Abstract
Mice are now commonly used as models of human cardiovascular diseases and conditions, but it is challenging to measure blood flow velocity in small vessels such as coronary arteries. Accordingly, we have developed a method using a 2 mm diameter 20 MHz pulsed Doppler probe applied to the chest of an anesthetized mouse to measure left main coronary blood flow velocity noninvasively. We also found that coronary flow velocity could be increased from baseline (B) to hyperemic (H) levels by changing the concentration of isoflurane gas anesthesia from 1% to 2.5% in oxygen and that the H levels are similar to or higher than those induced by adenosine. We used the ratio H/B to estimate coronary flow reserve (CFR) in young, adult, and old mice and in mice with atherosclerosis, coronary occlusion, pressure overload, and angiotensin infusion. We found that H/B increases with age from 2.4 (young) to 3.6 (old) and is reduced by all forms of coronary and vascular disease to as low as 1.1 by pressure overload. We conclude that CFR can be measured noninvasively and serially in mice as their cardiovascular systems adapt and remodel to various imposed or natural conditions, and that left main coronary flow reserve may be a good index of global cardiac function.
Keywords: Doppler ultrasound, noninvasive, cardiovascular physiology, hyperemia, blood flow, coronary circulation
I. Introduction
The noninvasive measurement of coronary blood flow by ultrasound is difficult in both man and animals because coronary arteries are small, are highly branched, lie deep within the chest, and are in constant motion due their attachment to the epicardial surface. Thus, measurements of coronary flow or velocity have required the use of invasive methods such as implantable flow probes [1] or coronary catheters [2]. In addition, coronary blood flow (even if it could be measured accurately) is often normal at rest even in the presence of severe coronary artery disease [2,3]. This problem is commonly addressed by administering a coronary vasodilator such as adenosine [4] to increase blood flow and then measuring the ratio of maximum hyperemic flow to resting baseline flow as an index of coronary flow reserve (CFR)[3]. Coronary flow reserve has been shown to be reduced in the presence of coronary lesions due to a reduction in hyperemic flow [3], and by other cardiac pathologies due to an increase in baseline flow[4].
The administration of a specific and maximal coronary vasodilator such as adenosine [4, 5] is necessary to evaluate coronary flow reserve, and this is much more problematic in mice where the veins are more difficult to cannulate and the tolerated doses and volumes are much smaller. Fortuitously, one of the most widely used anesthetic agents (isoflurane gas) is also a coronary vasodilator when administered at higher concentrations [6]. The use of an inhaled coronary vasodilator, if effective and well-tolerated, would greatly simplify the estimation of coronary flow reserve in mice and make the procedure truly noninvasive and amenable to high throughput.
Recently noninvasive Doppler ultrasound has been used to estimate coronary flow reserve in man using adenosine [7] and other agents to increase coronary flow. There have also been reports showing coronary flow velocity signals recorded noninvasively from mice [5]. Although the signals shown were identifiable and quantifiable, they were often of sub-optimal quality and fidelity. This encouraged us to test the feasibility of using a smaller and more focused Doppler probe to measure coronary flow velocity and to estimate reserve in mouse models of aging and cardiovascular abnormalities. Thus, we report here a noninvasive method using Doppler ultrasound [8] to measure left main coronary flow velocity at rest and during hyperemia induced by isoflurane in young, adult, and old mice, and in mice with atherosclerosis (ApoE−/−), coronary occlusion, pressure overload, and chronic angiotensin infusion.
II. Methods
Seven groups totaling 68 mice were studied following protocols approved by the Institutional Animal Care and Use Committees of Baylor College of Medicine and MD Anderson Cancer Center. The groups consisted of 10 young, 28 adult, and 10 old wild-type mice, and 20 old ApoE−/− mice. Mice were anesthetized in a closed chamber with 3% isoflurane in oxygen for 2 to 5 minutes until immobile. Each mouse was then removed, weighed, and taped supine to ECG electrodes on a heated (35–37 °C) procedure board with isoflurane supplied by a nose cone connected to an anesthesia machine. Next a 2 mm diameter 20 MHz Doppler probe was clamped to a micromanipulator attached to the procedure board as shown in Figure 1. The probe tip was placed on the left chest and pointed horizontally toward the origin of the left main coronary artery at a 2.5 mm depth setting. Coronary flow signals were identified on the Doppler spectral display by flow toward the probe peaking in early diastole as illustrated in Figure 2. In this orientation, the sound beam was nearly parallel to the axis of the left main coronary artery. The concentration of isoflurane was then reduced to 1% to lower coronary flow to a baseline level and a two-second sample of the ECG and the raw quadrature Doppler signals was acquired and stored in a computer file for later analysis [8]. Then the isoflurane level was increased to 2.5% to increase coronary flow, and when velocity was stabilized and optimized, more signals were stored. In 10 of the adult mice, the transverse aorta was then stenosed to 0.4 mm diameter to produce pressure overload; in 3 adult mice, the left anterior descending coronary artery was occluded to produce a myocardial infarction; and in 9 adult mice angiotensin was infused via an implantable pump for 6 wks to increase pressure and heart rate. In these mice the coronary measurements were repeated at intervals up to 6 wks. In another group of 6 normal adult mice, coronary velocity was measured with isoflurane at 1.5% before and after infusion of adenosine as a coronary vasodilator via the tail vein.
