Abstract
This pilot study compared the efficacy of orlistat as an adjunctive treatment for obesity between African American and Caucasian adolescents. 20 obese adolescents with obesity-related co-morbid conditions underwent measurements of body composition, glucose homeostasis by frequently sampled intravenous glucose tolerance test (FSIGT), and fasting lipids before and after 6-months’ treatment with orlistat 120mg TID in conjunction with a comprehensive behavioral program.
Weight (p< 0.05), BMI (p<0.001), total cholesterol (p<0.001), LDL cholesterol (p<0.001), fasting insulin (p<0.02) and fasting glucose (p<0.003) were lower after treatment. Insulin sensitivity, measured during the FSIGT, improved significantly (p<0.02), as did fasting indices such as the homeostasis model assessment for insulin resistance (p<0.01). African American subjects exhibited significantly less improvement in weight (p<0.05), BMI (p<0.01), waist circumference (p=0.03), and insulin sensitivity (p=0.05). Improvements in cholesterol were not significantly different between African Americans and Caucasians.
We conclude that Caucasians lost more weight and had greater improvements in insulin sensitivity than African Americans, but both exhibited improvements in plasma lipids. The true benefit of orlistat treatment over a comprehensive behavioral program remains to be determined in placebo-controlled trials.
Keywords: obesity, weight loss, adolescence, pharmacotherapy, orlistat, diabetes, lipids
Introduction
Excess weight among adolescents is an increasingly prevalent problem in the United States, especially among African American teenagers. The prevalence of overweight in adolescents, defined as a BMI greater than or equal to the age-and sex-specific 95th percentile for BMI, has risen from 6% in NHANES 1 (1971–1974) to 10.5% in NHANES III (1988–1994) and 15.5% in 1999–2000 1. For African American adolescents, the 1999–2000 prevalence rate is 23.6%, a 10% increase since NHANES III. With the rise in weight, the medical conditions associated with excess adiposity, especially Type 2 Diabetes, have also become more common among adolescents 2. As is the case for adults, conventional means of weight loss, consisting of healthy diet and moderate exercise, are not uniformly successful in adolescents 3–5. Therefore, it is important to explore the efficacy of pharmacotherapeutic agents for obesity in adolescents who already manifest the co-morbid conditions associated with obesity, particularly in African Americans, who are disproportionately affected by the complications of obesity and may have less response than Caucasians to obesity interventions 6–14.
Currently, there are no medications approved by the FDA for the treatment of obesity in adolescents less than 16 years of age. Orlistat is a gastric and pancreatic lipase inhibitor that reduces the absorption of triglyceride from the gastrointestinal tract 15, but is minimally absorbed itself 16. In adults, orlistat at the standard dose, 120 mg TID, inhibits approximately 30% of triglyceride absorption 15 with few adverse effects 17. In placebo-controlled studies, adults treated with orlistat for periods as long as 2 years exhibited greater average weight loss, better weight maintenance, lower total and LDL cholesterol, and, in those with type 2 diabetes, improved glycemic control 16–28.
We have previously presented preliminary results on the use of orlistat for 3 months in overweight adolescents 12–17 y of age that implied a favorable risk-benefit ratio in adolescents 29. Here we report the results of a 6-month pilot study conducted to examine the safety and efficacy of orlistat in African American and Caucasian adolescents.
Methods
Subjects
Twenty overweight African American and Caucasian adolescents (12–17 y) with mean body mass index (BMI) 44.1 ± 12.5 kg/m2, were recruited for a weight-loss study through newspaper advertisements and letters to local physicians (Table 1). Inclusion criteria were BMI greater than the NHANES I (1971–1974) 95th percentile for age, sex, and race 2, and the presence of at least one obesity-related co-morbid condition such as: hypertension, hyperinsulinemia (insulin ≥ 15 uU/L), impaired fasting glucose (glucose between 110–125 mg/dL), impaired glucose tolerance (glucose between 140–200 mg/dL 2h after), type 2 diabetes, or hyperlipidemia (total triglyceride ≥ 200 mg/dL, total cholesterol > 200 mg/dL, or LDL cholesterol ≥ 130 mg/dL). All subjects had unsuccessfully attempted weight reduction, although not all had previously participated in formal weight loss programs. Individuals were excluded if a significantly compromising pulmonary, hepatic, cardiac or musculoskeletal disorder was present. Additional exclusion criteria were a history of substance abuse or other psychiatric disorder that would impair compliance with the study protocol, the recent use of anorexiant medication, or significant weight loss (≥5%) in the previous 6 months. Each adolescent gave written assent, and a parent or guardian gave written consent, for protocol participation. The protocol was approved by the Institutional Review Board of the National Institute of Child Health and Human Development.
Table I.
Subject’s baseline demographics
| Sex* Race |
10 Female, 10 Male 10 Caucasian, 10 African-American |
|
|---|---|---|
| Mean ± SD | Range | |
| Height (cm) | 166.9 ± 10.4 | 151 – 192 |
| Age (y) | 14.6 ± 2.0 | 12.0 – 17.9 |
| Pubic Hair Tanner Stage | 4** | 1 – 5 |
| Girls' Breast Tanner Stage | 5** | 2 – 5 |
| Boys' Testicular Volume (mL) | 8.5 ± 6.5 | 2 – 20 |
| Co-morbidities associated with obesity: | Caucasians | African-American |
| Type 2 Diabetes | 0% | 10% |
| Glucose Intolerance | 10% | 0% |
| Hyperinsulinemia | 100% | 100% |
| Hyperlipidemia | 0% | 30% |
| Hypertension | 10% | 10% |
5 African Americans and 5 Caucasians were males and a similar number were females.
Median and modal value
Protocol
Subjects who met eligibility requirements were admitted to the Warren Magnuson Clinical Center at the NIH for inpatient evaluation at three time-points: before starting orlistat treatment (baseline), after 3 months, and again after 6 months of orlistat treatment. During the first hospitalization, a registered dietitian provided instruction on a 500 kcal-deficit diet containing no more than 30% of calories from fat. These instructions were reviewed during subsequent visits.
Subjects were prescribed orlistat, 120 mg TID with meals, plus a multivitamin supplement containing 5000 IU vitamin A (80% as retinol acetate, 20% as beta carotene), 400 IU vitamin D (as ergocalciferol), 30 IU vitamin E (as di-alpha tocopheryl acetate), and 25 mcg vitamin K (as phytonadione), to be taken at night. Orlistat was supplied by Roche Pharmaceuticals under a material transfer agreement. Concomitantly, subjects participated in a 12-week comprehensive behavioral program led by two registered dietitians, one of whom had graduate training in psychology, and a recreation therapist who was also a health fitness instructor. This program is described in depth elsewhere 29. Compliance was gauged through self-monitoring recorded in progress books returned to the group leaders each week. Points were awarded each week contingent on a minimum one-half pound weight loss and a completed progress book. After the first 3 months, subjects returned for follow-up evaluations monthly. Medication compliance was estimated by subject self-report and through counts of the unused medication returned at monthly intervals.
