The term cholestasis is derived from the Greek word “chole” which means bile and “stasis” meaning standing still. Cholestasis develops either due to a defect in bile synthesis, impairment in bile secretion or obstruction to bile flow. Cholestasis may occur as a result of an acute or chronic process involving either the extrahepatic or intrahepatic biliary tree (1). This syndrome is characterized by an elevated serum alkaline phosphatase (AP) and gamma-glutamyltransferase (GGT) out of proportion to elevation of aminotransferase enzymes (1). Clinically, pruritus and fatigue are the most common presenting symptoms of chronic cholestatic disorders. Key elements to the diagnostic workup include visualization of the biliary tree by cholangiography and evaluation of liver histology. Recent advances in understanding the genetic factors and immune mechanisms involved in the pathogenesis of cholestasis will hopefully lead to newer therapeutic interventions in the treatment of these diseases.
Representative case
A 68 yr old man presented with history of right upper quadrant pain. He described the pain as a dull aching sensation present intermittently for the past two years. He also reported a 20 pound weight loss and intermittent episodes of dark colored urine and pale stools. He denied taking any medications or excessive alcohol consumption. He had no other significant past medical history. The physical examination revealed jaundice and mild tenderness in the right upper quadrant but was otherwise normal. Laboratory tests revealed an elevated serum total bilirubin of 2.7mg/dl, AP of 437 U/L, alanine aminotransferase (ALT) of 85 U/L, aspartate aminotransferase (AST) 54 U/L. Computed tomography (CT) of the abdomen revealed mild prominence of extrahepatic bile ducts in the porta hepatis and intrahepatic biliary ductal dilation in both lobes with a patchy distribution; Endoscopic retrograde cholangiopraphy (ERCP) confirmed multifocal intrahepatic biliary ductal strictures with beading and focal dilatation and was reported to be consistent with sclerosing cholangitis. Cytology from the brushings was negative for malignancy. A liver biopsy revealed hepatic parenchyma with moderate to marked mixed portal inflammation, consisting of lymphocytes and plasma cells. Immunostain for IgG4 revealed numerous scattered IgG4-positive plasma cells in portal tracts up to 20/hpf. The serum IgG4 level was 320mg/dl. A diagnosis of IgG4-related autoimmune cholangitis was established and patient was started on corticosteroid therapy.
Physiology of Bile Formation
Bile acids are the predominant organic solutes in the bile and the driving force for the generation of bile flow. Conjugated bile acids, which represent the major fraction of bile acids in the blood, are transported across the basolateral membrane of hepatocytes by two distinct basolateral transporters: Na+-dependent transport represented by NTCP (SLC10A1, Sodium Taurocholate Co-Transporting polypeptide) and Na+-independent transport mediated by OATPs (organic anion transporting proteins OATP2, SLC21A6) (2). NTCP is exclusively expressed in hepatocytes and is involved in the transport of both conjugated and unconjugated bile acids (3). OATP on the other hand facilitates uptake of both conjugated and uncongugated bile salts, and also other organic anions and xenobiotics (4). Therefore these uptake and export transporters are essential not only for bile synthesis, but also hepatic elimination of various xenobiotics including bile salts, bilirubin, cholesterol and drugs. Bile acids are actively transported across the hepatocyte canalicular membrane into the bile by these transporters. Bile acid efflux is mediated by a superfamily of ATP- binding cassette (ABC) transporters. The predominant ABC transporter has been identified as BSEP (ABCB 11, bile salt export pump) which mediates export of bile acids (5). Some of the other ABC transporters identified include multidrug resistance associated protein MRP2 (ABCC2, efflux of multiple organic anions including bilirubin); multidrug resistance protein MDR1 (ABCB1, exports organic cation); and MDR2 (ABCB4, phospholipid export pump) (6). In addition MRP2 also plays an important role in detoxification via its role in transport of drugs including chemotherapeutic agents, antibiotics and toxins (7). MDR3 (multidrug resistance P-glycoprotein 3, ABCB4) is a special ABC transporter which flips phosphatidylcholine, a major lipid constituent, from the inner to the outer leaflet of the canalicular membrane into bile (8). The expression of these transporters is tightly controlled to prevent accumulation of toxic bile acids in hepatocytes. The primary hepatic bile excreted by the hepatocytes into the bile canaliculus undergoes modifications during its passage through the biliary tree. Cholangiocytes take up bile salts via ASBT (the apical sodium-dependent bile salt transporter ASBT (SLC10A2) allowing intrahepatic cycling of bile salts (9).
Furthermore, the cholangiocyte transporters, chloride-bicarbonate anion exchanger 2 (AE2, SLC10A2) and cystic fibrosis transmembrane conductance regulator (CFTR, ABCC7), add cholangiocellular bile to hepatocellular bile. However, the most critical step in bile acid homeostasis, and a major determinant of bile acid pool size, is the intestinal reabsorption of bile salts (10). Bile acids are re-absorbed into ileal columnar epithelium (via ASBT) and effluxed into the portal circulation (via MRP3) from where the hepatocytes then extract the bile salts completing the enterohepatic circulation (11). The bile acid transporters are subject to extensive regulation at both the transcriptional and posttranscriptional level (12). Nuclear receptors (NR) play an important role in bile acid metabolism and are activated by different compounds to promote transcription. The known NR include: farnesoid X receptor(FXR), pregnane X receptor(PXR), vitamin D receptor(VDR) and constitutive androstane(CAR) receptor. During cholestasis both FXR and PXR are activated, resulting in down regulation of CYP7A1 and thereby decreased conversion of cholesterol to bile acid and decreased bile acid synthesis (13). FXR is involved in up regulation of BSEP and down regulation of hepatic transporters NTCP. PXR activates Oatp2 whereas CAR regulates MRP2 and MPR3 (14). Taken together, activation of NR results in decreased bile acid synthesis and uptake and increased bile acid efflux, thereby preventing further accumulation of toxic bile salts in the hepatocytes.
Pattern of Liver Test Abnormalities in Cholestasis
Liver disease is often reflected by elevation in liver enzymes and/or liver function. The term “Liver function test (LFT)” is a misnomer as these tests do not reflect liver function and may be abnormal in conditions unrelated to liver diseases. “LFT’s” are often used to describe both serum liver enzymes (aminotransferases, AP) and true function tests such as prothrombin time, bilirubin and albumin. The pattern of liver enzyme abnormalities may help in characterizing the disease as hepatocellular, cholestatic, mixed or infiltrative (Table 1).
Table 1.
Categories based on liver tests
| Test | Liver Disease Category | |||
|---|---|---|---|---|
| Hepatocellular | Cholestatic | Infiltrative | Mixed | |
| ALT/AST | ++ | N/+ | N/+ | ++ |
| AP | N/+ | ++ | +/++ | ++ |
| TB | N/+ to ++ | N/+ to ++ | N/+ | N/+ to ++ |
ALT – alanine aminotransferase; AST – aspartate aminotransferase; AP – alkaline phosphatase; TB – total bilirubin, N – normal; + to ++ - degree of elevation
Persistent elevation of serum AP is frequently encountered and can pose a diagnostic dilemma. The highest concentration of AP is present in liver and bone; an elevated AP concentration is therefore generally attributed to either liver or bone disease. However, AP is also present in other organs including intestine, kidney, placenta and leucocytes. Serum AP levels vary with age, gender and blood type (15). Mild elevation of AP is seen in the first 3 months of life, puberty and a gradual increase is also noted between ages 40–65 years, especially in women. AP in adolescent boys may be 2–5 times greater than normal adults and correlates with bone growth (16). Likewise, African Americans have a 10–15% higher serum AP and smokers may have up to 10% higher AP compared to nonsmokers. Finally, individuals with Blood type O and B may have elevated AP following a fatty meal due to influx of intestinal AP. Some of the hepatic and extrahepatic causes of elevated and low AP are shown in Tables 2, 3 and 4.
Table 2.
Non-hepatic cause of elevated alkaline phosphatase
| Physiological | Pregnancy Adolescence Following a fatty meal in subjects with blood group O or B |
| Bone disease | Healing Fracture Paget’s Disease Osteomalacia Vitamin D insufficiency Rickets Malignancy: osteogenic sarcoma, metastatic |
| Renal | Renal Failure |
| Heart | Heart Failure |
| Endocrine | Hyperthyroid Hyperparathyroid |
| Malignancy | Lymphoma Leukemia Renal cell carcinoma Multiple endocrine neoplasia (MEN) II |
Table 3.
Causes of Cholestatic Liver Disease
| INTRAHEPATIC CHOLESTASIS | EXTRAHEPATIC CHOLESTASIS |
|---|---|
| Hepatitis: Viral (B,C), Alcoholic | Extrinsic Obstruction |
| Genetic: | - stones |
| - Benign recurrent intrahepatic cholestasis | Malignancy |
| - Progressive familial intrahepatic cholestasis | - Pancreas |
| - Dubin-Johnson, Rotor’s Syndrome | - Gall bladder |
| - Drugs and hebal remedies | - Metastatic |
| Pregnancy | - Cholangiocarcinoma |
| PBC | - Ampullary cancer |
| PSC | Pancreatitis |
| Granulomatous liver disease | Pancreatic pseudocyst |
| - Infections | Parasitic Infection |
| - Sarcoidosis | Secondary sclerosis (surgery, chemotherapy) |
| Infiltrative: | |
| - Amyloidosis | |
| - Lymphoma | |
| Idiopathic Adult Ductopenia | |
| Autoimmune Cholangitis (PSC-like) | |
| Autoimmune Cholangiopathy (PBC-like) | |
| Prolonged TPN | |
| Postoperative state | |
| Sepsis | |
| Malignancy | |
| - Hepatocellular | |
| - Metastatic | |
Table 4.
