Abstract
Aims
Professional practice guidelines recommend that pacemaker recipients be followed regularly. However, the majority of scheduled ambulatory visits is unproductive and imposes a heavy burden on the health-care system.
Methods and results
The COMPAS randomized, multicentre, non-inferiority trial examined the safety of long-term remote monitoring of pacemakers. Between December 2005 and January 2008, 538 patients were randomly assigned to remote monitoring follow-up (active group) vs. standard care (control group). The primary objective was to confirm that the proportion of patients who experienced at least one major adverse event (MAE), including all-cause death and hospitalizations for device-related or cardiovascular adverse events, was not >7% higher in the active than in the control group. MAE-free survivals and quality of life were compared in both groups. The characteristics of the study groups were similar. Over a follow-up of 18.3 months, 17.3% of patients in the active and 19.1% in the control group experienced at least one MAE (P < 0.01 for non-inferiority). Hospitalizations for atrial arrhythmias (6 vs. 18) and strokes (2 vs. 8) were fewer (P < 0.05), and the number of interim ambulatory visits was 56% lower (P < 0.001) in the active than the control group. Changes in pacemaker programming or drug regimens were made in 62% of visits in the active vs. 29% in the control group (P < 0.001). Quality of life remained unchanged in both groups.
Conclusion
Remote monitoring was a safe alternative to conventional care and significantly lowered the number of ambulatory visits during long-term follow-up of permanently paced patients.
ClinicalTrials.gov identifier: NCT00989326.
Keywords: Pacemaker follow-up, Remote monitoring, Telemedicine
Introduction
Professional practice guidelines recommend that pacemaker recipients be followed regularly.1,2 These regular office visits include examinations of device function and patient clinical status, and may prompt the reprogramming of the device or changes in medication regimens. The efficiency of this strategy is, however, questionable since, in the majority of scheduled visits, the device programming or drug regimen is left unchanged.3 To optimize the follow-up of device recipients, systems have been developed, which allow the automatic teletransmission of data stored in the devices' memories, including battery voltage, lead characteristics, and arrhythmias. The data sent by a home transmitter allow the remote surveillance of patients and eliminate unnecessary ambulatory visits. Clinical studies of these new systems have been conducted mostly in recipients of cardioverter defibrillators, and only a few studies of permanently paced patients have been published.4–10
The French randomized, multicentre ‘COMPArative follow-up Schedule with home monitoring’ (COMPAS) trial was conducted to evaluate the benefits of remote monitoring after first implantation or replacement of dual chamber pacemakers and, specifically, to determine whether remote monitoring could replace the standard long-term follow-up of patients with regard to the adverse events related or unrelated to the implanted devices.
Methods
Primary study objective
The primary objective of this non-inferiority trial was to confirm that the proportion of patients who experienced at least one major adverse event (MAE), including (i) death, (ii) hospitalization for complications related to the pacing system, and (iii) hospitalization for an adverse cardiovascular event, was not higher in the remote follow-up (active) than in the standard care (control) group.
Secondary objectives
The secondary objectives of the trial were to (i) compare the incidence of each MAE in both groups, (ii) measure the decrease in the number of in-office follow-ups conferred by remote monitoring, (iii) retrospectively analyse the delay in the management of adverse events in both study groups, (iv) compare the contributions of the follow-ups in both groups, and (v) examine the effect of remote monitoring on quality of life.11
Patient selection
Patients were eligible for inclusion in the COMPAS trial if (i) they had undergone implantation of a PHILOS II DR-T DDD pacemaker (Biotronik SE and Co. KG, Berlin, Germany) with an A/V bipolar lead, for standard pacing indications, at least 1 month earlier,1,2 and (ii) were able to (a) comply with the study protocol and (b) sign an informed consent. To optimize the safety of the trial and compliance with the protocol, the patients were excluded if their spontaneous ventricular rate was <30 b.p.m.
Telecardiology system
Home Monitoring® (Biotronik) is a system that automatically transmits the data stored in implantable devices to the Biotronik service centre, over a wireless global system for mobile communications network. After an automatic analysis, messages are posted daily on a secure Internet site accessible to the physician responsible for the patient's care. In case of clinical or technical anomaly (or both), the device emits additional warning messages, which are immediately forwarded by the service centre to the physician (Table 1).