Body composition
Weight was obtained weekly for the first 12 weeks, and then monthly, to the nearest 0.1 kg using a calibrated digital scale (Scale-Tronix, Wheaton, IL). Subjects were instructed to void before measurements were obtained, and measurements were made with the subject in minimal clothing and without shoes. Other anthropometric measurements were obtained at 3-month intervals. Height was obtained in triplicate using a stadiometer (Holtain Ltd., Crymych, Wales) calibrated to the nearest 1 mm. Body Mass Index (BMI) was calculated as the weight in kg divided by the height in meters, squared. Waist circumference was obtained with a flexible, non-stretching, tape measure 30. Subjects underwent air displacement plethysmography (ADP) to determine body density in the morning after an overnight fast (Life Measurement Instruments, Concord, CA), according to the manufacturer’s directions and procedures previously described 31–35. The validity of this instrument has been previously established 32, 33, 36, 37. Subjects were assessed in minimal clothing (either underwear or a tight-fitting bathing suit) and wearing a swim cap. The equation of Siri was used to estimate body adiposity from body density 38.
Resting Energy Expenditure (REE)
REE was assessed by open-circuit indirect calorimetry using a respiratory metabolic cart (Deltatrac II, SensorMedics Corp., Yorba Linda, CA). Before each test, the calorimeter was calibrated with a reference gas mixture (96% oxygen and 4% carbon dioxide). Subjects were studied in the morning after an overnight fast, during their inpatient hospitalizations. Subjects consumed a standardized diet consisting of 25 ± 3.7% calories from fat and 56.6 ± 2.6% calories from carbohydrate for 1–3 days before REE was determined. Before measurement, subjects were asked to void, and then returned to rest for a minimum of 30 minutes. Subjects were asked to minimize their movements, and were assessed while watching children’s videos. Measurements were recorded for a minimum of 30 minutes at one-minute intervals. The first five minutes (equilibration) and any value associated with excessive motion or a respiratory quotient (RQ) exceeding 0.95 were excluded from the analysis.
Laboratory Measurements
Blood specimens were collected for analysis of metabolic, hepatic, renal, and hematologic function: thyroid stimulating hormone; free T4; leptin; total, LDL, and HDL cholesterol; triglycerides; apolipoproteins; glycohemoglobin; glucose; insulin; vitamins A, D, E, and K; the minerals calcium, phosphorus, magnesium, zinc and iron; percent transferrin saturation; intact PTH; osteocalcin; and both total and bone-specific alkaline phosphatase. Mineral levels (except zinc), cholesterol, triglyceride, glucose, and transferrin were measured on a Hitachi 917 analyzer using reagents from Roche Diagnostics (Indianapolis, IN). Zinc analysis was performed at Mayo Medical Laboratories by inductively-coupled plasma emission spectroscopy 39. Plasma leptin was measured in duplicate by RIA (Linco, St. Louis, MO). Direct determinations of LDL- and HDL-cholesterol were performed on a Cobas FARA analyzer (Roche Diagnostics, Indianapolis, IN) using reagents from Sigma Chemical (St. Louis, MO). Apolipoproteins A1 and B were measured on an Array nephelometer (Beckman-Coulter, Brea, CA). Insulin and T3 levels were determined using an Immulite2000 machine (Diagnostic Product Corp., Los Angeles, CA). Vitamin A (retinol) was determined using a liquid-chromatographic assay 40. Vitamin D (cholecalciferol) was measured via RIA with a 125I-labeled tracer 41. Vitamin E (tocopherol) and vitamin K (phylloquinone) were determined by HPLC using post-column chemical reduction and fluorimetric detection 42, 43. Total and undercarboxylated osteocalcin were determined by RIA as described by Gundberg, et al 44. Intact PTH and TSH were measured on a Nichols Advantage (Nichols Institute Diagnostics, San Juan Capistrano, CA). Bone-specific alkaline phosphatase was measured by immunoradiometric assay (IRMA) (Esoterix Endocrine Sciences, Calabasas Hills, CA).
A 24-hr urine was collected for measurement of calcium, phosphorus, creatinine, and oxalate excretion. A 72-hour stool, whose beginning and end were detected by having subjects ingest methylene blue dye 45, was collected for measurement of fecal fat excretion. This analysis was performed as previously described by Mayo Medical Laboratories 46. Gallbladder and renal ultrasound were obtained by standard methodology on all subjects.
Frequently sampled intravenous glucose tolerance test (FSIGT)
At each inpatient evaluation, subjects underwent FSIGT. Dextrose, 0.3 g/kg, was administered intravenously as a smooth bolus over two minutes. Blood samples were collected through a second intravenous line for glucose and insulin at 2, 3, 4, 5, 6, 8, 10, 14, 19, 22, 24, 27, 30, 35, 40, 45, 50, 70, 90, 120, 150, 180, 210, 240, 270, and 300 minutes, and for C-peptide at 120 minute intervals. Insulin, 0.05 U/kg, was given intravenously as a bolus at 20 minutes. Insulin sensitivity and glucose effectiveness were assessed according to Bergman's minimal model 47 using the SAAM II program version 1.11 (SAAM Institute, Inc., Seattle, WA). Fasting values of insulin and glucose were analyzed by the homeostatic method of analysis for the Insulin Resistance Index (HOMA-IRI) and the percent beta-cell function 48, 49. The Quicki Index, another indicator of insulin resistance, (1 / log fasting glucose (mg/dL) + log fasting insulin (uU/mL)), was also calculated 50–52.
Monitoring of adverse events
Subjects were queried weekly about adverse events for the first 12 weeks, and monthly thereafter, with particular attention directed toward the expected gastrointestinal effects of orlistat. In addition, at 3-month intervals, a clinical pharmacist interviewed subjects with a comprehensive questionnaire employing a review-of-systems approach to identify expected and unexpected adverse events 53.
Statistical analysis
Parametric data were analyzed on a Macintosh G3 using StatView 5.0.1, and SuperAnova 1.11 software (Abacus Concepts, Inc., Berkeley, CA). All data were log-transformed before calculation of the geometric mean and standard deviation. Paired, two-tailed T-tests were conducted on the log-transformed data to determine differences between weights, blood lipids, glycemic indices, and other laboratory parameters at baseline versus 6 months of orlistat therapy. For the preplanned analysis between African-American and Caucasian subjects, data were expressed as the change from baseline due to the large differences in baseline values and analysis of covariance was used to determine the difference in response adjusted for the initial value at baseline. Analysis of covariance was also used to study differences from baseline and changes in REE adjusted for LBM and BFM at 3 months and six months. Least squares means are reported for ANCOVA results. Post-hoc tests were corrected for multiple comparisons using the Bonferroni-Holm procedure. Because three subjects (15%) withdrew from the study prior to completion of the 12-week treatment program, all comparisons are performed as intention-to-treat analyses. Data from each variable for which at least one follow-up measurement was available are reported as last observation carried forward. For log-transformed data, the back-transformed (geometric) means are reported.
Results
Subjects’ baseline demographic characteristics are given in Table 1. Height increased on average by 1.5 ± 1.8 cm over the 6-month treatment period (p<0.002). Height, age, and pubertal stage did not differ by race.