Low Alkaline Phosphatse
| Malnutrition |
| Wilsons Disease |
| Hypothyoidism |
| Zinc deficiency |
| Vitamin C deficiency |
| Low phosphorus level |
| Pernicious anemia |
Evaluation of Cholestasis
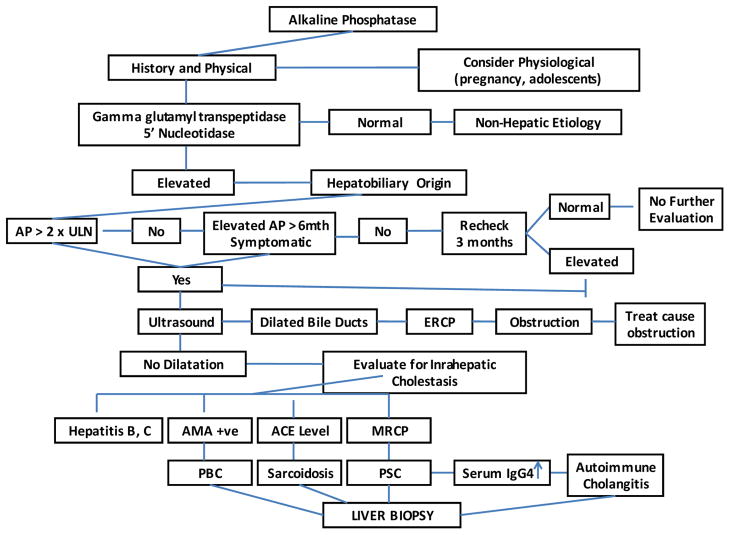
The first step in evaluating a patient with an elevated AP level is to try and identify the source of the AP. This can be done by either fractionation of isoenzymes by electrophoresis or obtaining 5’nucleotidase and gamma glutamyl transpeptidase (GGT) levels both of which are elevated in hepatobiliary disease (17). The next step is to determine whether cholestasis is secondary to intrahepatic or extrahepatic disease process. Cholestasis is considered intrahepatic when impairment in bile excretion occurs at the hepatocellular level and extrahepatic cholestasis refers to large bile duct involvement, either due to an intrinsic process or secondary to extrinsic compression (18). However, certain conditions like primary sclerosing cholangitis (PSC) may involve both intrahepatic and extrahepatic bile ducts (19).
History and Physical
A comprehensive history and physical examination is essential and may provide important clues that help with diagnosis. A history of painless jaundice with or without a palpable mass, strongly favors malignant biliary obstruction (20). Choledocholithiasis on the other hand frequently presents with abdominal pain and jaundice (21). Life threatening complications such as acute ascending cholangitis may occur in patients with common bile duct stones mandating urgent intervention. Cholangitis may occur due to partial or complete biliary obstruction and may present with the classic triad of fever with chills, right upper quadrant pain and jaundice (21). A history of previous biliary surgery and biliary manipulations increases the possibility of cholangitis in a patient with jaundice (22). History should also include details regarding alcohol consumption, recreational drug use and medications including recently discontinued medications, herbal and over the counter medications. The clinical setting in which cholestasis occurs should be considered. For example, cholestasis in a severely ill patient is likely secondary to sepsis (23). Finally, a family history of cholestatic liver disease may suggest a hereditary disorder (24).
The two most common symptoms suggestive of intrahepatic cholestasis include pruritus and fatigue. These symptoms however, are nonspecific and may be observed in extrahepatic cholestasis and other liver diseases such as viral and alcoholic hepatitis (25). Pruritus can be severe leading to impairment in quality of life. Scratching can result in skin mutilation and severe refractory itching maybe an indication for liver transplant (26). Pruritus in infants usually manifests as irritability and failure to thrive; while older children may experience poor school performance, sleep impairment and attention deficits (27). The mechanism of pruritus in liver disease is not entirely understood, and may be related to retention of bile salts or endogenous opioids (28). Fatigue is an intriguing but poorly recognized symptom in patients with chronic cholestatic liver disease. Fatigue can be very disabling, particularly in patients with primary biliary cirrhosis (PBC) (29).
Hypercholesterolemia is a common feature of intahepatic cholestasis (28). The abnormal lipotrotein observed in patients with cholestasis is lipoprotein X, which has low atherogenic potential (31). Clinically, lipid abnormalities may present with xanthomas (cholesterol deposition in tendon sheaths, bony prominences like elbow and knee, buttocks and peripheral nerves) and/or xanthelasmas (cholesterol deposits in the periorbital skin folds (32). Occasionally, xanthomas may develop in acute extrahepatic biliary obstruction, and are usually the eruptive type. Surgical removal of xanthelasmas is usually not effective and recurrence is typical. Malabsorption of fat soluble Vitamin E, D, K, A, is common. Careful attention to prevent and treat these vitamin deficiencies plays an important role in the treatment of chronic cholestatic diseases (33). Osteopenia and osteoporosis are seen in approximately 10–50% of patients with chronic cholestatic liver disease (34).
Investigation
Laboratory investigation should include complete blood count with differential, liver enzymes (aminotransferase enzymes and AP) GGT, liver synthetic function (albumin, bilirubin, prothrombin time), viral serologies, autoantibodies antinuclear antibody (ANA), smooth muscle antibody (SMA), anti mitochondrial antibody (AMA), pronuclear anti-neutrophil cytoplasmic antibody (p-ANCA) and immunoglobulin levels.
Transcutaneous abdominal ultrasound (TUS) is usually the first imaging modality to evaluate for intra and extrahepatic biliary dilatation (35). TUS is a noninvasive and relatively inexpensive test with a high degree of specificity to identify bile duct obstruction (36). However, TUS maybe technically difficult in obese patients and the pancreas is often difficult to image with the technique; furthermore, approximately 60 % of common bile duct (CBD) stones maybe missed by TUS. A CT scan is comparable to TUS in detection of stones and provides additional information regarding the liver parenchyma and may help identification of mass lesions, for example, a pancreatic tumor. CT scan is however not very helpful in delineating the biliary tree (37). A negative TUS and CT in a patient strongly suspected to have extrahepatic obstruction should be followed by further imaging. Magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound (EUS) are safe and accurate options for detection of lesions causing extrahepatic obstruction (38). The gold standard for visualizing the biliary tract is endoscopic cholangiopancreatography (ERCP). ERCP can identify the cause and level of obstruction; in addition, brushings and biopsy may be obtained and therapeutic interventions such as stone extraction, dilatation of biliary strictures and if required stent placement can be undertaken (39). Percutaneous transhepatic cholangiography may be required in patients with altered anatomy, such as a history of prior surgery such a gastrectomy or a choledochojejunostomy.
Once extrahepatic cholestasis is excluded, further workup of intrahepatic cholestasis is warranted. Certain autoantibodies are highly specific; a positive antimitochondrial antibody (AMA) is highly suggestive of PBC (40). The diagnostic criteria for PBC include an elevated AP, positive AMA and characteristic liver biopsy features, although liver biopsy is no longer considered essential for the diagnosis of PBC (41). Liver histology consistent with PBC in a patient with biochemical tests suggestive of PBC but a serologic pattern suggestive of autoimmune hepatitis (positive ANA or ASMA) is classified as autoimmune cholangiopathy (42). However, low titers of ANA and SMA may be seen in a number of conditions including PBC, PSC and other cholestatic diseases (43). In the absence of a positive AMA, MRCP is indicated to exclude PSC. The characteristic features of PSC include diffuse multifocal stricturing and dilatation involving the intrahepatic and/or extrahepatic ducts (44). In an appropriate clinical setting, an abnormal cholangiogram similar to PSC may indicate AIDS cholangiopathy or other secondary cholangitis (45). When diagnosis is unclear and/or for staging purpose, a percutaneous liver biopsy is indicated. The liver biopsy specimen should contain at least 10 portal tracts for accurate diagnosis. An algorithm for evaluating the adult patient with cholestasis is presented in Fig. 1.
Fig 1.
Propose algorithm for work up of elevated alkaline phosphatase
Genetic disorders of cholestasis
A number of genetic disorders characterized by defects in the hepatocellular transport system have been described. These include, among others, progressive familial intrahepatic cholestasis (PFIC), benign recurrent intrahepatic cholestasis (BRIC), cystic fibrosis and Dubin–Johnson syndrome (Table 5).
Table 5.
Hereditary Cholestatic Syndromes
| Protein/Gene | Chromosome | Clinical disease state | Lab findings | Characteristic Features |
|---|---|---|---|---|
| FIC1/ATP8B1 | 18q21 | PFIC1 | GGT low Serum bile acids increased | AR; severe pruritus; cholestasis; diarrhea; ductular proliferation absent |
| BRIC1 | GGT low | Periodic attacks of cholestasis | ||
| BSEP/ABCB11 | 2q24 | PFIC2 | GGT low Serum bile acids increased | AR; severe pruritus; cholestasis; ductular proliferation absent |
| BRIC2 | GGT low | Periodic attacks of cholestasis | ||
| ICP | GGT low or high | Cholestasis, pruritus in 3rd trimester of pregnancy | ||
| MDR3/ABCB4 | 7q21 | PFIC3 | GGT increased Bile acids normal | AR; moderate pruritus; cholestasis |
| ICP | ||||
| MRP2/ABCC2 | 10q24 | Dubin Johnson | Conjugated hyperbilirubinemia | Jaundice |
| CFTR/ABCC7 | 7q31 | Cystic fibrosis | Associated PSC | |
PFIC refers to a heterogeneous group of autosomal recessive cholestatic liver diseases which often present in the neonatal period or first year of life. Without a liver transplant, death from liver failure may occur in the first decade (24). Three types of PFIC have been described and the inheritance pattern of all three forms is autosomal recessive. The phenotypic findings of PFIC1 (Byler Disease) and PFIC2 (Byler syndrome) are similar. PFIC1 is due to a mutation in FIC1 (Familial intrahepatic cholestasis 1) located on chromosome 18q 21–22, whereas PFIC2 is due to mutation in the BSEP gene located on chromosome 2q 24 (46, 47). These children present with severe cholestasis, pruritus, elevated bile acids and interestingly a normal serum GGT level (48). PFIC2 patients usually have higher serum aminotransferase levels and are at an increased risk for development of hepatocellular carcinoma which may occur in the first or second decade of life (49). PFIC1 patients may demonstrate extrahepatic features such as watery diarrhea, bile acid malabsorption, pancreatitis and nephrolithiasis. PFIC 3 involves mutations in the ABCB4 gene, the gene that encodes multidrug resistance protein 3 (MDR3) (50). PFIC3 is characterized by markedly elevated serum GGT and usually present in later infancy or early childhood (48). Liver failure can occur in childhood or adulthood in people with PFIC3 (50). Obligate MDR3 heterozygous women have an increased risk for developing cholestasis during the 3rd trimester of pregnancy (51).
Benign recurrent intrahepatic cholestasis (BRIC) is a rare autosomal recessive or sporadic liver disease characterized by intermittent episodes of cholestasis. Two types of BRIC have been described. BRIC1 is due to mutations in FIC1 and BRIC2 due to BSEP mutation. This condition follows a benign course, with no progression to chronic liver disease (52, 53). During a cholestatic episode, both the serum bilirubin and AP are elevated, while GGT remains normal. Symptom free periods may last several weeks to months.
Other rare geneteic cholestatic conditions include Dubin-Johnson syndrome, a rare benign autosomal recessive, relapsing disorder caused by a MRP2 mutation (54). This condition is characterized by conjugated hyperbilirubinemia and an elevated GGT level. Cystic transmembrane regulator (CFTR) mutations results in impairment in chloride secretion and cholestasis (55). Finally, Alagille’s syndrome (due to a mutation in JAG1) is a rare condition characterized by hypoplasia of the intrahepatic bile ducts and other developmental defects (56).