Table 1.
Technical and clinical information transmitted daily, along with corresponding warnings in case of detected anomaly
| Daily transmission | Associated warning messages (warning level) | Device programming in both study groups |
|---|---|---|
| Technical information | DDD or DDDR mode | |
| Battery status | Elective replacement indicator (I) | |
| Atrial lead impedance | Atrial lead impedance <200 or >3000 Ω (I) | Automatic atrial lead test: ON |
| Ventricular lead impedance | Ventricular lead impedance <200 or >3000 Ω (I) | Automatic ventricular lead test: ON |
| R-wave safety margin | R-wave safety margin <50% (I) | |
| P-wave safety margin | P-wave safety margin <50% (I) | |
| Ventricular capture threshold | Autothreshold deactivated (I) | Autothreshold: ON |
| Ventricular threshold >4.8 V (I) | ||
| Variations in ventricular threshold >1 V (II) | ||
| No transmission | No transmission in last 14 days (I) | Transmission at 3:00 a.m. |
| Clinical information | ||
| Mean ventricular rate/24 h | ||
| Number and duration of atrial arrhythmia | Three consecutive episodes >18 h (I) | Mode switch: ON |
| First episode since onset of follow-up (II) | ||
| Peak ventricular rate during mode switch | IEGM recording for mode switch: ON | |
| Daily peak ventricular rate | IEGM recording for ventricular rate: ON | |
| Duration of episode with fastest ventricular rate | Ventricular rate >160 b.p.m. | |
| Number of ventricular episodes | More than eight consecutive PVC (II) | |
(I), warning level I prompting a mandatory interim follow-up; (II), warning level II prompting an optional interim follow-up; PVC, premature ventricular complex; IEGM, intracardiac electrogram.
Study protocol
COMPAS was a randomized, open-label, parallel-design trial, in which 43 French medical centres participated, including 30 public and 13 private institutions (see Supplementary material online, appendices). The trial protocol, which complied with the declaration of Helsinki, was reviewed and approved by the pertinent National Ethics Committees, and all patients granted their written, informed consent to participate. COMPAS was sponsored by Biotronik SE and Co. KG that participated to the study design and data monitoring. Patients who fulfilled the inclusion criteria and pacing indications were randomly assigned to an active vs. a control group at least 1 month after the pacemaker implantation.
The patients assigned to the active group were monitored daily by telecardiology. No interim visit was scheduled in the active group, but in case of device dysfunction (technical issue) or health event (medical issue), the cardiologist investigator was notified by e-mail, prompting the rescheduling of the next follow-up visit, if necessary. It is noteworthy, however, that remote monitoring was not a substitute for emergency medical services. The management of remote FU was organized in each centre either by direct access of the physician to the transmitted data or by delegation to an allied professional specifically educated to perform this task during office hours. No reaction time was imposed by the protocol, the patients were duly informed that the physician could only evaluate remote data during office hours and days. The events were assigned a level of warning. Level I prompted a mandatory interim follow-up to be scheduled as soon as possible. Level II prompted an optional interim follow-up left to the physician's decision (Table 1). Patients assigned to the control group were managed according to the usual practices of each participating medical centre, and the follow-up schedule was left to the physicians' discretion. So the dates of FU were not imposed by the protocol but physicians were encouraged to comply with guidelines. Both patient groups were followed for 18 months. At each follow-up visit, and at the end of the trial, 18 months after random assignment of the patient, the investigators interrogated the pacing system and recorded the possible occurrence of clinical events. We examined the contributions and reliability of remote monitoring in the early detection of MAE, and measured the number of contributory follow-ups, defined as a visit prompting a change in patient management or reprogramming of the pacemaker. Quality of life was assessed at the time of enrolment and at the end of the study by means of the SF-36 questionnaire.11
The patients assigned to the control group were also remotely monitored, though the data were not used for patient surveillance and were not made available online to the physician investigators. This allowed a retrospective comparison between the two groups of the delay between the remote monitoring warning message and subsequent medical interventions, such as (i) contributory interim follow-ups, as defined earlier, or (ii) hospitalizations.
