Abstract
Impairment of cognitive functions including hippocampus-dependent spatial learning and memory affects nearly half of the aged population. Age-related cognitive decline is associated with synaptic dysfunction that occurs in the absence of neuronal cell loss, suggesting that impaired neuronal signaling and plasticity may underlie age-related deficits of cognitive function. Expression of myelin-associated inhibitors (MAIs) of synaptic plasticity, including the ligands MAG, Nogo-A, and OMgp, and their common receptor, NgR1, was examined in hippocampal synaptosomes and CA1, CA3 and DG subregions derived from adult (12–13 months) and aged (26–28 months) Fischer 344 × Brown Norway rats. Rats were behaviorally phenotyped by Morris water maze testing and classified as aged cognitively intact (n=7–8) or aged cognitively impaired (n=7–10) relative to adults (n=5–7). MAI protein expression was induced in cognitively impaired, but not cognitively intact, aged rats and correlated with cognitive performance in individual rats. Immunohistochemical experiments demonstrated that upregulation of MAIs occurs, in part, in hippocampal neuronal axons and somata. While a number of pathways and processes are altered with brain aging, we report a coordinated induction of myelin-associated inhibitors of functional and structural plasticity only in cognitively impaired aged rats. Induction of MAIs may decrease stimulus-induced synaptic strengthening and structural remodeling, ultimately impairing synaptic mechanisms of spatial learning and memory and resulting in cognitive decline.
Keywords: myelin-associated glycoprotein, neurite outgrowth inhibitor A, oligodendrocyte myelin glycoprotein, Nogo-66 receptor, Fischer 344 × Brown Norway rat, Morris water maze
Introduction
Cognitive decline is a common complication of aging that impacts a variety of brain functions including processing speed, inductive reasoning, and spatial learning and memory (Hedden and Gabrieli 2004). Impairment of these functions diminishes health-span and decreases independence. Hippocampus-dependent spatial learning and memory is dramatically impaired in aging subjects experiencing cognitive decline, which can be disabling (Hedden and Gabrieli 2004). Currently, more than half of the otherwise healthy population over age 60 is affected by varying degrees of spatial learning and memory impairment, which increase in prevalence and severity with common conditions such as Type 2 diabetes, hypertension, and heart disease (Dahle et al. 2009;Okonkwo et al. 2010;Qiu et al. 2005). As the quality and availability of health care in developed countries continue to improve, the aged population is expected to continue to increase (Social Science Data Analysis Network 2010;Shrestha 2006). The prevalence of age-related cognitive decline is expected to rise concomitantly with the increase in our lifespan. As such, greater emphasis must be placed on understanding, preventing, and treating cognitive impairment. The neurobiological basis of n o nneurodegenerative cognitive decline, which occurs in the absence of neuronal cell death or neuropathology (Rapp and Gallagher 1996;Rapp et al. 2002;Rasmussen et al. 1996), remains to be determined but likely involves impaired hippocampal synaptic signaling and regulation [reviewed in (Hof and Morrison 2004)].
Impaired hippocampal function associated with aging is evident in humans (Schaie 1996), monkeys (Rapp and Amaral 1989), rats (Rapp and Gallagher 1996), and mice (Gower and Lamberty 1993). Alterations in neurobiologically-relevant processes, including decreased expression of synaptic machinery, increased oxidative stress, decreased glucose metabolism, and aberrant protein folding and trafficking are characteristic of the aging hippocampus [reviewed in (VanGuilder and Freeman 2011)]. Although these processes are important to healthy brain function, dysregulation of neurotransmission and synaptic plasticity is likely a more immediate cause of cognitive impairment. Electrophysiological studies of hippocampal function demonstrate signaling disruptions with aging and spatial learning and memory impairment, and are consistent with unstable encoding of spatial information. This instability manifests in decreased long-term potentiation, increased long-term depression, and errors in activation of spatiotemporal ensemble network sequences (Barnes et al. 1997;Kumar et al. 2007;Norris et al. 1996;Rosenzweig and Barnes 2003). These electrophysiological characteristics are associated with impaired neurotransmitter synthesis and receptor signaling, dysregulated neuronal gene and protein expression, and atypical synapse morphology (Burke and Barnes 2006;Liu et al. 2008;Poe et al. 2001;Shi et al. 2005).
We have previously reported the age-related downregulation of neurotransmission-associated proteins with roles in synaptic vesicle mobilization, release, and reuptake, in agreement with deficits of neurotransmission characteristic of hippocampal aging (VanGuilder et al. 2010). Additionally, we have described decreased expression of proteins that mediate activity-dependent plasticity [i.e., 14-3-3 theta (Skoulakis and Davis 1998), CamK2α (Lu and Hawkins 2006), and PSD-95 (Vickers et al. 2006)], and increased expression of modulators/stabilizers of neuronal and synaptic structure [i.e., MAP2 (Harada et al. 2002), drebrin (Majoul et al. 2007), Nogo-A (Zagrebelsky et al. 2010)], in hippocampal synaptosomes derived aged cognitively impaired rats compared to aged cognitively intact and adult rats (VanGuilder et al. 2011b). Together with demonstrated deficits of electrophysiological correlates of learning and memory in cognitively impaired rodents, these data further implicate age-associated, dysregulation of synaptic plasticity in cognitively impaired subjects as a potential basis of hippocampal dysfunction and impaired spatial learning and memory.
In recent years, the myelin-associated inhibitors (MAIs) myelin-associated glycoprotein (MAG), Nogo-A (neurite outgrowth inhibitor A), and oligodendrocyte myelin glycoprotein (OMgp) have emerged as potent inhibitors of neuronal outgrowth and structural plasticity (Akbik et al. 2011;Cafferty and Strittmatter 2006;Cafferty et al. 2010;Llorens et al. 2011). By signaling through one of their common receptors, NgR1 (Nogo-66 receptor 1), MAIs stabilize synaptic ultrastructure by modulating cytoskeletal rearrangements (Lee et al. 2008;Zagrebelsky et al. 2010), and suppress activity- and experience-dependent synaptic plasticity (Delekate et al. 2011;Raiker et al. 2010). These actions are similar to the effects of aging on hippocampal synapses, which include decreased functional (Barnes 2003;Kumar et al. 2007;Norris et al. 1996;Rosenzweig and Barnes 2003) and structural (Geinisman et al. 1986;Poe et al. 2001;Shi et al. 2005) plasticity consistent with impaired mechanisms of learning and memory. Additionally, increased MAI expression is implicated in a number of neurological conditions including ALS, AD, PD, MS, traumatic brain injury and stroke, which are associated with deficits of cognitive function and are exacerbated with increasing age (McDonald et al. 2011). However, the role of MAIs in non-injury-related and non-neurodegenerative cognitive decline has not been investigated. The aim of this work was to build on our previous finding that synaptosomal Nogo-A expression is upregulated only in cognitively impaired aged rats (VanGuilder et al. 2011b) by assessing hippocampal expression of MAIs in a well-characterized rodent model of cognitive decline and identifying potential relationships between protein-level expression of these plasticity-inhibiting factors and spatial learning and memory in individual animals.
Here, we describe protein-level induction of MAG, Nogo-A, OMgp, and NgR1 in synaptosomes derived from whole hippocampus, and in dissected hippocampal CA1, CA3 and DG subregions, that was observed only in aged rats with cognitive impairment, and demonstrate significant correlation of MAI expression levels with cognitive performance. We also demonstrate axonal localization of MAI ligands and the NgR1 receptor as a potential site of MAI-mediated suppression of functional and structural plasticity implicated in cognitive decline.
Methods
Animals
Male Fischer 344 × Brown Norway (F1) hybrid rats (see Table 1 for cohort information, described, in part, in references (VanGuilder et al. 2011a;VanGuilder et al. 2011b) were purchased from the National Institute on Aging colony maintained by Harlan Industries (Indianapolis, IN) and acclimatized in quarantine for two weeks upon arrival, as previously described (VanGuilder et al. 2010;VanGuilder et al. 2011b). Rats were housed in laminar-flow cages, with food (Purina Mills, Richmond, IN) and water available ad libitum, in the OUHSC specific pathogen-free Barrier Facility. In accordance with protocols commonly implemented in studies of aging and cognitive decline (VanGuilder et al. 2010;VanGuilder et al. 2011b;VanGuilder et al. 2011a;Kumar et al. 2011;Gomes da Silva et al. 2010;Freeman et al. 2009;Eckert et al. 2010;Adams et al. 2008), all rats were singly housed to minimize potential variables, such as differing degrees of social interaction and stressful interactions with cagemates, that can confound behavioral testing data. The Barrier Facility is maintained on a 12-hour light/dark cycle with temperature and humidity at constant levels. To minimize potential stress associated with handling during behavioral testing, all rats were handled daily by laboratory personnel for two weeks prior to cognitive assessment. During this acclimation period, rats were handled until they remained calm when picked up and held by multiple investigators, including the experimenter performing Morris water maze testing. Spatial learning and memory was assessed using Morris water maze testing, as described below. One week following conclusion of behavioral phenotyping, rats were sacrificed by decapitation without anesthesia to obviate potential acute effects of anesthetics on downstream endpoints including quantitation of gene and protein expression. Left and right hippocampi were individually dissected for preparation of synaptosomes [Cohort A (adult: n=5; aged intact: n=8; aged impaired: n=7)] or dissection of CA1, CA3 and DG subregions [Cohort B (adult: n=7; aged intact: n=7; aged impaired: n=10)]. A third cohort of cognitively-assessed rats [Cohort C (adult: n=9; aged intact: n=10; aged impaired: n=5)] was perfusion-fixed under ketamine/xylazine anesthesia by transcardial perfusion with 6U/mL heparin (sodium salt) in phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in PBS (pH 7.4). At sacrifice, all rats were examined for exclusionary criteria (e.g., pituitary tumors, cortical atrophy, gross nephropathy) that could confound behavioral and molecular analyses. The OUHSC animal facilities are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and all animal procedures were approved by the Institutional Animal Care and Use Committee in compliance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and the National Research Council's Guide for the Care and Use of Laboratory Animals.
Table 1.
Animal cohort information
| Cohort | Group | Age (months) | n | Sample Type | Endpoints |
|---|---|---|---|---|---|
| Aa | Adult | 12 | 5 | Synaptosome preparations (whole hippocampus) | Spatial learning and memory, protein/mRNA expression |
| Aged Intact | 28 | 8 | |||
| Aged Impaired | 28 | 7 | |||
| Bb | Adult | 12 | 7 | Hippocampal subregion dissections (CA1, CA3, DG) | Spatial learning and memory, protein/mRNA expression |
| Aged Intact | 26 | 7 | |||
| Aged Impaired | 26 | 10 | |||
| Ca,b | Adult | 13 | 9 | Perfusion-fixed sagittal hemispheres (cryosectioned) | Spatial learning and memory, protein localization |
| Aged Intact | 26 | 10 | |||
| Aged Impaired | 26 | 5 |
Also described in VanGuilder et al., 2011b
Also described in VanGuilder et al., 2011a
Morris water maze testing
Hippocampus-dependent spatial learning and memory was evaluated by Morris water maze as previously described (VanGuilder et al. 2011a;VanGuilder et al. 2011b). A round water maze tank (diameter: 1.7m; height: 0.6m) was filled to a depth of 25cm with water rendered opaque with non-toxic, water-based white food coloring, and was surrounded by a curtain with fixed-position visual reference cues. A retractable escape platform (12cm2) was submerged 2cm below the surface of the water, and a center-mounted camera mounted above the maze pool provided visual input to an automated tracking system (Noldus Ethovision XT, Wageningen, Netherlands) used for analysis of maze performance measures.
The escape platform location was maintained in a fixed position throughout the acquisition phase. Task acquisition was conducted over eight days in four individual two-day blocks (Figure 1). Each acquisition block consisted of five 60s trials performed over two days (2–3 trials/day) to avoid fatiguing the rats, with starting positions pseudo-randomized to ensure that rats learned to associate visual cues with the escape platform location rather than following a consistent path from a single starting location. Rats' path length to the escape platform was the dependent measure in acquisition trials, with decreasing path length indicating improving maze performance. Probe trials were interpolated between acquisition blocks to increase the accuracy of cognitive assessment measures throughout maze testing, as previously described by ourselves and others (VanGuilder et al. 2011b;VanGuilder et al. 2011a;Stranahan et al. 2011;Gallagher et al. 1993;Rapp and Gallagher 1996;Nieves-Martinez et al. 2010;Geinisman et al. 2004;Nicholson et al. 2004).
Figure 1. Timecourse of behavioral testing.
Upon arrival to the OUHSC Barrier Facility, rats were quarantined for two weeks, then acclimated to handling by investigators for two weeks. Morris water maze testing was performed in four blocks, each consisting of five acquisition trials and a probe trial performed by a single investigator. Each block was performed over two days (~3 trials/day) for a total of eight days of testing. After conclusion of behavioral testing, rats received a series of three swim trials with a visible platform to test for deficits of visual function.
After completion of each two-day acquisition block (i.e., after every five acquisition trials), the escape platform was lowered to an inaccessible depth, and a probe trial was performed [for a total of four probe trials, performed on testing days two, four, six, and eight (Figure 1)]. Rats were placed into the maze and allowed 30s to locate the escape platform location based on the surrounding reference cues. During each probe trial, the average distance to the platform location over the 30s trial was calculated. After the last probe trial, a composite “mean proximity to the platform location” measure was generated by calculating the mathematical mean of the average distance to the platform location measured in all four probe trials for each animal. This “mean proximity” measure reflects each animal's average performance in four individual probe trials, and is used to classify aged rats as cognitively intact or cognitively impaired. The composite mean proximity value is also used for statistical correlation analyses of cognitive performance and protein expression. Mean swim velocity was also measured to ensure that motor deficits did not confound maze performance. Memory extinction between blocks was prevented by replacing the escape platform and allowing rats 60s to re-locate its position in the context of the surrounding visual cues. One day following conclusion of maze testing, visual performance was evaluated over four consecutive swim trials with the escape platform visible to ensure visual deficits did not confound maze performance. To decrease the likelihood that molecular alterations potentially induced with maze testing did not confound downstream biochemical analyses, rats were sacrificed one week following conclusion of testing.