Primary Biliary Cirrhosis or Chronic Nonsuppurative Destructive Cholangitis
The exact etiology of PBC is not known; however it is believed that PBC develops when a genetically susceptible individual is exposed to an environmental trigger (57). Evidence that genetic factors play a major role is based on high concordance rate of 63% in monozygotic twins versus 0% in dizygotic twins and 5–6% of patients have a first degree relative with PBC (58). A link between PBC and HLA-DR8 and HLA-DPB1 and other genetic variants of HLA class II suggest an inherited immune system abnormality resulting in inability to suppress the inflammatory injury contributing to biliary cell lysis observed in PBC (59). Xenobiotics (drugs, pesticides, cosmetics) and certain infections (Chlamydia pneumoniae, Escherichia coli, Retrovirus, Novosphingobium aromaticivorans, Lactobacilli) are thought to play a role in the pathogenesis of PBC by either possibly triggering an autoimmune reaction or by causing a direct toxic effect (60, 61). Smoking is a possible risk factor for the development of PBC and possibly accelerates progression (62).
Clinical features
PBC is more common among women, with female: male ratio of 10: 1(62). Nearly 60 % are asymptomatic at the time of diagnosis and are incidentally detected to have an elevated serum AP level (63). Fatigue and pruritus are common symptoms and may be present in 40% to 80% of patients; although these symptoms do not correlate with the severity of liver disease (29, 64). Fat-soluble vitamin deficiencies (vitamins A, D, E, and K) and osteoporosis on the other hand are usually seen in patients with advanced PBC (34, 65).
Diagnosis
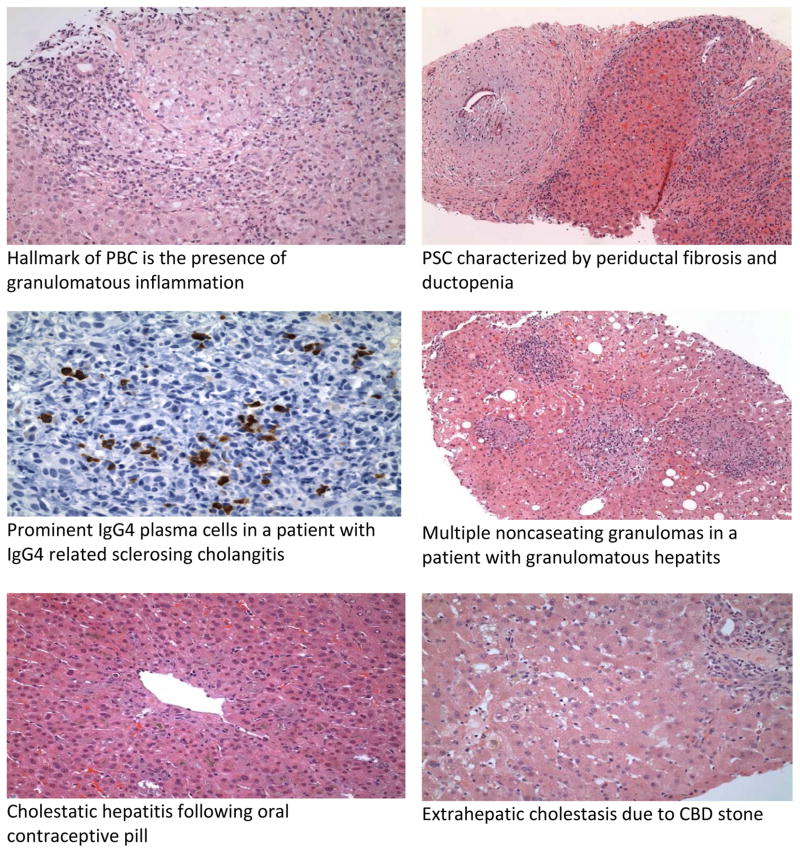
PBC is characterized by a cholestatic pattern of liver enzyme abnormalities with a predominant elevation of serum AP and GGT. An elevated serum bilirubin is an independent predictor of survival (66). However, the hallmark of PBC is presence of AMA which is positive in 90–95% of patients with a sensitivity of 98% and specificity 96 %( 40). ANA may be present in 20–50% of patients with PBC (68). The role of imaging in PBC is mainly to exclude possible biliary obstruction, cirrhosis and screen for hepatocellular carcinoma in patients with advanced PBC and cirrhosis. The characteristic histology in PBC is the “florid duct lesion” with involvement of interlobular bile ducts, inflammation and bile duct injury. The infiltrate comprises of plasma cells, macrophages and eosinophils. Noncaseating granulomas may be present (69). Occasionally the only finding on histology maybe ductopenia defined as absence of bile ducts in greater than 50% of portal tracts (Fig 2).
Fig 2.
Histological features in cholestatic liver disease
Treatment
Ursodeoxycholic acid (UDCA) is the only FDA-approved drug for PBC and is used at a dose 13–15mg/kg/day. UDCA improves liver tests including serum bilirubin and other serum biochemical markers as well as liver histology (70). When used in early stages of PBC, UDCA halts progression of disease. Individuals with a favorable biochemical response have a significantly reduced likelihood of needing a liver transplant and reduced mortality from decompensated liver disease (71). Liver transplantation is reserved for patients with decompensated PBC. Liver transplant does not change the AMA positive status even though only 20% have recurrence of PBC at 5 years post transplant (72).
Autoimmune Cholangiopathy (AIC)
AIC is the term used to describe the 5% to 8% of AMA-negative PBC patients. AIC is characterized by a cholestatic liver enzymes pattern, liver histology consistent with PBC and a negative AMA (73). Hypergammaglobinemia and positive ANA and SMA may be present. AIC is treated similarly to PBC and may represent a single disease with a variable autoantibody profile. The autoantibodies found in autoimmune cholestatic conditions are shown in Table 6.
Table 6.
Autoimmune Antibodies in Cholestatic Liver Disease
| PBC | AIC | PSC | IgG4-SC | |
|---|---|---|---|---|
| ANA | 20–50% | 79–100% | 7–77% | 43–80% |
| SMA | 20% | 50% | 15–20% | < 5% |
| AMA | 95% | 0 | 0 | 0 |
| p-ANCA | 3–33% | unknown | 80% | <5% |
| Ig | IgM | IgG | IgG, IgM | IgG |
PBC –primary biliary cirrhosis; AIC- autoimmune cholangiopathy; PSC – primary sclerosing cholangitis; IgG4-SC – IgG4 relates sclerosing cholangitis; ANA – anti nuclear antibody; SMA – smooth muscle antibody; p-ANCA perinuclear anti-neutrophilic cytoplasmic antibody; Ig - immunoglobulin
Primary Sclerosing Cholangitis (PSC)
PSC is a chronic cholestatic liver disease characterized by concentric and obliterative fibrosis of the intrahepatic and/or extrahepatic bile ducts which may progress to biliary cirrhosis (74). Although the first case of PSC was most likely described in 1929 in a patient with ulcerative colitis who developed cirrhosis, it was only in 1960 that the term “primary sclerosing cholangitis” was coined (75). PSC is frequently associated with inflammatory bowel disease (IBD), commonly ulcerative colitis (UC) (76). Approximately 62–73% of patients with PSC have UC and 3–4% of UC patients develop PSC. Interestingly, even though IBD is more frequent in women, PSC has a 2:1 male predominance (77). The median age of onset is about 40 years and it is more common among non-smokers compared to smokers (78).
PSC is a multifactorial disease. Environmental factors and genetic predisposition likely plays a role as siblings of patients with PSC have a prevalence of 1.5%; approximately a 100 fold increased risk (79) The major histocompatibility complex (MHC) encodes human leukocyte antigen (HLA) class I and class II molecules and MHC class I chain-like alpha-molecules (MICA). MICA has a role in the innate immune response as ligands for natural killer cells. Polymorphisms in MICA * 008 have been associated with PSC (80). Furthermore there is an increased prevalence of HLA-B8, HLA-DR3, and HLA-DRw52a in patients with PSC; with HLA-B8 in up to 60 to 80% of PSC patients (81). More recently, several genome wide-association studies ( GWAS) have demonstrated that, outside of the HLA complex, at least 3 non-HLA susceptibility loci (chromosome 13q31, chromosome 2q35, and chromosome 3p21), all three of which are also implicated in UC, may contribute towards risk of development of PSC (82).
The association of PSC and UC, has led to considerations of both infectious and autoimmune contributions to the pathogenesis; PSC like UC, is an autoimmune disease and bacterial or viral antigens may enter the portal circulation through an inflamed colonic wall triggering an immune reaction (83–85). The autoimmne hypothesis is further supported by the presence of autoantibodies, such as p-ANCA or ANA in PSC patients.(86). However, unlike other autoimmune conditions, PSC has a male predominance and does not respond to corticosteroids (87).
Clinical features
PSC should be suspected in any patient with IBD presenting with cholestatic liver enzyme abnormalities. Similar to PBC, the majority of patients are asymptomatic at the time of presentation (88). The most common symptoms at presentation include intermittent episodes of fatigue, pruritus, and/or abdominal discomfort. During the initial course of the disease, most symptoms resolve spontaneously (89). The serum AP and bilirubn may also fluctuate, indicating transient biliary obstruction from biliary sludge or stones. Episodes of fever, chills and jaundice may reflect bacterial cholangitis from biliary obstruction rather than advanced disease. Persistent jaundice suggests advanced PSC and should raise the suspicion of cholangiocarcinoma (CCA).
Diagnosis
ERCP has traditionally been the gold standard for the diagnosis of PSC; however MRCP is increasingly being used a noninvasive alternative to ERCP (90). ERCP should currently be reserved for situations where an intervention such as dilatation and stenting of biliary stricture is anticipated or if MRCP technique is sub-optimal for diagnosis. ERCP is also indicated if a dominant stricture is present to obtain cytologic brushings to rule out CCA (91). On cholangiography, the strictures are short and alternate with dilated segments producing characteristic ‘beaded’ appearance (92). The chatacteristic histologic finding in PSC is “onion skin” fibrosis pattern present in 7–50% (93).(Fig 2). This describes the periductal concentric fibrosis that occurs around the interlobular and septal bile ducts, resulting in ductpenia and bile duct proliferation. The classical PSC finding of obliterative fibrous cholangitis which represents concentric fibrosis with obliterative cholangitis is present in less than 10% patients with PSC(93). Conditions that result in secondary sclerosing cholangits should be ruled out prior to making a diagnosis of PSC. These include intraductal stones, ischemic cholangiopathy, bile duct injury from intra-arterial chemotherapy, AIDS cholangiopathy, recurrent pyogenic cholangitis and bile duct injury during surgery (94).
