | 0.15 |
Significant at 5% level (p ≤ 0.05)
The patient medical history and comorbidity status for subjects in the active surveillance and treatment groups are in Table 2. Of note, the two groups only differed in regards to ECOG PS (p=0.01). Patients with ECOG scores < 2 were more likely to undergo treatment whereas those with ECOG scores ≥ 2 were more likely to undergo active surveillance. These two cohorts did not differ in terms of overall renal function (p=0.46) or CI (0.69). A total of 45% and 48% of patients in the active surveillance and treatment cohorts had a CI ≥ 2, respectively.
Table 2.
Medical history and comorbidities – Active surveillance versus Treatment
| Characteristic | Active surveillance (n=73) |
Treatment (n=131) |
P- value |
|---|---|---|---|
| BMI (kg/m2) ± standard deviation | 30 ± 7.8 | 32 ± 8.3 | 0.13 |
| Prior renal cell carcinoma (%) | 2 (14) | 12 (86) | 0.09 |
| Hypertension (%) | 40 (37) | 68 (63) | 0.69 |
| Diabetes (%) | 18 (36) | 32 (64) | 0.97 |
| Coronary artery disease (%) | 13 (46) | 15 (54) | 0.21 |
| eGFR (ml/min) ± standard deviation | 76.2 ± 21.7 | 78.8 ± 26.2 | 0.46 |
| eGFR ≤ 60 ml/min (%) | 15 (34) | 29 (66) | 0.91 |
| Charlson Comorbidity Index | |||
| < 2 (%) | 40 (37) | 68 (63) | 0.69 |
| ≥ 2 (%) | 33 (34) | 63 (66) | |
| ECOG Performance Status | |||
| < 2 (%) | 57 (33) | 117 (67) | 0.01* |
| ≥ 2 (%) | 14 (64) | 8 (36) |
BMI = body mass index; ECOG = Eastern Cooperative Oncology Group; eGFR = estimated glomerular filtration rate
Significant at 5% level (p ≤ 0.05)
Tumor and surgeon characteristics are shown in Table 3. Patients with multifocal lesions were more likely to undergo active surveillance (p=0.03). Ten out of the 11 patients with a solitary kidney underwent treatment (p=0.10). These 10 patients were more likely to have a prior history of renal cell carcinoma (p<0.01) and more likely to undergo a biopsy (p=0.05) than patients with 2 kidneys treated for a small renal mass, although they did not differ in terms of renal function, performance status, or tumor size. In general, a smaller tumor size favored active surveillance (p < 0.01) as did an entirely endophytic lesion on imaging (p=0.04). A total of 78 patients (38.2%) underwent a biopsy, of which 12 (15.4%) were either benign or oncocytoma. Specifically, 41% of active surveillance patients and 37% of patients undergoing treatment had a biopsy (p=0.53). Surgeons were categorized by which surgical approach they utilize for small renal masses the majority (i.e., greater than 50%) of the time. Using this method, our active surveillance cohort comprised 4 “robotic”, 3 “laparoscopic”, and 2 “open” surgeons. Patients managed by surgeons who performed primarily open surgical partial nephrectomy were more likely to enter active surveillance (p=0.01).
Table 3.
Tumor and surgeon characteristics – Active surveillance versus Treatment
| Characteristic | Active surveillance (n=73) |
Treatment (n=131) |
P- value |
|---|---|---|---|
| Multifocal lesions (%) | 12 (60) | 8 (40) | 0.03* |
| Solitary kidney (%) | 1 (9) | 10 (91) | 0.10 |
| Tumor size (cm) ± standard deviation |
2.3 ±1.2 | 2.9 ± 1.3 | <0.01* |
| Nephrometry scorea | |||
| Low, 4-6 (%) | 26 (38) | 42 (62) | 0.14 |
| Moderate, 7-9 (%) | 28 (29) | 69 (71) | |
| High, 10-12 (%) | 14 (45) | 17 (55) | |
| Exophytic lesiona | |||
| ≤ 50% (%) | 18 (26) | 51 (74) | 0.04* |
| < 50% (%) | 30 (36) | 53 (64) | |
| Entirely endophytic (%) | 21 (50) | 21 (50) | |
| Anterior/Posteriora | |||
| Anterior (%) | 29 (37) | 49 (63) | 0.56 |
| Posterior (%) | 33 (36) | 59 (64) | |
| Neither (%) | 7(26) | 20 (74) | |
| Kidney biopsy (%) | 30 (38.5) | 48 (61.5) | 0.53 |
| Surgeon | |||
| Robotic (%) | 19 (26) | 55 (74) | 0.01* |
| Laparoscopic (%) | 28 (35) | 52 (65) | |
| Open (%) | 26 (52) | 24 (48) |
Significant at 5% level (p ≤ 0.05)
In all, 8 cases were missing nephrometry scores, 10 cases were missing information about the exophytic nature of the lesion, and 7 cases were missing information about the anterior/posterior location due to missing/incomplete radiographic imaging or reports
On multivariable analysis, only tumor size and surgeon’s primary approach were significant factors. A patient with a tumor between 2 and <4cm was 73% less likely to undergo active surveillance than one with a lesion <2cm (odds ratio (OR) 0.27; 95% confidence interval [CI], 0.12-0.60). Surgeons primarily using an open approach were more likely to choose active surveillance compared to those using a robotic approach (OR 4.47; CI, 1.76-11.37), whereas no difference in active surveillance rates was observed among those who generally use a laparoscopic versus a robotic approach (OR 1.45; CI, 0.61-3.44).