Seventy-five percent of subjects (15/20) completed 6 months of treatment. One subject cited intolerance of gastrointestinal effects as the reason for withdrawing from the study. Subjects reported taking 81% (range: 0 – 100%) of prescribed orlistat doses on average. Two subjects had stopped taking the medication completely by 5 months of treatment. African-American subjects reported missing 19.0 ± 17.0 capsules/mo (23% of doses), while the Caucasian subjects reported missing 12.5 ± 15.6 capsules/mo (15% of doses) on average over 6 mo of orlistat treatment (p=0.36). Reasons given for noncompliance with the thrice-daily orlistat regimen were: having skipped a meal (e.g., not having time to eat), not wanting to take medication at school, snacking instead of eating a full meal, or eating a fat-free meal. The accuracy of these self-reports was generally supported by actual returned capsule counts, which quantified average medication compliance at 75% of prescribed doses (range 0–100%). There were no significant differences in actual capsule counts between African-American and Caucasian subjects (p=0.29). When actual capsule counts were compared to self-reports, subjects significantly under-estimated the number of capsules they had missed (p<0.02).
Fecal fat excretion increased from 5.41 ± 4.01 gm (5.8% of ingested fat kcal) to 17.16 ± 12.87 gm (35.2% of fat kcal) with 6 months of orlistat treatment (p<0.001). Reports of the adverse effects experienced, obtained weekly for the first 3 months of the study period, found the gastrointestinal effects of orlistat were generally mild and transient. The most frequently cited effects were: fatty or oily stools (85% of subjects), oily spotting on clothing (60%), increased flatulence (55%), and increased defecation (50%). A detailed description of the adverse effects was previously reported 29.
There were no significant changes in the serum levels of the fat-soluble vitamins A, E (after correcting tocopherol levels for the change in lipid concentrations), or K. A small, but significant, decrease in 25-hydroxy vitamin D levels was seen at 1 month (14.9 ± 6.8 vs. 10.6 ± 3.9, p<0.02) that was ameliorated within two months after extra vitamin D supplementation (50,000 IU/d for 1 month) was given to 3 subjects 54.
Serum iron levels rose slightly for the group as a whole from 52.1 ± 1.5 to 62.3 ± 1.6 mg/dL (p<0.002), but stayed well within the normal range (50 – 120 mg/dL). There was also a small but significant rise in percent transferrin saturation from 15 to 18% (p=0.006). There were no significant changes observed in ionized calcium, phosphorous, magnesium, zinc, intact PTH, osteocalcin, bone-specific alkaline phosphatase, TSH, free T4, apolipoproteins A1 and B, or triglycerides during the study. In addition, the gallbladder and renal ultrasound studies found no abnormalities, either at baseline or at follow-up visits.
In the group as a whole, subjects’ weight decreased on average from 117.6 ± 1.4 to 112.2 ± 1.4 kg (range +11.7 to −25.5 kg, p < 0.02) or 3.5 ± 6.0% (calculated from raw data) of original body weight. Waist circumference decreased from 112.2 ± 1.1 to 109.6 ±1.2 cm (p = 0.02), and mean BMI decreased from 42.7 ± 1.3 to 40.7 ± 1.4 kg/m2 (p = 0.001) after 6 months of treatment. However, both body fat mass and lean body mass as determined by air displacement plethysmography were not different (p>0.3). At the end of 6 months of treatment, six subjects (30% of total, 83% Caucasian, 67% female) had lost > 5% of initial body weight, and three of these six subjects (15% of total, 100% Caucasian, 67% female) had lost > 10% of initial body weight. REE, 2042 ± 1.3 kcal/d at baseline, decreased significantly to 1820 ±1.3 kcal/d (p < 0.001, adjusted for body fat mass and lean body mass) at 3 months, but returned to 1950 ± 1.3 kcal/d at 6 months (p=NS vs. baseline measurement). Fasting serum leptin, 30.9 ± 1.6 ng/mL at baseline, fell to 22.4 ± 1.8 ng/mL (p=0.004) at 3 months and remained significantly lower, 23.5 ± 1.8 ng/mL (p=0.03), at 6 months.
When the data were separated according to race (Table 2), at baseline, African American subjects were significantly more obese than Caucasian subjects (BMI 50.3 ± 1.3 vs. 36.0 ± 1.2 kg/m2, p=0.003). There was also a uniform and significant difference in response to the intervention between African American and Caucasian subjects. Caucasian subjects exhibited greater decreases in weight (−7.86 vs. +0.36 kg, p<0.05), BMI (−7.95 vs. −2.14 kg/m2, p=0.003), BMI-SD (−1.10 vs. +0.29, p<0.03), waist circumference (−8.65 vs. −1.59, p=0.03), and leptin (−16.8 vs. −6.5, p=0.09) at 6 months. After an initial slight decrease, African Americans’ weight and BMI returned to baseline, while waist circumference increased above baseline, at 6 months. When one African American subject who gained 11.7 kg during the program was excluded, there was still little weight loss among African American subjects (−1.1 ±1.5 kg, p=NS). Lean body mass did not change significantly in either Caucasians or African Americans. Mean REE (adjusted for LBM and BFM) was significantly lower in African American vs. Caucasian subjects (p<0.05) at baseline and 3 months, but this difference was no longer significant at 6 months. The change from baseline REE at 6 months adjusted for LBM and BFM, however, showed a significantly greater decrease in African Americans’ REE (African Americans −217 ± 108 kcal/d or −11.5 ± 4.6% vs. Caucasians –54 ± 105 kcal/d or –1.2%, p=0.01). Change in REE was related to change in change in weight in both African Americans (r = 0.60, p = 0.09) and Caucasians (r = 0.63, p = 0.09), but these trends did not reach statistical significance. However, the slope of the association for African Americans showed a trend to be steeper than that of Caucasians (−26.6 ± 13.4 vs. −17.5 ± 8.7, p = 0.092).
Table 2.
Effects of weight-loss program including orlistat on body composition and metabolism.
| Race | African American | Caucasian | ||||
|---|---|---|---|---|---|---|
| Variable | Baseline | 3 month | 6 month | Baseline | 3 month | 6 month |
| Weight (kg) | 136.7 ± 1.4 | 134.2 ± 1.4 | 135.7 ± 1.4 | 99.3 ± 1.4 | 93.5 ± 1.4‡ | 92.9 ± 1.3† |
| BMI (kg/m2) | 50.3 ± 1.3 | 48.9 ± 1.3† | 49.2 ± 1.3 | 36.2 ± 1.2 | 33.5 ± 1.2† | 33.2 ± 1.2‡ |
| BMI SD Score | 7.7 ± 1.0 | 7.4 ±1.1* | 8.7 ± 1.4 | 4.5 ± 0.8 | 3.8 0.5‡ | 3.4 ± 0.8† |
| BFM (kg) | 68.4 ± 1.4 | 67.3 ± 1.6 | 70.1 ± 1.6 | 45.0 ± 1.4 | 37.7 ±1.3† | 36.3 ± 1.3† |
| LBM (kg) | 66.8 ± 1.3 | 69.1 ± 1.2 | 73.5 ± 1.2 | 53.1 ± 1.3 | 50.3 ± 1.2 | 50.6 ± 1.2 |
| WC (cm) | 121.8 ± 1.2 | 122.3 ± 1.2 | 122.5 ± 1.2 | 104.3 ± 1.2 | 97.9 ± 1.1† | 96.3 ± 1.1‡ |
| REE (kcal/d) | 1923 ± 1.1 | 1710 ± 1.1† | 1790 ± 1.2 | 2183 ± 1.1 | 1963 ± 1.1* | 2099 ± 1.2 |
| Leptin (ng/mL) | 34.7 ± 1.7 | 29.8 ± 1.8 | 31.1 ± 1.6 | 28.5 ± 1.6 | 18.1 ± 1.6* | 18.5 ± 1.8* |
p < 0.05
p < 0.01
p < 0.001 vs. baseline.