Data collection, management, monitoring, and analyses
The data were collected at each medical centre on case report forms completed by the investigators and dedicated staff, under the supervision of study coordinators and with the assistance of study monitors assigned by the sponsor. Specific responsibilities of the study coordinators included (a) the verification, along with the study monitors, of the completeness and accuracy of the case report forms, (b) analysis of the data in collaboration with the investigators, (c) support of the publication and presentation of the study results, and (d) preparation of the adverse event report forms and supporting information for evaluation by a member of the Safety Monitoring Committee, composed of three expert cardiologists who did not participate in the trial and were unaware of the random patient assignment to the active vs. the control group. The adverse event report forms and supporting documentation were reviewed by a member of the Safety Monitoring Committee for confirmation of the investigator's classification of seriousness and outcome. Discordant classifications were reviewed by all members of the committee and final classifications were reached by consensus. The statistical analyses were performed by a biostatistician employed by the sponsor.
Statistical analyses
The size of the randomized population was calculated to reach a >80% power to confirm the non-inferiority of remote monitoring compared with standard follow-ups, assuming a 7% incidence of primary study endpoint in both groups and with a non-inferiority margin of 7%, a 5% significance level, and assuming a 10% rate of non-compliance. The patients were randomly assigned to an active vs. a control group, in even blocks among study centres. The baseline characteristics of the two study groups were compared by two-sided χ2 test for nominal, qualitative variables, and by Student's t-test for normally distributed, quantitative, continuous and discrete variables. The normal distribution of variables was verified, using the Kolmogorov–Smirnov and Shapiro–Wilk tests. Survival free from MAE were estimated by the Kaplan–Meier method and compared, using the log-rank test. Cox proportional hazard regression analysis was used to estimate the likelihood of survival, after verifying the proportional hazard assumption. Cox model was also used to verify the non-inferiority of remote monitoring vs. primary care in various patient subgroups. The event rates of each component of the composite primary study objective were also measured separately. Odds (OR) and hazard (HR) ratios were calculated and the confidence intervals (CI) were evaluated with Miettinen's method. Friedman's non-parametric, repeated-measures analysis was used to compare the SF-36-scale and summary scores. We estimated the number of follow-ups per patient-year, with 95% CI, in each study group, assuming a Poisson distribution. A P-value < 0.05 was considered significant. The SPSS statistical software, version 18.0 (SPSS Institute, Inc., Chicago, IL, USA) was used for all analyses.
Results
Patient population
Between December 2005 and January 2008, 538 patients (mean age = 76 ± 9 years, 65% men) were enrolled in the trial, of whom 269 were assigned to the active and 269 to the control group. The baseline clinical characteristics of the two study groups, including antithrombotic regimens, were similar (Table 2). The proportions of first implants (88 vs. 87%) were also similar in both groups. After 44 patients (8.2%) withdrew their consent to participate in the study (no compliance with remote monitoring system or study (n = 40) or patient relocation (n = 4), the final analysis included 494 patients, of whom 248 were assigned to the active, and 246 to the control group. The mean duration of follow-up was 18.3 ± 3.3 months.
Table 2.