Patients with PSC are at risk for development of a number of malignancies. PSC patients have a 5–10% life time risk for developing CCA (95). Risk factors for CCA include older age at time of diagnosis of PSC, longer duration of IBD, smoking and alcohol use and patients who have undergone proctocolectomy (95). An annual MRI/MRCP or ultrasound along with CA19-9 is recommended for screening for CCA (96). PSC patients also have increased prevalence of gallbladder cancer, colorectal cancer or dysplasia and hepatocellular cancer (97). Therefore an annual colonoscopy with multiple biopsy is recommended for screening purposes (98).
Treatment
UDCA has been evaluated for the treatment of PSC (99). Results from studies using low dose UDCA were inconclusive and a high dose (28–30mg/kg/day) trial was discontinued early because of development of serious adverse events including cirrhosis, death or liver transplant (100).. UDCA at a dose up to 13–15 mg/kg per day, may show improvement in some features of cholestasis, but does not have survival benefit or delay in need for transplant (101). As a result, UDCA is not recommended for the treatment of PSC (102).
IgG4-related sclerosing cholangitis (IgG4-SC)
‘IgG4-related sclerosing disease’ is a systemic inflammatory condition characterized by IgG4-positive plasma cells and T-lymphocyte infiltration frequently involving multiple organs (103). The tissues commonly involved include the pancreas, bile duct, gall bladder, kidney, retroperitoneum, and salivary glands (104). IgG4-related sclerosing cholangitis (IgG4-SC) is a variant of this condition characterized by bile duct involvement (105). IgG4-SC is associated with autoimmune pancreatitis (AIP) in 80–90% of patients (105).
Clinical Features
IgG4-SC clinically and cholangiographically resembles PSC. It is however important to differenciate the two conditions as IgG4-SC is responsive to steroid therapy (106). Table 7 outlines some of the differences between the two conditions.
Table 7.
Differences Between PSC and IgG4-SC
| PSC | IgG4-SC | |
|---|---|---|
| Age | 25–45yrs | 65yrs |
| Male Gender | 65% | 80% |
| Association with IBD | Present | Absent |
| Other organ involvement | No | Pancreas frequently involved |
| p-ANCA | Positive | Less common |
| Elevated serum IgG4 | 7–9% | 70% |
| Histology | Ductopenia Periductal concentric fibrosis |
Abundant IgG4 + plasma cells Periportal fibroinflammatory nodules |
| Cholangiogram | Multifocal “beaded”; pruned tree appearance of intrahepatic and extrahepatic ducts | Segmental, long strictures with prestenotic dilatation, distal CBD involvement, pancreatic duct involvement |
| Response to Steroid | No | Yes |
PSC –primary sclerosing cholangitis; IgG4-SC – IgG4 related sclerosing cholangitis
Diagnosis
IgG4-SC is relatively easy to diagnose when associated with AIP. AIP manifests with typical radiologic features such as “sausage-like diffuse pancreatic swelling with peripancreatic capsule-like rim, and irregular narrowing of the pancreatic duct” along with an elevated serum IgG4 level (107). Ghazale et al have shown that in AIP, an IgG4 over 140mg/dl had a sensitivity and specificity of 76% and 93% respectively, and when the cutoff was raised to 280mg/dl, the sensitivity and specificity was 53% and 99% respectively (108). However, 9% of patients with PSC may also have elevated IgG4 levels. IgG4-SC patients may be associated with hypergammaglobulinemia, positive ANA in 40–50%, positive rheumatoid factor in about 20% and eosinophilia in 15% (108)
The cholangiographic features in IgG4-SC is classified into 4 types (109)
-
Type 1
Stenosis occurs in the lower part of the common bile duct; often misdiagnosed as pancreatic cancer
-
Type 2
Diffuse stenosis involving intrahepatic and extrahepatic bile ducts; closely resembles PSC.
-
Type 3
Stenosis involving the hilar region and the lower part of the common bile duct; often misdiagnosed as CCA
-
Type 4
Stenosis only in the hilar hepatic region; often misdiagnosed as CCA.
The gold standard for the diagnosis of IgG4-SC is histology showing the characteristic diffuse lymphoplasmacytic infiltration, infiltration by IgG4+ plasma cells and obliterative phlebitis which is distinct from the ductopenia and periductal concentric fibrosis seen in PSC(111) (Fig 2).
Treatment
This disease may be characterized by spontaneous relapsing and remitting course, but is very responsive to corticosteroid therapy and steroids can induce remission (111). There is no concensus regarding dose and duration of steroid therapy, but an initial high dose of prednisone 40mgs/day for 3–4 weeks followed by a tapering course guided by biochemical and radiological response should be considered (93). Steroid sparing immunosuppressive therapy with azathioprine and mycophenolate mofetil and rituximab maybe sometimes indicated for patients with a relapsing course requiring chronic immunosuppressive therapy (112).
Hepatic Sarcoidosis
Sarcoidosis is a multisystemic disease of unknown etiology characterized by the presence of non-caseating granulomata (113). Sarcoidosis primarily affects the lungs and lymph nodes but can involve almost every organ including the liver (114). Hepatic sarcoid usually affects individuals between 20–40years of age; African-Americans have a two to three fold increased risk of developing sarcoidosis compared to Caucasians (115). African-Americans also may have a more chronic and severe form of disease (115).
Clinical Features
Most patients with hepatic sarcoid are asymptomatic and only 5–30% present with symptoms such as nausea, vomiting, abdominal pain, fever with night sweats, myalgia and possible weight loss (116). Approximately 50–80% of patients with systemic sarcoidosis have hepatic involvement (117).
Diagnosis
The most common biochemical abnormality noted in hepatic sarcoid is an elevated AP and GGT and the extent of elevation may reflect severity of fibrosis (118). Serum Angiotensin-converting enzyme (ACE) level is almost always elevated in hepatic sarcoid and is associated with an active disease process(118). Other granulomatous conditions have not been associated with significant ACE level elevation (119). Hepatic nodules are usually diffuse, multiple and small, usually approximately 1.0 cm in diameter (120). On CT imaging, these nodules generally appear as multiple low –attenuating, non-conteast enhancing lesions and on MRI they appear as hypodense lesions on T2 weighed sequence without contrast enhancement(120,121). The main histological features in hepatic sarcoid are noncaseating granulomas which consist of multinucleated giant cells (Fig 2). Granulomas are typically in the portal and periportal areas (122). In more advanced cases, chronic cholestasis, ductopenia and cirrhosis may be evident (122).A number of other conditions may be associated with granulomas including PBC, lymphoma, tuberculosis among others (123).
Treatment
Corticosteroids is the main stay of treatment for hepatic sarcoidosis(124).There is general consensus that treatment is not required in the absence of symptoms or biochemical abnormalities. In patients with symptoms such as fever and abdominal pain, low dose prednisone 10–15mg/day is often associated with clinical response (125). Steroid therapy also appear to improve liver tests in those with mild to moderate enzyme elevation (124). However, biochemical response does not correlate with histologic response and progression to cirrhosis may occur (124). In advanced hepatic sarcoiosis, steroids are unlikely to have any significant benefit (126). Methotrexate, hydroxychloroquine, azathioprine, and cyclophosphamide have been used as steroid sparing agents and have been shown to be of some benefit (126).. UDCA has also been to be useful in patients with chronic cholestasis and may improve clinical and biochemical abnormalities (127).
Portal hypetension (PHT) is a rare complication of hepatic sarcoid and warrants mention. PHT may occur with or without cirrhosis (128). In the absence of cirrhosis, PHT may be a result of obstruction of portal flow by granulomas, arteriovenous shunts that increase portal blood flow, granulomatous phlebitis causing portal and hepatic vein occlusion resulting in ischemia and/or extrinsic hepatic vein obstruction by granulomas resulting in Budd –Chiari syndrome (128,129). Liver transplantation may be the ultimate treatment option in these patients (130).
Parenteral nutrition – associated liver disease (PNALD)
Parenteral nutrition (PN) induced hepatobiliary disturbance is more common in children, particularly premature infants compared to adults (131). This condition should be suspected in patients on PN who develop hyperbilirubinemia and liver enzyme elevation after rulling our drug induced liver injury, sepsis and extrahepatic obstruction. Liver enzyme elevation is usually observed within 1–4 weeks after initiation of PN and in most instances improves in spite of continued PN (132). Two patterns of injury maybe seen with PNALD: steatosis and steatohepatitis or cholestasis (133). Steatosis is more common in adults while cholestasis occurs in children (133). PNALD range from mild liver enzyme elevation with resolution following cessation of PN, to steatosis and steatohepatitis, cholestasis and in a few patients may progress to severe hepatobiliary damage and cirrhosis (134)
The etiology of PNALD is multifactorial. Decreased gallbladder emptying resulting in cholelithiasis, bacterial overgrowth due to the unused gut leading to gut translocation of endotoxins into the portal circulation and/or amino acids in PN solution are all thought to contribute to the development of PNALD (133–135). Immaturity of the biliary secretory system plays a major role in the development of cholestasis in premature infants (136)
PNALD can be prevented by early resumption of oral or enteral intake, prevention of hypoxia, prompt treatment of sepsis, treating hypoproteinemia and avoidance of hepatotoxic drugs. Using a cyclic PN schedule and decreasing excess amino acids in PN will also likely prevent liver injury (137).
Treatment
Treatment options for PNALD are limited. UDCA has been shown to be possibly effective in PNALD (138). Bowel decontamination with antibiotics such as metronidazole may be helpful (139). Intestinal transplantation, frequently along with liver transplantation, is reserved for patients with short bowel syndrome dependent on PN who develop overt or pending liver failure or other life threatening complications from PN (140).
Intrahepatic Cholestasis Of Pregnancy (ICP)
ICP is defined as pruritus and elevated serum bile acid levels in otherwise healthy pregnant women (141). ICP usually manifests around 25 to 32 weeks of gestation, resolves following delivery and tends to recur in approximately 45%–70% of subsequent pregnancies (142).
Clinical Features
The main manifestation of ICP is pruritus which affects all parts of the body and is frequently worse at night. Occasionally patients may present with steatorrhea (143). Jaundice occurs in less than 25% of patients (144). Hormonal factors (both estrogen and progesterone), genetic and environmental factors likely influence the development of ICP (145). MDR3 mutations account for approximately 15% of cases of ICP (146).
Diagnosis
The diagnosis of ICP is one of exclusion and all other causes of hepatic impairment should be considered first. Prompt resolution of symptoms following delivery favors a diagnosis of ICP. Serum AP will be elevated but is of limited diagnostic value in pregnancy. Aminotransferase enzyme elevation is usually mild to moderate and patients may infrequently have hyperbilirubinemia although serum bilirubin level usually less than 5 mg/dL (147). Serum bile acid (BA) level is a more sensitive and specific biochemical marker of ICP, both for diagnosis and subsequent monitoring (148) Specifically, serum cholic acid level is increased resulting in an increased cholic/chenodeoxycholic acid ratio; this is the most sensitive test for diagnosis of ICP (149)
Treatment
UDCA is the treatment of choice for ICP and has been shown to not only control symptoms, but also reduce both serum bilirubin and BA in maternal and cord blood (150). More importantly, no maternal or fetal adverse effects have been noted (150). Dexamethasone is occasionally used but is less effective compared to UDCA (151).