Baseline: n=20; 3, 6 month: n=17 Change in variables was adjusted for baseline value. BFM, body fat mass; LBM, lean body mass; WC, waist circumference; REE, resting energy expenditure. Geometric means ± SD are reported except REE values are least squares means adjusted for LBM and BFM. Adjusted REE in African Americans was significantly lower than in Caucasians (p<0.05) at 0 and 3 months.
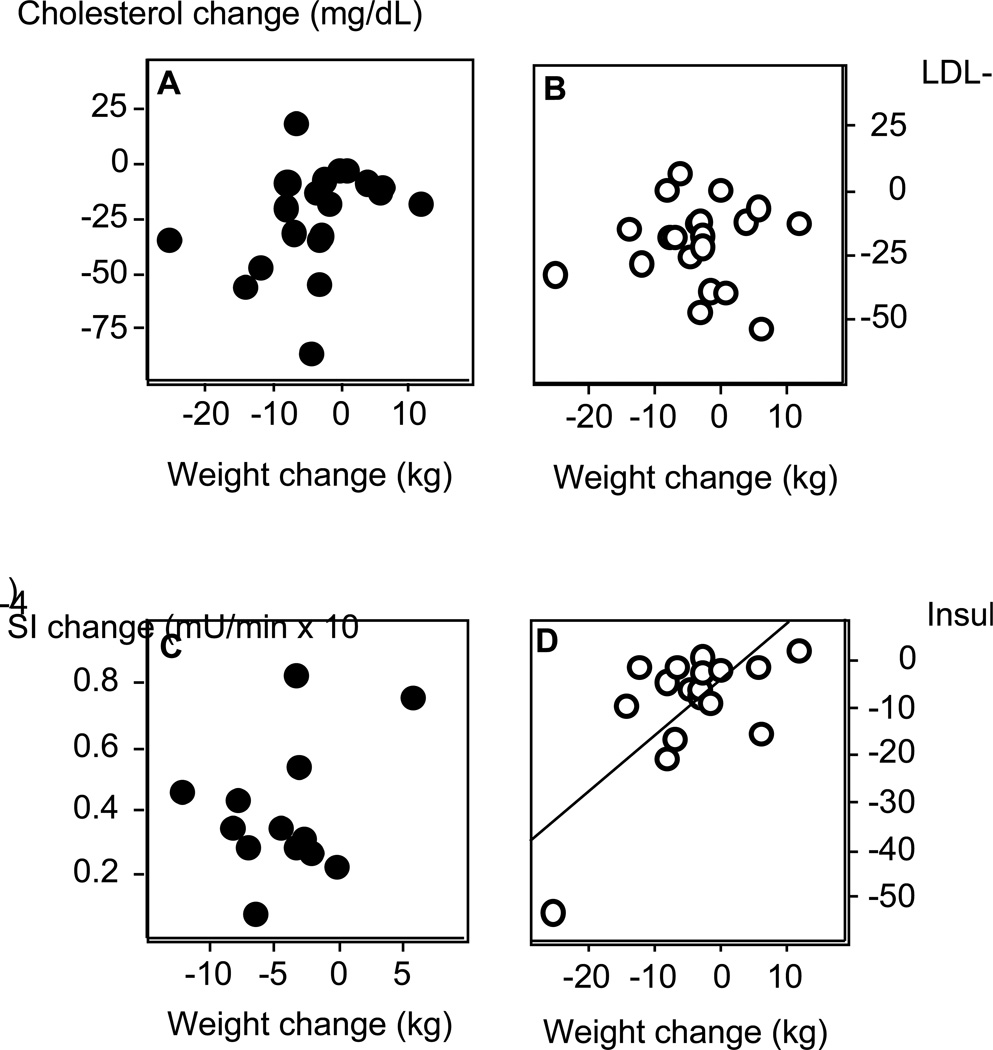
In the group as a whole, total-cholesterol and LDL-cholesterol decreased from 173.8 ± 1.2 to 151.4 ± 4.6, p<0.0001; and 117.5 ± 1.3 to 97.7 ± 1.2, p<0.0001, respectively, throughout the 6-month period. HDL-cholesterol, HDL/LDL ratio, and serum triglycerides remained unchanged. The changes in total- and LDL-cholesterol measurements were not significantly correlated with the amount of weight lost (Figure 1, all r≤0.35, p≥0.14). When the data were separated by race, both African American and Caucasian subjects exhibited significant decreases in total- and LDL-cholesterol with orlistat treatment (Table 3). These differences were not correlated with weight loss in either group (all p>0.30). HDL-cholesterol, HDL/LDL ratio, and serum triglycerides did not change significantly, nor were there any significant differences in these indices between African American and Caucasian subjects.
Figure 1.
Correlations between weight loss and: A. change in total cholesterol (r = −0.35, p = 0.14); B. change in LDL-cholesterol (r = −0.27. p = 0.91); C. change in insulin sensitivity (SI) from frequently sampled intravenous glucose tolerance test (r = −0.67, p = 0.01); D. change in fasting insulin (r = 0.61, p = 0.007). N= 20 for total and LDL-cholesterol (last observation carried forward); 17 for insulin; 13 for SI.
Table 3.
Effects of weight-loss program including orlistat on lipid metabolism
| Race | African American | Caucasian | ||||
|---|---|---|---|---|---|---|
| Variable | Baseline | 3 month | 6 month | Baseline | 3 month | 6 month |
| Cholesterol (mg/dL) | 171.1 ± 1.1 | 158.6 ± 1.2 | 156.2 ± 1.1* | 177.0 ± 1.3 | 148.8 ± 1.2‡ | 148.6 ± 1.2‡ |
| LDL-Cholesterol (mg/dL) | 118.7 ± 1.3 | 104.0 ± 1.4* | 101.3 ± 1.3* | 114.3 ± 1.3 | 95.1 ± 1.2† | 95.0 ± 1.2† |
| HDL-Cholesterol (mg/dL) | 49.1 ± 1.2 | 45.5 ± 1.2 | 46.0 ± 1.2 | 41.7 ± 1.3 | 39.4 ± 1.2 | 39.7 ± 1.2 |
| HDL/LDL ratio | 0.41 ± 1.6 | 0.44 ± 1.5 | 0.45 ± 1.5 | 0.36 ± 1.4 | 0.41 ± 1.3 | 0.42 ± 1.3 |
p < .05
p < .01
p < .001 vs. baseline.
