Letter to the Editor
The development of increasingly immunocompromised mice has allowed for improved engraftment of human tissue in xenograft hosts. NOD/SCID IL2Rgamma-c null mice (NSG) represent the latest iterative attempt to limit murine innate and acquired immune function, thus preventing immune surveillance during xenografting. NSG mice have severe T- and B-cell impairment, as well as a complete absence of NK-cells.(1–2) Carroll and colleagues have recently reported in Leukemia the engraftment of human acute myeloid leukemia (AML) cells in the bone marrow of NSG mice, suggesting that NSG mice may be more permissive to human malignant myeloid cell engraftment than previous xenograft hosts.(3) To date, there has been only limited success in establishing a robust xenograft model of low-risk myelodysplastic syndromes (MDS) despite the use of a variety of immune deficient and transgenic mice.(4–7) In an attempt to improve upon these models, we tested whether the increased deficiency in NK cell function in NSG mice might improve the myeloid cell engraftment of MDS samples with low myeloblast counts and provide a useful pre-clinical model of low-risk MDS.
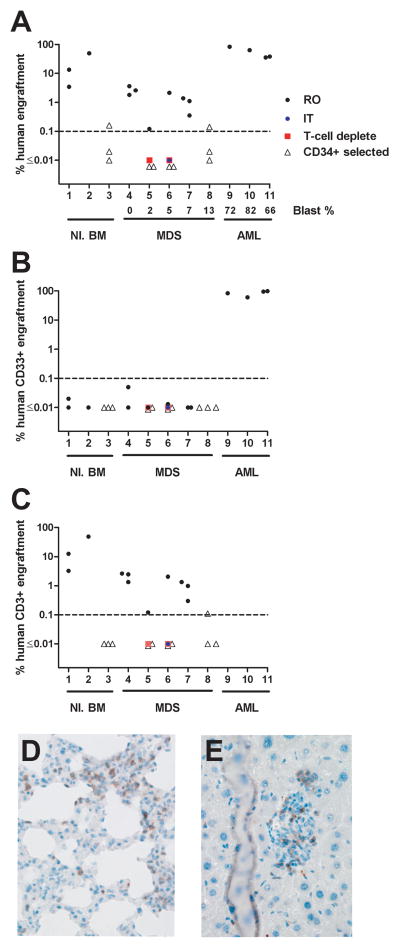
Thirty-four NSG mice were injected with unfractionated, T-cell depleted, or CD34+ purified bone marrow cells from consented normal donors (3 samples into 2–3 mice each), patients with acute myeloid leukemia (AML) (4 samples into 1–2 mice each), or patients with MDS and bone marrow myeloblast counts ranging from 0–13% (5 samples into 3–5 mice each) 24 hours after recipient mice received 250 cGy total body irradiation (Figure 1A). All samples were previously cryopreserved and engraftment was defined as >0.1% human CD45+ cells. Tri-lineage hematopoietic engraftment of human cells was analyzed in the peripheral blood, bone marrow and spleens of NSG mice 12 weeks post-injection, or when an animal was moribund (Figure 1A–C and data not shown). Bone marrow engraftment was significantly higher in NSG mice following retro-orbital (RO) injection of unfractionated normal bone marrow compared to MDS bone marrow cells (average 22.3% vs. 1.6%, respectively, p= 0.03). All mice receiving normal or MDS cells had < 0.1% human CD33+ cells in their bone marrow (Figure 1B), and only MDS sample #4 had any splenic CD33+ engraftment in two mice (0.38–0.78%). The majority of engrafted human cells in all organs were CD3+ (normal bone marrow median CD3+ = 97.6%, range 83–99.6%; MDS median CD3+ = 98.5%, range 72.3–100%) (Figure 1C). Several engrafted mice became moribund with weight loss, ruffled fur, and failure to thrive. Pathologic examination of the lung and liver from a MDS injected moribund mouse revealed human CD3+ cell infiltration, consistent with graft versus host disease (GVHD) (Figure 1D–E).