Cholestasis Related to Sepsis
Sepsis is the most common etiology of jaundice and cholestasis in the ICU setting (152). Cholestatic jaundice may complicate both gram positive and gram negative bacteremia, E.coli being the commonest organism linked to this condition (153). This syndrome accounts for a third of neonatal jaundice (152). Sepsis induced liver disease may be a primary hepatic dysfunction which occurs immediately following shock as a consequence of hypotension and/or hypoxia and is manifested by significant elevation in aminotransferase enzymes. Secondary hepatic dysfunction occurs due to Kupffer cell activation by bacteria and endotoxins and release of inflammatory mediators (154).
Intrahepatic cholestasis related to sepsis is characterized by disproportionate elevation in serum bilirubin compared to serum AP. Serum AP is usually 2–3 times above the upper limit of normal. Serum aminotransferases are usually less than 2 times the upper limit of normal (155). Biliary obstruction and hetabobiliary infection should be ruled out prior to making a diagnosis of sepsis related cholestasis. Unlike other cholestatic conditions, pruritus is not a major manifestation of cholestasis associated with sepsis. Histologically, bile is seen in bile canaliculi and hepatocytes. Hyperplasia of Kupffer cells and apoptotoc bodies may be present on histology (152).
Aggressive supportive measures and treatment of underlying infection are the mainstay of treatment of cholestasis of sepsis. At present there are insufficient data regarding regarding the use of UDCA in the treatment of sepsis related cholestasis (156).
Drug Induced Liver Disease (DILI)
DILI is probably the most common cause of cholestatic liver disease and accounts for 40% of adults presenting with hepatitis (157). Hepatoxocity can occur following use of prescription drugs, over the counter medications, toxins and herbal medications and accounts for about 10% of all adverse drug reaction (158). DILI may manifest as pure cholestasis due to an abnormality in canalicular bile flow or a mixed hepatocellular cholestatic pattern (159). The pathogenesis of drug-induced liver injury is poorly understood. In genetically susceptible individuals, a direct injury triggered by a drug or its metabolites may initiate both an innate and adaptive immune response (160). Certain drugs also directly bind to intracellular proteins or inhibit mitochondrial function resulting in decreased energy production and eventually, activation of apoptotic pathways resulting in programmed cell death (161). In a subset of patients, inhibition of hepatobiliary transporter systems by a drug may be a major factor in pathogenesis of cholestasis. Studies support the role of mutations in the ABCB11 and ABCB4 genes encoding BSEP and MDR3 in DILI (162). Drugs that affect canalicular membrane transport pumps such as MDR3 also can interrupt bile flow causing cholestasis.
Clinical Features
DILI may present with nonspecific symptoms such as abdominal pain, nausea and fatigue or as an acute illness with jaundice(163). Chronic drug-induced cholestasis may present with pruritus. DILI often resolves following withdrawal of the offending drug but occasionally can cause significant bile duct damage and chronic liver disease (163). Table 8 outlines some of the manifestations of DILI. DILI should be suspected in individuals with no known medical illness who present with symptoms and abnormal liver tests shortly after initiation of a medication, with prompt improvement following withdrawal of the drug.
Table 8.
Clinical Characteristics of Drug Induced Liver Injury
| Subclinical | Granulomatous hepatitis |
| ALT < 3 x ULN | Noncaseating granulomas |
| Usually benign | Usually asymptomatic |
| Resolves weeks to months after stopping drug | Hepatocellular or cholestatic pattern |
| Eg – Sulfonamide, Salicylate | Eg - Allopurinol, Carbamazepine, Cephalexin |
| Acute Liver Injury | Chronic Hepatic Injury |
| Acute hepatitis – resembles viral hepatitis | Chronic hepatitis – resembles autoimmne hepatitis |
| Cytotoxic hepatocelluar injury | Extrahepatic features common |
| High mortality if acute liver failure develops | (arthralgia, eosinophilia, rash) |
| Eg - Acetaminophen | Eg. Methydopa, Diclofenac |
| Cholestatic Injury – resembles extrahepatic | Chronic Steatosis |
| May take months for jaundice to improve | Usually macrovesicular |
| Eg - Amoxicillin-clavulanate | Eg - Valproate, Amiodarone |
| Mixed | Cirrhosis |
| Increased risk of chronic liver disease | Eg - Methotrexate, Azathioprine, OCP |
| Eg - Phenytoin | |
| Acute Steatosis | Phospholipodosis |
| Usually microvesicular | High incidence of cirrhosis |
| Eg - Amiodarone, Zidovudine, Herbal remedies | Eg - Amiodarone, Chloroquine |
| Chronic Cholestasis | Vascular Disease |
| Chronic intrahepatic –resembles PBC | Hepatic vein thrombosis : |
| Ususally resolves after stopping drug. | Thrombosis of hepatic vein or inferior vena cava |
| Eg - Amitriptyline, Ampicillin, Chlorpromazine | Eg - OCP |
| Vanishing bile duct syndrome | Sinusoidal obstruction syndrome (SOS) |
| Ductopenia | Clinically resemble Budd-chiari |
| C progress cirrhosis | Eg - Azathioprine, Vitamin A |
| Eg -Amoxicillin, Clindamycin, Carbamazepine | Peliosis hepatis: |
| Biliary sclerosis - resembles PSC | Multiple, small, blood filled cavities in hepatic parenchyma |
| Eg - Intra-arterial infusion 5-flurodexoyuridine | Eg- Anabolic steroids, arsenic |
Diagnosis
It may not always be possible to establish a temporal relationship between drug exposure and clinical presentation. When a single agent is involved, diagnosis may be relatively simple but most individuals are on multiple medications with potential for hepatotoxicity. Furthermore, other liver conditions such as autoimmune hepatitis or nonalcoholic steatohepatitis may have a similar clinical presentation. A detailed history including the duration, dose, route of administration, prior exposure to drug, use of over-the counter medications and herbal medications should be obtained. Laboratory studies to assess degree of liver enzyme elevation and synthetic liver function should be performed. A liver biopsy remains an important tool for the diagnosis although it is not always necessary(164) (Fig 2).
Treatment
Treatment of DILI is early recognition and withdrawal of the offending drug. Specific treatment is limited to acetaminophen and valproic acid overdose which are treated with specific antidotes N-acetylcysteine and L-carnitine respectively (165). In individuals with a predominant cholestatic pattern, ursodeoxycholic acid has been used (166). It is important to remember that acute liver failure in this setting has a mortality rate of over 80% without liver transplant and should prompt an early transplant referral.
Extrahepatic Cholestasis
Choledocholithiasis and Acute Ascending Cholangitis
Cholelithiasis affects approximately 10% of adults in the United States, and 10–20% of patients with symptomatic choleithiasis have concomitant choledocholithiasis (167). A CBD stone may result in biliary obstruction and bile stasis resulting in the life threatening complication of acute ascending cholangitis. Biliary manipulation during ERCP is the second most common cause of acute cholangitis (168).
Clinical Features
Choledocholithaisis may be asymptomatic and one-third of patients may have normal laboratory values (169). Symptomatic stones can present with RUQ pain, fever and jaundice. This triad of symptoms is present in approximately 55–70% of patients with acute cholangitis (170). The pentad which includes hypotension and altered mental status is seen in less than 5–7%.
Diagnosis
90% of patients with obstruction from stones will have an elevated AP and GGT (170). Bile cultures are positive in 80% to 100% of patients who have cholangitis, and blood cultures may be positive in 20% to 70% (171). TUS remains the initial screening test for CBD stones with a sensitivity and specificity of 25–60% and 95–100% respectively (172). MRCP has evolved into an accurate noninvasive modality for diagnosis of CBD stones with sensitivity and specificity comparable to ERCP (85% and 93% vs 93–98% and 97–100% respectively) (173). MRCP however may be falsely negative in patients with stones smaller than 6mm. Endoscopic ultrasound (EUS) is also comparable to ERCP for detection of stones and does not carry the risk of pancreatitis and cholngitis (173). The main advantage of ERCP over MRCP is that ERCP is both a diagnostic tool but can be used for therapeutic interventions such as stone retrieval (169).
Choledochal cyst
This is a rare condition characterized by cystic dilatation of intrahepatic ducts, extrahepatic ducts or both (174). This condition is more common among Asians compared to Caucasians with a female preponderance (F: M is 4:1) (174) Reflux of pancreatic secretion into CBD results in damage to bile ducts and recurrent cholangitis, pancreatitis, chronic inflammation and fibrosis (175).
Clinical Features
Most patients are diagnosed in childhood and the classic triad of abdominal pain, jaundice, and a palpable right upper quadrant abdominal mass is seen in only 10–20% of adults (176).
Diagnosis
No specific laboratory tests help in the diagnosis. TUS is the initial test of choice, although MRI and MRCP is preferred as they delineate the biliary tree, define the extent of involvement of bile ducts and also provide information on surrounding parenchyma (177). On MRCP, choledochal cysts appear as a markedly dilated CBD with saccular formation. On histology, the cyst wall appears thin and fibrous and adults may have evidence of biliary cirrhosis (178). The most dreaded complication of choledochal cyst is CCA which occurs in approximately 20% of adults and therefore surgical resection is recommended for the treatment of choledochal cysts, except Type 3 (choledochocele) which carries a low risk of CCA (179,180).
Benign And Malignant Biliary Stricture
Benign biliary stricture may occur in patients with previous history of surgery resulting in bile duct injury, history of PSC, recurrent choledocholithiasis, chronic pancreatitis, and history of blunt trauma to the abdomen and following liver transplant. In approximately 30% of patients the injury to bile ducts during surgery is unrecognized and patients may present many years later with biliary strictures (181). Anastomotic strictures following liver transplant usually occur about 2–6 months following surgery (182). Chronic pancreatitis accounts for 9–10% of benign strictures which occur in the intrapancreatic section of the CBD (183).
Pancreatic cancer is the commonest cause of malignant biliary stricture (184). Other conditions that cause malignant stricture include CCA, Gall Bladder tumor and ampullary tumor. CCA or biliary tree cancers can involve the intrahepatic or extrahepatic ducts. Klatskin tumors are CCA that occurs at the bifurcation of the right and left main bile ducts or proximal hepatic ducts (185). Malignant strictures can be due to either a primary bile duct cancer causing narrowing within the bile ducts or extrinsic compression by a tumor in an adjacent organ such as the head of the pancreas.