Cognitive stratification of aged rats
Probe trial data were used to classify aged rats as cognitively intact or cognitively impaired relative to the adult group. A composite mean proximity to the escape platform measure, reflecting the average distance to the platform across all four probe trials, was used as the primary measure of cognitive status, as previously described (VanGuilder et al. 2011b). Aged rats performing within the range of adults were classified as cognitively intact, while those with inferior performance were classified as cognitively impaired. To verify that the probe trial performance of adult and aged, cognitively intact rats was indeed superior to that of the aged, cognitively impaired group, individual probe trial and mean probe trial data were analyzed by one-way ANOVA with Student Newman Keuls post hoc testing. Cognitive stratification by probe trial performance allowed retrospective analysis of group-based acquisition phase data. To ascertain that all rats (adult, aged cognitively intact, aged cognitively impaired) successfully acquired the task, data were statistically analyzed across acquisition blocks by group using oneway repeated measures ANOVA with Holm-Sidak post hoc testing. Differences between groups in individual acquisition blocks were evaluated by one-way ANOVA with Student Newman Keuls post hoc testing.
Synaptosome isolation
Synaptosome fractions were prepared from dissected hippocampi as previously described (VanGuilder et al. 2010;VanGuilder et al. 2011b). Left and right hippocampi were individually dissected into ice-cold sucrose buffer (320mM sucrose, 4mM HEPES, 1mM Na3VO4, pH 7.4) and incubated on ice for 30min with buffer exchanged every ten minutes. Hippocampi were homogenized in 8mL sucrose buffer with a mechanically-driven douce homogenizer, and nuclear, cytoskeletal, and synaptosomal fractions were separated by differential centrifugation. Synaptosome-containing fractions were resuspended in lysis buffer for protein isolation or TRI-Reagent (Molecular Research Center, Cincinnati, OH) for RNA isolation. Enrichment of synaptic elements and depletion of nuclear material was confirmed by immunoblotting.
Subregion dissection
Hippocampal CA1, CA3 and DG subregions were individually dissected from left and right hippocampi. Briefly, hippocampi were hemisected and the dorsomedial portion was further dissected into four blocks perpendicular to the longitudinal axis. From these blocks, CA3 was isolated by cutting along the edge of the DG. CA1 and DG were isolated by cutting along the hippocampal fissure as described previously (Newton et al. 2005;VanGuilder et al. 2011a).
Protein isolation and immunoblotting
Soluble protein was extracted by homogenizing synaptosome and subregion samples in a detergent-based protein lysis buffer containing protease and phosphatase inhibitors (100mM NaCl, 20mM HEPES, 1mM EDTA, 1mM dithiothreitol, 1.0% Tween20, 1mM Na3VO4, 1 Complete Mini EDTA-Free Protease Inhibitor Cocktail Tablet (Roche Applied Science, Indianapolis, IN) for every 10 mL lysis buffer) using a bead mill (Retsch TissueLyzer II, Qiagen, Valencia, CA). Homogenates were incubated at 4°C with gentle rocking for 15min, and insoluble protein was removed by centrifugation (10,000 × g, 15min, 4°C). The soluble protein-containing supernatant was collected, and protein concentrations were determined by bicinchoninic acid quantitation (Pierce, Rockford, IL).
Protein-level expression of MAG, Nogo-A, OMgp, NgR1, and GAP-43 was determined using standard immunoblotting methods (VanGuilder et al. 2010;VanGuilder et al. 2011b), with the age and cognitive status of samples masked to investigators. Protein samples were adjusted to a concentration of 2μg/μL in protein lysis buffer and LDS sample buffer (Invitrogen, Carlsbad, CA) in volumes sufficient to perform all immunoblot analyses and to confirm equal protein content, from one sample preparation as previously described (VanGuilder et al. 2010). 10μg of each prepared protein sample was denatured at 95°C prior to SDS-PAGE separation using Criterion Tris-HCl precast 4–20% acrylamide gradient gels (BioRad, Hercules, CA). For each sample type (synaptosome preparations, CA1, CA3, and DG subregions) a gel containing all study samples was stained with Deep Purple total protein stain (GE Healthcare, Piscataway, NJ) and quantitated by whole-lane digital densitometry (ImageQuant TL, Molecular Dynamics, Sunnyvale, CA) to ensure equal protein content between samples. Beta actin immunoblot quantitation was also performed to confirm equal protein content. For immunoblotting, proteins were transferred to PVDF membranes (HyBond, GE Healthcare), blocked with 5% nonfat milk in PBS containing 1% Tween-20 (PBST), and incubated with primary antibodies (Table 2). Membranes were washed with PBST, incubated with species-appropriate secondary antibodies (Table 2), and visualized with enhanced chemiluminescence substrate (GE Healthcare). Immunoreactive bands were imaged on film, digitized at a resolution of 800d.p.i., and quantitated using automated digital densitometry software with rolling ball background subtraction (ImageQuant TL).
Table 2.
Antibody information
| Target | Supplier | Catalog # | Host | Use | Methoda |
|---|---|---|---|---|---|
| MAG (myelin-associated glycoprotein, Siglec 4a) | Neuromics | gt15152 | goat | primary | IB/IHC |
| Nogo-A (neurite outgrowth inhibitor A, reticulon 4) | Millipore | ab5888 | rabbit | primary | IB |
| Nogo-A (neurite outgrowth inhibitor A, reticulon 4) | Abcam | ab32298 | rabbit | primary | IHC |
| OMgp (oligodendrocyte-myelin glycoprotein) | Abcam | ab32760 | rabbit | primary | IB/IHC |
| NgR1 (Nogo-66 receptor 1, reticulon 4 receptor) | Santa Cruz Biotechnology | sc25659 | rabbit | primary | IB/IHC |
| GAP-43 (growth-associated protein 43) | Abcam | ab12274 | rabbit | primary | IB |
| Beta actin | Chemicon | mab1501 | mouse | primary | IB |
| NFh (heavy chain neurofilament) | Sigma | no142 | mouse | primary | IHC |
| Goat IgG (HRP-conjugated) | Santa Cruz Biotechnology | sc2768 | rabbit | secondary | IB |
| Rabbit IgG (HRP-conjugated) | GE Healthcare | na934V | donkey | secondary | IB |
| Goat [F(ab')2] DyLight 549 | Jackson ImmunoResearch | 705506147 | donkey | secondary | IHC |
| Rabbit [F(ab')2] DyLight 649 | Jackson ImmunoResearch | 711496152 | donkey | secondary | IHC |
| Mouse [F(ab')2] DyLight 488 | Jackson ImmunoResearch | 715486150 | goat | secondary | IHC |
IB: immunoblotting; IHC: immunohistochemistry
Immunoblot data were normalized to corresponding whole-lane densitometric volumes of total protein-stained gels as previously described (VanGuilder et al. 2010;VanGuilder et al. 2011b), and were statistically analyzed by one-way ANOVA with Student Newman Keuls post hoc testing. Relationships between protein expression and cognitive performance were evaluated by Pearson product moment correlation (normal data distributions) or Spearman rank order correlation (non-normal data distributions) using SigmaStat 3.5 software (Systat Software, San Jose, CA).
RNA isolation and RT-qPCR
Synaptosome and subregion samples were homogenized in TRI-Reagent using a bead mill as previously described (Rountree et al. 2010;VanGuilder et al. 2011a). RNA was isolated by addition of 10% BCP and standard phase separation, and was precipitated overnight at −20°C in isopropanol. RNA was purified using the RNease Mini kit (Qiagen), and assessed for quality and quantity by microfluidics chip (2100 Expert Bioanalyzer NanoChip, Agilent, Palo Alto, CA) and spectrometry (NanoDrop ND1000, Thermo Scientific, Wilmington, DE), respectively.
Gene expression levels were quantitated by RT-qPCR performed as previously described (Brucklacher et al. 2008;Freeman et al. 2010;VanGuilder et al. 2011a), with samples de-identified to investigators. Briefly, cDNA was synthesized from purified RNA using the High-capacity cDNA Reverse Transcription kit (Applied Biosciences, Foster City, CA). RT-qPCR analysis of MAG, Nogo-A, OMgp, and NgR1 was performed using TaqMan Assay-On-Demand gene-specific primers/probe assays (Applied Biosystems) with β-actin as the endogenous control (Table 3). Experiments were performed and analyzed with a 7900HT Sequence Detection System (Applied Biosystems) and SDS 2.2.2 software using the 2−ΔΔCt analysis method. Statistical analysis was performed by one-way ANOVA.
Table 3.
mRNA expression of myelin-associated inhibitors
| Gene Expression (% of Adult mean, ± SEM) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CA1 | CA3 | DG | |||||||||
| Protein name | Gene Symbol | TaqMan AOD | Adult | Aged Intact | Aged Impaired | Adult | Aged Intact | Aged Impaired | Adult | Aged Intact | Aged Impaired |
| Myelin-associated glycoprotein | MAG, Siglec-4 | Rn02586362 | 100 ± 13.8 | 101 ± 8.3 | 109 ± 5.0 | 100 ± 10.1 | 115 ± 6.7 | 105 ± 6.5 | 100 ± 12.2 | 107 ± 12.4 | 138 ± 15.1 |
| Oligodendrocyte myelin glycoprotein | OMgp, MOG | Rn00575354 | 100 ± 13.4 | 79 ± 6.8 | 99 ± 13.1 | 100 ± 12.7 | 124 ± 8.9 | 104 ± 5.4 | 100 ± 11.3 | 123 ± 10.9 | 127 ± 14.5 |
| Neurite outgrowth inhibitor | Nogo-A, Rtn4 | Rn01429676 | 100 ± 3.9 | 71 ± 7.8 | 83 ± 9.4 | 100 ± 6.8 | 118 ± 8.4 | 105 ± 9.9 | 100 ± 10.9 | 52 ± 5.9* | 87 ± 13.9 |
| Nogo-66 receptor 1 | NgR1, Rtn4r | Rn00586061 | 100 ± 9.6 | 114 ± 4.8 | 109 ± 9.1 | 100 ± 9.6 | 114 ± 4.9 | 109 ± 9.1 | 100 ± 18.1 | 66 ± 14.2 | 111 ± 17.5 |
| β-actin | Actb | Rn00667869 | - | - | - | - | - | - | - | - | - |
Immunohistochemical localization
Following perfusion, rats used for immunohistochemistry experiments were decapitated and their brains extracted, hemisected sagittally, immersion-fixed in 4% paraformaldehyde (pH 7.4), and impregnated with 30% sucrose in PBS, as described previously (VanGuilder et al. 2011b). Tissues were embedded in Tissue-Tek optimal cutting temperature compound (Sakura Finetek, Torrance, CA), frozen in isopentane on dry ice, and stored at −80°C.
Brains from three rats from each group (adult, aged cognitively intact, aged cognitively impaired) were de-identified to investigators prior to immunohistochemical localization experiments and visualization by confocal microscopy. Tissues were cryosectioned (12μm; HN 505E, Microm International, Walldorf, Germany) in the sagittal plane and sections were collected on FisherBrand SuperFrost Plus glass slides (Fisher Scientific, Pittsburg, PA), postfixed with 2% paraformaldehyde in PBS, and blocked with 10% donkey serum (Jackson ImmunoResearch, West Grove, PA) in PBS containing 0.1% Triton X-100. Slides were incubated with primary antibodies (Table 2) in blocking solution overnight at 4°C, washed to remove unbound antibody, and incubated with species-appropriate fluorescence-conjugated secondary antibodies (Table 2) in blocking buffer. To control for non-specific secondary antibody signals and background fluorescence, a negative control was included with primary antibodies omitted. Nuclei were stained with Hoechst 33258 (5μg/mL, Invitrogen). After washing, slides were coverslipped with Aqua Poly/Mount (Polysciences, Warrington, PA) and dried prior to imaging.
Images were acquired using a confocal laser scanning microscope (Leica TCS SP2 AOBS, Exton, PA) equipped with UV-diode (Hoechst, 405nm), argon (488nm), and helium-neon (546nm and 649nm) lasers. To characterize MAI expression within hippocampal subregions, tissue sections were imaged as 1024×1024 pixel, 3.9μm series of 6 optical sections (step size = 0.65 μm) acquired using a 20× oil-immersion objective with 2×2 line and frame averaging to decrease noise, using identical laser settings optimized for each antibody. High-resolution imaging of Hoechst nuclear staining and neurofilament/MAI immunoreactivity was performed using a 63× oil-immersion objective with 2×2 line and frame averaging, with identical laser settings optimized for each antibody. All images are presented as average projections of z-stacks. Additional noise reduction and background subtraction were performed using Adobe Photoshop CS4 software (Adobe Systems, San Jose, CA), with equal adjustments applied to all images of a given antibody. 20× images of MAI distribution within hippocampal subregions were converted to inverted grayscale to enhance contrast in monochrome images.