Change in variables was adjusted for baseline value. Geometric means ± SD are reported.
Indicators of insulin resistance also improved with 6 months of treatment. Fasting insulin and the insulin/glucose ratio decreased (24.5 ± 1.8 to 17.8 ± 1.7 and 0.26 ± 1.84 to 0.19 ± 1.79 uU/mL, respectively, both p=0.002). The Quicki Index, increased significantly, from 0.29 ± 1.08 to 0.31 ± 1.07 (p<0.003), and the HOMA-IRI fell significantly (from 5.54 ± 1.80 to 3.94 ± 1.68, p=0.002). Insulin sensitivity (SI), derived from the FSIGT, increased an average of 49% (from 0.70 ± 2.20 to 1.05 ± 1.69 µU/min × 10-4, p=0.007); while the acute insulin response to glucose (AIRG) remained essentially unchanged. A calculated indicator of endocrine pancreatic function also improved: the percent beta-cell function decreased from 312 ± 2 to 213 ± 2 %, p=0.006). These improvements in glycemic control were all significantly correlated with weight loss (Figure 1, all r’s > 0.45, p<0.05).
When the data were separated by race, fasting insulin, the I/G ratio, HOMA IRI and SI improved significantly in both African American and Caucasian subjects (Table 4). However, baseline insulin (p=0.05) and HOMA IRI (p=0.04) were higher, and the Quicki Index and SI (p=0.02) were lower among the African American subjects. The direction and degree of change in these four indices followed the changes in body weight. Caucasians had greater improvements in insulin (−14.28 vs. –6.57 uU/mL, p<0.03), HOMA IRI (−3.26 vs. −1.75, p=0.07), Quicki Index (−0.032 vs. −0.055, p=0.02), and SI (+3.38 × 10−5 vs. –1.45 × 10−5 mU/mL•min−1, p=0.05). The Quicki Index and the percent beta-cell function improved significantly (p<0.03 and p<0.01, respectively) only among Caucasian subjects. The fasting glucose and the AIRG did not change significantly when the group was separated by race.
Table 4.
Effects of orlistat on glucose metabolism.
| Race | African American | Caucasian | ||||
|---|---|---|---|---|---|---|
| Variable | Baseline | 3 month |
6 month | Baseline | 3 month | 6 month |
| Glucose (mg/dL) | 94.6 ± 1.2 | 89.5 ± 1.1 | 90.0 ± 1.1 | 89.9 ± 1.0 | 92.4 ± 1.1 | 91.7 ± 1.1 |
| Insulin (UU/mL) | 31.1 ± 1.5 | 28.0 ± 1.6 | 23.0 ± 1.7* | 18.5 ± 1.9 | 13.0 ± 1.5 | 12.4 ± 1.4* |
| I/G Ratio | 0.33 ± 1.60 | 0.31 ± 1.62 | 0.26 ± 1.79* | 0.20 ± 1.97 | 0.14 ± 1.59* | 0.14 ± 1.50† |
| Quicki Index | 0.29 ±1.05 | 0.29 ±1.06 | 0.30 ±1.07 | 0.31 ±1.08 | 0.32 ±1.05 | 0.33 ±1.04* |
| SI (mU/min) | 0.45 ± 1.93 | 0.73 ± 1.56* | 0.74 ± 1.39* | 1.15 ± 1.89 | 1.32 ± 1.88* | 1.50 ± 1.57* |
| AIRG (pmol/L) | 1588 ± 1.5 | 1598 ± 1.5 | 1470 ± 1.4 | 1329 ± 1.0 | 1336 ± 1.0 | 1380 ± 1.0 |
| IRI | 7.2 ± 1.5 | 6.2 ± 1.6 | 5.1 ± 1.7* | 4.1 ± 1.9 | 3.0 ± 1.5 | 2.8 ± 1.4* |
| % beta-cell function | 455 ± 275 | 446 ± 241 | 368 ±204 | 343 ±376 | 185 ±109 | 190 ±116 |
p < .05
p < .01
p < .001
p < .0001 vs. baseline values.
I/G ratio: insulin/glucose ratio; SI: insulin sensitivity; AIRG: acute insulin response to glucose; IRI: insulin resistance index.
Discussion
This study explored the efficacy of 6 months of orlistat in conjunction with a psychoeducational program in an adolescent population. Because of the open-label nature of the study, conclusions cannot be drawn as to the efficacy of orlistat over the psychoeducational program. However, in general, orlistat was well–tolerated, and no unanticipated adverse events were observed. Orlistat was taken regularly by most subjects, and only one subject left the study due to orlistat’s gastrointestinal effects. Significant weight reductions were observed in Caucasian subjects, and there were improvements in some of the co-morbid conditions associated with obesity in both African American and Caucasian participants.
The increases in serum iron and percent transferrin saturation observed during the study may be due to the effects of the multivitamin supplement given as part of the treatment regimen, and may be an indication of subject compliance with this prescription. The lack of change in other hormones (except leptin and insulin), enzymes, and minerals suggests that there were no immediate adverse effects of orlistat on adrenal, gonadal, thyroid and bone metabolism. The lack of change in urine chemistries and ultrasound results indicates that there were no acute adverse effects of orlistat on the gallbladder or kidneys of adolescents.
The mean weight loss achieved in this study, 3.75 kg, is lower than the mean weight losses, ranging from 3.9 – 10.3 kg, achieved in the clinical trials on adult subjects taking orlistat for 1 to 2 years 17, 18, 20–23, 25, 26, 55. There may be several potential reasons for the lesser efficacy of orlistat observed in the present study. First, some of the adolescents in this study had not completed their growth; therefore, some weight change may have been due to bone growth and increases in height. Second, most studies of adults were designed with 4–6 week lead-in periods prior to randomization during which potential subjects were given placebo plus diet and exercise instruction. These lead-in periods allow a specific level of medication compliance, usually 70–80%, and initial weight loss to become inclusion criteria. Therefore, these adult studies may have eliminated some subjects expected to have difficulty with the protocol. The present study design did not contain a lead-in period. Consequently, our sample contained some adolescents who remained in the study until its completion even though they stopped taking their medication completely and/or consistently gained weight. Third, the average BMI in most studies of adults is 30–35 kg/m2, whereas the average BMI in this study was 43 kg/m2. The greater BMI of the adolescents studied is primarily due to the fact that, unlike previous adult studies, the present investigation required subjects to have a medical complication of their obesity, and therefore recruited subjects with greater adiposity. Our results may therefore be more representative of orlistat’s effectiveness among the severely overweight adolescents for whom pharmacotherapy would be recommended.