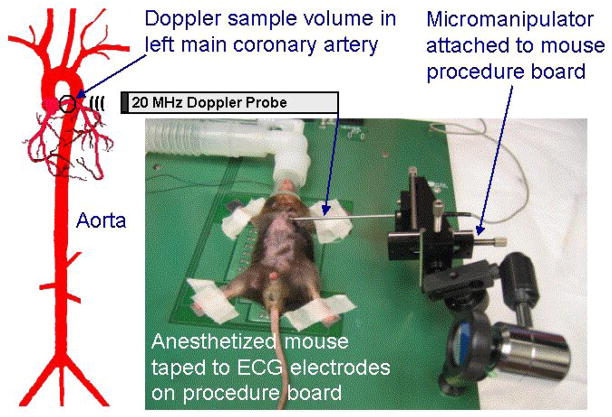
Figure 1.
Drawing showing a Doppler sample volume placed over the left main coronary artery of a mouse and a photo showing a mouse and a Doppler probe held in a micromanipulator.
Figure 2.
Simultaneous ECG and velocity signals from a mouse showing the waveform and timing of coronary flow with respect to aortic and carotid flows. The vertical bars show the beginning and end of ejection. Coronary flow occurs primarily during diastole.
The Doppler instrumentation consisted of a 2 mm diameter 20 MHz single-element ultrasonic transducer focused at 4 mm and connected to a 20 MHz pulsed Doppler instrument both of which were constructed in our laboratory [8]. The Doppler instrument was optimized for use in mice by setting the burst length to 8 cycles (400 ns) and the pulse repetition frequency to 125 kHz. These settings allow the measurement of velocities as high as 4.5 m/s at a maximum sample volume depth of 6 mm. The quadrature audio signals from the pulsed Doppler were connected to an Indus Doppler Signal Processing Workstation.
Data (quadrature audio Doppler signals and lead 2 ECG) were sampled at 125 kHz and stored in 2-second files on a personal computer for later analysis. During analysis the Doppler signals were processed by a complex fast Fourier transform (FFT), displayed on the workstation, and the peak spectral frequency or envelope of the spectrum was converted to velocity (V) using the Doppler equation: V = cΔf/focosθ, where c is the speed of sound in blood (~1,570 m/s), Δf is the Doppler frequency, fo is the ultrasonic frequency (20 MH z), and θ is the angle between the sound beam and the direction of flow (0o). In some mice we measured the spectral peak diastolic velocity (V PD), and in others the spectral peak velocity averaged over the cardiac cycle (V AVG). The hyperemic (H) to baseline (B) ratio of coronary flow (H/B) was calculated as the ratio of V PD or V AVG at the highest flow attained during the high level of isoflurane to V PD or V AVG at the minimum baseline flow obtained at the lowest level of isoflurane. Data are presented as mean +/− SEM, and statistical significance is defined as P < 0.05.
III. Results
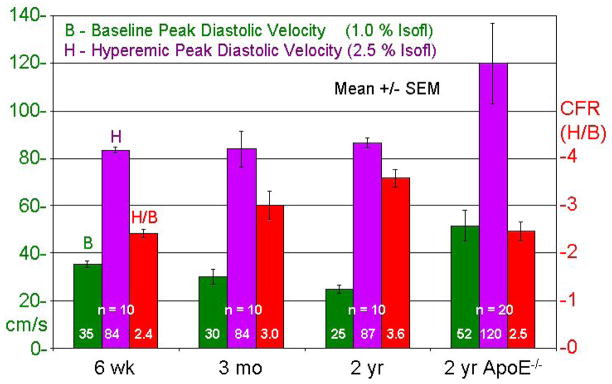
High quality coronary flow velocity signals were obtained from all animals up to 5 times under baseline and hyperemic flow conditions as illustrated in Figure 3. Figure 4 shows B and H (based on V PD), and H/B in young, adult, old, and ApoE−/− mice. Baseline velocities were statistically different between the groups, but hyperemic velocities in the wild-type groups were similar. The H/B ratio increased significantly with age, but was significantly lower in the ApoE−/− mice than in the age-matched old mice. In addition, baseline and hyperemic coronary velocities were significantly higher and with much more scatter in ApoE−/− mice.