Results
Behavioral stratification of adult and aged rats by Morris water maze performance
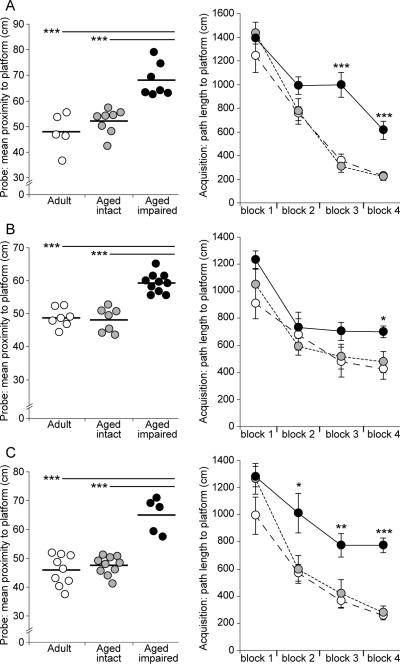
Hippocampus-dependent cognitive performance of adult and aged Fischer 344 × Brown Norway rats was assessed by Morris water maze testing, and aged animals were assigned to cognitively intact or cognitively impaired groups based on mean proximity to the escape platform during probe trials as previously described (Freeman et al. 2009;VanGuilder et al. 2011b). Stratification of aged rats into cognitively intact and cognitively impaired groups was evident in the three cohorts included in this study (Table 1). Statistical analysis confirmed that spatial learning and memory was superior in adult and aged cognitively intact rats compared to aged cognitively impaired rats (p<0.001, one-way ANOVA with Student Newman Keuls post hoc testing). In the cohort used for preparation of hippocampal synaptosomes (Cohort A, Figure 2A, left), mean proximity-to-platform measures of adult (48±1.9 cm) and aged intact (52±2.4 cm) were significantly lower than aged impaired rats (68±1.6cm). Similar differences were observed in the cohort used for dissection of hippocampal subregions (Cohort B, Figure 2B, left), in which the performance of aged impaired rats (59±0.9 cm) was significantly inferior to that of adult (48±1.1 cm) and aged intact (48±1.4 cm) rats. The cohort used for immunohistochemical localization of target proteins (Cohort C, Figure 2C, left) also demonstrated performance-based stratification, with significant differences evident between the mean proximity-to-platform measures of aged impaired (65±2.5 cm) rats and that of both adult (46±3.8 cm) and aged intact (47±3.4 cm) rats. No differences in mean proximity to the platform location were detected between adult and aged cognitively intact rats in any cohort.
Figure 2. Deficits of spatial learning and memory manifest in a subset of aged rats.
Three independent cohorts of adult (12–13 months) and aged (26–28 months) male Fischer 344 × Brown Norway rats were assessed for hippocampus-dependent cognitive function by Morris water maze testing. Based on probe trial performance (mean proximity to platform), aged rats were classified as cognitively intact or cognitively impaired relative to the adult group mean. In all three cohorts, statistical testing verified that the performance of both adult and aged cognitively intact rats was significantly superior to aged impaired rats (left, A: cohort A; B: cohort B; C: cohort C). Points represent individual animals and horizontal bars indicate group means; ***p<0.001, ANOVA with Student Newman Keuls post hoc testing. Retrospective analysis of segregated group acquisition data revealed that while all groups improve performance across blocks (repeated measures ANOVA p<0.05, statistics not shown), the acquisition performance of aged rats classified as cognitively impaired maintained significantly longer path-to-platform lengths than adult and aged intact rats across training blocks (right, A–C). Acquisition of aged intact and adult rats was not different on any training block. *p<0.05, **p<0.01, ***p<0.001, one way ANOVA with Student Newman Keuls post hoc testing.
Retrospective analysis of group-based acquisition data demonstrated that while all groups learned the task over the four progressive acquisition blocks in all three cohorts (repeated measures ANOVA, p<0.05), aged cognitively impaired rats maintained longer path lengths to the escape platform than adult and aged intact rats (Figure 2A–C, right panels). Significantly inferior performance was evident in aged impaired rats compared to both adult and aged intact rats at the end of task acquisition (p<0.001 to p<0.05, one-way ANOVA with Student Newman Keuls post hoc testing), suggesting that adult and aged intact rats learned the maze task more effectively than aged impaired rats. Inferior performance in aged, cognitively impaired rats was not associated with decreased swim velocity (cm/s) compared to adults or aged intact rats, as no differences in average swim speed were apparent between adult, aged intact, and aged impaired rats in Cohort A (adult: 19 ± 1.04 cm/s; aged intact: 20.9 ± 0.62 cm/s; aged impaired: 19.2 ± 0.97 cm/s), Cohort B (adult: 19.5 ± 0.91 cm/s; aged intact: 19.7 ± 0.70 cm/s; aged impaired: 19.2 ± 0.97 cm/s), or Cohort C (adult: 20.1 ± 0.63 cm/s; aged intact: 18.9 ± 0.68 cm/s; aged impaired: 18.9 ± 0.49 cm/s).
Immunoblot assessment of myelin-associated inhibitors
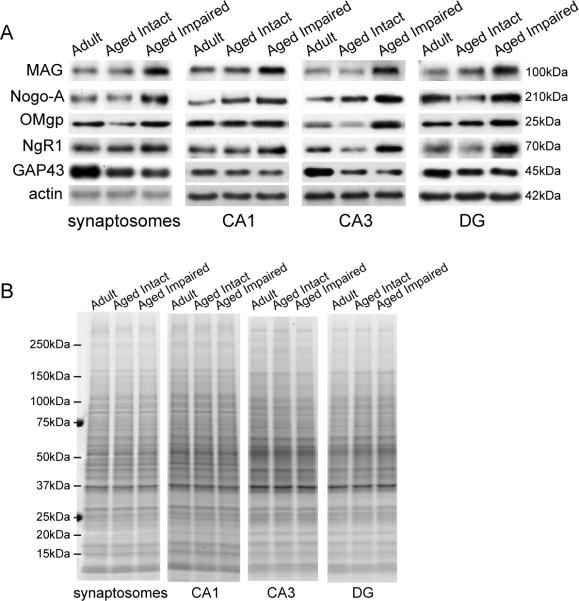
Immunoblot analysis was performed to assess protein content of MAI ligands [MAG (myelin-associated glycoprotein), Nogo-A (neurite outgrowth inhibitor A), and OMgp (oligodendrocyte myelin glycoprotein)] and a well-characterized MAI receptor [NgR1 (Nogo-66 receptor 1)]. GAP-43 (growth-associated protein 43) was assessed as a marker of neuronal outgrowth, and beta actin quantitation was included in immunoblot analysis as an unchanged control. Representative bands from immunoblot analysis of these proteins are depicted in Figure 3A. MAG immunoreactivity was apparent as a single band at approximately 100kDa. Two Nogo-A-immunoreactive bands were evident: a very faint band was observed at ~120kDa, and a darker, heavier band was observed at ~210kDa. Only the 210kDa band (shown) was consistently detected in all samples and at intensities suitable for quantitation. A single OMgp-immunoreactive band was detected ~25kDa in all samples. Multiple faint, variable bands were apparent on anti-NgR1 immunoblots, between 25kDa and 37kDa, but were inconsistent. A prominent NgR1-immunoreactive single band (shown) was detected at 70kDa. MAG, Nogo-A, OMgp, and NgR1 immunoblots suggested increased protein expression in aged, cognitively impaired rats. Immunoblotting for the neuronal regeneration marker GAP-43 produced a single band at ~45kDa and appeared to decrease in intensity in aged rats compared to adults. Beta actin immunoblotting was performed separately to confirm equal protein loading, and produced a single band at 42kDa that was consistent across all animal groups. Immunoblot data were normalized to total-protein stained gels (Figure 3B) generated from the same sample preparations as those used for protein quantitation. No differences in total protein content were apparent between animal groups, and whole-lane densitometry confirmed equal protein loading.
Figure 3. Immunoblot assessment of hippocampal MAI expression.
(A) Hippocampal synaptosomes and dissected subregions were immunoblotted for the MAI ligands MAG, Nogo-A, and OMgp and their common receptor, NgR1. MAI expression was increased in aged, cognitively impaired rats compared to adult and aged intact rats. Immunoblotting for the neuronal growth-associated protein GAP-43 indicated an age-related decrease in GAP-43 expression. Actin immunoblotting was included as an unchanged control to ensure equal loading. (B) Gels loaded with an aliquot of each sample used in immunoblot analysis were stained for total protein content to ensure equivalent protein amounts in all samples.
Hippocampal MAG expression is significantly upregulated with cognitive decline
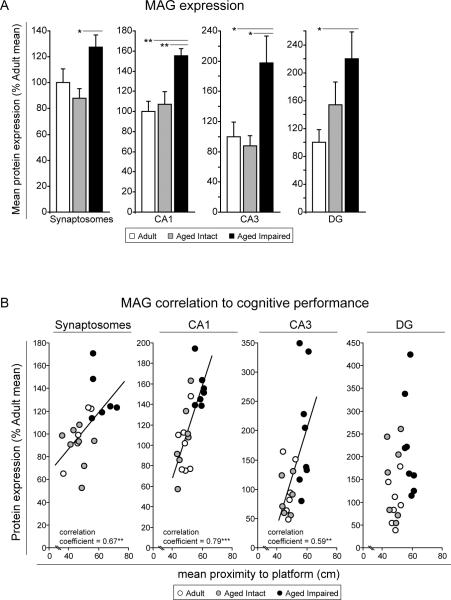
Protein expression of myelin-associated glycoprotein (MAG) in synaptosomal preparations derived from whole hippocampus, and dissected CA1, CA3 and DG hippocampal subregions were quantified by digital densitometry (Figure 4A). In aged, cognitively impaired rats, MAG content was increased by nearly 50% in synaptosomes (p<0.05) and CA1 (p<0.01), and DG, and by 220% in CA3 (p<0.05), compared to aged cognitively intact rats (one-way ANOVA with Student Newman Keuls post hoc testing). Significant elevation of MAG was also evident in aged impaired rats compared to adults in CA1 (p<0.01), CA3 (p<0.05) and DG (p<0.05). No differences in MAG expression were detected between adult and aged intact rats. Potential correlation between cognitive impairment and increased MAG expression in individual rats was evaluated by Pearson (normal distributions) or Spearman (non-normal distributions) correlation analyses (Figure 4B). Significant correlations between MAG content and mean proximity-to-platform probe trial measures were identified in synaptosomes (p<0.01), and CA1 (p<0.001) and CA3 (p<0.01) subregions, indicating that higher MAG expression in hippocampal synapses and pyramidal cell-containing subregions is associated with poorer spatial learning and memory.
Figure 4. Induction of hippocampal MAG expression with cognitive decline.
(A) Protein-level MAG expression in aged, cognitively impaired rats was significantly upregulated in hippocampal synapses, and CA1 and CA3 subregions compared to aged intact rats, and in CA1, CA3 and DG compared to adults. *p<0.05, **p<0.01; one-way ANOVA with Student Newman Keuls post hoc testing; n=5–10/group. (B) Across individual animals, MAG expression in synaptosomes and CA1 and CA3 subregions correlated with probe trial measures of cognitive performance, indicating a relationship between increasing MAG protein content and decreasing spatial learning and memory ability. **p<0.01, ***p<0.001, Pearson (normally-distributed data) or Spearman (non-normally-distributed data) correlation; n=5–10/group.
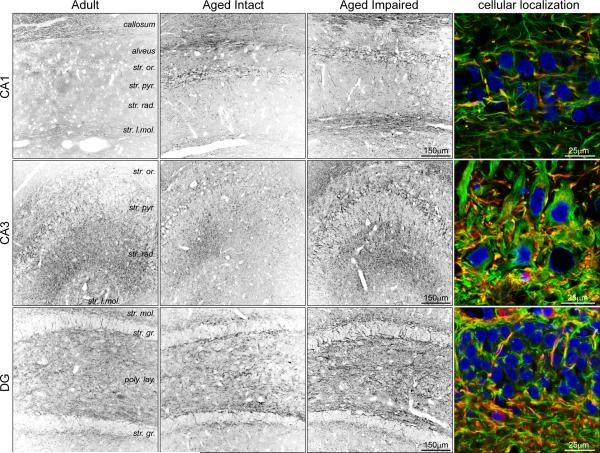
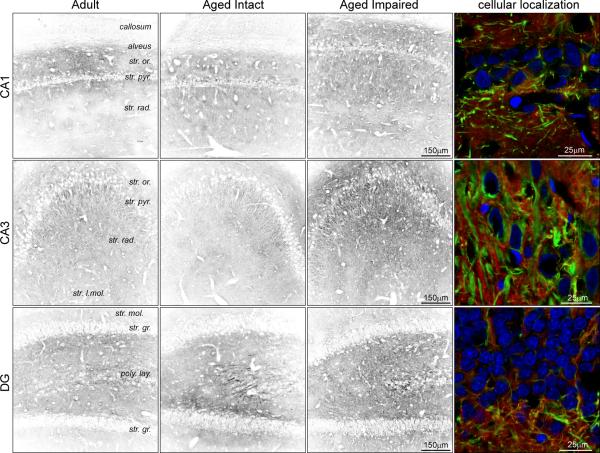
To extend our immunoblot characterization of MAG expression, immunohistochemical localization experiments were performed (Figure 5). MAG immunoreactivity was evident throughout the hippocampus as diffuse staining in the neuropil, and was also associated with puncta and cellular processes in synapse-containing strata. Significant MAG immunoreactivity was also apparent in the heavily-myelinated corpus callosum. In the hippocampus, MAG expression appeared to be higher in the cellular processes of synapse- and projection fiber-containing layers of CA1 (e.g., stratum lacunosum-moleculare) CA3 (e.g., stratum radiatum) and DG (e.g., polymorphic layer) than in CA1, which is consistent with the higher degree of myelination evident in these regions in the mature hippocampus (Meier et al. 2004). Perinuclear staining was also evident in the pyramidal cell layer of CA1 and CA3, but not the DG granule cell layer. In DG, MAG-immunoreactive processes traversed the granule cell layer between the polymorphic and molecular layers. In agreement with immunoblot data, hippocampal immunoreactivity to MAG was increased in tissue sections from aged, cognitively impaired rats compared to aged intact and adult rats. Since MAIs are expressed predominantly by myelinating oligodendrocytes ensheathing neuronal axons, MAG immunoreactivity was co-imaged with heavy chain neurofilament (NFh) at high magnification to provide a cytoarchitectural reference for visualization of MAG expression at the cellular level (Figure 5, right column). MAG expression was observed in close proximity to axons (MAG+/NFh−), as expected, with a greater density of peri-axonal MAG immunoreactivity in CA3 and DG. A degree of colocalization of MAG and NFh was also evident (MAG+/NFh+), suggesting that MAG may be expressed by hippocampal neurons in addition to myelinating oligodendrocytes. Increased periaxonal immunoreactivity was evident in CA1, CA3 and DG of aged impaired rats compared to aged intact and adult rats (Supplemental Figure 1). Assessment of the negative control, incubated with secondary antibodies but with primary antibodies omitted, revealed no non-specific signals and minimal background fluorescence, demonstrating the specificity of MAI visualization (Supplemental Figure 2).