When separated by race, the mean weight loss among Caucasian subjects, 7.3 kg (3.5% of initial body weight), was similar to the weight loss achieved in studies on adults. Conversely, African American subjects lost only 0.8 kg (0.6% of initial body weight. There are several potential explanations for this difference. It is possible that because the African American adolescents were more severely overweight at baseline (136.7 ± 1.4 vs. 99.3 ± 1.4 kg) in the present study, they may have had more difficulty losing weight because their movement was restricted by their weight and they could not fully utilize exercise in their weight loss efforts. There may also have been sociocultural factors that made the weight reduction program used less efficacious for African Americans 6, 56–59. Another possible explanation for the lower weight loss among African American subjects may lie in the lower REE that has been described for African Americans. African American children have lower REE than Caucasians 60–67. In our study, not only was the REE of the African American subjects lower at baseline, the decrease in REE (adjusted for LBM and BFM) was 162 kcal/24 hr larger among African Americans than among Caucasians at 6 months, despite the fact that the African American subjects lost less weight. Thus, the REE of African American subjects decreased more per kg of weight loss. Similar findings have been obtained in adult African Americans 68. In a study of 109 obese females (24 African American, 85 Caucasian), REE showed a greater decrease (p = 0.02) among African American subjects, 9.9 ± 7.3%, compared to Caucasian subjects, 6.3 ± 7.4%, despite a larger weight loss among the Caucasian subjects (17.0 ± 5.7% compared to 14.2 ± 5.7%, p = 0.04). They also found a stronger relationship between change in REE and weight loss in African Americans (r = 0.49) than Caucasians (r = 0.38). These results suggest that African Americans respond differently to energy restriction and may be at a metabolic disadvantage when attempting weight loss. However, others have suggested that there may be differences in metabolically active organ mass of African Americans and Caucasians that may explain the differences in REE as resulting from considering all lean body mass as metabolically equivalent 61. At present, it remains unclear whether differences in REE can account in part for the difficulties African Americans may experience when attempting weight reduction.
The modest weight reductions among adolescents in the present study improved obesity-related comorbid conditions. As seen in studies on adults 24, 69, 70 there were improvements in the cardiac risk factors, total – and LDL-cholesterol that were weight loss-independent. Orlistat may also have had a positive effect on indicators of insulin resistance. At 6 months, the degree of improvement in SI was substantial in both African American and Caucasian subjects, and the serum insulin and IRI was lower among African American subjects despite little weight loss. These results suggest a potential weight-independent effect, in addition to the weight-dependent effects, of orlistat on indices of insulin resistance.
This study is limited by its open-label design and small sample size. We cannot separate the effects of the behavioral weight loss program from the effects of orlistat. It is possible that these effects could have been achieved through the use of the comprehensive behavioral weight loss program alone. These limitations prevent delineation of the magnitude of orlistat’s effects on obese adolescents’ body weight apart from the psychoeducational program. However, these limitations do not preclude an examination of the relative efficacy of a behavioral program plus orlistat for African American and Caucasian adolescents.
In conclusion, compared to Caucasians, African American adolescents lost less weight in a comprehensive weight management program including orlistat, but this study’s small sample size is insufficient to determine with certainty which adolescents are most likely to benefit from such treatment. Our findings support the need for a randomized, placebo-controlled, trial of orlistat in a large, multiracial adolescent population to determine whether orlistat has an effect independent of a behavioral program and is, therefore, helpful as an adjunct to behavioral treatment of obesity.
Acknowledgments
Supported by NICHD grant Z01-HD-00641 (JAY) and the National Center on Minority Health and Health Disparities, NIH (JAY)
Footnotes
Commissioned Officers in the United States Public Health Service
References
- 1.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 2.Must A, Strauss RS. Risks and consequences of childhood and adolescent obesity. Int J Obes Relat Metab Disord. 1999;23(Suppl 2):S2–S11. doi: 10.1038/sj.ijo.0800852. [DOI] [PubMed] [Google Scholar]
- 3.Maffeis C. Aetiology of overweight and obesity in children and adolescents. Eur J Pediatr. 2000;159(Suppl 1):S35–S44. doi: 10.1007/pl00014361. [DOI] [PubMed] [Google Scholar]
- 4.Jeffery RW, Drewnowski A, Epstein LH, et al. Long-term maintenance of weight loss: current status. Health Psychol. 2000;19:5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- 5.Haddock C, Shadish W, Klesges R, Stein R. Treatments for childhood and adolescent obesity. Annals of Behavioral Medicine. 1994;16:235–244. [Google Scholar]
- 6.Kumanyika SK, Obarzanek E, Stevens VJ, Hebert PR, Whelton PK. Weight-loss experience of black and white participants in NHLBI-sponsored clinical trials. Am J Clin Nutr. 1991;53:1631S–1638S. doi: 10.1093/ajcn/53.6.1631S. [DOI] [PubMed] [Google Scholar]
- 7.Wing RR, Anglin K. Effectiveness of a behavioral weight control program for blacks and whites with NIDDM. Diabetes Care. 1996;19:409–413. doi: 10.2337/diacare.19.5.409. [DOI] [PubMed] [Google Scholar]
- 8.Yanovski SZ, Gormally JF, Leser MS. The effects of binge eating and race on weight loss during very low calorie diet. Obes Res. 1993;1:15. doi: 10.1002/j.1550-8528.1994.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 9.Sugerman HJ, Londrey GL, Kellum JM, et al. Weight loss with vertical banded gastroplasty and Roux-Y gastric bypass for morbid obesity with selective versus random assignment. Am J Surg. 1989;157:93–102. doi: 10.1016/0002-9610(89)90427-3. [DOI] [PubMed] [Google Scholar]
- 10.Brown CD, Higgins M, Donato KA, et al. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8:605–619. doi: 10.1038/oby.2000.79. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999;22:345–354. doi: 10.2337/diacare.22.2.345. [DOI] [PubMed] [Google Scholar]