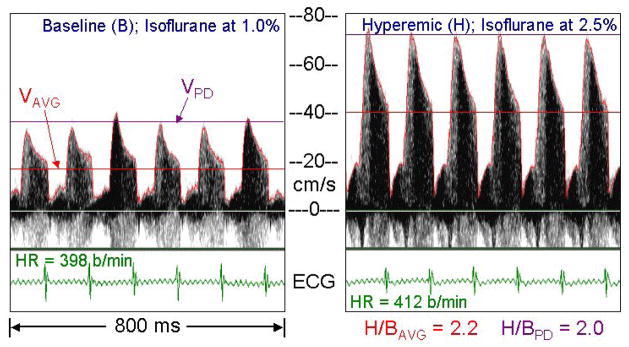
Figure 3.
Left main coronary velocity from a mouse anesthetized at low and high levels of isoflurane showing its selective coronary vasodilator effects with minimal changes in heart rate (HR). The red curves are the envelopes of the spectra, the red lines (VAVG) are the means of the envelopes over a cardiac cycle, and the violet lines (VPD) are the peak diastolic velocity.
Figure 4.
Baseline (B) and hyperemic (H) velocities and H/B for young, adult, old, and ApoE−/− mice based on peak diastolic velocities.
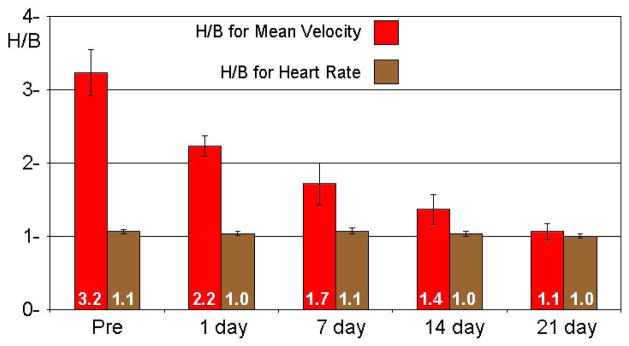
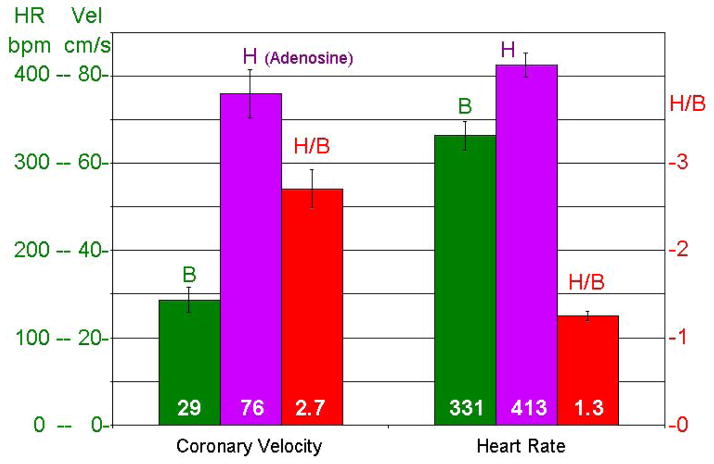
Figure 5 shows H/B for average or mean coronary velocity and heart rate at the two concentrations of isoflurane in 10 mice before and at 1, 7, 14, and 21 days after aortic banding. The response of velocity (H/B) decreased progressively after banding and remodeling while the heart rate response to isoflurane was minimal and did not change after banding. Figure 6 shows the responses of velocity and heart rate to adenosine in 6 normal adult mice with isoflurane kept at 1.5%.
Figure 5.
Hyperemic/Baseline (H/B) for mean coronary velocity and heart rate in 10 mice before and up to 21 days after transverse aortic banding.
Figure 6.
Baseline, Hyperemic, and H/B ratios for coronary velocity and heart rate in 6 mice before (B) and after (H) adenosine infusion.
Figure 7 shows B, H, and H/B for peak diastolic coronary velocity in several groups of mice by age (6 wk, 3 mo, or 2 yr), genetic background (ApoE), or intervention (transverse aortic band, LAD coronary occlusion, or angiotensin infusion). In general, H/B was reduced by all of the interventions studied.
Figure 7.