Figure 5. Hippocampal localization of MAG induction.
MAG immunoreactivity was distributed throughout the neuropil, and was also evident in projections and puncta, particularly in stratum lacunosum of CA1 and stratum radiatum of CA3. Perinuclear staining was also evident in the pyramidal cell layer. MAG immunoreactivity was increased in intensity in aged cognitively impaired rats compared to aged intact and adult rats. High-resolution visualization of MAG immunoreactivity and NFh-expressing axons demonstrated both periaxonal and axonal localization of MAG. Blue: Hoechst; Green: NFh; Red: MAG.
Increased hippocampal expression of Nogo-A occurs in cognitively impaired aged rats
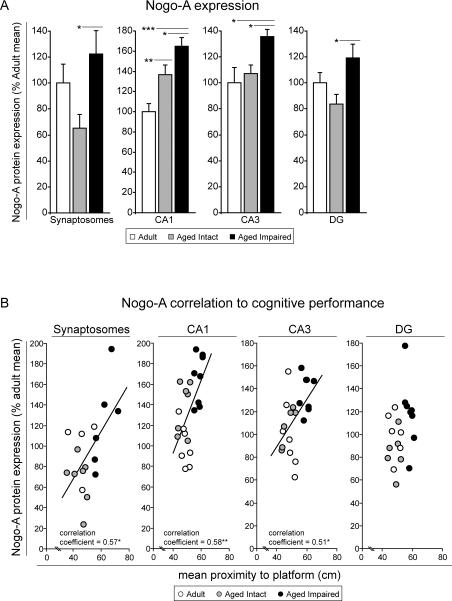
Immunoblot quantitation of neurite outgrowth inhibitor A (Nogo-A) content in whole-hippocampus synaptosomes and unfractionated CA1, CA3 and DG subregions derived from adult and aged, behaviorally phenotyped rats demonstrated significant induction of Nogo-A protein with cognitive impairment (Figure 6A). As previously described, synaptosomal expression of Nogo-A was increased by more than 80% (p<0.05) in aged cognitively impaired rats compared to aged intact rats (VanGuilder et al. 2011b), but was not different from adult rat levels. Nogo-A upregulation was also observed in CA1 (20%, p<0.05), CA3 (20%, p<0.05) and DG (40%, p<0.05) of aged impaired rats compared to aged intact rats. Significant increases in Nogo-A content were also evident between aged impaired rats and adult rats in CA1 (70%, p<0.001) and CA3 (35%, p<0.05), and between aged intact and adult rats in CA1 (35%, p<0.01). Among individual animals, Nogo-A content was significantly correlated with mean proximity-to-platform measures, demonstrating a relationship between increased Nogo-A expression and decreased cognitive performance (Figure 6B). Correlations were identified in whole-hippocampus synaptosome preparations (p<0.05), CA1 (p<0.01), and CA3 (p<0.01).
Figure 6. Hippocampal Nogo-A expression is increased with cognitive impairment in aged rats.
(A) In aged cognitively impaired rats, Nogo-A expression was significantly increased in synaptosomes as well as CA1, CA3 and DG subregions compared to aged intact rats, and in CA1 and in CA3 compared to adults. *p<0.05, **p<0.01, ***p<0.001; one-way ANOVA with Student Newman Keuls post hoc testing; n=5–10/group. (B) In whole-hippocampal synaptosomes and CA1 and CA3, but not DG, significant correlation between Nogo-A expression and mean probe trial proximity-to-platform measures of cognitive function were detected. *p<0.05, **p<0.01, ***p<0.001, Pearson (normally-distributed data) or Spearman (non-normally-distributed data) correlation; n=5–10/group.
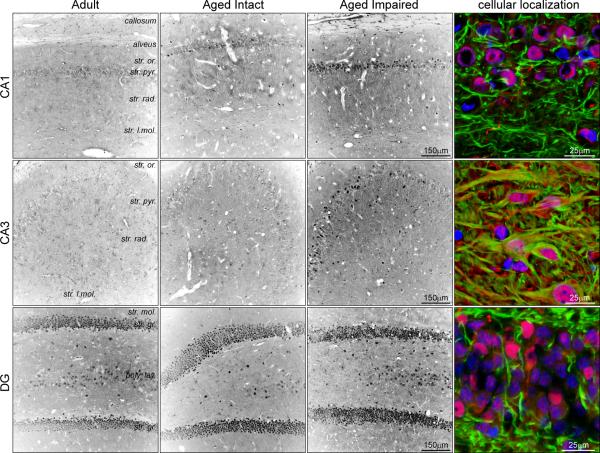
Immunohistochemical visualization demonstrated Nogo-A expression throughout the hippocampus (Figure 7). Punctate and projection-associated Nogo-A immunoreactivity was evident throughout synapse-containing hippocampal layers, particularly in CA1 and CA3 stratum radiatum. Additionally, in agreement with previous reports (Jin et al. 2003;Zhou et al. 2003), nuclear Nogo-A staining was observed throughout the hippocampus, and was concentrated in the pyramidal and granule cell layers. In aged impaired rats, enhanced Nogo-A staining was apparent throughout all subregions compared to aged intact and adult rats. Increased nuclear immunoreactivity was evident both in the number of Nogo-A immunoreactive nuclei and in the intensity of nuclear staining. Similarly, in aged impaired rats, Nogo-A immunoreactivity associated with cellular projections was both darker and more frequently observed, particulary in CA1 and CA3. Nogo-A immunoreactivity in the corpos callosum also appeared to be increased in aged cognitively impaired rats, in which Nogo-A+ cells (presumably oligodendrocytes) exhibited increased somatic and projection-associated staining. Co-staining experiments visualizing axon cytoarchitecture (NFh immunoreactivity) and Nogo-A demonstrated NFh−, putatively oligodendrocytic, Nogo-A immunoreactivity in processes near axons in CA1 and DG (Figure 7, right column). Compared to aged intact and adult rats, Nogo-A staining in aged impaired rats was more intense throughout CA3, and was observed in both NFh− and NFh+ projections (Supplemental Figure 2), suggesting both oligodendrocytic and neuronal expression of hippocampal Nogo-A. A subset of Nogo-A-immunoreactive nuclei in CA3 also appeared to be neuronal, as indicated by somatic NFh immunoreactivity.
Figure 7. Localization of hippocampal Nogo-A.
The distribution of Nogo-A protein was assessed by immunohistochemical visualization in adult, aged cognitively intact and aged cognitively impaired rats. Punctate and process-associated Nogo-A immunoreactivity was evident in CA1 and CA3 stratum radiatum. Additionally, a number of Nogo-A+ nuclei were observed in the pyramidal and granule cell layers. In aged impaired rats, Nogo-A staining in both nuclei and projections was increase compared to adult and aged intact rats. Counterstaining with NFh demonstrated that the majority of Nogo-A+ processes are non-axonal (putatively oligodendrocytic), although a number of NFh+/Nogo-A+ axons were observed in CA3. Additionally, several Nogo-A+ nuclei were surrounded by somatic NFh immunoreactivity, indicating neuronal Nogo-A expression. Blue: Hoechst; Green: NFh; Red: Nogo-A.
Induction of hippocampal OMgp expression occurs with age-related cognitive impairment
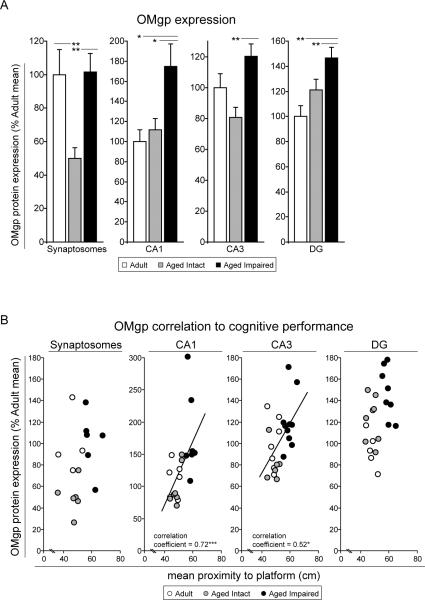
To assess expression of oligodendrocyte myelin glycoprotein (OMgp) in hippocampus with advanced aging and age-related cognitive decline, immunoblot analysis of synaptosomal preparations and CA1, CA3, and DG subregions was performed (Figure 8A). Similarly to MAG and Nogo-A, OMgp was significantly upregulated in hippocampal samples derived from aged cognitively impaired rats compared to adult and aged cognitively intact rats. The greatest impairment-related induction in OMgp expression was observed in synaptosomes derived from whole hippocampus. Compared to aged intact rats, synaptosomal OMgp in aged impaired rats was increased two-fold (p<0.01). Interestingly, OMgp was decreased by 50% in aged intact rats compared to adults (p<0.01), although no differences in synaptosomal OMgp expression were observed between adult and aged impaired rats and no generalized effect of aging (i.e., all aged vs. adult) was apparent. OMgp was similarly upregulated in CA1 (60%, p<0.05), CA3 (50%, p<0.01), and DG (25%, p<0.01) of aged impaired rats compared to aged intact rats. Significant elevation of OMgp was also evident in CA1 (70%, p<0.05) and DG (45%, p<0.01) of aged impaired rats compared to adults. OMgp content was correlated to cognitive impairment in CA1 (p<0.001) and CA3 (p<0.05), with higher OMgp expression associated with greater mean proximity-to-platform probe trial performance measures (Figure 8B).
Figure 8. Upregulation of hippocampal OMgp with age-related cognitive decline.
(A) OMgp protein expression was significantly induced in hippocampal synaptosomes, and CA1, CA3 and DG subregions in cognitively impaired rats compared to aged cognitively intact rats, and in CA1 and DG compared to adults. *p<0.05, **p<0.01; one-way ANOVA with Student Newman Keuls post hoc testing; n=5–10/group. (B) In CA1 and CA3, OMgp expresson correlated with Morris water maze probe trial performance, demonstrating a relationship between upregulated OMgp expression and decreased spatial learning and memory. *p<0.05, ***p<0.001, Pearson (normally-distributed data) or Spearman (non-normally-distributed data) correlation; n=5–10/group.
OMgp distribution throughout the hippocampus in adult, aged cognitively intact and aged cognitively impaired rats was characterized by immunohistochemical localization experiments (Figure 9). OMgp immunoreactivity was distributed throughout the hippocampus, with concentrated areas of staining in processes and, more diffusely, in regions surrounding OMgp-negative processes (e.g., CA3 stratum radiatum) and somata (e.g., CA1 stratum pyramidale, DG stratum granulosum). Punctate OMgp immunoreactivity was also observed throughout the neuropil, and was particularly evident in the stratum radiatum and stratum oriens of CA3 in aged impaired rats. Interestingly, in the hilus of DG, processes highly immunoreactive to OMgp were evident in aged intact and aged impaired, but not adult, rats. In aged, cognitively impaired rats, immunoreactivity was increased in CA1, CA3 and DG compared to adult and aged intact rats. Immunohistochemical visualization of OMgp and NFh-immunoreactive axons demonstrated little to no colocalization, demonstrating that hippocampal OMgp is expressed around axons, presumably by myelinating oligodendrocytes (Figure 9, right column). At the cellular level, diffuse OMgp staining was evident throughout the peri-axonal space. A small number of NFh−, OMgp-immunoreactive processes were also observed in CA3 and DG. Both diffuse and projection-associated, periaxonal OMgp staining was increased in aged impaired rats compared to aged intact and adult rats (Supplemental Figure 3).
Figure 9. Hippocampal localization of OMgp upregulation.
Immunohistochemical experiments were performed to assess the localization of OMgp in adult, aged cognitively intact and aged cognitively impaired rats. OMgp immunoreactivity was distributed throughout the hippocampus, and was concentrated in CA3 stratum radiatum as well as the CA1 pyramidal cell layer and DG granule cell layer. Visualization of OMgp in the context of NFh+ axonal cytoarchitecture demonstrated that OMgp is expressed around, but not in, axons, and localizes to projections in CA3 and DG. Blue: Hoechst; Green: NFh; Red: OMgp.