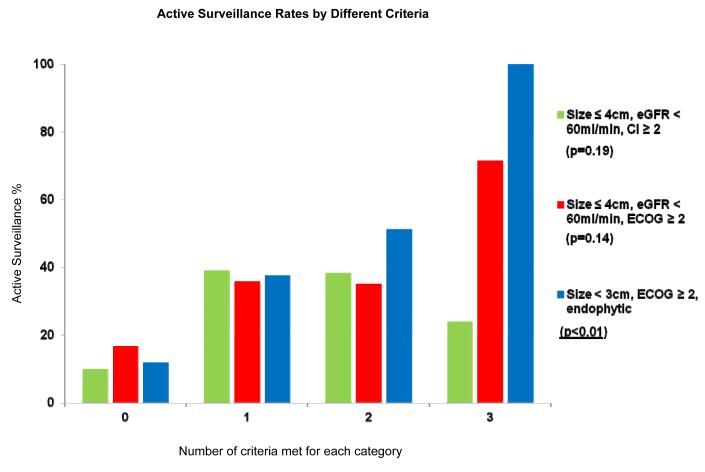
Comparisons of active surveillance rates based on different sets of criteria are shown in Figure 1. The characteristics most predictive of active surveillance are tumor size < 3cm, ECOG PS ≥ 2, and an endophytic lesion (p < 0.01).
Figure 1.
Comparison of active surveillance rates based on different criteria CI = Charlson Comorbidity Index; ECOG = Eastern Cooperative Oncology Group; eGFR = estimated glomerular filtration rate The green column represents the three criteria thought to be most predictive of active surveillance, a priori. The next two columns show other criteria chosen based on our own analyses and also the active surveillance literature. The blue column represents the most predictive characteristics of active surveillance in our study (i.e., tumor size < 3cm, ECOG ≥ 2, and an endophytic lesion). Individuals have a greater chance of undergoing active surveillance the more of these characteristics they possess.
COMMENT
At our institution, patients with clinical T1 lesions were more likely to undergo active surveillance compared to treatment if they lived closer to the hospital (< 60 miles), had poorer performance status (ECOG PS ≥ 2), smaller tumors (tumor size < 3cm), multifocal lesions, endophytic lesions, or were seen by a surgeon who uses primarily open surgery. Contrary to the factors felt to be the most predictive a priori (i.e., tumor size ≤ 4cm, CI ≥ 2 or ECOG PS ≥ 2, and eGFR < 60ml/min), the most predictive criteria of active surveillance were tumor size < 3cm, ECOG PS ≥ 2, and an endophytic lesion.
According to the American Urological Association guidelines for the management of clinical T1 renal lesions, active surveillance is a reasonable treatment option for patients with decreased life expectancy or extensive comorbidities.17 Generally, renal lesions suspicious for cancer based on imaging are resected soon after detection. This treatment paradigm is based on the assumption that the early removal of these lesions translates into improved cancer-specific survival.2 However, mortality rates for kidney cancer have steadily increased over the past several years.18 Further, one-third of elderly patients with a small renal mass will die of other causes.19 Thus, the early treatment of all small renal masses may not be necessary.