Summary of Baseline (B), Hyperemic (H), and H/B values of peak diastolic coronary velocity using isoflurane at 1% and 2.5% in several groups of mice by age or intervention.
IV. Discussion
In this report we demonstrated a simple and noninvasive method based on Doppler ultrasound to measure coronary flow velocity in mice under baseline and hyperemic conditions created by changing the concentration of isoflurane gas and documented differences in H/B as an estimate of coronary flow reserve (CFR) due to age, atherosclerosis, aortic constriction, coronary occlusion, or angiotensin infusion. We were able to obtain adequate coronary velocity signals in all mice at all time points without image guidance, and the average time to complete a measurement was less than 25 minutes per animal.
The waveform of epicardial coronary arterial flow in humans and other animals has a characteristic shape and timing which is easy to recognize, and the same is true in mice as illustrated in Figure 2. Coronary flow starts up when aortic flow ceases, reaches a peak of 25–50 cm/s in early diastole, and then decays. At the start of isovolumic contraction, flow drops to nearly zero followed by a significantly lower systolic component. After locating a coronary velocity signal, we adjusted the probe position and sample volume depth to maximize the Doppler frequency and signal strength. This procedure ensured a sample volume location close to the origin of the left coronary artery in normal mice and at any lesion of the proximal left main coronary artery in the ApoE−/− mice. The small variation in peak diastolic hyperemic velocities in the normal young, adult, and old mice (range: 73 to 95 cm/s) suggests that the sample volume was in a consistent position in all mice.
In some of the mice in this report we measured the diastolic peak of the spectral envelope of coronary velocity and in others we measured the mean of the envelope averaged over the cardiac cycle. Peak diastolic velocity is often used in the calculation of CFR, but in the banded mice there was a significant increase in the systolic component of coronary flow. In these mice we found that using peak diastolic instead of mean velocity could under-estimate CFR by 1.5% to 14.6%.
The coronary vasodilator properties of isoflurane have been known for many years, but it has not been used for estimating CFR. We found that using step changes in concentration from 1% to 2.5% elicited maximum changes in coronary flow velocity with minimal (5%) changes in heart rate. At concentrations below 1%, the mice became aroused and above 2.5% there were no further increases in coronary flow velocity. Figure 6 shows that adenosine administered as an infusion via the tail vein increases in left main coronary velocity to a value similar to that at the higher level of isoflurane. The H/B levels we measured in adult mice (3.2+/−0.3) are higher than those reported by Wikstrom, et al [9] and other groups using adenosine (1.9+/−0.2), and are closer the values reported for man (2.5–5.0) [4]. It is likely in these other studies that the baseline levels for coronary velocity were higher than true baseline because isoflurane was used at levels between 1% and 2% for anesthesia, and the investigators were unaware of the coronary vasodilator effect. Indeed, it is difficult to overestimate CFR given the difficulty in forcing coronary flow below minimum or above maximum. This suggests that the ratio of hyperemic to baseline coronary velocity in response to high and low levels of isoflurane may be valid index of CFR in mice and that isoflurane may be superior to adenosine as a specific coronary vasodilator in mice.
We found that CFR was increased with age in healthy mice and was decreased with atherosclerosis, pressure overload hypertrophy, LAD coronary artery occlusion, and chronic angiotensin infusion. We also found increases in both baseline and hyperemic velocity in many ApoE mice consistent with the presence of non-flow limiting lesions in the left main coronary artery. In man CFR generally decreases with age, but it is hard to eliminate the effects of underlying disease processes which increase in severity with age. The decreases in CFR with disease in mice are consistent with observations in man [4].
In the absence of coronary artery disease (ApoE or LAD Occl), or changes in arterial pressure which dilate the left main coronary artery (TA Band), peak hyperemic diastolic velocity is nearly the same (~80 cm/s) in all mice studied. When the left main coronary artery is dilated (TA Band), H is reduced; when it is constricted by disease (ApoE), H is increased; and when the perfusion bed is reduced (LAD Occl), H is also reduced. This suggests that maximum coronary perfusion or volume flow is unchanged by loading.
Baseline coronary flow reflects baseline cardiac loading, and baseline velocity was more variable in the groups studied. In the TA Band and Angiotensin mice, B was higher than in the age-matched 3mo old mice because of higher loading. It is interesting to note that B in the LAD Occl mice is similar to B in the 3mo old mice despite the reduction in perfusion territory. This suggests that the perfused part of the occluded heart requires the same amount of blood flow as the whole heart to supply the same baseline demand.