- 12.Potts JL, Thomas J. Traditional coronary risk factors in African Americans. Am J Med Sci. 1999;317:189–192. doi: 10.1097/00000441-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Fagot-Campagna A. Emergence of type 2 diabetes mellitus in children: epidemiological evidence. J Pediatr Endocrinol Metab. 2000;13:1395–1402. doi: 10.1515/jpem-2000-s613. [DOI] [PubMed] [Google Scholar]
- 14.Dietz W. Focus group data pertinent to the prevention of obesity in African Americans. Am J Med Sci. 2001;322:286–289. [PubMed] [Google Scholar]
- 15.Mittendorfer B, Ostlund RJ, Patterson B, Klein S. Orlistat inhibits dietary cholesterol absorption. Obes Res. 2001;9:599–604. doi: 10.1038/oby.2001.79. [DOI] [PubMed] [Google Scholar]
- 16.Van Gaal LF, Broom JI, Enzi G, Toplak H. Efficacy and tolerability of orlistat in the treatment of obesity: a 6-month dose-ranging study. Orlistat Dose-Ranging Study Group. Eur J Clin Pharmacol. 1998;54:125–132. doi: 10.1007/s002280050433. [DOI] [PubMed] [Google Scholar]
- 17.Davidson MH, Hauptman J, DiGirolamo M, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA. 1999;281:235–242. doi: 10.1001/jama.281.3.235. [DOI] [PubMed] [Google Scholar]
- 18.Rossner S, Sjostrom L, Noack R, Meinders AE, Noseda G. Weight loss, weight maintenance, and improved cardiovascular risk factors after 2 years treatment with orlistat for obesity. European Orlistat Obesity Study Group. Obes Res. 2000;8:49–61. doi: 10.1038/oby.2000.8. [DOI] [PubMed] [Google Scholar]
- 19.Drent ML, van der Veen EA. First clinical studies with orlistat: a short review. Obes Res. 1995;3(Suppl 4):623S–625S. doi: 10.1002/j.1550-8528.1995.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 20.Finer N, James WP, Kopelman PG, Lean ME, Williams G. One-year treatment of obesity: a randomized, double-blind, placebo-controlled, multicentre study of orlistat, a gastrointestinal lipase inhibitor. Int J Obes Relat Metab Disord. 2000;24:306–313. doi: 10.1038/sj.ijo.0801128. [DOI] [PubMed] [Google Scholar]
- 21.Hauptman J, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med. 2000;9:160–167. doi: 10.1001/archfami.9.2.160. [DOI] [PubMed] [Google Scholar]
- 22.Hollander PA, Elbein SC, Hirsch IB, et al. Role of orlistat in the treatment of obese patients with type 2 diabetes. A 1-year randomized double-blind study. Diabetes Care. 1998;21:1288–1294. doi: 10.2337/diacare.21.8.1288. [DOI] [PubMed] [Google Scholar]
- 23.James WP, Avenell A, Broom J, Whitehead J. A one-year trial to assess the value of orlistat in the management of obesity. Int J Obes Relat Metab Disord. 1997;21(Suppl 3):S24–S30. [PubMed] [Google Scholar]
- 24.Karhunen L, Franssila-Kallunki A, Rissanen P, et al. Effect of orlistat treatment on body composition and resting energy expenditure during a two-year weight-reduction programme in obese Finns. Int J Obes Relat Metab Disord. 2000;24:1567–1572. doi: 10.1038/sj.ijo.0801443. [DOI] [PubMed] [Google Scholar]
- 25.Lindgarde F. The effect of orlistat on body weight and coronary heart disease risk profile in obese patients: the Swedish Multimorbidity Study. J Intern Med. 2000;248:245–254. doi: 10.1046/j.1365-2796.2000.00720.x. [DOI] [PubMed] [Google Scholar]
- 26.Sjostrom L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352:167–172. doi: 10.1016/s0140-6736(97)11509-4. [DOI] [PubMed] [Google Scholar]
- 27.Zavoral JH. Treatment with orlistat reduces cardiovascular risk in obese patients. J Hypertens. 1998;16:2013–2017. doi: 10.1097/00004872-199816121-00024. [DOI] [PubMed] [Google Scholar]
- 28.Muls E, Kolanowski J, Scheen A, Van Gaal L. The effects of orlistat on weight and on serum lipids in obese patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled, multicentre study. Int J Obes Relat Metab Disord. 2001;25:1713–1721. doi: 10.1038/sj.ijo.0801814. [DOI] [PubMed] [Google Scholar]
- 29.McDuffie J, Calis K, Uwaifo G, et al. Three-month tolerability of orlistat in adolescents with obesity-related comorbid conditions. Obes Res. 2002;10:642–650. doi: 10.1038/oby.2002.87. [DOI] [PubMed] [Google Scholar]
- 30.Lohman T, Roche A, Martorell R. Anthropometric standardization reference manual. Champagne, IL: Human Kinetics Books; 1988. [Google Scholar]
- 31.Dempster P, Aitkens S. A new air displacement method for the determination of human body composition. Med Sci Sports Exerc. 1995;27:1692–1697. [PubMed] [Google Scholar]
- 32.McCrory MA, Gomez TD, Bernauer EM, Mole PA. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med Sci Sports Exerc. 1995;27:1686–1691. [PubMed] [Google Scholar]
- 33.Levenhagen DK, Borel MJ, Welch DC, et al. A comparison of air displacement plethysmography with three other techniques to determine body fat in healthy adults. J Parenter Enteral Nutr. 1999;23:293–299. doi: 10.1177/0148607199023005293. [DOI] [PubMed] [Google Scholar]
- 34.Biaggi RR, Vollman MW, Nies MA, et al. Comparison of air-displacement plethysmography with hydrostatic weighing and bioelectrical impedance analysis for the assessment of body composition in healthy adults. Am J Clin Nutr. 1999;69:898–903. doi: 10.1093/ajcn/69.5.898. [DOI] [PubMed] [Google Scholar]
- 35.Lockner DW, Heyward VH, Baumgartner RN, Jenkins KA. Comparison of air-displacement plethysmography, hydrodensitometry, and dual X-ray absorptiometry for assessing body composition of children 10 to 18 years of age. Ann N Y Acad Sci. 2000;904:72–78. doi: 10.1111/j.1749-6632.2000.tb06423.x. [DOI] [PubMed] [Google Scholar]
- 36.Dewit O, Fuller NJ, Fewtrell MS, Elia M, Wells JC. Whole body air displacement plethysmography compared with hydrodensitometry for body composition analysis. Arch Dis Child. 2000;82:159–164. doi: 10.1136/adc.82.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nunez C, Kovera AJ, Pietrobelli A, et al. Body composition in children and adults by air displacement plethysmography. Eur J Clin Nutr. 1999;53:382–387. doi: 10.1038/sj.ejcn.1600735. [DOI] [PubMed] [Google Scholar]
- 38.Siri W. Body composition from fluid spaces and density: analysis of methods. In: Brozek J, Honschel A, editors. Techniques for measuring body composition. Washington, D.C: National Academy of Sciences/National Research Council; 1961. pp. 223–224. [Google Scholar]
- 39.Nixon DE, Moyer TP, Johnson P, et al. Routine measurement of calcium, magnesium, copper, zinc, and iron in urine and serum by inductively coupled plasma emission spectroscopy. Clin Chem. 1986;32:1660–1665. [PubMed] [Google Scholar]
- 40.McClean SW, Ruddel ME, Gross EG, DeGiovanna JJ, Peck GL. Liquid-chromatographic assay for retinol (vitamin A) and retinol analogs in therapeutic trials. Clin Chem. 1982;28:693–696. [PubMed] [Google Scholar]
- 41.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39:529–533. [PubMed] [Google Scholar]