NgR1 expression is upregulated with cognitive decline
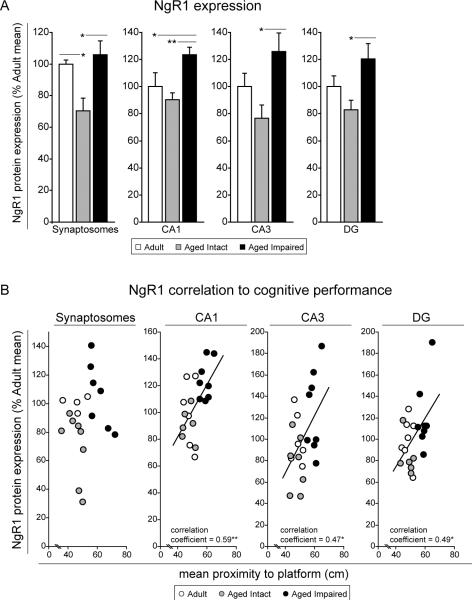
The myelin-associated inhibitors MAG, Nogo-A and OMgp signal through common receptors including the Nogo-66 receptor (NgR1). NgR1 protein expression was quantified in hippocampal synaptosomes and subregions to identify potential regulation with aging or cognitive decline (Figure 10A). In whole-hippocampal synaptosomes, NgR1 was increased by 60% (p<0.05) in aged impaired rats compared to aged intact rats. NgR1 expression was not different between aged impaired and adult rats, but was decreased by 30% (p<0.05) in aged intact rats compared to adults. Age-related cognitive decline-associated induction of NgR1 was observed in CA1 (30%, p<0.01), CA3 (60%, p<0.05), and DG (30%, p<0.05) compared to aged intact rats, and in CA1 (20%, p<0.05) compared to adults. Significant correlation of NgR1 expression levels and cognitive impairment were observed in all three hippocampal subregions assessed (Figure 10B). In CA1 (p<0.01), CA3 (p<0.05) and DG (p<0.05), increased NgR1 expression was associated with increased mean proximity-to-platform measures (i.e., with decreasing cognitive performance).
Figure 10. Hippocampal NgR1 expression is increased with cognitive impairment in aged rats.
(A) Protein-level NgR1 expression was significantly increased in aged rats with cognitive impairment compared to age-matched, cognitively intact rats in hippocampal synapses, and CA1, CA3, and DG subregions. Induction of NgR1 was also observed in aged impaired rats compared to adult rats in CA1. No generalized regulation of NgR1 content was evident with aging. *p<0.05, **p<0.01; one-way ANOVA with Student Newman Keuls post hoc testing; n=5–10/group. (B) NgR1 expression in individual rats correlated with probe trial measures of cognitive performance, demonstrating a relationship between upregulated NgR1 and decreased spatial learning and memory. *p<0.05, **p<0.01, Pearson (normally-distributed data) or Spearman (non-normally-distributed data) correlation; n=5–10/group.
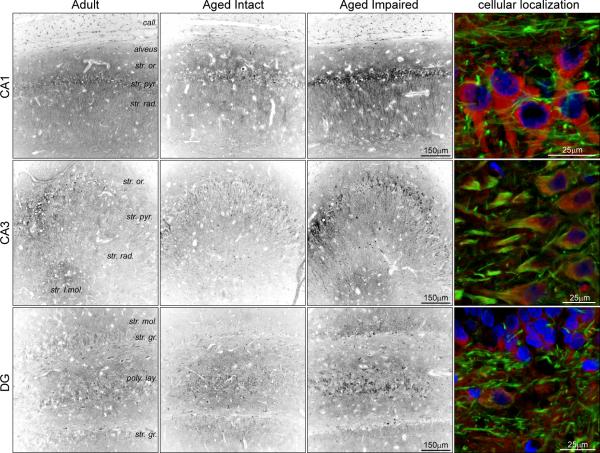
Immunohistochemical assessment of NgR1 distribution in the hippocampus (Figure 11) was performed to extend immunoblot quantitation of NgR1 protein in hippocampal subregions and synapses. Perinuclear somatic NgR1 staining was distributed throughout the corpus callosum, and increased in immunoreactivity with cognitive impairment. In the hippocampus, diffuse NgR1 immunoreactivity was observed in all subregions assessed, but was most prominent as somatic staining in the pyramidal cell layer in CA1 and CA3. Interestingly, this immunoreactivity was enhanced in CA2. In stratum radiatum, particularly in aged impaired rats, distinct cellular processes originating from the pyramidal cell layer projected through the stratum radiatum to the stratum lacunosum-moleculare. In DG, NgR1 immunoreactivity was observed in granule cell and polymorphic layers, with both somatic and diffuse, punctate staining evident. The intensity of NgR1 staining in CA1, CA3 and DG recapitulated the quantitative immunoblot demonstration of increased NgR1 expression in aged cognitively impaired rats. Higher-magnification visualization of NgR1 immunoreactivity demonstrated neuronal expression of NgR1, particularly in somata and proximal axon segments of NFh-expressing neurons in DG and CA3 (Figure 11, right column). Interestingly, in CA1, NgR1-immunoreactivity was apparent in cell bodies and NFh− processes, although NFh+ projections were frequently observed in close proximity to the NgR1 immunoreactive somata. Increased NgR1 immunoreactivity at the cellular level was apparent in aged impaired rats compared to adult and aged intact rats (Supplemental Figure 4).
Figure 11. Localization of increased hippocampal NgR1.
The distribution of the MAI receptor NgR1 was assessed in adult, aged intact and aged impaired rats. Diffuse NgR1 immunoreactivity was observed throughout the hippocampus, and concentrated areas of somatic NgR1 staining were observed in pyramidal cell layer in CA1 and CA3. Distinct cellular processes originating from the pyramidal cell layer were highly immunoreactive to NgR1. Enhanced NgR1 immunoreactivity was evident in hippocampus of aged cognitively impaired rats compared to adult and aged intact rats. Higher magnification imaging of NgR1 immunoreactivity demonstrated somatic and axonal expression of NFh+ neurons in CA3 and DG, and large NFh− somata and proximal projections in the CA1 pyramidal cell layer. Blue: Hoechst; Green: NFh; Red: NgR1.
Protein-level induction of myelin-associated inhibitor pathway components is not regulated at the level of transcript content
To assess potential transcript-level regulation of increased MAG, Nogo-A, OMgp, and NgR1 protein expression with age-related cognitive decline, mRNA expression was quantitated by qPCR of synaptosomes prepared from whole-hippocampus and of CA1, CA3 and DG subregions (Table 3). No differences in mRNA content were observed between aged intact and aged impaired rats, or between aged and adult rats with the exception of Nogo-A. Nogo-A transcript levels were decreased by nearly 50% in DG of aged intact rats compared to adults (p<0.05, ANOVA with Student Newman Keuls post hoc testing). In agreement with a previous report of selective downregulation of Nogo-A transcript in the aging hippocampus (Trifunovski et al. 2006), we observed a trend toward decreased Nogo-A mRNA in CA1 and DG of aged rats compared to adults (~25% decrease, p=0.09, two-tailed t-test, all aged vs. adult).
GAP-43 protein expression decreases with advanced aging but not cognitive decline
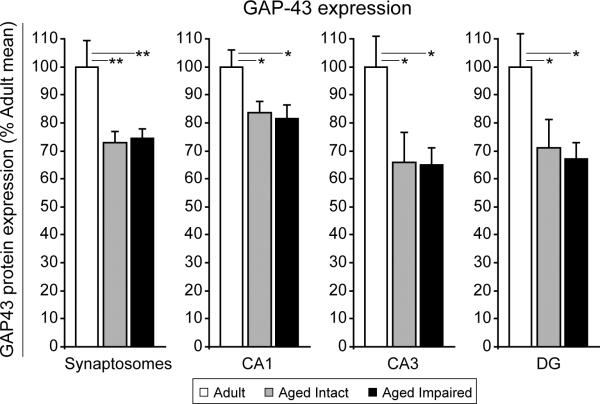
To determine whether increased MAI expression is associated with decreased neuronal sprouting, protein expression of the neuronal regeneration-associated protein GAP-43 (growth-associated protein 43) (Thompson et al. 2006b;Schmidt-Kastner et al. 1997) was assessed by immunoblotting in both hippocampal synaptosomes and hippocampal subregion dissections (Figure 12). GAP-43 expression was significantly decreased in all aged subjects regardless of cognitive status, and was not differentially expressed between aged intact and aged impaired rats. Synaptosomal GAP-43 was decreased by ~25% in both aged intact and aged impaired rats compared to adults (p<0.01, one-way ANOVA with Student Newman Keuls post hoc testing). In discrete hippocampal subregions dissected from aged intact and aged impaired rats, GAP-43 protein content was decreased by ~20% (CA1, p<0.05) to more than 30% (CA3 and DG, p<0.05) compared to expression in adults. There was no correlation between GAP-43 expression and cognitive performance.
Figure 12. Hippocampal GAP-43 expression decreases with advanced aging.
Immunoblotting for the neuronal growth/regeneration-associated protein GAP-43 in hippocampal synaptosome preparations and subregion dissections demonstrated an age-related decrease in protein expression. Interestingly, GAP-43 expression was not different between aged, cognitively intact and aged, cognitively impaired rats. *p<0.05, **p<0.01; one-way ANOVA with Student Newman Keuls post hoc testing; n=5–10/group.
Discussion
Aging is a primary risk factor for impairment of cognitive functions including hippocampus-dependent spatial learning and memory. In both humans and animal models, deficits of spatial learning and memory manifest in only approximately half of aged subjects, indicating that age-related alterations in hippocampal cytostructure and neurochemical composition are, by themselves, insufficient to cause cognitive decline. We have previously reported that neurotransmission-regulating proteins decrease in expression with aging and are not further regulated with cognitive decline (VanGuilder et al. 2010;VanGuilder et al. 2011b). Additionally, differential regulation of synaptic proteins with roles in functional and structural plasticity occurs only with age-related cognitive impairment rather than as a general effect of aging (VanGuilder et al. 2011b). Aberrant functional and structural plasticity are characteristic of aging and cognitive decline (Boric et al. 2008;Norris et al. 1996;O'Callaghan et al. 2009;Platano et al. 2008;Poe et al. 2001;Ramsey et al. 2004;Shi et al. 2005;Tombaugh et al. 2002;Wang et al. 2006), but the molecular mechanisms of these deficits remain to be identified. Building on reports implicating myelin-associated inhibitors (MAIs) in suppression of neuronal plasticity (Bongiorno and Petratos 2010;Llorens et al. 2011;Schwab et al. 2006) and extending our initial finding that hippocampal synaptic expression of Nogo-A is increased with cognitive impairment (VanGuilder et al. 2011b), we investigated the expression of MAI ligands (MAG, Nogo-A, OMgp) and a shared receptor (NgR1) in a well-characterized rodent model of age-related cognitive decline.
The MAI ligands MAG, Nogo-A and OMgp are expressed by myelinating oligodendrocytes, but also localize to neuronal plasma membranes, axons, dendrites, synaptic terminals, and somata (Gil et al. 2006;Jin et al. 2003;Kuramoto et al. 1997;Mingorance-Le Meur et al. 2007;Raiker et al. 2010;Wang et al. 2002). In the current study, we observed MAI expression both diffusely distributed throughout the hippocampus and in close proximity to axons, as expected. Further, expression of Nogo-A and, to a lesser degree, NgR1, in the corpus callosum was evident both in distinct cells and their projections, while MAG expression was evident in numerous processes in the callosum and alveus. As expected (Peng et al. 2011;Wang et al. 2002), NgR1 expression was evident in neuronal somata and axons, in agreement with its function as a receptor for ligands present on adjacent myelin. All three MAI ligands investigated (MAG, Nogo-A, and OMgp) were highly expressed in the more heavily myelinated CA3 stratum radiatum (Meier et al. 2004). Together, this indicates a high level of MAI expression in densely myelinated structures, in agreement with the literature (Huber et al. 2002;Park et al. 2006;Stolt et al. 2002). Interestingly, increased MAG and Nogo-A immunoreactivity was also observed in axons, predominantly in CA3 stratum radiatum, and, unlike MAG and OMgp, Nogo-A was expressed in neuronal nuclei in the pyramidal and granule cell layers. It is possible that the nuclear Nogo-A localization reflects Nogo-B cross-reactivity, which has been previously reported in hippocampal pyramidal cells (Huber et al. 2002), and future experiments are required to confirm the specificity of Nogo-A staining. The function of nuclear Nogo is not yet clear, although immunogold labeling at the electron microscopic level has demonstrated that Nogo-A is associated with chromatin, indicating a potential regulatory role in gene transcription (Jin et al. 2003). MAG, Nogo-A and OMgp signal through NgR1 to inhibit structural remodeling of synapses and regeneration/outgrowth of axons (Lauren et al. 2007;Akbik et al. 2011;Llorens et al. 2011). Additionally, Nogo-A functions in a cell-autonomous, non-NgR1-mediated manner to regulate dendrite structure (e.g., in hippocampal pyramidal neurons) (Zagrebelsky et al. 2010). Interestingly, we have also observed age-associated hippocampal upregulation in axons and pyramidal cell bodies of another neuronal MAI receptor, the immunological paired immunoglobulin receptor B (PirB) (personal communication), through which these three ligands also signal to inhibit neuronal plasticity (Atwal et al. 2008;Llorens et al. 2011). The functional redundancy of the MAI ligands and their convergence on two distinct receptors, NgR1 and PirB, indicates a potential synergistic action of the MAI pathway in nonneurodegenerative cognitive impairment that requires further characterization.
