Abstract
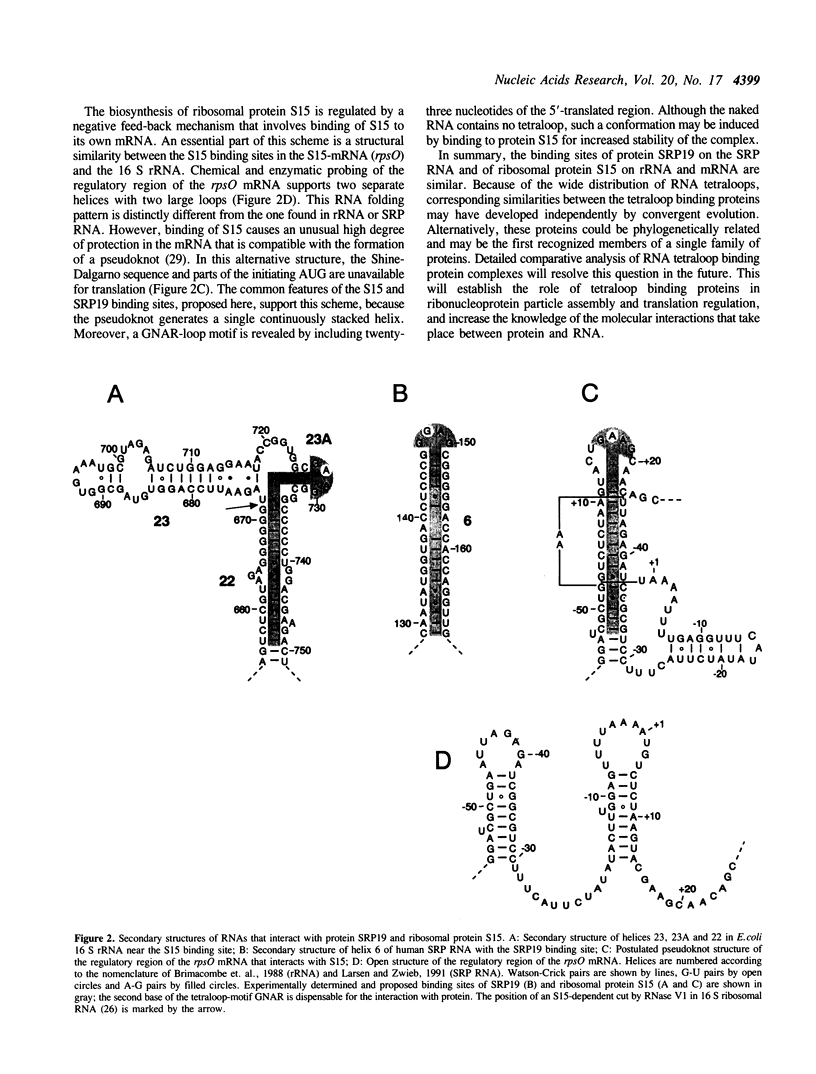
A group of RNA binding proteins, termed tetraloop binding proteins, includes ribosomal protein S15 and protein SRP19 of signal recognition particle. They are primary RNA binding proteins, recognize RNA tetranucleotide loops with a GNAR consensus motif, and require a helical region located adjacent to the tetraloop. Closely related RNA structures that fit these criteria appear in helix 6 of SRP RNA, in helices 22 and 23A of 16 S ribosomal RNA, and, as a pseudoknot, in the regulatory region of the rpsO gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhin M. R., Alblas J., van Duin J. Secondary structure at the 3' terminal region of RNA coliphages: comparison with tRNA. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):110–118. doi: 10.1016/0167-4781(90)90150-z. [DOI] [PubMed] [Google Scholar]
- Andreazzoli M., Gerbi S. A. Changes in 7SL RNA conformation during the signal recognition particle cycle. EMBO J. 1991 Apr;10(4):767–777. doi: 10.1002/j.1460-2075.1991.tb08008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimacombe R., Atmadja J., Stiege W., Schüler D. A detailed model of the three-dimensional structure of Escherichia coli 16 S ribosomal RNA in situ in the 30 S subunit. J Mol Biol. 1988 Jan 5;199(1):115–136. doi: 10.1016/0022-2836(88)90383-x. [DOI] [PubMed] [Google Scholar]
- Cheong C., Varani G., Tinoco I., Jr Solution structure of an unusually stable RNA hairpin, 5'GGAC(UUCG)GUCC. Nature. 1990 Aug 16;346(6285):680–682. doi: 10.1038/346680a0. [DOI] [PubMed] [Google Scholar]
- Dang H., Ellis S. R. Structural and functional analyses of a yeast mitochondrial ribosomal protein homologous to ribosomal protein S15 of Escherichia coli. Nucleic Acids Res. 1990 Dec 11;18(23):6895–6901. doi: 10.1093/nar/18.23.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro-Novick S., Honma M., Beckwith J. The product of gene secC is involved in the synthesis of exported proteins in E. coli. Cell. 1984 Aug;38(1):211–217. doi: 10.1016/0092-8674(84)90542-7. [DOI] [PubMed] [Google Scholar]
- Fitzky B., Subramanian A. R. Nucleotide sequence and map positions of the duplicated gene for chloroplast ribosomal protein S15 in Zea mays (maize). Nucleic Acids Res. 1990 Jun 11;18(11):3407–3407. doi: 10.1093/nar/18.11.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. D., Mattaj I. W., Rio D. C. RNA-protein interactions. Cell. 1991 Dec 20;67(6):1041–1046. doi: 10.1016/0092-8674(91)90282-4. [DOI] [PubMed] [Google Scholar]
- Held W. A., Ballou B., Mizushima S., Nomura M. Assembly mapping of 30 S ribosomal proteins from Escherichia coli. Further studies. J Biol Chem. 1974 May 25;249(10):3103–3111. [PubMed] [Google Scholar]
- Heus H. A., Pardi A. Structural features that give rise to the unusual stability of RNA hairpins containing GNRA loops. Science. 1991 Jul 12;253(5016):191–194. doi: 10.1126/science.1712983. [DOI] [PubMed] [Google Scholar]
- Hiratsuka J., Shimada H., Whittier R., Ishibashi T., Sakamoto M., Mori M., Kondo C., Honji Y., Sun C. R., Meng B. Y. The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol Gen Genet. 1989 Jun;217(2-3):185–194. doi: 10.1007/BF02464880. [DOI] [PubMed] [Google Scholar]