Underlying heart disease, disease manifestations, and electrocardiographic indications for pacing in each study group
| Active group (n= 269) | Control group (n= 269) | P-value | |
|---|---|---|---|
| Underlying heart disease | |||
| Primary conduction system disease | 92 (34.2) | 80 (29.7) | 0.27 |
| Hypertension | 71 (26.4) | 58 (21.6) | 0.19 |
| Cardiomyopathy | |||
| Ischaemic | 54 (20.0) | 55 (20.4) | 0.91 |
| Non-ischaemic | 15 (5.6) | 12 (4.5) | 0.55 |
| Valvular heart disease | 12 (4.5) | 15 (5.6) | 0.55 |
| Other | 41 (15.2) | 56 (20.8) | 0.09 |
| None | 48 (17.8) | 50 (18.6) | |
| Disease manifestations | |||
| None | 36 (13.4) | 22 (8.2) | 0.05 |
| Syncope | 120 (44.6) | 134 (49.8) | 0.23 |
| Dyspnoea | 55 (20.4) | 57 (21.2) | 0.83 |
| Lightheadedness | 23 (8.5) | 26 (9.7) | 0.65 |
| Fatigue/weakness | 25 (9.3) | 29 (10.8) | 0.57 |
| Palpitation | 19 (7.1) | 19 (7.1) | 1.00 |
| Others | 37 (13.8) | 39 (14.5) | 0.80 |
| Pacing indications | |||
| Sinus node disease | 69 (25.6) | 73 (27.1) | 0.69 |
| Atrioventricular block | |||
| First or second degree | 98 (36.4) | 93 (34.6) | 0.65 |
| Third degree | 79 (29.4) | 93 (34.6) | 0.20 |
| Bundle branch block | 11 (4.1) | 4 (1.5) | 0.07 |
| Others | 12 (4.5) | 6 (2.2) | 0.15 |
| History of atrial arrhythmia | 26 (9.7) | 29 (10.8) | 0.67 |
| Antithrombotic therapy | 131 (48.7) | 133 (49.4) | 0.86 |
Values are numbers (%) of patients in corresponding group.
Analysis of major adverse clinical events
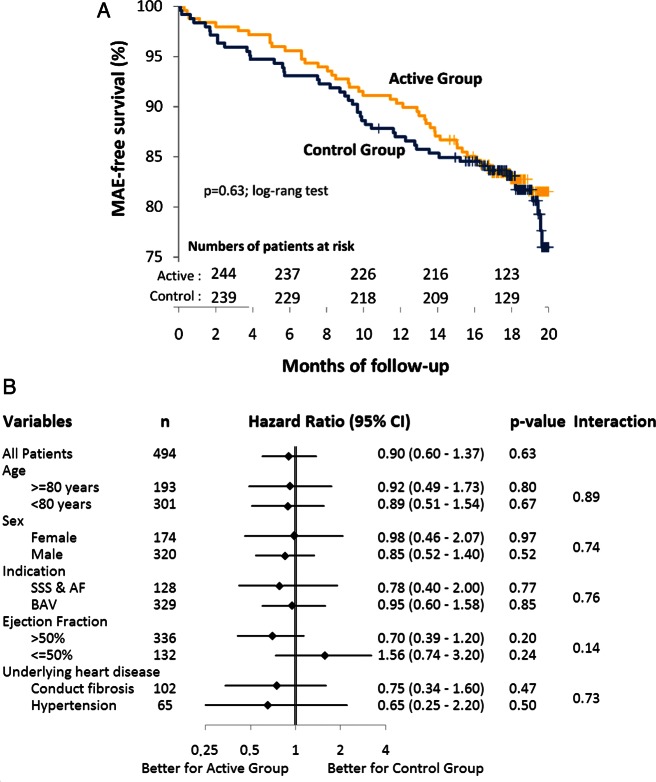
The cumulative, 18-month MAE-free survival of each study group is shown in Figure 1A. By the end of the trial, 90 patients (18.2%) had experienced an MAE, including 43 patients (17.3%) in the active group and 47 (19.1%) in the control group (HR 0.90; 95% CI: 0.59–1.41; P = 0.63), confirming the non-inferiority of remote monitoring compared with standard care with pre-specified difference margin of 7%. That non-inferiority was further confirmed among selected patient subgroups (Figure 1B).
Figure 1.
(A) Cumulative survival free from MAE in each study group estimated by Kaplan–Meier analysis. (B) Outcomes of the test of non-inferiority among various patient subgroups.
In a separate analysis of each component of the composite MAE, 18 patients (7.3%) died in the active, vs. 13 (5.3%) in the control group (P = 0.37). In the active group, 3 patients died from heart failure and 15 from non-cardiovascular causes, while in the control group, 4 patients died from strokes and 9 from non-cardiovascular causes. Hospitalizations for management of adverse cardiovascular events were recorded in 29 patients in the active (11.7%) vs. 32 patients in the control (11.8%) group (P = 0.66). Finally, one patient in the active (0.4%), vs. seven patients in the control (2.8%) group were hospitalized for complications related to the pacing system (OR = 0.14; 95% CI: 0.02–0.86; P = 0.03).