Others have found that cardiac function as measured by ejection fraction and diameter shortening fraction was maintained after banding for up to 4 weeks after which the mice went into decompensated failure as evidenced by ventricular dilation and reduced shortening fraction at 5 weeks with 60% dying within 8 weeks. It is thought that resting cardiac function is maintained as long as CFR is over 1.0, and that when CFR drops below 1.0, heart failure ensues [10]. The trend in CFR shown in Figure 5 suggest that these mice may have gone into decompensated failure beyond 3 weeks.
V. Conclusions
We have shown here the serial and repeated measurement of coronary flow velocity in mice. The use of isoflurane gas at low and high concentrations as a coronary vasodilator when coupled with a method such as Doppler ultrasound to measure left main coronary flow or velocity provides a convenient and noninvasive method to estimate global coronary flow reserve in mice. Coronary flow reserve increases with age, is reduced by several models of cardiovascular disease, and is virtually eliminated 21 days after transverse aortic banding. The data presented show that the murine left ventricle and its coronary circulation respond similarly to those of man and dogs when the heart is subjected to coronary stenosis, occlusion, or chronic pressure overload. The reductions in coronary reserve suggest similar reductions in cardiac reserve which when exhausted is a prelude to decompensated heart failure. Thus, left main coronary flow reserve may serve as an index of global cardiac functional reserve.
Acknowledgments
The authors wish to acknowledge the contributions of Thuy Pham, Jennifer Pocius, Ross Hartley, and James Brooks for technical, surgical, and editorial assistance.
Footnotes
Presented at the 32nd International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC2010) held in Buenos Aires, Argentina, Aug. 31 – Sep. 4, 2010.
References
- 1.Hartley CJ, Cole JS. An ultrasonic pulsed doppler system for measuring blood flow in small vessels. J Appl Physiol. 1974;37:626–629. doi: 10.1152/jappl.1974.37.4.626. [DOI] [PubMed] [Google Scholar]
- 2.Cole JS, Hartley CJ. The pulsed Doppler coronary artery catheter: Preliminary report of a new technique for measuring rapid changes in coronary artery flow velocity in man. Circulation. 1977;56:18–25. doi: 10.1161/01.cir.56.1.18. [DOI] [PubMed] [Google Scholar]
- 3.Gould LK, Lipscomb K, Hamilton GW. Physiologic basis for assessing critical coronary stenosis: Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol. 1974;33:87–94. doi: 10.1016/0002-9149(74)90743-7. [DOI] [PubMed] [Google Scholar]
- 4.Marcus ML. The coronary circulation in health and disease. New York: McGraw-Hill; 1983. [Google Scholar]
- 5.Wikstrom J, Gronros J, Bergstrom G, Gan LM. Functional and morphologic imaging of coronary atherosclerosis in living mice using high-resolution color Doppler echocardiography and ultrasound biomicroscopy. J A C C. 2005;46:720–727. doi: 10.1016/j.jacc.2005.04.053. [DOI] [PubMed] [Google Scholar]
- 6.Reiz S, Balfors E, Sorensen MB, Ariola S, Friedman A, Truedsson H. Isoflurane - a powerful coronary vasodilator in patients with coronary artery disease. Anesthesiology. 1983;59:91–97. doi: 10.1097/00000542-198308000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Neishi Y, Akasaka T, Tsukiji M, Kume T, Wada N, Watanabe N, Kawamoto T, Kaji S, Yoshida K. Reduced coronary flow reserve in patients with congestive heart failure assessed by transthoracic Doppler echocardiography. J Am Soc Echocard. 2005;18:15–19. doi: 10.1016/j.echo.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Hartley CJ, Reddy AK, Madala S, Martin-McNulty B, Vergona R, Sullivan ME, Halks-Miller M, Taffet GE, Michael LH, Entman ML, Wang YX. Hemodynamic changes in apolipoprotein E-knockout mice. Am J Physiol Heart Circ Physiol. 2000;279:H2326–H2334. doi: 10.1152/ajpheart.2000.279.5.H2326. [DOI] [PubMed] [Google Scholar]
- 9.Wikstrom J, Gronros J, Gan LM. Adenosine induces dilation of epicardial coronary arteries in mice - Relationship between coronary flow velocity reserve and coronary flow reserve in vivo using transthoracic echocardiography. Ultrasound in Med & Biol. 2008;34:1053–1062. doi: 10.1016/j.ultrasmedbio.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Vatner SF, Hittinger L. Coronary vascular mechanisms involved in decompensation from hypertrophy to heart failure. J Am Coll Cardiol. 1993;22:34A–40A. doi: 10.1016/0735-1097(93)90460-i. [DOI] [PubMed] [Google Scholar]