Clinical Features
Benign strictures maybe asymptomatic, although cholangitis is a common presentation. Patients with pancreatic cancer usually present with painless jaundice. Jaundice is also the commonest symptoms in patients with CCA. Decreased appetite, weight loss and epigastric pain radiating to the back should raise a suspicion of a malignant process.
Diagnosis
Extrahepatic cholestasis presents with elevated AP and GGT with normal or near normal aminotransferase enzymes. Hyperbilrubinemia may be significant in patients with pancreatic cancer with serum bilirubin often higher than 20mg/dl (186). A serum CA19-9 above 100U/ml has a sensitivity and specificity of 75% and 80% respectively for CCA in patients with PSC (186). As discussed earlier, workup for extrahepatic obstruction includes imaging such as TUS or CT, followed by MRCP or ERCP depending on the clinical situation and need for possibly therapeutic interventions. When malignancy is suspected, brushings during ERCP of biliary tree for cytology should be obtained. Advanced techniques such as digital image analysis (DIA) and fluorescence in situ hybridization (FISH) have greatly increased sensitivity and specificity for detection of CCA (187).
Management of Cholestasis
Pruritus
This is a challenging clinical problem which often complicates chronic cholestatic liver disease. Cholestyramine is the first line agent in patients with moderate pruritus and the recommended dose is 4–16gm per day in divided doses (188). Cholestyramine is a resin that binds to bile salts in the small intestine thereby interrupting enterohepatic circulation and decreasing its reabsorption by approximately 90% (188). Cholestyramine however is unpalatable and interferes with absorption of other drugs such as digoxin, warfarin and thiazides (189). Rifampin has also been shown to be useful in the treatment of pruritus. Rifampin decreases hepatic uptake and facilitates renal elimination of bile acids (190). The recommended dose is 300–600mg/day. Liver tests should be monitored closely while on rifampin as severe hepatitis may sometimes occur (191). There have been a few small studies that report benefit with Phenobarbital; however it is less effective compared to rifampin and cholestyramine and is therefore not recommended for treatment of pruritus (192). The antidepressants sertraline and paroxetine have been shown to be useful in a few trials (193,194). Increased opioidergic neurotransmission in the brain may have a role in the pathogenesis of pruritus. Opiod receptor antagonist naloxone, nalmefene and naltrexone have been shown to cause substantial relief of symptoms (191). The main drawback for their use however is the potential for opiod withdrawal (195). The role of UDCA has been discussed above under specific cholestatic diseases. Plasmapheresis should be reserved for patients with refractory disabling pruritus when all other treatment options are ineffective (196). Finally, liver transplantation is an indication for severe pruritus secondary to cholestatic liver disease (197).
Fatigue
No therapy has been found to be effective in the treatment of fatigue. UDCA has not been shown to be useful and fatigue may persist even after liver transplant, although its intensity may be reduced (198,199). CNS stimulants such as modafinil and low dose amitryptyline may be beneficial in some patients (200).
Osteoporosis
Calcium and Vitamin D should be recommended to all patients with chronic cholestatic liver disease in order to prevent metabolic bone disease. Once osteoporosis is established, bisphosphonates are recommended. In a study comparing etidronate and alendronate, the latter was found to be more effective in increasing bone mass (201).
Fat soluble vitamin deficiencies
All patients with chronic cholestatic liver disease should have annual vitamin A, D, E, K levels monitored and appropriate treatment should be initiated if found to be deficient.
Hyperlipidemia
The utility of cholestyramine for the treatment of hyperlipidemia in this group of patients has not been established. UDCA has been shown to significantly reduce total cholesterol, VLDL and LDL cholesterol but has no effect on triglyceride or HDL cholesterol, and the maximum benefit was seen in patients with higher baseline cholesterol and higher serum bilirubin values (202). Clofibrate, due to an unknown mechanism, results in paradoxical increase in cholesterol in patients with PBC and therefore should not be used for the treatment of hyperlipidemia in these patients (203). Statins have been shown to be effective in reducing cholesterol with frequent monitoring of liver enzymes and should possibly be avoided in patients with severe cholestatic disease (204).Plasmapheresis is reserved for patients with very high serum cholesterol ; approximately 6gms of cholesterol can be removed by a single plasma exchange resulting in decrease in serum cholesterol levels, resorption of xanthomata, decrease in of the pain caused by xanthomatous neuropathy and also improvement in pruritusassociated with cholestasis (205).
Outcome in the Representative Case
The patient was started on prednisone 40mgs once a day, which was slowly tapered based on his clinical response. He achieved complete biochemical response within 3–4 months of therapy. The patient is currently on a maintenance dose of 10mg/day per day and his APU/L is 90, ALT 31mg/dl and AST 35mg/dl.
Conclusion
Although the causes of cholestasis are numerous, a systematic step by step work up starting with a comprehensive history and physical and complemented by laboratory testing and appropriate imaging, results in an accurate diagnosis in almost all patients.
Acknowledgments
We acknowledge the help of Dr, Anjali D’Souza towards this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Asma Siddique, Fellow in Hepatology.
Kris V. Kowdley, Center for Liver Disease, Virginia Mason Medical Center, Digestive Disease Institute, Seattle, WA.
References
- 1.Scharschmidt BF, Goldberg HI, Schmid R. Current concepts in diagnosis. Approach to the patient with cholestatic jaundice. N Engl J Med. 1983 Jun 23;308(25):1515–9. doi: 10.1056/NEJM198306233082507. [DOI] [PubMed] [Google Scholar]
- 2.Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003 Apr;83(2):633–71. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- 3.Hagenbuch B, Dawson P. The sodium bile salt cotransport family SLC10. Pflugers Arch. 2004 Feb;447( 5):566–70. doi: 10.1007/s00424-003-1130-z. [DOI] [PubMed] [Google Scholar]
- 4.Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011 Mar;63(1):157–81. doi: 10.1124/pr.110.002857. [DOI] [PubMed] [Google Scholar]
- 5.Arrese M, Trauner M. Molecular aspects of bile formation and cholestasis. Trends Mol Med. 2003 Dec;9(12):558–64. doi: 10.1016/j.molmed.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Kipp H, Arias IM. Intracellular trafficking and regulation of canalicular ATP-binding cassette transporters. Semin Liver Dis. 2000;20(3):339–51. doi: 10.1055/s-2000-9388. [DOI] [PubMed] [Google Scholar]
- 7.Gerk PM, Vore M. Regulation of expression of the multidrug resistance-associated protein 2 (MRP2) and its role in drug disposition. J Pharmacol Exp Ther. 2002 Aug;302(2):407–15. doi: 10.1124/jpet.102.035014. [DOI] [PubMed] [Google Scholar]
- 8.Kullak-Ublick GA, Beuers U, Paumgartner G. Hepatobiliary transport. J Hepatol. 2000;32(1 Suppl):3–18. doi: 10.1016/s0168-8278(00)80411-0. [DOI] [PubMed] [Google Scholar]
- 9.Lazaridis KN, Pham L, Tietz P, et al. Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodium-dependent bile acid transporter. J Clin Invest. 1997 Dec 1;100(11):2714–21. doi: 10.1172/JCI119816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu G, Shneider BL, Shefer S, et al. Ileal bile acid transport regulates bile acid pool, synthesis, and plasma cholesterol levels differently in cholesterol-fed rats and rabbits. J Lipid Res. 2000 Feb;41(2):298–304. [PubMed] [Google Scholar]
- 11.Rost D, Mahner S, Sugiyama Y, et al. Expression and localization of the multidrug resistance-associated protein 3 in rat small and large intestine. Am J Physiol Gastrointest Liver Physiol. 2002 Apr;282(4):G720–6. doi: 10.1152/ajpgi.00318.2001. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Chen J, Hollister K, et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999 May;(3):543–53. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 13.Ananthanarayanan M, Balasubramanian N, Makishima M, et al. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem. 2001 Aug 3;276(31):28857–65. doi: 10.1074/jbc.M011610200. [DOI] [PubMed] [Google Scholar]
- 14.Cheng X, Maher J, Dieter MZ, et al. Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab Dispos. 2005 Sep;33(9):1276–82. doi: 10.1124/dmd.105.003988. [DOI] [PubMed] [Google Scholar]
- 15.Gordon T. Factors associated with serum alkaline phosphatase level. Arch Pathol Lab Med. 1993 Feb;117(2):187–90. [PubMed] [Google Scholar]
- 16.Schiele F, Henny J, Hitz J, et al. Total bone and liver alkaline phosphatases in plasma: biological variations and reference limits. Clin Chem. 1983 Apr;29(4):634–41. [PubMed] [Google Scholar]
- 17.Connell MD, Dinwoodie AJ. Diagnostic use of serum alkaline phosphatase isoenzymes and 5-nucleotidase. Clin Chim Acta. 1970 Nov;30(2):235–41. doi: 10.1016/0009-8981(70)90108-7. [DOI] [PubMed] [Google Scholar]
- 18.McIntyre N. Cholestasis. In: Bircher J, et al., editors. Oxford textbook of Clinical Hepatology. 2. Oxford: Oxford Medical. Publications; 1999. pp. 1574–9. [Google Scholar]
- 19.Maggs JR, Chapman RW. An update on primary sclerosing cholangitis. Curr Opin Gastroenterol. 2008 May;24(3):377–83. doi: 10.1097/MOG.0b013e3282f9e239. [DOI] [PubMed] [Google Scholar]