- Ibrahimi I., Gentz R. A functional interaction between the signal peptide and the translation apparatus is detected by the use of a single point mutation which blocks translocation across mammalian endoplasmic reticulum. J Biol Chem. 1987 Jul 25;262(21):10189–10194. [PubMed] [Google Scholar]
- Jacquier A., Michel F. Multiple exon-binding sites in class II self-splicing introns. Cell. 1987 Jul 3;50(1):17–29. doi: 10.1016/0092-8674(87)90658-1. [DOI] [PubMed] [Google Scholar]
- Larsen N., Zwieb C. SRP-RNA sequence alignment and secondary structure. Nucleic Acids Res. 1991 Jan 25;19(2):209–215. doi: 10.1093/nar/19.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingelbach K., Zwieb C., Webb J. R., Marshallsay C., Hoben P. J., Walter P., Dobberstein B. Isolation and characterization of a cDNA clone encoding the 19 kDa protein of signal recognition particle (SRP): expression and binding to 7SL RNA. Nucleic Acids Res. 1988 Oct 25;16(20):9431–9442. doi: 10.1093/nar/16.20.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima S., Nomura M. Assembly mapping of 30S ribosomal proteins from E. coli. Nature. 1970 Jun 27;226(5252):1214–1214. doi: 10.1038/2261214a0. [DOI] [PubMed] [Google Scholar]
- Morinaga T., Funatsu G., Funatsu M., Wittman H. G. Primary structure of the 16S rRNA binding protein S15 from Escherichia coli ribosomes. FEBS Lett. 1976 May 1;64(2):307–309. doi: 10.1016/0014-5793(76)80316-x. [DOI] [PubMed] [Google Scholar]
- Mougel M., Eyermann F., Westhof E., Romby P., Expert-Bezançon A., Ebel J. P., Ehresmann B., Ehresmann C. Binding of Escherichia coli ribosomal protein S8 to 16 S rRNA. A model for the interaction and the tertiary structure of the RNA binding site. J Mol Biol. 1987 Nov 5;198(1):91–107. doi: 10.1016/0022-2836(87)90460-8. [DOI] [PubMed] [Google Scholar]
- Mougel M., Philippe C., Ebel J. P., Ehresmann B., Ehresmann C. The E. coli 16S rRNA binding site of ribosomal protein S15: higher-order structure in the absence and in the presence of the protein. Nucleic Acids Res. 1988 Apr 11;16(7):2825–2839. doi: 10.1093/nar/16.7.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace N. R., Smith D. K., Olsen G. J., James B. D. Phylogenetic comparative analysis and the secondary structure of ribonuclease P RNA--a review. Gene. 1989 Oct 15;82(1):65–75. doi: 10.1016/0378-1119(89)90031-0. [DOI] [PubMed] [Google Scholar]
- Philippe C., Portier C., Mougel M., Grunberg-Manago M., Ebel J. P., Ehresmann B., Ehresmann C. Target site of Escherichia coli ribosomal protein S15 on its messenger RNA. Conformation and interaction with the protein. J Mol Biol. 1990 Jan 20;211(2):415–426. doi: 10.1016/0022-2836(90)90362-P. [DOI] [PubMed] [Google Scholar]
- Régnier P., Grunberg-Manago M., Portier C. Nucleotide sequence of the pnp gene of Escherichia coli encoding polynucleotide phosphorylase. Homology of the primary structure of the protein with the RNA-binding domain of ribosomal protein S1. J Biol Chem. 1987 Jan 5;262(1):63–68. [PubMed] [Google Scholar]