MAIs cause growth cone collapse (Chivatakarn et al. 2007;Lee et al. 2008), reduce neurite and axon growth (DeBellard et al. 1996;Ji et al. 2008;Kottis et al. 2002;Li et al. 1996;Mingorance et al. 2005;Schwab et al. 2006), and modulate cytoskeletal remodeling dynamics to stabilize synaptic structure (Hsieh et al. 2006;Blochlinger et al. 2001;Zagrebelsky et al. 2010). Nogo-A, however, is reported to be a more potent negative regulator of structural plasticity than either MAG or OMgp (Cafferty et al. 2010;Li et al. 1996). This has been most clearly demonstrated through evaluation of axon growth inhibition in single Nogo-A, double MAG/OMgp, and triple Nogo-A/MAG/OMgp knockout mice (Cafferty et al. 2010). It is also possible that increased nuclear expression of Nogo contributes to suppression of hippocampal plasticity by influencing expression of plasticity-associated genes (Jin et al. 2003). NgR1 also modulates synaptic structure in the mature CNS (Raiker et al. 2010), in part by regulating dendritic spine shape (Zagrebelsky et al. 2010) and inhibiting axon branching (Lee et al. 2008;Petrinovic et al. 2010). Over-expression of Nogo-A is reported to downregulate expression of synaptic scaffolding proteins and trigger synaptic disassembly (Aloy et al. 2006), while inhibition of Nogo-A action increases axon complexity and shifts synaptic morphology to more plastic phenotypes (Zagrebelsky et al. 2010). Pharmacological suppression of NgR1 signaling by antibody- and peptide-based blockade of MAI binding induces formation of functional synapses and reorganization of axon arbors (Hanell et al. 2010;Li et al. 2004;Mehta et al. 2007). Cognitive impairment in aged subjects is associated with a preferential loss of functional high-efficiency synapses including multiple-spine bouton and perforated phenotypes, which are necessary for spatial learning and memory formation (Burke and Barnes 2006;Geinisman et al. 1986;Poe et al. 2001;Shi et al. 2005). In the Fischer 344 × Brown Norway rat model of cognitive decline used in the present studies, improvement in spatial learning and memory through IGF-1 administration is associated with increases in hippocampal populations of perforated and multiple-spine bouton synapses (Poe et al. 2001;Shi et al. 2005). This suggests that inhibition of synaptic structural remodeling analogous to, and potentially induced by, MAI action plays a key role in age-related cognitive decline.
Abnormal electrophysiological activity, including suppressed LTP and increased LTD (Barnes et al. 1997;Barnes 2003;Norris et al. 1996) is a hallmark of aging and cognitive decline. The functional effects of MAI signaling in the healthy and injured CNS range from dysregulating electrophysiological correlates of activity-dependent synaptic plasticity to impairing learning and memory. MAI/NgR1 signaling suppresses activity- and experience-dependent functional plasticity both in vivo and in vitro (Delekate et al. 2011;Lee et al. 2008;Raiker et al. 2010). Nogo-A in particular is consistently reported to influence synaptic signaling strength (Bongiorno and Petratos 2010). For example, pharmacological induction of hippocampal Nogo-A decreases NMDA receptor-dependent long-term potentiation (LTP) (Raiker et al. 2010), while genetic deletion and antibody-based neutralization of Nogo-A increases hippocampal LTP (Delekate et al. 2011;Lee et al. 2008). Similar, although attenuated, effects are associated with suppression of MAG and OMgp action (Cafferty et al. 2010;Raiker et al. 2010). Likewise, genetic deletion of NgR1 enhances LTP and decreases long-term depression (LTD) at hippocampal Schaffer collateral synapses (Lee et al. 2008), further indicating a role of MAI-NgR1 signaling in neuronal mechanisms of cognition and behavior.
While this is the first characterization of MAIs in age-related cognitive decline, the literature describes a clear relationship between MAI expression and behavioral impairments. Transgenic induction of NgR1 expression causes deficits of hippocampus-dependent swim maze performance that is reversible through blockade of NgR1 transgene expression (Karlen et al. 2009), suggesting a direct role of MAI signaling in spatial learning and memory. Genetic deletion and antibody-based neutralization of MAIs, on the other hand, improves both behavioral recovery following spinal cord lesion and functional recovery following ischemic or traumatic brain injury (Walmsley and Mir 2007). In models of ischemic or traumatic CNS injury affecting the brain and spinal cord, suppression of the MAI signaling pathway increases regeneration and facilitates recovery of motor function, as assessed by forelimb reaching tasks (Li and Strittmatter 2003;Papadopoulos et al. 2002;Seymour et al. 2005;Tsai et al. 2007;Wiessner et al. 2003). Similarly, recovery of cognitive function, including spatial learning and memory, following stroke or traumatic brain injury is enhanced by antagonism of NgR1 or Nogo-A (Gillani et al. 2010;Hanell et al. 2010;Lenzlinger et al. 2005;Marklund et al. 2007;Thompson et al. 2006a). The correlation of MAG, Nogo-A, OMgp, and NgR1 protein levels in hippocampal synapses and subregions with Morris water maze performance that we have observed suggests that a similar relationship exists between increased MAI expression and the decreased spatial learning and memory ability associated with cognitive decline. Induction of MAIs across CA1, CA3 and DG may impair plasticity at multiple points of spatial learning and memory pathways (e.g., Schaffer collateral synapses on CA1 pyramidal cells, DG mossy fiber synapses on CA3 pyramidal cells) (Hanell et al. 2010;Huebner et al. 2011).
The regulatory mechanisms of protein-level MAI and NgR1 expression are not fully understood; however, transcript-level expression of Nogo-A and NgR1 has been reported to decrease in hippocampal subregions throughout development and aging (Josephson et al. 2003;Trifunovski et al. 2006). While we observed a trend toward decreased Nogo-A mRNA expression in CA1 and DG, this trend did not reach statistical significance, and transcript content for MAG, OMgp and NgR1 was likewise unchanged with aging or cognitive decline, suggesting that a post-transcriptional regulatory mechanism underlies cognitive impairment-associated induction of MAI protein expression. Interestingly, MAI and NgR1 expression is downregulated by neuronal activity (Josephson et al. 2003;Karlen et al. 2009;Trifunovski et al. 2004;Mingorance et al. 2004), Conversely, antagonism of MAI-NgR1 signaling by infusion of antibodies and soluble decoy receptors both downregulates MAI expression and increases neuronal activity (Weinmann et al. 2006;Zhou et al. 2003;Peng et al. 2010). This complex feedback mechanism is thought to release MAI-mediated inhibition and facilitate structural and functional synaptic plasticity of neurons (Karlen et al. 2009;Schwab et al. 2006). Suppression of MAI signaling in the mature brain increases neuronal activity and exerts long-lasting improvements in behavioral and biochemical correlates of cognitive function that persist for weeks to months following treatment (Seymour et al. 2005;Gillani et al. 2010;Thompson et al. 2006a;Weinmann et al. 2006;Wiessner et al. 2003). The chronic beneficial effects of suppressed MAI pathway function may in fact reflect restoration of the regulatory balance between synaptic signaling and MAI expression. The induction of MAG, Nogo-A, OMgp and NgR1 only in cognitively impaired aged rats, and not age-matched cognitively intact rats, reported here may contribute to a feed-forward cycle of decreased synaptic signaling and increased synaptic suppression by the MAI pathway, and likely reflects deficits of neuronal activity characteristic of hippocampal aging and cognitive impairment.
MAI-blocking interventions have been proposed as potential therapeutic approaches to improve cognitive function following CNS injury (Cao et al. 2010;McDonald et al. 2011;McGee and Strittmatter 2003;Starkey and Schwab 2011). Together with the data described here, this suggests that suppression of MAI signaling through pharmacological neutralization of ligands or antagonism of the NgR1 receptor may be a promising therapy for age-related deficits of cognitive function, particularly since MAI induction was evident only in cognitively impaired aged rats. Correlation of MAG, Nogo-A, OMgp, and NgR1 expression in hippocampal synapses and in both pyramidal cell- and granule cell-containing hippocampal subregions with deficits of spatial learning and memory further supports a novel, widespread function of the MAI pathway in hippocampal dysfunction and cognitive decline associated with aging that could be targeted by pharmacological approaches. Unlike targeting proteins and signaling pathways generally regulated with advanced aging (e.g., neurotransmission-regulating proteins, immune response and inflammatory signaling pathways) and which may serve compensatory functions not directly associated with cognitive impairment, targeting MAIs to improve cognitive function may prove to be a more specific approach. Since the model of cognitive decline described here replicates human cognitive impairment with normative aging, which occurs without gross neuronal loss or neurodegenerative damage, the potential mechanistic role of MAI induction likely involves inhibition of synaptic plasticity (Lee et al. 2008;Raiker et al. 2010), rather than the significant axon outgrowth observed in stroke and lesion models (Blochlinger et al. 2001;Hanell et al. 2010;Papadopoulos et al. 2002;Tsai et al. 2007). This is further supported by the demonstration of that activity-dependent synaptic plasticity-associated protein expression (VanGuilder et al. 2011b) and populations of high-efficiency synapses (Geinisman et al. 1986;Nicholson et al. 2004;Poe et al. 2001;Shi et al. 2005), decrease in models of age-related cognitive decline, while expression of the regeneration- and outgrowth- associated protein GAP-43 is unchanged between aged intact and aged impaired rats.
The data presented here implicate the MAI ligands MAG, Nogo-A, and OMgp, and their shared receptor, NgR1, as a potential molecular mechanism of age-related cognitive decline through suppression of both functional and structural plasticity of hippocampal synapses. Induction of this inhibitory pathway likely exerts additive effects with additional cognitive decline-associated alterations in bioenergetic metabolism, glutamate regulation, immediate-early gene activation, and activity-related and structural synaptic plasticity (Poon et al. 2006;Rowe et al. 2007;VanGuilder et al. 2011b), among others. Future functional studies antagonizing NgR1 signaling or MAI ligand binding are required to define the specific role of MAIs in age-related cognitive decline. Additionally, whether the cognitive decline-associated upregulation of MAIs inhibits the synaptic plasticity necessary for activity-dependent synaptic strengthening, or merely reflects a response to decreased neuronal activity (Karlen et al. 2009;Schwab et al. 2006), remains to be determined. With the known improvements in cognitive function that occur through blocking MAI ligand expression/action (Hanell et al. 2010;Lenzlinger et al. 2005;Marklund et al. 2007;Thompson et al. 2006a;Wiessner et al. 2003), and impairment of spatial learning and memory by transgenic induction of NgR1 alone (Karlen et al. 2009), future studies will need to directly determine whether hippocampal upregulation of MAG, Nogo-A, OMgp, and NgR1 in aged, cognitively impaired rats is a causative factor in age-related cognitive decline.
Supplementary Material
Acknowledgments
This work was supported by NIA grants R01AG026607 and P01AG11370 to W.E.S., and generous support from the Donald W. Reynolds Foundation. We thank Julie Farley for invaluable assistance with behavioral phenotyping, Han Yan and Junie P. Warrington for performing hippocampal subregion dissections, and Carol Danvers for technical assistance.