- 42.Davidson KW, Sadowski JA. Determination of vitamin K compounds in plasma or serum by high-performance liquid chromatography using postcolumn chemical reduction and fluorimetric detection. Methods Enzymol. 1997;282:408–421. doi: 10.1016/s0076-6879(97)82124-6. [DOI] [PubMed] [Google Scholar]
- 43.Jansson L, Nilsson B, Lindgren R. Quantitation of serum tocopherols by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1980;181:242–247. doi: 10.1016/s0378-4347(00)81609-6. [DOI] [PubMed] [Google Scholar]
- 44.Gundberg CM, Nieman SD, Abrams S, Rosen H. Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J Clin Endocrinol Metab. 1998;83:3258–3266. doi: 10.1210/jcem.83.9.5126. [DOI] [PubMed] [Google Scholar]
- 45.DiSanto AR, Wagner JG. Pharmacokinetics of highly ionized drugs. II. Methylene blue--absorption, metabolism, and excretion in man and dog after oral administration. J Pharm Sci. 1972;61:1086–1090. doi: 10.1002/jps.2600610710. [DOI] [PubMed] [Google Scholar]
- 46.Ellefson R, Caraway W. Lipids and Lipoproteins. In: Tietz N, editor. Fundamentals of Clinical Chemsitry. Philadelphia: W.B. Saunders Company; 1994. pp. 524–527. [Google Scholar]
- 47.Bergman RN. Lilly lecture 1989. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes. 1989;38:1512–1527. doi: 10.2337/diab.38.12.1512. [DOI] [PubMed] [Google Scholar]
- 48.Boden G, Reichard G, Hoeldtke R, Rezvzni I, Owen O. Severe insulin-induced hypoglycemia experienced by patients with type 2 diabetes associated with deficiencies in the release of counterregulatory hormones. N Eng J Med. 1981;305:1200–1205. doi: 10.1056/NEJM198111123052007. [DOI] [PubMed] [Google Scholar]
- 49.Hepburn D, MacLeod K, Pell A, Scougal I, Frier B. Frequency and symptoms of hypoglycemia experienced by patients with type 2 diabetes treated with insulin. Diabetic Medicine. 1993;10:231–237. doi: 10.1111/j.1464-5491.1993.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 50.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 51.Quon MJ. QUICKI is a useful and accurate index of insulin sensitivity. J Clin Endocrinol Metab. 2002;87:949–950. doi: 10.1210/jcem.87.2.8223. [DOI] [PubMed] [Google Scholar]
- 52.Hrebicek J, Janout V, Malincikova J, Horakova D, Cizek L. Detection of insulin resistance by simple quantitative insulin sensitivity check index QUICKI for epidemiological assessment and prevention. J Clin Endocrinol Metab. 2002;87:144–147. doi: 10.1210/jcem.87.1.8292. [DOI] [PubMed] [Google Scholar]
- 53.Corso DM, Pucino F, DeLeo JM, Calis KA, Gallelli JF. Development of a questionnaire for detecting potential adverse drug reactions. Ann Pharmacother. 1992;26:890–896. doi: 10.1177/106002809202600704. [DOI] [PubMed] [Google Scholar]
- 54.McDuffie J, Calis K, Booth S, Uwaifo G, Yanovski J. Effects of Orlistat on Fat-Soluble Vitamins in Obese Adolescents. Pharmacotherapy. 2002;22:814–822. doi: 10.1592/phco.22.11.814.33627. [DOI] [PubMed] [Google Scholar]
- 55.Rosenfalck AM, Hendel H, Rasmussen MH, et al. Minor long-term changes in weight have beneficial effects on insulin sensitivity and beta-cell function in obese subjects. Diabetes Obes Metab. 2002;4:19–28. doi: 10.1046/j.1463-1326.2002.00161.x. [DOI] [PubMed] [Google Scholar]
- 56.Gordon-Larsen P. Obesity-related knowledge, attitudes, and behaviors in obese and non-obese urban Philadelphia female adolescents. Obes Res. 2001;9:112–118. doi: 10.1038/oby.2001.14. [DOI] [PubMed] [Google Scholar]
- 57.Kimm SY, Barton BA, Berhane K, Ross JW, Payne GH, Schreiber GB. Self-esteem and adiposity in black and white girls: the NHLBI Growth and Health Study. Ann Epidemiol. 1997;7:550–560. doi: 10.1016/s1047-2797(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 58.Davis SP, Northington L, Kolar K. Cultural considerations for treatment of childhood obesity. J Cult Divers. 2000;7:128–132. [PubMed] [Google Scholar]
- 59.Adams K, Sargent RG, Thompson SH, Richter D, Corwin SJ, Rogan TJ. A study of body weight concerns and weight control practices of 4th and 7th grade adolescents. Ethn Health. 2000;5:79–94. doi: 10.1080/13557850050007374. [DOI] [PubMed] [Google Scholar]
- 60.Gannon B, DiPietro L, Poehlman ET. Do African Americans have lower energy expenditure than Caucasians? Int J Obes Relat Metab Disord. 2000;24:4–13. doi: 10.1038/sj.ijo.0801115. [DOI] [PubMed] [Google Scholar]
- 61.Hunter GR, Weinsier RL, Darnell BE, Zuckerman PA, Goran MI. Racial differences in energy expenditure and aerobic fitness in premenopausal women. Am J Clin Nutr. 2000;71:500–506. doi: 10.1093/ajcn/71.2.500. [DOI] [PubMed] [Google Scholar]
- 62.Wong WW, Butte NF, Ellis KJ, et al. Pubertal African-American girls expend less energy at rest and during physical activity than Caucasian girls. J Clin Endocrinol Metab. 1999;84:906–911. doi: 10.1210/jcem.84.3.5517. [DOI] [PubMed] [Google Scholar]
- 63.Yanovski JA. Resting energy expenditure in African American and white children. Am J Clin Nutr. 2001;73:149–150. doi: 10.1093/ajcn/73.2.149. [DOI] [PubMed] [Google Scholar]
- 64.Tershakovec AM, Kuppler KM, Zemel B, Stallings VA. Age, sex, ethnicity, body composition, and resting energy expenditure of obese African American and white children and adolescents. Am J Clin Nutr. 2002;75:867–871. doi: 10.1093/ajcn/75.5.867. [DOI] [PubMed] [Google Scholar]
- 65.Arslanian S, Suproasongsin C, Janosky J. Insulin secretion and sensitivity in black versus white prepubertal healthy children. J Clin Endocrinol Metab. 1997;82:1923–1927. doi: 10.1210/jcem.82.6.4002. [DOI] [PubMed] [Google Scholar]
- 66.Yanovski SZ, Reynolds JC, Boyle AJ, Yanovski JA. Resting metabolic rate in African-American and Caucasian girls. Obes Res. 1997;5:321–325. doi: 10.1002/j.1550-8528.1997.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 67.Sun M, Gower BA, Bartolucci AA, Hunter GR, Figueroa-Colon R, Goran MI. A longitudinal study of resting energy expenditure relative to body composition during puberty in African American and white children. Am J Clin Nutr. 2001;73:308–315. doi: 10.1093/ajcn/73.2.308. [DOI] [PubMed] [Google Scholar]
- 68.Foster GD, Wadden TA, Swain RM, Anderson DA, Vogt RA. Changes in resting energy expenditure after weight loss in obese African American and white women. Am J Clin Nutr. 1999;69:13–17. doi: 10.1093/ajcn/69.1.13. [DOI] [PubMed] [Google Scholar]
- 69.Heck AM, Yanovski JA, Calis KA. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy. 2000;20:270–279. doi: 10.1592/phco.20.4.270.34882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tonstad S, Pometta D, Erkelens DW, et al. The effect of the gastrointestinal lipase inhibitor, orlistat, on serum lipids and lipoproteins in patients with primary hyperlipidaemia. Eur J Clin Pharmacol. 1994;46:405–410. doi: 10.1007/BF00191901. [DOI] [PubMed] [Google Scholar]