A rare double mutation in the glucocerebrosidase gene, [D409H; H255Q], has recently been described principally in patients with Gaucher disease from the Balkan region (1, 2). This double mutation, occurring exclusively in cis, is associated with a particularly severe phenotype. Mutation H255Q has not been found independently of the D409H mutation. Further, the fact that these two point mutations occur in cis is especially noteworthy because D409H is present in the glucocerebrosidase pseudogene, while H255Q is not (3). This would suggest that the allele arose as a result of two separate mutations.
We previously described one infant of Greek descent with type 2 Gaucher disease, reporting his genotype as RecTL/H255Q (4). The RecTL recombination mutation includes D409H, so our initial interpretation of sequencing data was that the D409H mutation was part of the recombinant allele. However, subsequent subcloning and sequencing of each allele separately, reveals that the D409H mutation was actually on the same allele as the H255Q allele. The recombination on the second allele occurred downstream of D409; therefore, the correct genotype of this patient is [D409H; H255Q]/RecNcil. This possibility was also suggested by Santamaria et.al.(2).
D409H is an important Gaucher mutation because it is associated with an atypical phenotype, encompassing cardiac calcifications or fibrosis, hydrocephalus, slow horizontal eye movements, and dysmorphic features (5). However, mutant alleles with D409H and H255Q together also appear to confer a specific, albeit different phenotype, characterized by progressive neurologic disease and an early demise. The mechanisms by which these mutations affect the enzymatic activity or potentially interfere with interactions between this protein and others are still unknown.
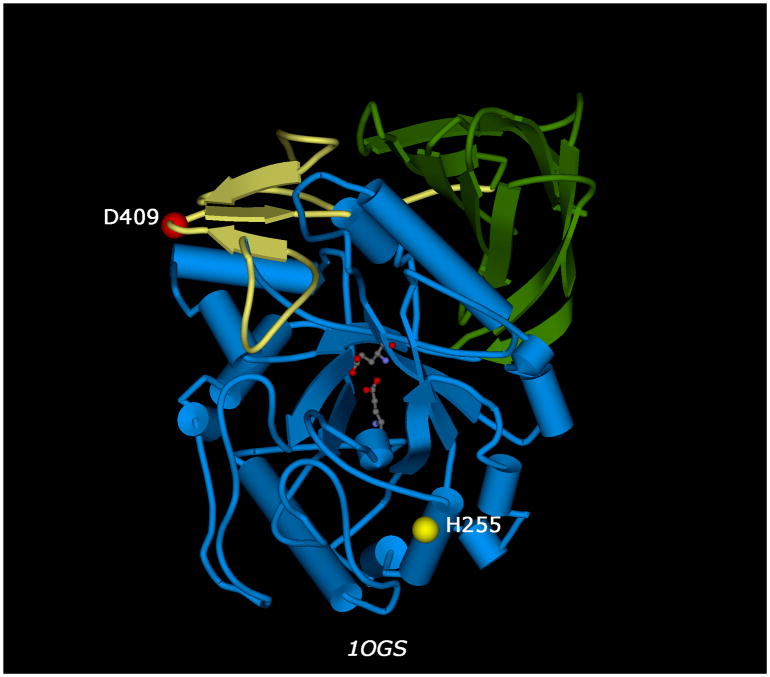
Using the available three-dimensional structure of glucocerebrosidase (6), we attempted to explore the effects of these mutations on the protein structure. In the wild type protein, D409 is located on the surface of the protein, in a loop region between two strands of a three-stranded antiparallel β-sheet, while H255 is located in one of the α-helices comprising the TIM barrel that is part of the catalytic domain of the molecule (Figure 1). Amino acids D409 and H255 are situated on opposite sides of the protein and are both distal to the catalytic core; therefore, these two residues are not able to interact with one another, nor would they be likely to play any direct role in catalysis.
Fig. 1.