ABBREVIATIONS
- MAI
myelin-associated inhibitor
- MAG
myelin-associated glycoprotein
- Nogo-A
neurite outgrowth inhibitor A
- OMgp
oligodendrocyte myelin glycoprotein
- NgR1
Nogo-66 receptor
- LTP
long-term potentiation
- LTD
long-term depression
- CA1
Cornu ammonis area 1
- CA3
Cornu ammonis area 3
- DG
dentate gyrus
- ANOVA
analysis of variance
- CNS
central nervous system
References
- Adams MM, Shi L, Linville MC, Forbes ME, Long AB, Bennett C, Newton IG, Carter CS, Sonntag WE, Riddle DR, Brunso-Bechtold JK. Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning ability. Exp. Neurol. 2008;211:141–149. doi: 10.1016/j.expneurol.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbik F, Cafferty WB, Strittmatter SM. Myelin associated inhibitors: A link between injury-induced and experience-dependent plasticity. Exp. Neurol. 2011 doi: 10.1016/j.expneurol.2011.06.006. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloy EM, Weinmann O, Pot C, Kasper H, Dodd DA, Rulicke T, Rossi F, Schwab ME. Synaptic destabilization by neuronal Nogo-A. Brain Cell Biol. 2006;35:137–156. doi: 10.1007/s11068-007-9014-3. [DOI] [PubMed] [Google Scholar]
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Long-term potentiation and the ageing brain. Philos. Trans. R. Soc. Lond B Biol. Sci. 2003;358:765–772. doi: 10.1098/rstb.2002.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, Suster MS, Shen J, McNaughton BL. Multistability of cognitive maps in the hippocampus of old rats. Nature. 1997;388:272–275. doi: 10.1038/40859. [DOI] [PubMed] [Google Scholar]
- Blochlinger S, Weinmann O, Schwab ME, Thallmair M. Neuronal plasticity and formation of new synaptic contacts follow pyramidal lesions and neutralization of Nogo-A: a light and electron microscopic study in the pontine nuclei of adult rats. J. Comp Neurol. 2001;433:426–436. doi: 10.1002/cne.1150. [DOI] [PubMed] [Google Scholar]
- Bongiorno D, Petratos S. Molecular regulation of Nogo-A in neural cells: Novel insights into structure and function. Int. J. Biochem. Cell Biol. 2010;42:1072–1075. doi: 10.1016/j.biocel.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Boric K, Munoz P, Gallagher M, Kirkwood A. Potential adaptive function for altered long-term potentiation mechanisms in aging hippocampus. J. Neurosci. 2008;28:8034–8039. doi: 10.1523/JNEUROSCI.2036-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucklacher RM, Patel KM, VanGuilder HD, Bixler GV, Barber AJ, Antonetti DA, Lin CM, LaNoue KF, Gardner TW, Bronson SK, Freeman WM. Whole genome assessment of the retinal response to diabetes reveals a progressive neurovascular inflammatory response. BMC. Med. Genomics. 2008;1:26. doi: 10.1186/1755-8794-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, Duffy P, Huebner E, Strittmatter SM. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J. Neurosci. 2010;30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, Strittmatter SM. The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J. Neurosci. 2006;26:12242–12250. doi: 10.1523/JNEUROSCI.3827-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Gao Y, Deng K, Williams G, Doherty P, Walsh FS. Receptors for myelin inhibitors: Structures and therapeutic opportunities. Mol. Cell Neurosci. 2010;43:1–14. doi: 10.1016/j.mcn.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Chivatakarn O, Kaneko S, He Z, Tessier-Lavigne M, Giger RJ. The Nogo-66 receptor NgR1 is required only for the acute growth cone-collapsing but not the chronic growth-inhibitory actions of myelin inhibitors. J. Neurosci. 2007;27:7117–7124. doi: 10.1523/JNEUROSCI.1541-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahle CL, Jacobs BS, Raz N. Aging, vascular risk, and cognition: blood glucose, pulse pressure, and cognitive performance in healthy adults. Psychol. Aging. 2009;24:154–162. doi: 10.1037/a0014283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBellard ME, Tang S, Mukhopadhyay G, Shen YJ, Filbin MT. Myelin-associated glycoprotein inhibits axonal regeneration from a variety of neurons via interaction with a sialoglycoprotein. Mol. Cell Neurosci. 1996;7:89–101. doi: 10.1006/mcne.1996.0007. [DOI] [PubMed] [Google Scholar]
- Delekate A, Zagrebelsky M, Kramer S, Schwab ME, Korte M. NogoA restricts synaptic plasticity in the adult hippocampus on a fast time scale. Proc. Natl. Acad. Sci. U. S. A. 2011;108:2569–2574. doi: 10.1073/pnas.1013322108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MJ, Bilkey DK, Abraham WC. Altered plasticity in hippocampal CA1, but not dentate gyrus, following long-term environmental enrichment. J. Neurophysiol. 2010;103:3320–3329. doi: 10.1152/jn.01037.2009. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Bixler GV, Brucklacher RM, Lin CM, Patel KM, VanGuilder HD, LaNoue KF, Kimball SR, Barber AJ, Antonetti DA, Gardner TW, Bronson SK. A multistep validation process of biomarkers for preclinical drug development. Pharmacogenomics. J. 2010;10:385–395. doi: 10.1038/tpj.2009.60. [DOI] [PubMed] [Google Scholar]
- Freeman WM, VanGuilder HD, Bennett C, Sonntag WE. Cognitive performance and age-related changes in the hippocampal proteome. Neuroscience. 2009;159:183–195. doi: 10.1016/j.neuroscience.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav. Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F. Loss of perforated synapses in the dentate gyrus: morphological substrate of memory deficit in aged rats. Proc. Natl. Acad. Sci. U. S. A. 1986;83:3027–3031. doi: 10.1073/pnas.83.9.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, Ganeshina O, Yoshida R, Berry RW, Disterhoft JF, Gallagher M. Aging, spatial learning, and total synapse number in the rat CA1 stratum radiatum. Neurobiol. Aging. 2004;25:407–416. doi: 10.1016/j.neurobiolaging.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Gil V, Nicolas O, Mingorance A, Urena JM, Tang BL, Hirata T, Saez-Valero J, Ferrer I, Soriano E, del Rio JA. Nogo-A expression in the human hippocampus in normal aging and in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006;65:433–444. doi: 10.1097/01.jnen.0000222894.59293.98. [DOI] [PubMed] [Google Scholar]
- Gillani RL, Tsai SY, Wallace DG, O'Brien TE, Arhebamen E, Tole M, Schwab ME, Kartje GL. Cognitive recovery in the aged rat after stroke and anti-Nogo-A immunotherapy. Behav. Brain Res. 2010;208:415–424. doi: 10.1016/j.bbr.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes da Silva S, Unsain N, Masco DH, Toscano-Silva M, de Amorim HA, Silva Araujo BH, Simoes PS, da GN-M, Mortara RA, Scorza FA, Cavalheiro EA, Arida RM. Early exercise promotes positive hippocampal plasticity and improves spatial memory in the adult life of rats. Hippocampus. 2010 doi: 10.1002/hipo.20903. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Gower AJ, Lamberty Y. The aged mouse as a model of cognitive decline with special emphasis on studies in NMRI mice. Behav. Brain Res. 1993;57:163–173. doi: 10.1016/0166-4328(93)90132-a. [DOI] [PubMed] [Google Scholar]
- Hanell A, Clausen F, Bjork M, Jansson K, Philipson O, Nilsson LN, Hillered L, Weinreb PH, Lee D, McIntosh TK, Gimbel DA, Strittmatter SM, Marklund N. Genetic deletion and pharmacological inhibition of Nogo-66 receptor impairs cognitive outcome after traumatic brain injury in mice. J. Neurotrauma. 2010;27:1297–1309. doi: 10.1089/neu.2009.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A, Teng J, Takei Y, Oguchi K, Hirokawa N. MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J. Cell Biol. 2002;158:541–549. doi: 10.1083/jcb.200110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat. Rev. Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Hof PR, Morrison JH. The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci. 2004;27:607–613. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Hsieh SH, Ferraro GB, Fournier AE. Myelin-associated inhibitors regulate cofilin phosphorylation and neuronal inhibition through LIM kinase and Slingshot phosphatase. J. Neurosci. 2006;26:1006–1015. doi: 10.1523/JNEUROSCI.2806-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AB, Weinmann O, Brosamle C, Oertle T, Schwab ME. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J. Neurosci. 2002;22:3553–3567. doi: 10.1523/JNEUROSCI.22-09-03553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner EA, Kim BG, Duffy PJ, Brown RH, Strittmatter SM. A Multi-domain Fragment of Nogo-A Protein Is a Potent Inhibitor of Cortical Axon Regeneration via Nogo Receptor 1. J. Biol. Chem. 2011;286:18026–18036. doi: 10.1074/jbc.M110.208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B, Case LC, Liu K, Shao Z, Lee X, Yang Z, Wang J, Tian T, Shulga-Morskaya S, Scott M, He Z, Relton JK, Mi S. Assessment of functional recovery and axonal sprouting in oligodendrocyte-myelin glycoprotein (OMgp) null mice after spinal cord injury. Mol. Cell Neurosci. 2008;39:258–267. doi: 10.1016/j.mcn.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin WL, Liu YY, Liu HL, Yang H, Wang Y, Jiao XY, Ju G. Intraneuronal localization of Nogo-A in the rat. J. Comp Neurol. 2003;458:1–10. doi: 10.1002/cne.10547. [DOI] [PubMed] [Google Scholar]
- Josephson A, Trifunovski A, Scheele C, Widenfalk J, Wahlestedt C, Brene S, Olson L, Spenger C. Activity-induced and developmental downregulation of the Nogo receptor. Cell Tissue Res. 2003;311:333–342. doi: 10.1007/s00441-002-0695-8. [DOI] [PubMed] [Google Scholar]
- Karlen A, Karlsson TE, Mattsson A, Lundstromer K, Codeluppi S, Pham TM, Backman CM, Ogren SO, Aberg E, Hoffman AF, Sherling MA, Lupica CR, Hoffer BJ, Spenger C, Josephson A, Brene S, Olson L. Nogo receptor 1 regulates formation of lasting memories. Proc. Natl. Acad. Sci. U. S. A. 2009;106:20476–20481. doi: 10.1073/pnas.0905390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottis V, Thibault P, Mikol D, Xiao ZC, Zhang R, Dergham P, Braun PE. Oligodendrocyte-myelin glycoprotein (OMgp) is an inhibitor of neurite outgrowth. J. Neurochem. 2002;82:1566–1569. doi: 10.1046/j.1471-4159.2002.01146.x. [DOI] [PubMed] [Google Scholar]
- Kumar A, Rani A, Tchigranova O, Lee WH, Foster TC. Influence of late-life exposure to environmental enrichment or exercise on hippocampal function and CA1 senescent physiology. Neurobiol. Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.06.023. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Thinschmidt JS, Foster TC, King MA. Aging effects on the limits and stability of long-term synaptic potentiation and depression in rat hippocampal area CA1. J. Neurophysiol. 2007;98:594–601. doi: 10.1152/jn.00249.2007. [DOI] [PubMed] [Google Scholar]
- Kuramoto H, Hozumi I, Inuzuka T, Sato S. Occurrence of myelin-associated glycoprotein (MAG)-like immunoreactivity in some nervous, endocrine, and immune-related cells of the rat. An immunohistochemical study. Mol. Chem. Neuropathol. 1997;31:85–94. doi: 10.1007/BF02815163. [DOI] [PubMed] [Google Scholar]
- Lauren J, Hu F, Chin J, Liao J, Airaksinen MS, Strittmatter SM. Characterization of myelin ligand complexes with neuronal Nogo-66 receptor family members. J. Biol. Chem. 2007;282:5715–5725. doi: 10.1074/jbc.M609797200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Raiker SJ, Venkatesh K, Geary R, Robak LA, Zhang Y, Yeh HH, Shrager P, Giger RJ. Synaptic function for the Nogo-66 receptor NgR1: regulation of dendritic spine morphology and activity-dependent synaptic strength. J. Neurosci. 2008;28:2753–2765. doi: 10.1523/JNEUROSCI.5586-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzlinger PM, Shimizu S, Marklund N, Thompson HJ, Schwab ME, Saatman KE, Hoover RC, Bareyre FM, Motta M, Luginbuhl A, Pape R, Clouse AK, Morganti-Kossmann C, McIntosh TK. Delayed inhibition of Nogo-A does not alter injury-induced axonal sprouting but enhances recovery of cognitive function following experimental traumatic brain injury in rats. Neuroscience. 2005;134:1047–1056. doi: 10.1016/j.neuroscience.2005.04.048. [DOI] [PubMed] [Google Scholar]
- Li M, Shibata A, Li C, Braun PE, McKerracher L, Roder J, Kater SB, David S. Myelin-associated glycoprotein inhibits neurite/axon growth and causes growth cone collapse. J. Neurosci. Res. 1996;46:404–414. doi: 10.1002/(SICI)1097-4547(19961115)46:4<404::AID-JNR2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J. Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Walus L, Rabacchi SA, Jirik A, Chang E, Schauer J, Zheng BH, Benedetti NJ, Liu BP, Choi E, Worley D, Silvian L, Mo W, Mullen C, Yang W, Strittmatter SM, Sah DW, Pepinsky B, Lee DH. A neutralizing anti-Nogo66 receptor monoclonal antibody reverses inhibition of neurite outgrowth by central nervous system myelin. J. Biol. Chem. 2004;279:43780–43788. doi: 10.1074/jbc.M401803200. [DOI] [PubMed] [Google Scholar]
- Liu P, Smith PF, Darlington CL. Glutamate receptor subunits expression in memory-associated brain structures: regional variations and effects of aging. Synapse. 2008;62:834–841. doi: 10.1002/syn.20563. [DOI] [PubMed] [Google Scholar]
- Llorens F, Gil V, del Rio JA. Emerging functions of myelin-associated proteins during development, neuronal plasticity, and neurodegeneration. FASEB J. 2011;25:463–475. doi: 10.1096/fj.10-162792. [DOI] [PubMed] [Google Scholar]
- Lu FM, Hawkins RD. Presynaptic and postsynaptic Ca(2+) and CamKII contribute to long-term potentiation at synapses between individual CA3 neurons. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4264–4269. doi: 10.1073/pnas.0508162103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majoul I, Shirao T, Sekino Y, Duden R. Many faces of drebrin: from building dendritic spines and stabilizing gap junctions to shaping neurite-like cell processes. Histochem. Cell Biol. 2007;127:355–361. doi: 10.1007/s00418-007-0273-y. [DOI] [PubMed] [Google Scholar]
- Marklund N, Bareyre FM, Royo NC, Thompson HJ, Mir AK, Grady MS, Schwab ME, McIntosh TK. Cognitive outcome following brain injury and treatment with an inhibitor of Nogo-A in association with an attenuated downregulation of hippocampal growth-associated protein-43 expression. J. Neurosurg. 2007;107:844–853. doi: 10.3171/JNS-07/10/0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CL, Bandtlow C, Reindl M. Targeting the Nogo receptor complex in diseases of the central nervous system. Curr. Med. Chem. 2011;18:234–244. doi: 10.2174/092986711794088326. [DOI] [PubMed] [Google Scholar]
- McGee AW, Strittmatter SM. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26:193–198. doi: 10.1016/S0166-2236(03)00062-6. [DOI] [PubMed] [Google Scholar]