The types and numbers of MAE, and the number of patients who suffered at least one MAE in the overall population and in each study group, are listed in Table 3. Hospitalizations related to atrial arrhythmias and strokes were observed in six patients (six events: four atrial arrhythmias and two strokes) in the active, vs. 17 patients (18 events: 10 atrial arrhythmias and 8 strokes) in the control group (OR = 0.33; 95% CI: 0.14–0.87; P = 0.02). All other between-groups differences were statistically non-significant.
Table 3.
Major adverse events in the entire patient population and in each study group
| All patients, (n= 494) | Active group, (n= 248) | Control group, (n= 246) | |
|---|---|---|---|
| Deaths | |||
| Stroke | 4 | 0 | 4 |
| Heart failure | 3 | 3 | 0 |
| Pulmonary disease | 3 | 1 | 2 |
| Cancer | 9 | 6 | 3 |
| Other non-cardiac causes | 12 | 8 | 4 |
| All deaths | 31 | 18 | 13 |
| Hospitalizations for cardiovascular adverse events | |||
| Ventricular arrhythmia | 2/2 | 1/1 | 1/1 |
| Atrial arrhythmia, strokea, or both | 24/23 | 6/6 | 18/17* |
| Heart failure | 24/19 | 18/13 | 6/6 |
| Acute coronary syndrome | 12/11 | 6/5 | 6/6 |
| Others | 8/8 | 6/6 | 2/2 |
| All hospitalizations for cardiovascular adverse events | 70/61 | 37/29 | 33/32 |
| Hospitalizations for device-related adverse events | |||
| Infection, extrusion | 4/4 | 0 | 4/4 |
| Lead dislodgment | 2/2 | 0 | 2/2 |
| Venous thrombosis | 3/2 | 2/1 | 1/1 |
| High ventricular threshold | 1/1 | 0 | 1/1 |
| All hospitalizations for device-related adverse events | 10/8 | 2/1 | 8/7 |
| All adverse eventsb | 104/90 | 54/43 | 50/47 |
Values are numbers of adverse events/patients; the comparisons between both groups for a given type of event were calculated on the first event in the case of multiple events per patient.
aAn ischaemic aetiology of the stroke and history of atrial arrhythmia were confirmed in all patients.
bSome patients had multiple events in different categories, so the total numbers of adverse events do no add up.
*P < 0.05.
Interim follow-ups
The mean number of interim follow-ups per patient-year was 0.51 ± 0.71 (95% CI: 0.43–0.59) in the active group, vs. 1.15 ± 1.07 (95% CI: 1.03–1.27) in the control group, a 56% difference (95% CI: −61 to −48; P < 0.001; Figure 2). After the last scheduled follow-up, the between-groups difference was −36% (95% CI −43 to −28; P < 0.001); the mean number of follow-ups per patient-year was 1.04 ± 1.02 (95% CI: 0.94–1.14) in the active group vs. 1.63 ± 1.28 (95% CI: 1.50–1.76) in the control group. Based on the total number of visits recorded in each group, the difference became statistically significant past the sixth month of follow-up (Figure 2). On this curve, the 6-month increments of the control group demonstrate that the patients in the control group were usually followed every 6 months.
Figure 2.
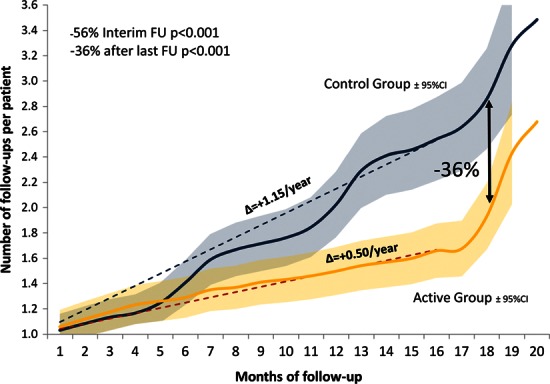
Number of follow-ups per patient. The blue and orange curves indicate the number of follow-ups per patient in the control and the active groups, respectively. The shaded areas represent the 95% CI. At 18 months, the number of follow-ups was 36% lower in the active than in the control group. The slopes (▵) of the dotted line indicate the mean number of visits per year.