- Römisch K., Webb J., Lingelbach K., Gausepohl H., Dobberstein B. The 54-kD protein of signal recognition particle contains a methionine-rich RNA binding domain. J Cell Biol. 1990 Nov;111(5 Pt 1):1793–1802. doi: 10.1083/jcb.111.5.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P. J., Beckwith J. Genetic analysis of protein export in Escherichia coli. Annu Rev Genet. 1990;24:215–248. doi: 10.1146/annurev.ge.24.120190.001243. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern S., Powers T., Changchien L. M., Noller H. F. RNA-protein interactions in 30S ribosomal subunits: folding and function of 16S rRNA. Science. 1989 May 19;244(4906):783–790. doi: 10.1126/science.2658053. [DOI] [PubMed] [Google Scholar]
- Stirling C. J., Hewitt E. W. The S. cerevisiae SEC65 gene encodes a component of yeast signal recognition particle with homology to human SRP19. Nature. 1992 Apr 9;356(6369):534–537. doi: 10.1038/356534a0. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Olvera J., Wool I. G. The primary structure of rat ribosomal protein S13. Biochem Biophys Res Commun. 1990 Sep 14;171(2):519–524. doi: 10.1016/0006-291x(90)91176-s. [DOI] [PubMed] [Google Scholar]
- Svensson P., Changchien L. M., Craven G. R., Noller H. F. Interaction of ribosomal proteins, S6, S8, S15 and S18 with the central domain of 16 S ribosomal RNA. J Mol Biol. 1988 Mar 20;200(2):301–308. doi: 10.1016/0022-2836(88)90242-2. [DOI] [PubMed] [Google Scholar]
- Takata R., Mukai T., Aoyagi M., Hori K. Nucleotide sequence of the gene for Escherichia coli ribosomal protein S15 (rpsO). Mol Gen Genet. 1984;197(2):225–229. doi: 10.1007/BF00330967. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C. Tetraloops and RNA folding. Nature. 1990 Aug 16;346(6285):613–614. doi: 10.1038/346613a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Disassembly and reconstitution of signal recognition particle. Cell. 1983 Sep;34(2):525–533. doi: 10.1016/0092-8674(83)90385-9. [DOI] [PubMed] [Google Scholar]
- Waring R. B., Davies R. W. Assessment of a model for intron RNA secondary structure relevant to RNA self-splicing--a review. Gene. 1984 Jun;28(3):277–291. doi: 10.1016/0378-1119(84)90145-8. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Hayashi S., Wu H. C. Synthesis and export of the outer membrane lipoprotein in Escherichia coli mutants defective in generalized protein export. J Bacteriol. 1988 Sep;170(9):4001–4007. doi: 10.1128/jb.170.9.4001-4007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Winker S., Gutell R. R. Architecture of ribosomal RNA: constraints on the sequence of "tetra-loops". Proc Natl Acad Sci U S A. 1990 Nov;87(21):8467–8471. doi: 10.1073/pnas.87.21.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieb C. A basic region neighboring the lysine-rich C-terminus of protein SRP19 is required for binding to signal recognition particle RNA. Biochem Cell Biol. 1991 Sep;69(9):649–654. doi: 10.1139/o91-096. [DOI] [PubMed] [Google Scholar]
- Zwieb C., Larsen N. The signal recognition particle (SRP) database. Nucleic Acids Res. 1992 May 11;20 (Suppl):2207–2207. doi: 10.1093/nar/20.suppl.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieb C. Structure and function of signal recognition particle RNA. Prog Nucleic Acid Res Mol Biol. 1989;37:207–234. doi: 10.1016/s0079-6603(08)60699-6. [DOI] [PubMed] [Google Scholar]
- Zwieb C., Ullu E. Identification of dynamic sequences in the central domain of 7SL RNA. Nucleic Acids Res. 1986 Jun 11;14(11):4639–4657. doi: 10.1093/nar/14.11.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]