Figure 1.
Engraftment of human bone marrow cells into NSG mice. 5 × 105 – 5 × 106 total bone marrow cells (5 × 106 cells for AML samples), 1.8–5 × 106 T-cell depleted bone marrow cells, or 5 × 104 – 2 × 106 CD34+ selected cells were administered to NSG mice by retro-orbital (RO) injection, or 3.3–8 × 105 total bone marrow cells administered by intra-tibial (IT) injection. Engraftment was assessed by flow cytometry of bone marrow cells at 7 – 12 weeks post-xenografting for murine CD45, human CD45, human CD3, CD19 and CD33. A. Percentage of human CD45+ cells in bone marrow cells at 7 – 12 weeks post-xenografting. B. Percentage of human CD33+ cells in bone marrow cells at 7 – 12 weeks post-xenografting. C. Percentage of human CD3+ cells in bone marrow cells at 7 – 12 weeks post-xenografting. D. Immunohistochemistry of xenograft lung tissue stained with anti-human CD3ε (clone F7.2.38, Dako) consistent with GVHD. E. Immunohistochemistry of xenograft liver tissue stained with anti-human CD3 consistent with GVHD. Blast %= percent of myeloblast in the bone marrow.
We attempted to improve engraftment of human myeloid cells by injecting whole bone marrow cells intra-tibial (IT) (to abrogate potential trafficking/homing defects), by T-cell depleting bone marrow samples (to limit the number of T-cells that may contribute to graft versus host disease), and by injecting CD34+ purified cells. Four out of five animals that received an intra-tibial injection of bone marrow cells died unexpectedly 40–56 days after injection (MDS samples #5, 6). The remaining IT injected mouse from MDS sample #6 had 0.12% peripheral blood human engraftment at 6 weeks (100% T-cells), suggesting that GVHD may have contributed to the death of the other IT injected mice. Next, we RO injected T-cell depleted bone marrow cells (>2 log reduction in T-cells) from MDS samples #5 and #6. Both samples had 0.2% human cell engraftment in the spleen, but no engraftment in the peripheral blood or bone marrow. Surprisingly, engrafted human cells were predominantly CD3+ (70–80%), suggesting that T-cell expansion in NSG mice is robust even when less than 900 T-cells were injected. Finally, we injected CD34+ purified cells (autoMACS, Miltenyi) from normal or MDS bone marrow cells. One mouse receiving normal CD34+ bone marrow cells had 0.17% bone marrow engraftment which consisted predominantly of B-cells (95%). CD34+ purified cells from three MDS samples (#5, 6, 8) were injected RO, and only MDS sample #8 (13% myeloblasts) had human cell engraftment in the bone marrow (0.16%, 17.7% of engrafted cells were CD33+ following injection of 2 × 106 cells)(Figure 1A–B). To ensure that the viability of CD34+ injected cells was not compromised during the purification process, we assessed their growth in normoxic methylcellulose culture (H4544, Stem Cell Technologies). CD34+ purified cells from the normal bone marrow and MDS sample #8 produced 52 and 14 CFU per 1,000 cells plated, respectively. Interphase FISH analysis from unfractionated and CD34+ purified methylcellulose colonies from MDS sample #8 detected trisomy 8 in 190/200 and 188/200 cells, respectively, indicating that the CD34+ purified cells were clonal. In contrast, CD34+ cells from MDS samples #5 and #6 did not produce methylcellulose colonies, which may reflect the variable growth of MDS cells in normoxic vs. hypoxic (3% oxygen) conditions. Collectively, these results indicate that intra-tibial injection, T-cell depletion, or CD34+ purification do not significantly improve the engraftment of low blast count MDS samples in NSG mice.