Among 371 interim follow-ups in the control group, 308 (83.0%) were scheduled visits, 21 (5.7%) were prompted by a patient call, 9 (2.4%) were prompted by a general practitioner's call, and 33 (8.9%) were scheduled before, during or after a hospitalization. Among 167 interim follow-up in the active group, 73 (43.7%) were prompted by remote monitoring, 30 (17.9%) by a patient call, 21 (12.6%) by a general practitioner's call, and 43 (25.9%) were scheduled before, during or after a hospitalization. Out of 73 follow-ups prompted by remote monitoring (i) 34 (47%) were for clinical events; including 29 (40%) automatic mode switches and 5 (7%) critical changes in ventricular rate; and (ii) 39 (53%) were for technical events, including 25 (34%) changes in capture threshold; 2 (3%) change in lead impedance, 5 (7%) P- or R-wave amplitude safety margin; 7 (9%) transmission difficulties.
In the active group, 126 patients (50.8%) needed no interim follow-up throughout the 18 months of follow-up.
Contributions of follow-ups
In the control group, 71% of interim follow-ups were non-contributory vs. 38% in the active group (P < 0.001). Limiting the analysis to the interim follow-ups prompted by remote monitoring, only 26% were non-contributory. At the 18-month scheduled follow-ups, 79% were non-contributory in the control vs. 73% in the active group (P = 0.13).
Detection of major adverse events
Both in the active (24 of 39 events) and in the control (28 of 41 events), the majority of cardiovascular or device-related MAE coincided with emergent hospitalizations. The other 15 events in the active group were detected by remote monitoring (n = 7) or during an interim follow-up prompted by a patient's call or a physician (n = 7) or at the 18-month follow-up (n = 1). The other 13 events in the control group were detected during an interim follow-up prompted by a patient's call or a physician (n = 7) or during a scheduled follow-up (n = 6). The seven MAE detected by remote monitoring in the active group prompted hospitalizations for atrial arrhythmias (n = 3), heart failure (n = 3), or complications of valvular heart disease (n = 1).
A retrospective analysis in the control group revealed that 10 events were detectable by remote monitoring including warning messages for (i) automatic mode switches preceding eight hospitalizations for atrial arrhythmias or heart failure, and (ii) less than safety margin of the P-wave amplitude preceding two hospitalizations for dislodgement of atrial leads.
Medical interventions delay
The retrospective comparison between the two study groups of the delay between the remote monitoring warning message and subsequent medical interventions revealed that 34 interim follow-ups or hospitalizations were detectable by remote monitoring in the active group vs. 15 in the control group. Therefore, the median delay in medical intervention with inter-quartile was 17 (4; 48) days in the active group vs. 139 (33; 201) days in the control group, representing a mean 117-day gain in the event detection (95% CI: 49–184 days; P = 0.001).
Quality of life
At the time of enrolment in the trial, the mean physical, psychological, and overall SF-36 quality of life scores in the active group were 64 ± 22, 68 ± 20, and 68 ± 21, respectively, vs. 64 ± 21, 69 ± 20, and 69 ± 20 in the control group. At last follow-up, the mean physical, psychological, and overall scores in the active group were 65 ± 23, 68 ± 21, and 68 ± 22, respectively, vs. 67 ± 20, 71 ± 17, and 71 ± 19 in the control group. These between- and within-groups differences were all statistically non-significant.
Discussion
In the COMPAS trial, remote monitoring of pacemaker recipients (i) was as safe as conventional, scheduled follow-ups, (ii) enabled the early detection of a variety of adverse events, and (iii) decreased significantly the number of ambulatory follow-up visits.