- 20.Robinson JR, Moyer CA. Methods for differential diagnosis of painless jaundice. Surg Gynecol Obstet. 1947 Oct;85(4):535–40. [PubMed] [Google Scholar]
- 21.Center SA. Diseases of the gallbladder and biliary tree. Vet Clin North Am Small Anim Pract. 2009 May;39( 3):543–98. doi: 10.1016/j.cvsm.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Lillemoe KD. Surgical treatment of biliary tract infections. Am Surg. 2000 Feb;66(2):138–44. [PubMed] [Google Scholar]
- 23.Bansal V, Schuchert VD. Jaundice in the intensive care unit. Surg Clin North Am. 2006;86:1495–502. doi: 10.1016/j.suc.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Cavestro GM, Frulloni L, Cerati E, et al. Progressive familial intrahepatic cholestasis. Acta Biomed. 2002;73(3–4):53–6. [PubMed] [Google Scholar]
- 25.Fisher DA, Wright TL. Pruritus as a symptom of hepatitis C. J Am Acad Dermatol. 1994 Apr;30(4):629–32. doi: 10.1016/s0190-9622(94)70072-9. [DOI] [PubMed] [Google Scholar]
- 26.Jones EA, Bergasa NV. The pathogenesis and treatment of pruritus and fatigue in patients with PBC. Eur J Gastroenterol Hepatol. 1999 Jun;11(6):623–31. doi: 10.1097/00042737-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Bergasa NV. Pruritus in chronic liver disease: mechanisms and treatment. Curr Gastroenterol Rep. 2004 Feb;6(1):10–6. doi: 10.1007/s11894-004-0020-7. [DOI] [PubMed] [Google Scholar]
- 28.Jones EA, Bergasa NV. The pruritus of cholestasis: from bile acids to opiate agonists. Hepatology. 1990 May;11(5):884–7. doi: 10.1002/hep.1840110526. [DOI] [PubMed] [Google Scholar]
- 29.Milkiewicz P, Heathcote EJ. Fatigue in chronic cholestasis. Gut. 2004 Apr;53(4):475–7. doi: 10.1136/gut.2003.025155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talwalkar JA, Lindor KD. Primary biliary cirrhosis. Lancet. 2003 Jul 5;362(9377):53–61. doi: 10.1016/S0140-6736(03)13808-1. [DOI] [PubMed] [Google Scholar]
- 31.Walli AK, Seidel D. Role of lipoprotein-X in the pathogenesis of cholestatic hypercholesterolemia. Uptake of lipoprotein-X and its effect on 3-hydroxy-3-methylglutaryl coenzyme A reductase and chylomicron remnant removal in human fibroblasts, lymphocytes, and in the rat. J Clin Invest. 1984 Sep;74(3):867–79. doi: 10.1172/JCI111504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crippin JS, Lindor KD, Jorgensen R, et al. Hypercholesterolemia and atherosclerosis in primary biliary cirrhosis: what is the risk? Hepatology. 1992 May;15(5):858–62. doi: 10.1002/hep.1840150518. [DOI] [PubMed] [Google Scholar]
- 33.Phillips JR, Angulo P, Petterson T, et al. Fat-soluble vitamin levels in patients with primary biliary cirrhosis. Am J Gastroenterol. 2001 Sep;96(9):2745–50. doi: 10.1111/j.1572-0241.2001.04134.x. [DOI] [PubMed] [Google Scholar]
- 34.Isaia G, Di Stefano M, Roggia C, et al. Bone disorders in cholestatic liver diseases. In: Ardissone P, Rosina F, editors. Forum (Genova) 1. Vol. 8. 1998. Jan-Mar. pp. 28–38. [PubMed] [Google Scholar]
- 35.Lui P, Ng HS, Teh LB, et al. Ultrasonography in the diagnosis of cholestatic jaundice. Ann Acad Med Singapore. 1986 Apr;15(2):182–5. [PubMed] [Google Scholar]
- 36.Shea JA, Berlin JA, Escarce JJ, et al. Revised estimates of diagnostic test sensitivity and specificity in suspected biliary tract disease. Arch Intern Med. 1994 Nov 28;154(22):2573–81. [PubMed] [Google Scholar]
- 37.Balci NC, Befeler AS, Leiva P, et al. Imaging of liver disease: comparison between quadruple-phase multidetector computed tomography and magnetic resonance imaging. J Gastroenterol Hepatol. 2008 Oct;23(10):1520–7. doi: 10.1111/j.1440-1746.2008.05434.x. [DOI] [PubMed] [Google Scholar]
- 38.Varghese JC, Liddell RP, Farrell MA, et al. Diagnostic accuracy of magnetic resonance cholangiopancreatography and ultrasound compared with direct cholangiography in the detection of choledocholithiasis. Clin Radiol. 2000 Jan;55( 1):25–35. doi: 10.1053/crad.1999.0319. [DOI] [PubMed] [Google Scholar]
- 39.NIH state-of-the-science statement on endoscopic retrograde cholangiopancreatography (ERCP) for diagnosis and therapy. NIH Consens State Sci Statements. 2002;19:1–26. [PubMed] [Google Scholar]
- 40.Berg PA, Klein R. Antimitochondrial antibodies in primary biliary cirrhosis and other disorders: definition and clinical relevance. Dig Dis. 1992;10:85–101. doi: 10.1159/000171347. [DOI] [PubMed] [Google Scholar]
- 41.Poupon R, Chazouillères O, Poupon RE. Chronic cholestatic diseases. J Hepatol. 2000;32(1 Suppl):129–40. doi: 10.1016/s0168-8278(00)80421-3. [DOI] [PubMed] [Google Scholar]
- 42.Heathcote J. Autoimmune cholangitis. Gut. 1997 Apr;40(4):440– 2. doi: 10.1136/gut.40.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strassburg CP, Manns MP. Antinuclear antibody (ANA) patterns in hepatic and extrahepatic autoimmune disease. J Hepatol. 1999 Oct;31(4):751. doi: 10.1016/s0168-8278(99)80358-4. [DOI] [PubMed] [Google Scholar]
- 44.Charatcharoenwitthaya P, Lindor KD. Primary sclerosing cholangitis: diagnosis and management. Curr Gastroenterol Rep. 2006 Feb;8(1):75–82. doi: 10.1007/s11894-006-0067-8. [DOI] [PubMed] [Google Scholar]
- 45.Kariv R, Konikoff FM. Sclerosing cholangitis--primary, secondary and more. Isr Med Assoc J. 2002 Dec;4(12):1141–2. [PubMed] [Google Scholar]
- 46.Bull LN, Carlton VE, Stricker NL, et al. Genetic and morphological findings in progressive familial intrahepatic cholestasis (Byler disease [PFIC-1] and Byler syndrome): evidence for heterogeneity. Hepatology. 1997 Jul;26(1):155–64. doi: 10.1002/hep.510260121. [DOI] [PubMed] [Google Scholar]
- 47.Strautnieks SS, Kagalwalla AF, Tanner MS, et al. Identification of a locus for progressive familial intrahepatic cholestasis PFIC2 on chromosome 2q24. Am J Hum Genet. 1997 Sep;61(3):630–3. doi: 10.1086/515501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jansen PL, Müller MM. Progressive familial intrahepatic cholestasis types 1, 2, and 3. Gut. 1998 Jun;42(6):766–7. doi: 10.1136/gut.42.6.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knisely AS, Strautnieks SS, Meier Y, et al. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology. 2006 Aug;44(2):478–86. doi: 10.1002/hep.21287. [DOI] [PubMed] [Google Scholar]
- 50.Jacquemin E, De Vree JM, Cresteil D, et al. The wide spectrum of multidrug resistance 3 deficiency: from neonatal cholestasis to cirrhosis of adulthood. Gastroenterology. 2001 May;120(6):1448–58. doi: 10.1053/gast.2001.23984. [DOI] [PubMed] [Google Scholar]
- 51.Dixon PH, Weerasekera N, Linton KJ, et al. Heterozygous MDR3 missense mutation associated with intrahepatic cholestasis of pregnancy: evidence for a defect in protein trafficking. Hum Mol Genet. 2000 May 1;9(8):1209–17. doi: 10.1093/hmg/9.8.1209. [DOI] [PubMed] [Google Scholar]
- 52.Bull LN, Juijn JA, Liao M, et al. Fine-resolution mapping by haplotype evaluation: the examples of PFIC1 and BRIC. Hum Genet. 1999 Mar;104(3):241–8. doi: 10.1007/pl00008714. [DOI] [PubMed] [Google Scholar]
- 53.van Mil SW, van der Woerd WL, van der Brugge G, et al. Benign recurrent intrahepatic cholestasis type 2 is caused by mutations in ABCB11. Gastroenterology. 2004 Aug;127(2):379–84. doi: 10.1053/j.gastro.2004.04.065. [DOI] [PubMed] [Google Scholar]
- 54.Keitel V, Nies AT, Brom M, et al. A common Dubin-Johnson syndrome mutation impairs protein maturation and transport activity of MRP2 (ABCC2) Am J Physiol Gastrointest Liver Physiol. 2003 Jan;284(1):G165–74. doi: 10.1152/ajpgi.00362.2002. [DOI] [PubMed] [Google Scholar]
- 55.Colombo C. Liver disease in cystic fibrosis. Curr Opin Pulm Med. 2007 Nov;13(6):529–36. doi: 10.1097/MCP.0b013e3282f10a16. [DOI] [PubMed] [Google Scholar]
- 56.Colliton RP, Bason L, Lu FM, et al. Mutation analysis of Jagged1 (JAG1) in Alagille syndrome patients. Hum Mutat. 2001 Feb;17(2):151–2. doi: 10.1002/1098-1004(200102)17:2<151::AID-HUMU8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 57.Jones DE. Pathogenesis of primary biliary cirrhosis. Gut. 2007 Nov;56(11):1615–24. doi: 10.1136/gut.2007.122150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones DE, Watt FE, Metcalf JV, et al. Familial primary biliary cirrhosis reassessed: a geographically-based population study. J Hepatol. 1999 Mar;30(3):402–7. doi: 10.1016/s0168-8278(99)80097-x. [DOI] [PubMed] [Google Scholar]
- 59.Bloom S, Fleming K, Chapman R. Adhesion molecule expression in primary sclerosing cholangitis and primary biliary cirrhosis. Gut. 1995 Apr;36(4):604–9. doi: 10.1136/gut.36.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Griem P, Wulferink M, Sachs B, et al. Allergic and autoimmune reactions to xenobiotics: how do they arise? Immunol Today. 1998 Mar;19(3):133–41. doi: 10.1016/s0167-5699(97)01219-x. [DOI] [PubMed] [Google Scholar]
- 61.Varyani FK, West J, Card TR. An increased risk of urinary tract infection precedes development of primary biliary cirrhosis. BMC Gastroenterol. 2011 Aug 26;11:95. doi: 10.1186/1471-230X-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gershwin ME, Selmi C, Worman HJ, et al. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology. 2005 Nov;42(5):1194–202. doi: 10.1002/hep.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prince MI, Chetwynd A, Craig WL, et al. Asymptomatic primary biliary cirrhosis: clinical features, prognosis, and symptom progression in a large population based cohort. Gut. 2004 Jun;53(6):865–70. doi: 10.1136/gut.2003.023937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Newton JL. Fatigue in primary biliary cirrhosis. Clin Liver Dis. 2008 May;12(2):367–83. doi: 10.1016/j.cld.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 65.Levy C, Lindor KD. Management of osteoporosis, fat-soluble vitamin deficiencies, and hyperlipidemia in primary biliary cirrhosis. Clin Liver Dis. 2003 Nov;7(4):901–10. doi: 10.1016/s1089-3261(03)00097-7. [DOI] [PubMed] [Google Scholar]