In contrast to low-risk MDS cells, three human AML samples showed robust engraftment in the bone marrow of NSG mice (mean 1.6% versus 55.3%, respectively, p<0.0001), with the majority of engrafted AML cells being CD33+ (60.5–98.5%). One mouse injected with a fourth AML sample died at day 35. This mouse had 10% human peripheral blood cell engraftment (100% T-cells), suggesting GVHD may have contributed to its death.
In summary, we were unable to establish a robust low-risk MDS xenograft model using NSG mice despite their profound immune deficiency. NSG mice had consistent CD3+ cell engraftment/expansion with evidence of human CD3+ cell organ infiltration in some mice. Our results highlight the challenges of creating a xenograft model of low-risk MDS and demonstrate that even small numbers of residual T-cells are capable of significant expansion in NSG mice leading to morbidity and mortality. Better, but still limited, success has been noted with MDS samples that contain increased blast counts (>10%) and with the co-injection of MDS bone marrow cells with stromal cell lines.(4–7) This suggests that clonal MDS cells may be dependent on cell extrinsic factors for survival, such as cell-cell interaction within the bone marrow niche and appropriate cytokine support. We suggest that future experiments evaluating the engraftment of low-risk MDS should focus on providing an appropriate micro-environment in NSG mice. This may include the addition of stromal cells and/or species specific cytokines, or the injection of cells into neonatal NSG mice.(8)
Acknowledgments
We thank Tim Ley, Tim Graubert, Dan Link and Gerry Linette for helpful scientific discussions and reagents. This work was supported by National Institutes of Health Grant HL082973 and the Howard Hughes Medical Institute Physician-Scientist Early Career Award (MJW).
Reference List
- 1.Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995 Jan 1;154(1):180–191. [PubMed] [Google Scholar]
- 2.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005 May 15;174(10):6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez PV, Perry RL, Sarry JE, Perl AE, Murphy K, Swider CR, et al. A robust xenotransplantation model for acute myeloid leukemia. Leukemia. 2009 Nov;23(11):2109–2117. doi: 10.1038/leu.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim DK, Kojima M, Fukushima T, Miyasaka M, Nakauchi H. Engraftment of human myelodysplastic syndrome derived cell line in transgenic severe combined immunodeficient (TG-SCID) mice expressing human GM-CSF and IL-3. Eur J Haematol. 1998 Aug;61(2):93–99. doi: 10.1111/j.1600-0609.1998.tb01067.x. [DOI] [PubMed] [Google Scholar]
- 5.Benito AI, Bryant E, Loken MR, Sale GE, Nash RA, John Gass M, et al. NOD/SCID mice transplanted with marrow from patients with myelodysplastic syndrome (MDS) show long-term propagation of normal but not clonal human precursors. Leuk Res. 2003 May;27( 5):425–436. doi: 10.1016/s0145-2126(02)00221-7. [DOI] [PubMed] [Google Scholar]
- 6.Thanopoulou E, Cashman J, Kakagianne T, Eaves A, Zoumbos N, Eaves C. Engraftment of NOD/SCID-beta2 microglobulin null mice with multilineage neoplastic cells from patients with myelodysplastic syndrome. Blood. 2004 Jun 1;103(11):4285–4293. doi: 10.1182/blood-2003-09-3192. [DOI] [PubMed] [Google Scholar]
- 7.Kerbauy DM, Lesnikov V, Torok-Storb B, Bryant E, Deeg HJ. Engraftment of distinct clonal MDS-derived hematopoietic precursors in NOD/SCID-beta2-microglobulin-deficient mice after intramedullary transplantation of hematopoietic and stromal cells. Blood. 2004 Oct 1;104(7):2202–2203. doi: 10.1182/blood-2004-04-1518. [DOI] [PubMed] [Google Scholar]
- 8.Saito Y, Uchida N, Tanaka S, Suzuki N, Tomizawa-Murasawa M, Sone A, et al. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat Biotechnol. 2010 Mar;28(3):275–280. doi: 10.1038/nbt.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]