The increasing rate of cardiac device implantations is increasing the burden of specialized medical centres to a point of becoming unmanageable. Remote monitoring offers an alternate management strategy, which decreases the need for follow-up visits. The safety and clinical benefits conferred by a systematic application of this technology needs to be thoroughly validated. While the TRUST trial confirmed that remote monitoring of ICD recipients was as safe and effective as standard care,12 no clinical trial had examined its performance in permanently paced patients. By its non-inferiority design, the COMPAS trial confirmed the safety of remote monitoring in this specific population. In addition, although the study was not powered to make these comparisons, significant differences were observed between the two study groups in the rates of hospitalizations for the management of atrial arrhythmias and strokes. Several follow-ups prompted by remote monitoring, which enabled the early detection and management of atrial arrhythmias in the active group, may have prevented the development of more serious adverse events.13
The a posteriori analysis of all daily transmissions by remote monitoring, including those recorded in the control group, revealed a considerably longer delay in medical interventions in the control than in the active group, representing a temporal gain of nearly 120 days. It is noted that in the active group, this delay was left free to each physician according to the emergency of the warning message and his knowledge of the patient profile, so the delay could be more or less prompt. The retrospective analysis of AWARE, including over 11 000 patients from 23 countries, found similarly that, compared with conventional biannual scheduled visits, remote monitoring advanced the detection of adverse events by a mean of 154 days,14 a time interval similar to the 144 days observed in COMPAS.
While one might argue with the selection of devices,15 all patients enrolled in the COMPAS trial were recipients of dual chamber pacemakers. The atrial lead, by enabling the detection of atrial arrhythmias (a function unavailable in single chamber pacemakers), may have played a role in the added value of remote monitoring. Moreover, for safety reason, the study included only non-pacemaker-dependent patients, but now it would be interesting to evaluate remote monitoring for patients with spontaneous ventricular rates <30 b.p.m.
The majority of adverse events in COMPAS was clinical, and few technical events, such as lead dysfunction, were observed, probably because the follow-up was limited to 18 months. A longer period of observation would have allowed the detection of a higher number of technical adverse events and would have included the management of the end of pacemaker battery life. The difference of complications related to the pacing system observed in COMPAS is difficult to interpret due to the few number of events. Furthermore, by study design, the total number of follow-ups over 18 months was 36% lower in the active than in the control group. Before the final 18-month follow-up imposed by the protocol, the decrease reached nearly 60%. Finally, over 70% of the routine ambulatory visits in the control group were non-contributory, whereas the much less frequent and unscheduled follow-ups prompted by remote monitoring in the active group were usually medically appropriate.
The results of this trial confirmed that remote monitoring is not a substitute for an emergency system, as it prompted only a few hospitalizations. However, it safely eliminated unnecessary follow-up visits and allowed the early detection of events, prompting more appropriate follow-up visits. It is noteworthy that over 40% of the overall population transmitted no warning message. A similar 47% of patients regularly monitored remotely transmitted no warning messages in the AWARE and 40% in the OEDIPE studies.5,14 Moreover, over 50% of patients in the active group needed no interim follow-ups. The observations made in this trial might soon set a new standard of care for the follow-up of pacemaker recipients.
The important reduction in FU suggests evaluating economic implications. The results of this costs analysis will be shown in a forthcoming publication.
Conclusions
In the COMPAS trial, long-term remote monitoring of pacemaker recipients was a safe substitute for conventional follow-ups, decreased the number of ambulatory visits and enabled the early detection of important clinical and device-related adverse events.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This study was supported by an unrestricted grant from Biotronik SE and Co. KG. Funding to pay the Open Access publication charges for this article was provided by Biotronik.
Conflict of interest: P.M., MD has received consulting fees and research grants from Biotronik, Boston-Guidant, Medtronic, St Jude Medical, Sorin Group. A.D.C., MD has received consulting fees from St Jude Medical, Medtronic, Biotronik and Boston Scientific and has received research support from St Jude Medical, Medtronic, Biotronik, Boston Scientific and Sorin Group. J.C.D., MD has received speaker honoraria and consulting fees from Medtronic, Sorin group and St Jude Medical. All other authors do not have any potential conflicts of interest.
Supplementary Material
Acknowledgements
The authors thank Mr Nicolas Canot, Clinical Study Manager, Mr Xavier Laroche, Home-Monitoring Manager, and Ms Sophie Fauquembergue, Clinical Study Engineer for their assistance in the conduct of the COMPAS trial, and Rodolphe Ruffy, MD, for his assistance in the preparation of the manuscript.