In this context, active surveillance is an attractive treatment option for patients with certain characteristics. Patients undergoing active surveillance are typically older with several comorbidities.20,21 Our results support these findings in that active surveillance patients are more likely to have an ECOG PS ≥ 2. Insofar as a CI ≥ 2 is not associated with active surveillance, we suspect that the ECOG score reflects the clinical impression of the patient’s condition more so than the CI, and thus, plays more of a role in the clinical decision-making process. However, whether ECOG PS is in fact a more appropriate measure than CI remains to be seen. Of those undergoing active surveillance, the average patient age was younger (63 ± 14 years) than that reported in prior studies.11,21 Reasons that may explain this observation include seeing patients with poorer overall health or patients with more complex renal lesions. As a tertiary center with a large referral base, both of these contentions are realistic. In addition, patients on active surveillance tended to live closer to the hospital (i.e., <60 miles). Surveillance may be seen as inconvenient to patients traveling greater distances. Interestingly, worsening renal function was not predictive of active surveillance, which may be attributed to the increasing ability to perform nephron-sparing surgery in patients with marginal renal function.
In addition to these patient factors, treatment-related risks encompass an important facet in choosing active surveillance. Some treatment-related factors, such as tumor size, are well-established.11,21 Consistent with prior studies, our average tumor size among active surveillance subjects was 2.3cm. Not surprisingly, patients with multifocal lesions were more likely to undergo active surveillance. Multiple lesions increase the risk of renal failure from extirpative treatment and thus, make active surveillance an attractive option for many of these patients. Other treatment-related risks, however, have generally been less appreciated. Heretofore, anatomic characteristics (e.g., the location or depth of the lesion) have not impacted the use of active surveillance11 and, indeed, were not considered in our initial determination of “ideal” criteria.13 However, in analyzing our data, we found that certain anatomic factors, such as an endophytic lesion, were associated with active surveillance (p=0.04). This observation corroborates findings from a survey of American Urologic Association members about the treatment of patients with small renal masses in which active surveillance was chosen more frequently for perihilar tumors compared to mid kidney or polar lesions.16 Collectively, these findings suggest that anatomic characteristics are important but so far underappreciated in the decision-making process.
The role of tumor factors may be influenced by surgeon preferences. The analysis of surgeon based on primary surgical approach (i.e., open, laparoscopic, robotic) demonstrates that at our institution “open” surgeons favored active surveillance. This may reflect undesirable variation, but more likely represents referral patterns. For instance, patients with more complex lesions may be preferentially referred to open surgeons. This trend may only hold true in academic settings or larger practices in which surgeons are more subspecialized in their practices.
In evaluating candidates for active surveillance we postulated, a priori, that the most predictive factors would be tumor size ≤ 4cm, CI ≥ 2 or ECOG PS ≥ 2, and GFR < 60ml/min. In contrast to these “ideal” criteria, however, we found tumor size < 3cm, ECOG PS ≥ 2, and an endophytic lesion to be most predictive of active surveillance after performing sensitivity analyses. While certain anatomic characteristics may or may not prohibit a partial nephrectomy depending on surgeon skill level or experience (e.g., perihilar location or polarity), it is generally agreed upon that a partial nephrectomy is more challenging for endophytic lesions. This may explain why an endophytic lesion is associated with active surveillance as opposed to the overall nephrometry score. In this cohort, it seems that treatment-related risks were more heavily weighted in patients with complex tumors.
In interpreting our findings, it is important to consider several limitations. First, although our data was collected prospectively, this is an observational study and is subject to potential confounders. We attempted to minimize this effect by including several factors in our multivariable analysis. Second, our small renal mass database comprises all clinical T1 masses, which includes T1b lesions (i.e., between 4cm and 7cm). Factors that may influence surveillance in a patient with a 6cm lesion are different from those in a patient with a 1cm lesion. However, although we included all clinical T1 lesions, only 11% of patients undergoing active surveillance and 15% of patients undergoing treatment had lesions > 4cm, which are comparable to the percentages observed in other active surveillance studies.11,20,21 Third, our findings may not be generalizable to all patients with small renal masses. Our patient population comprises referred patients who often have multiple comorbidities as well as more complex renal lesions. As such, there may be an inclination towards higher rates of active surveillance. However, evidence suggests that fellowship-trained and academic urologists do not prefer active surveillance more than those in the community.16 Third, there are inherent surgical approach selection biases as our surgeons who primarily perform open partial nephrectomy are referred the more complex renal masses; this, at least in part, may explain their greater tendency toward active surveillance. Given the large number of surgeons in our study, it is difficult to analyze each surgeon individually to determine their active surveillance tendencies. However, it is important to note that surgeons who prefer the open approach choose active surveillance more frequently. The relationship between individual surgeons and active surveillance deserves further attention. Fourth, in performing the sensitivity analyses, some of the subgroups were composed of a small number of patients. These results require validation in future studies. Lastly, our small renal mass database began accruing patients in June, 2009. As of now, more extended follow up is necessary to assess the association between active surveillance and disease-specific outcomes, such as rates of progression to metastatic disease, cancer-specific and overall mortality. These outcomes will help better judge which patients are appropriate for active surveillance.