- Mehta NR, Lopez PH, Vyas AA, Schnaar RL. Gangliosides and Nogo receptors independently mediate myelin-associated glycoprotein inhibition of neurite outgrowth in different nerve cells. J. Biol. Chem. 2007;282:27875–27886. doi: 10.1074/jbc.M704055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S, Brauer AU, Heimrich B, Nitsch R, Savaskan NE. Myelination in the hippocampus during development and following lesion. Cell Mol. Life Sci. 2004;61:1082–1094. doi: 10.1007/s00018-004-3469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingorance A, Fontana X, Sole M, Burgaya F, Urena JM, Teng FY, Tang BL, Hunt D, Anderson PN, Bethea JR, Schwab ME, Soriano E, del Rio JA. Regulation of Nogo and Nogo receptor during the development of the entorhino-hippocampal pathway and after adult hippocampal lesions. Mol. Cell Neurosci. 2004;26:34–49. doi: 10.1016/j.mcn.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Mingorance A, Fontana X, Soriano E, del Rio JA. Overexpression of myelin-associated glycoprotein after axotomy of the perforant pathway. Mol. Cell Neurosci. 2005;29:471–483. doi: 10.1016/j.mcn.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Mingorance-Le Meur A, Zheng B, Soriano E, del Rio JA. Involvement of the myelin-associated inhibitor Nogo-A in early cortical development and neuronal maturation. Cereb. Cortex. 2007;17:2375–2386. doi: 10.1093/cercor/bhl146. [DOI] [PubMed] [Google Scholar]
- Newton IG, Forbes ME, Legault C, Johnson JE, Brunso-Bechtold JK, Riddle DR. Caloric restriction does not reverse aging-related changes in hippocampal BDNF. Neurobiol. Aging. 2005;26:683–688. doi: 10.1016/j.neurobiolaging.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Yoshida R, Berry RW, Gallagher M, Geinisman Y. Reduction in size of perforated postsynaptic densities in hippocampal axospinous synapses and age-related spatial learning impairments. J. Neurosci. 2004;24:7648–7653. doi: 10.1523/JNEUROSCI.1725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieves-Martinez E, Sonntag WE, Wilson A, Donahue A, Molina DP, Brunso-Bechtold J, Nicolle MM. Early-onset GH deficiency results in spatial memory impairment in mid-life and is prevented by GH supplementation. J. Endocrinol. 2010;204:31–36. doi: 10.1677/JOE-09-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J. Neurosci. 1996;16:5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan RM, Griffin EW, Kelly AM. Long-term treadmill exposure protects against age-related neurodegenerative change in the rat hippocampus. Hippocampus. 2009;19:1019–1029. doi: 10.1002/hipo.20591. [DOI] [PubMed] [Google Scholar]
- Okonkwo OC, Cohen RA, Gunstad J, Tremont G, Alosco ML, Poppas A. Longitudinal trajectories of cognitive decline among older adults with cardiovascular disease. Cerebrovasc. Dis. 2010;30:362–373. doi: 10.1159/000319564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos CM, Tsai SY, Alsbiei T, O'Brien TE, Schwab ME, Kartje GL. Functional recovery and neuroanatomical plasticity following middle cerebral artery occlusion and IN-1 antibody treatment in the adult rat. Ann. Neurol. 2002;51:433–441. doi: 10.1002/ana.10144. [DOI] [PubMed] [Google Scholar]
- Park JH, Gimbel DA, GrandPre T, Lee JK, Kim JE, Li W, Lee DH, Strittmatter SM. Alzheimer precursor protein interaction with the Nogo-66 receptor reduces amyloid-beta plaque deposition. J. Neurosci. 2006;26:1386–1395. doi: 10.1523/JNEUROSCI.3291-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Kim J, Zhou Z, Fink DJ, Mata M. Neuronal Nogo-A regulates glutamate receptor subunit expression in hippocampal neurons. J. Neurochem. 2011;119:1183–1193. doi: 10.1111/j.1471-4159.2011.07520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Zhou Z, Hu J, Fink DJ, Mata M. Soluble Nogo receptor down-regulates expression of neuronal Nogo-A to enhance axonal regeneration. J. Biol. Chem. 2010;285:2783–2795. doi: 10.1074/jbc.M109.046425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrinovic MM, Duncan CS, Bourikas D, Weinman O, Montani L, Schroeter A, Maerki D, Sommer L, Stoeckli ET, Schwab ME. Neuronal Nogo-A regulates neurite fasciculation, branching and extension in the developing nervous system. Development. 2010;137:2539–2550. doi: 10.1242/dev.048371. [DOI] [PubMed] [Google Scholar]
- Platano D, Fattoretti P, Balietti M, Giorgetti B, Casoli T, Di SG, Bertoni-Freddari C, Aicardi G. Synaptic remodeling in hippocampal CA1 region of aged rats correlates with better memory performance in passive avoidance test. Rejuvenation. Res. 2008;11:341–348. doi: 10.1089/rej.2008.0725. [DOI] [PubMed] [Google Scholar]
- Poe BH, Linville C, Riddle DR, Sonntag WE, Brunso-Bechtold JK. Effects of age and insulin-like growth factor-1 on neuron and synapse numbers in area CA3 of hippocampus. Neuroscience. 2001;107:231–238. doi: 10.1016/s0306-4522(01)00341-4. [DOI] [PubMed] [Google Scholar]
- Poon HF, Shepherd HM, Reed TT, Calabrese V, Stella AM, Pennisi G, Cai J, Pierce WM, Klein JB, Butterfield DA. Proteomics analysis provides insight into caloric restriction mediated oxidation and expression of brain proteins associated with age-related impaired cellular processes: Mitochondrial dysfunction, glutamate dysregulation and impaired protein synthesis. Neurobiol. Aging. 2006;27:1020–1034. doi: 10.1016/j.neurobiolaging.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- Raiker SJ, Lee H, Baldwin KT, Duan Y, Shrager P, Giger RJ. Oligodendrocyte-myelin glycoprotein and Nogo negatively regulate activity-dependent synaptic plasticity. J. Neurosci. 2010;30:12432–12445. doi: 10.1523/JNEUROSCI.0895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey MM, Weiner JL, Moore TP, Carter CS, Sonntag WE. Growth hormone treatment attenuates age-related changes in hippocampal short-term plasticity and spatial learning. Neuroscience. 2004;129:119–127. doi: 10.1016/j.neuroscience.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Amaral DG. Evidence for task-dependent memory dysfunction in the aged monkey. J. Neurosci. 1989;9:3568–3576. doi: 10.1523/JNEUROSCI.09-10-03568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Deroche PS, Mao Y, Burwell RD. Neuron number in the parahippocampal region is preserved in aged rats with spatial learning deficits. Cereb. Cortex. 2002;12:1171–1179. doi: 10.1093/cercor/12.11.1171. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen T, Schliemann T, Sorensen JC, Zimmer J, West MJ. Memory impaired aged rats: no loss of principal hippocampal and subicular neurons. Neurobiol. Aging. 1996;17:143–147. doi: 10.1016/0197-4580(95)02032-2. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog. Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Rountree CB, Van Kirk CA, You H, Ding W, Dang H, VanGuilder HD, Freeman WM. Clinical application for the preservation of phospho-proteins through in-situ tissue stabilization. Proteome. Sci. 2010;8:61–20. doi: 10.1186/1477-5956-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe WB, Blalock EM, Chen KC, Kadish I, Wang D, Barrett JE, Thibault O, Porter NM, Rose GM, Landfield PW. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J. Neurosci. 2007;27:3098–3110. doi: 10.1523/JNEUROSCI.4163-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW. Intellectual Development in Adulthood: The Seattle Longitudinal Study. Cambridge University Press; Cambridge: 1996. [Google Scholar]
- Schmidt-Kastner R, Bedard A, Hakim A. Transient expression of GAP-43 within the hippocampus after global brain ischemia in rat. Cell Tissue Res. 1997;288:225–238. doi: 10.1007/s004410050808. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Tuli SK, Failli V. The Nogo receptor complex: confining molecules to molecular mechanisms. Trends Mol. Med. 2006;12:293–297. doi: 10.1016/j.molmed.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Seymour AB, Andrews EM, Tsai SY, Markus TM, Bollnow MR, Brenneman MM, O'Brien TE, Castro AJ, Schwab ME, Kartje GL. Delayed treatment with monoclonal antibody IN-1 1 week after stroke results in recovery of function and corticorubral plasticity in adult rats. J. Cereb. Blood Flow Metab. 2005;25:1366–1375. doi: 10.1038/sj.jcbfm.9600134. [DOI] [PubMed] [Google Scholar]
- Shi L, Linville MC, Tucker EW, Sonntag WE, Brunso-Bechtold JK. Differential effects of aging and insulin-like growth factor-1 on synapses in CA1 of rat hippocampus. Cereb. Cortex. 2005;15:571–577. doi: 10.1093/cercor/bhh158. [DOI] [PubMed] [Google Scholar]
- Shrestha L. The Changing Demographic Profile of the United States; CRS Report for Congress. 2006 http://www.fas.org/sgp/crs/misc/RL32701.pdf.
- Skoulakis EM, Davis RL. 14-3-3 proteins in neuronal development and function. Mol. Neurobiol. 1998;16:269–284. doi: 10.1007/BF02741386. [DOI] [PubMed] [Google Scholar]
- Social Science Data Analysis Network United States Age Distribution, U.S. census data. CensusScope. 2010 http://www.censusscope.org/us/chart_age.html.
- Starkey ML, Schwab ME. Anti-Nogo-A and training: Can one plus one equal three? Exp. Neurol. 2011 doi: 10.1016/j.expneurol.2011.04.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Rehberg S, Ader M, Lommes P, Riethmacher D, Schachner M, Bartsch U, Wegner M. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 2002;16:165–170. doi: 10.1101/gad.215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Haberman RP, Gallagher M. Cognitive decline is associated with reduced reelin expression in the entorhinal cortex of aged rats. Cereb. Cortex. 2011;21:392–400. doi: 10.1093/cercor/bhq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson HJ, Marklund N, LeBold DG, Morales DM, Keck CA, Vinson M, Royo NC, Grundy R, McIntosh TK. Tissue sparing and functional recovery following experimental traumatic brain injury is provided by treatment with an anti-myelin-associated glycoprotein antibody. Eur. J. Neurosci. 2006a;24:3063–3072. doi: 10.1111/j.1460-9568.2006.05197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SN, Gibson TR, Thompson BM, Deng Y, Hall ED. Relationship of calpain-mediated proteolysis to the expression of axonal and synaptic plasticity markers following traumatic brain injury in mice. Exp. Neurol. 2006b;201:253–265. doi: 10.1016/j.expneurol.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Rowe WB, Chow AR, Michael TH, Rose GM. Theta-frequency synaptic potentiation in CA1 in vitro distinguishes cognitively impaired from unimpaired aged Fischer 344 rats. J. Neurosci. 2002;22:9932–9940. doi: 10.1523/JNEUROSCI.22-22-09932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifunovski A, Josephson A, Bickford PC, Olson L, Brene S. Selective decline of Nogo mRNA in the aging brain. Neuroreport. 2006;17:913–916. doi: 10.1097/01.wnr.0000221831.95598.a3. [DOI] [PubMed] [Google Scholar]
- Trifunovski A, Josephson A, Ringman A, Brene S, Spenger C, Olson L. Neuronal activity-induced regulation of Lingo-1. Neuroreport. 2004;15:2397–2400. doi: 10.1097/00001756-200410250-00019. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Markus TM, Andrews EM, Cheatwood JL, Emerick AJ, Mir AK, Schwab ME, Kartje GL. Intrathecal treatment with anti-Nogo-A antibody improves functional recovery in adult rats after stroke. Exp. Brain Res. 2007;182:261–266. doi: 10.1007/s00221-007-1067-0. [DOI] [PubMed] [Google Scholar]
- VanGuilder HD, Bixler GV, Brucklacher RM, Farley JA, Yan H, Warrington JP, Sonntag WE, Freeman WM. Concurrent hippocampal induction of MHC II pathway components and glial activation with advanced aging is not correlated with cognitive impairment. J. Neuroinflammation. 2011a;8:138–159. doi: 10.1186/1742-2094-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGuilder HD, Farley JA, Yan H, Van Kirk CA, Mitschelen M, Sonntag WE, Freeman WM. Hippocampal dysregulation of synaptic plasticity-associated proteins with age-related cognitive decline. Neurobiol. Dis. 2011b;43:201–212. doi: 10.1016/j.nbd.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGuilder HD, Freeman WM. The hippocampal neuroproteome with aging and cognitive decline: past progress and future directions. Front Aging Neurosci. 2011;3:8. doi: 10.3389/fnagi.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGuilder HD, Yan H, Farley JA, Sonntag WE, Freeman WM. Aging alters the expression of neurotransmission-regulating proteins in the hippocampal synaptoproteome. J. Neurochem. 2010;113:1577–1588. doi: 10.1111/j.1471-4159.2010.06719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers CA, Stephens B, Bowen J, Arbuthnott GW, Grant SG, Ingham CA. Neurone specific regulation of dendritic spines in vivo by post synaptic density 95 protein (PSD-95) Brain Res. 2006;1090:89–98. doi: 10.1016/j.brainres.2006.03.075. [DOI] [PubMed] [Google Scholar]
- Walmsley AR, Mir AK. Targeting the Nogo-A signalling pathway to promote recovery following acute CNS injury. Curr. Pharm. Des. 2007;13:2470–2484. doi: 10.2174/138161207781368611. [DOI] [PubMed] [Google Scholar]
- Wang X, Chun SJ, Treloar H, Vartanian T, Greer CA, Strittmatter SM. Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact. J. Neurosci. 2002;22:5505–5515. doi: 10.1523/JNEUROSCI.22-13-05505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang L, Wu J, Cai J. The in vivo synaptic plasticity mechanism of EGb 761-induced enhancement of spatial learning and memory in aged rats. Br. J. Pharmacol. 2006;148:147–153. doi: 10.1038/sj.bjp.0706720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann O, Schnell L, Ghosh A, Montani L, Wiessner C, Wannier T, Rouiller E, Mir A, Schwab ME. Intrathecally infused antibodies against Nogo-A penetrate the CNS and downregulate the endogenous neurite growth inhibitor Nogo-A. Mol. Cell Neurosci. 2006;32:161–173. doi: 10.1016/j.mcn.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Wiessner C, Bareyre FM, Allegrini PR, Mir AK, Frentzel S, Zurini M, Schnell L, Oertle T, Schwab ME. Anti-Nogo-A antibody infusion 24 hours after experimental stroke improved behavioral outcome and corticospinal plasticity in normotensive and spontaneously hypertensive rats. J. Cereb. Blood Flow Metab. 2003;23:154–165. doi: 10.1097/01.WCB.0000040400.30600.AF. [DOI] [PubMed] [Google Scholar]
- Zagrebelsky M, Schweigreiter R, Bandtlow CE, Schwab ME, Korte M. Nogo-A stabilizes the architecture of hippocampal neurons. J. Neurosci. 2010;30:13220–13234. doi: 10.1523/JNEUROSCI.1044-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Li Y, Nanda A, Zhang JH. HBO suppresses Nogo-A, Ng-R, or RhoA expression in the cerebral cortex after global ischemia. Biochem. Biophys. Res. Commun. 2003;309:368–376. doi: 10.1016/j.bbrc.2003.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.