References
- 1.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices); American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002. Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;27:e1–e62. doi: 10.1016/j.jacc.2008.02.032. 51. [DOI] [PubMed] [Google Scholar]
- 2.Vardas PE, Auricchio A, Blanc JJ European Society of Cardiology; European Heart Rhythm Association. Guidelines for cardiac pacing and cardiac resynchronization therapy. The Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Developed in collaboration with the European Heart Rhythm Association. Europace. 2007;9:959–998. doi: 10.1093/europace/eum189. [DOI] [PubMed] [Google Scholar]
- 3.Heidbüchel H, Lioen P, Foulon S, Huybrechts W, Ector J, Willems R, Ector H. Potential role of remote monitoring for scheduled and unscheduled evaluations of patients with an implantable defibrillator. Europace. 2008;10:351–357. doi: 10.1093/europace/eun010. [DOI] [PubMed] [Google Scholar]
- 4.Wallbrück K, Stellbrink C, Santini M, Gill J, Hartmann A, Wunderlich E. The value of permanent follow-up of implantable pacemakers—first results of an European trial. Biomed Tech (Berl) 2002;47:950–953. doi: 10.1515/bmte.2002.47.s1b.950. [DOI] [PubMed] [Google Scholar]
- 5.Halimi F, Clémenty J, Attuel P, Dessenne X, Amara W OEDIPE trial Investigators. Optimized post-operative surveillance of permanent pacemakers by home monitoring: the OEDIPE trial. Europace. 2008;10:1392–1399. doi: 10.1093/europace/eun250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricci RP, Morichelli L, Santini M. Home monitoring remote control of pacemaker and implantable cardioverter defibrillator patients in clinical practice: impact on medical management and health-care resource utilization. Europace. 2008;10:164–170. doi: 10.1093/europace/eum289. [DOI] [PubMed] [Google Scholar]
- 7.Cleland JG, Louis AA, Rigby AS, Janssens U, Balk AH TEN-HMS Investigators. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: the Trans-European Network—Home Care Management System (TEN-HMS) Study. J Am Coll Cardiol. 2005;45:1654–1664. doi: 10.1016/j.jacc.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 8.Brugada P. What evidence do we have to replace in-hospital implantable cardioverter defibrillator follow-up? Clin Res Cardiol. 2006;95:III3–III9. doi: 10.1007/s00392-006-1302-x. [DOI] [PubMed] [Google Scholar]
- 9.Ricci RP, Russo M, Santini M. Management of atrial fibrillation—what are the possibilities of early detection with home-monitoring? Clin Res Cardiol. 2006;95:III10–III16. doi: 10.1007/s00392-006-1303-9. [DOI] [PubMed] [Google Scholar]
- 10.Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH CONNECT Investigators. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) Trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. 2011;57:1181–1189. doi: 10.1016/j.jacc.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Ware JE, Jr, Sherbourne CD. The MOS 36-Item short-form health survey (SF-36). Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 12.Varma N, Epstein AE, Irimpen A, Schweikert R, Love C TRUST Investigators. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation. 2010;122:325–332. doi: 10.1161/CIRCULATIONAHA.110.937409. [DOI] [PubMed] [Google Scholar]
- 13.Ricci RP, Morichelli L, Santini M. Remote control of implanted devices through Home Monitoring technology improves detection and clinical management of atrial fibrillation. Europace. 2009;11:54–61. doi: 10.1093/europace/eun303. [DOI] [PubMed] [Google Scholar]
- 14.Lazarus A. Remote, wireless, ambulatory monitoring of implantable pacemakers, cardioverter defibrillators, and cardiac resynchronization therapy systems: analysis of a worldwide database. Pacing Clin Electrophysiol. 2007;30:S2–S12. doi: 10.1111/j.1540-8159.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- 15.Toff WD, Camm AJ, Skehan JD United Kingdom Pacing and Cardiovascular Events Trial Investigators. Single-chamber vs. dual-chamber pacing for high-grade atrioventricular block. N Engl J Med. 2005;353:145–155. doi: 10.1056/NEJMoa042283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.