Despite these limitations, this study highlights specific patient and tumor characteristics that predict the current use of active surveillance for small renal masses at an academic institution. As the incidence of kidney cancer continues to rise, active surveillance will likely play an expanding role in the management of small renal masses. Accordingly, better understanding the drivers of active surveillance, and the ability to more appropriately select patients for this treatment strategy is crucial. To better risk-stratify patients with small renal masses, our practice has begun to routinely discuss a percutaneous biopsy with patients who have a lesion ≤ 4cm. In our study, nearly 40% of patients underwent a biopsy, of which 15% were either benign or oncocytoma. Obtaining a renal biopsy provides an opportunity to identify benign or indolent cancerous lesions, which may obviate the risks of surgical intervention in a subset of patients with small renal masses.4
CONCLUSIONS
Patient, tumor, and surgeon characteristics all influence active surveillance for small renal masses. Based on sensitivity analyses, active surveillance was driven by tumor size <3cm, poor performance status (i.e., ECOG PS ≥ 2), and an endophytic lesion.
Acknowledgments
Bruce Jacobs is supported by funding from the National Institutes of Health T32 Training Grant NIH 5 T32 DK007782-12
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Parsons JK, Schoenberg MS, Carter HB. Incidental renal tumors: casting doubt on the efficacy of early intervention. Urology. 2001;57:1013–1015. doi: 10.1016/s0090-4295(01)00991-8. [DOI] [PubMed] [Google Scholar]
- 3.Chow WH, Devesa SS, Warren JL, et al. Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 4.Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 5.Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol. 2001;166:6–18. [PubMed] [Google Scholar]
- 6.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau WK, Blute ML, Weaver AL, et al. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc. 2000;75:1236–1242. doi: 10.4065/75.12.1236. [DOI] [PubMed] [Google Scholar]
- 8.Thompson RH, Boorjian SA, Lohse CM, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008;179:468–471. doi: 10.1016/j.juro.2007.09.077. discussion 472-473. [DOI] [PubMed] [Google Scholar]
- 9.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 10.Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective randomized EORTC intergroup phase 3 study comparing the complications of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2007;51:1606–1615. doi: 10.1016/j.eururo.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Chawla SN, Crispen PL, Hanlon AL, et al. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol. 2006;175:425–431. doi: 10.1016/S0022-5347(05)00148-5. [DOI] [PubMed] [Google Scholar]
- 12.Crispen PL, Viterbo R, Fox EB, et al. Delayed intervention of sporadic renal masses undergoing active surveillance. Cancer. 2008;112:1051–1057. doi: 10.1002/cncr.23268. [DOI] [PubMed] [Google Scholar]
- 13.Wolf JS, Jr., Hollenbeck BK. A plea for judicious treatment of small renal masses. Urol Oncol. 2007;25:281–283. doi: 10.1016/j.urolonc.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Roux AV Diez, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 15.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844–853. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 16.Breau RH, Crispen PL, Jenkins SM, et al. Treatment of patients with small renal masses: a survey of the American Urological Association. J Urol. 2010;185:407–413. doi: 10.1016/j.juro.2010.09.092. [DOI] [PubMed] [Google Scholar]
- 17.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271–1279. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Ries LAG, Krapcho M, Stinchcomb DG, et al., editors. SEER Cancer Statistics Review, 1975-2005. National Cancer Institute; 2008. [Google Scholar]
- 19.Hollingsworth JM, Miller DC, Daignault S, et al. Five-year survival after surgical treatment for kidney cancer: a population-based competing risk analysis. Cancer. 2007;109:1763–1768. doi: 10.1002/cncr.22600. [DOI] [PubMed] [Google Scholar]
- 20.Haramis G, Mues AC, Rosales JC, et al. Natural History of Renal Cortical Neoplasms During Active Surveillance With Follow-up Longer Than 5 Years. Urology. 2011;77:787–791. doi: 10.1016/j.urology.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 21.Rosales JC, Haramis G, Moreno J, et al. Active surveillance for renal cortical neoplasms. J Urol. 2010;183:1698–1702. doi: 10.1016/j.juro.2010.01.024. [DOI] [PubMed] [Google Scholar]