Three-dimensional structure of human glucocerbrosidase (pdb| 1OGS). The alpha-carbons of mutated residues D409 and H255 are depicted as red and yellow balls, respectively. The glutamates of the central catalytic core are depicted as ball-and-stick representations. Structural domains, as defined by Dvir et al. (6), are colored yellow (domain I), green (domain II, immunoglobulin-like), and blue (domain III, catalytic TIM barrel).
Additionally, we generated a three-dimensional molecular model of the double mutant using the MODELLER package (7), as implemented within Discovery Studio (Accelrys, San Diego, CA). MODELLER was run in fully automated mode with a high optimization level. The resulting energy-minimized three-dimensional model of the mutated molecule gives no indication that either the D409H or the H255Q mutation grossly disrupts the overall structure of the protein (data not shown). Both of the mutations are well-accommodated sterically, with the H255Q substitution predicted to preserve the helical conformation at its position as in the wild-type protein. Because the mutations affect surface residues with fully or partially charged side-chains, D409H and H255Q may alter important electrostatic interactions that maintain the integrity of the catalytic domain or that are involved in a molecular interface. Recently, it was suggested that P415R, a mutation in the same region as D409, interferes with binding to LIMP-2, which has been shown to be the receptor responsible for trafficking of glucocerebrosidase to lysosomes (8). Given the lack of a definitive structure-based explanation for the phenotype observed in individuals with this rare double mutation, we propose that the impact of D409H/H255Q allele on higher-order interactions warrants further investigation.
These observations underscore the necessity for the careful molecular dissection of mutant alleles carrying D409H, as two very different complex alleles are associated with this mutation. The complexity of the double mutation makes it all the more difficult to determine the molecular etiology underlying the resulting severity of the Gaucher phenotype. Nonetheless, an accurate description of these unusual alleles is essential for genotype-phenotype studies or for better-predicting patient prognosis.
Acknowledgments
This research was funded by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
References
- 1.Michelakakakis H, Moraitou M, Dimitriou E, et al. Homozygosity for the double D409H+H255Q allele in type II Gaucher disease. J Inherit Metab Dis. 2006;29:59. doi: 10.1007/s10545-006-0316-x. [DOI] [PubMed] [Google Scholar]
- 2.Santamaria R, Michelakakis H, Moraitou M, et al. Haplotype analysis suggests a single Balkan origin for the Gaucher disease [D409H; H255Q] double mutant allele. Hum Mutat. 2008;29:E58–67. doi: 10.1002/humu.20776. [DOI] [PubMed] [Google Scholar]
- 3.Hruska KS, LaMarca ME, Scott CR, Sidransky E. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA) Hum Mutat. 2008;29:567–583. doi: 10.1002/humu.20676. [DOI] [PubMed] [Google Scholar]
- 4.Stone DL, Tayebi N, Orvisky E, et al. Glucocerebrosidase gene mutations in patients with type 2 Gaucher disease. Hum Mutat. 2000;15:181–8. doi: 10.1002/(SICI)1098-1004(200002)15:2<181::AID-HUMU7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 5.Abrahamov A, Elstein D, Gross-Tsur V, et al. Gaucher's disease variant characterised by progressive calcification of heart valves and unique genotype. Lancet. 1995;346:1000–3. doi: 10.1016/s0140-6736(95)91688-1. [DOI] [PubMed] [Google Scholar]
- 6.Dvir H, Harel M, McCarthy AA, et al. X-ray structure of human acid-beta-glucosidase, the defective enzyme in Gaucher disease. EMBO Rep. 2003;4:704–9. doi: 10.1038/sj.embor.embor873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sali A, Potterton L, Yuan F, van Vlijmen H, Karplus M. Evaluation of comparative protein modeling by MODELLER. Proteins. 1995;23:318–326. doi: 10.1002/prot.340230306. [DOI] [PubMed] [Google Scholar]
- 8.Reczek D, Schwake M, Schroder J, et al. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell. 2007;131:770–83. doi: 10.1016/j.cell.2007.10.018. [DOI] [PubMed] [Google Scholar]