- 66.Shapiro JM, Smith H, Schaffner F. Serum bilirubin: a prognostic factor in primary biliary cirrhosis. Gut. 1979 Feb;20(2):137–40. doi: 10.1136/gut.20.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leung PS, Coppel RL, Ansari A, et al. Antimitochondrial antibodies in primary biliary cirrhosis. Semin Liver Dis. 1997;17:61–69. doi: 10.1055/s-2007-1007183. [DOI] [PubMed] [Google Scholar]
- 68.Drebber U, Mueller JJ, Klein E, et al. Liver biopsy in primary biliary cirrhosis: clinicopathological data and stage. Pathol Int. 2009 Aug;59(8):546–54. doi: 10.1111/j.1440-1827.2009.02405.x. [DOI] [PubMed] [Google Scholar]
- 69.Ludwig J, Dickson ER, McDonald GS. Virchows Arch A Pathol Anat Histol. 1978 Aug 22;379(2):103–12. doi: 10.1007/BF00432479. [DOI] [PubMed] [Google Scholar]
- 70.Leuschner U, Fischer H, Kurtz W, et al. Ursodeoxycholic acid in primary biliary cirrhosis: results of a controlled double-blind trial. Gastroenterology. 1989 Nov;97(5):1268–74. doi: 10.1016/0016-5085(89)91698-3. [DOI] [PubMed] [Google Scholar]
- 71.Poupon RE, Lindor KD, Parés A, et al. Combined analysis of the effect of treatment with ursodeoxycholic acid on histologic progression in primary biliary cirrhosis. J Hepatol. 2003 Jul;39(1):12–6. doi: 10.1016/s0168-8278(03)00192-2. [DOI] [PubMed] [Google Scholar]
- 72.MacQuillan GC, Neuberger J. Liver transplantation for primary biliary cirrhosis. Clinics in liver disease. 2003 Nov;7(4):941–56. doi: 10.1016/s1089-3261(03)00099-0. [DOI] [PubMed] [Google Scholar]
- 73.Lacerda MA, Ludwig J, Dickson ER, et al. Antimitochondrial antibody-negative primary biliary cirrhosis. Am J Gastroenterol. 1995 Feb;90(2):247–9. [PubMed] [Google Scholar]
- 74.Broome U, Olsson R, Loof L, et al. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996 Apr;38(4):610–615. doi: 10.1136/gut.38.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bargen J. Complications and sequelae of chronic ulcerativecolitis. Ann Intern Med. 1929;3:335–52. [Google Scholar]
- 76.Schrumpf E, Boberg KM. Epidemiology of primary sclerosingcholangitis. Best Pract Res Clin Gastroenterol. 2001 Aug;15(4):553–62. doi: 10.1053/bega.2001.0204. [DOI] [PubMed] [Google Scholar]
- 77.Mendes FD, Lindor KD. Primary sclerosing cholangitis. Clin Liver Dis. 2004 Feb;8(1):195–211. doi: 10.1016/S1089-3261(03)00127-2. [DOI] [PubMed] [Google Scholar]
- 78.Loftus EV, Jr, Sandborn WJ, Tremaine WJ, et al. Primary sclerosing cholangitisis associated with nonsmoking: a case-control study. Gastroenterology. 1996 May;110(5):1496–502. doi: 10.1053/gast.1996.v110.pm8613055. [DOI] [PubMed] [Google Scholar]
- 79.Bergquist A, Montgomery SM, Bahmanyar S, et al. Increased risk of primary sclerosing cholangitis and ulcerative colitis in first-degree relatives of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2008 Aug;6(8):939–43. doi: 10.1016/j.cgh.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 80.Norris S, Kondeatis E, Collins R, et al. Mapping MHC-encoded susceptibility and resistance in primary sclerosing cholangitis: the role of MICA polymorphism. Gastroenterology. 2001 May;120(6):1475–82. doi: 10.1053/gast.2001.24041. [DOI] [PubMed] [Google Scholar]
- 81.van Milligen de Wit AW, van Deventer SJ, Tytgat GN. Immunogenetic aspects of primary sclerosing cholangitis: implications for therapeutic strategies. Am J Gastroenterol. 1995 Jun;90(6):893–900. [PubMed] [Google Scholar]
- 82.Karlsen TH, Franke A, Melum E, et al. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology. 2010 Mar;138(3):1102–11. doi: 10.1053/j.gastro.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 83.Krasinskas AM, Yao Y, Randhawa P, et al. Helicobacter pylori may play a contributory role in the pathogenesis of primary sclerosing cholangitis. Dig Dis Sci. 2007 Sep;52(9):2265–70. doi: 10.1007/s10620-007-9803-7. [DOI] [PubMed] [Google Scholar]
- 84.Mehal WZ, Hattersley AT, Chapman RW, et al. A survey of cytomegalovirus (CMV) DNA in primary sclerosing cholangitis (PSC) liver tissues using a sensitive polymerase chain reaction (PCR) based assay. J Hepatol. 1992 Jul;15(3):396–9. doi: 10.1016/0168-8278(92)90076-2. [DOI] [PubMed] [Google Scholar]
- 85.Kulaksiz H, Rudolph G, Kloeters-Plachky P, et al. Biliary Candida infections in primary sclerosing cholangitis. J Hepatol. 2006 Nov;45(5):711–6. doi: 10.1016/j.jhep.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 86.Terjung B, Worman HJ. Anti-neutrophil antibodies in primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2001 Aug;15:629–42. doi: 10.1053/bega.2001.0209. [DOI] [PubMed] [Google Scholar]
- 87.Worthington J, Cullen S, Chapman R. Immunopathogenesis of primary sclerosing cholangitis. Clin Rev Allergy Immunol. 2005 Apr;28:93–103. doi: 10.1385/CRIAI:28:2:093. [DOI] [PubMed] [Google Scholar]
- 88.Okolicsanyi L, Fabris L, Viaggi S, et al. Primary sclerosing cholangitis: Clinical presentation, natural history and prognostic variables: An Italian multicentre study. The Italian PSC Study Group. Eur J Gastroenterol Hepatol. 1996 Jul;8(7):685–91. [PubMed] [Google Scholar]
- 89.Olsson R, Broome U, Danielsson A, et al. Spontaneous course of symptoms in primary sclerosing cholangitis: Relationships with biochemical and histological features. Hepatogastroenterology. 1999 Jan-Feb;46(25):136–41. [PubMed] [Google Scholar]
- 90.Ciocirlan M, Ponchon T. Diagnostic endoscopic retrograde cholangiopancreatography. Endoscopy. 2004 Feb;36(2):137–46. doi: 10.1055/s-2004-814181. [DOI] [PubMed] [Google Scholar]
- 91.Furmanczyk PS, Grieco VS, Agoff SN. Biliary brush cytology and the detection of cholangiocarcinoma in primary sclerosing cholangitis: evaluation of specific cytomorphologic features and CA19-9 levels. Am J Clin Pathol. 2005 Sep;124(3):355–60. doi: 10.1309/J030-JYPW-KQTH-CLNJ. [DOI] [PubMed] [Google Scholar]
- 92.Angulo P, Pearce DH, Johnson CD, et al. Magnetic resonance cholangiography in patients with biliary disease: its role in primary sclerosing cholangitis. J Hepatol. 2000 Oct;33(4):520–7. doi: 10.1034/j.1600-0641.2000.033004520.x. [DOI] [PubMed] [Google Scholar]
- 93.Burak KW, Angulo P, Lindor KD. Is there a role for liver biopsy in primary sclerosing cholangitis? Am J Gastroenterol. 2003 May;98(5):1155–8. doi: 10.1111/j.1572-0241.2003.07401.x. [DOI] [PubMed] [Google Scholar]
- 94.Abdalian R, Heathcote EJ. Sclerosing cholangitis: a focus on secondary causes. Hepatology. 2006 Nov;44(5):1063–74. doi: 10.1002/hep.21405. [DOI] [PubMed] [Google Scholar]
- 95.Burak K, Angulo P, Pasha TM, et al. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004 Mar;99(3):523–6. doi: 10.1111/j.1572-0241.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- 96.Charatcharoenwitthaya P, Enders FB, Halling KC, et al. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008 Oct;48(4):1106–17. doi: 10.1002/hep.22441. [DOI] [PubMed] [Google Scholar]
- 97.Bergquist A, Ekbom A, Olsson R, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002 Mar;36(3):321–7. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 98.Thackeray EW, Charatcharoenwitthaya P, Elfaki D, et al. Colon neoplasms develop early in the course of inflammatory bowel disease and primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2011 Jan;9(1):52–6. doi: 10.1016/j.cgh.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 99.Mitchell SA, Bansi DS, Hunt N, et al. A preliminary trial of high-dose ursodeoxycholic acid in primary sclerosing cholangitis. Gastroenterology. 2001 Oct;121(4):900–7. doi: 10.1053/gast.2001.27965. [DOI] [PubMed] [Google Scholar]
- 100.Imam MH, Sinakos E, Gossard AA, et al. High-dose ursodeoxycholic acid increases risk of adverse outcomes in patients with early stage primary sclerosing cholangitis. Aliment Pharmacol Ther. 2011 Nov;34(10):1185–92. doi: 10.1111/j.1365-2036.2011.04863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997 Mar 6;336(10):691–5. doi: 10.1056/NEJM199703063361003. [DOI] [PubMed] [Google Scholar]
- 102.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010 Feb;51(2):660–78. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 103.Takuma K, Kamisawa T, Igarashi Y. Autoimmune pancreatitis and IgG4-related sclerosing cholangitis. Curr Opin Rheumatol. 2011 Jan;23(1):80–7. doi: 10.1097/BOR.0b013e3283412f60. [DOI] [PubMed] [Google Scholar]
- 104.Sugumar A, Chari ST. Autoimmune pancreatitis. J Gastroenterol Hepatol. 2011 Sep;26(9):1368–73. doi: 10.1111/j.1440-1746.2011.06843.x. [DOI] [PubMed] [Google Scholar]
- 105.Ghazale A, Chari ST, Zhang L, et al. Immunoglobulin G4-Associated Cholangitis: clinical Profile and Response to Therapy. Gastroenterology. 2008 Mar;134(3):706–15. doi: 10.1053/j.gastro.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 106.Björnsson E, Chari S, Silveira M, et al. Primary sclerosing cholangitis associated with elevated immunoglobulin G4: clinical characteristics and response to therapy. Am J Ther. 2011 May;18(3):198–205. doi: 10.1097/MJT.0b013e3181c9dac6. [DOI] [PubMed] [Google Scholar]
- 107.Sahani DV, Kalva SP, Farrell J, et al. Autoimmune pancreatitis: imaging features. Radiology. 2004 Nov;233(2):345–52. doi: 10.1148/radiol.2332031436. [DOI] [PubMed] [Google Scholar]
- 108.Okazaki K, Uchida K, Ohana M, et al. Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology. 2000 Mar;118(3):573–81. doi: 10.1016/s0016-5085(00)70264-2. [DOI] [PubMed] [Google Scholar]
- 109.Nakazawa T, Ohara H, Sano H, et al. Schematic classification of sclerosing cholangitis with autoimmune pancreatitis by cholangiography. Pancreas. 2006 Mar;32(2):229. doi: 10.1097/01.mpa.0000202941.85955.07. [DOI] [PubMed] [Google Scholar]
- 110.Zen Y, Fujii T, Sato Y, et al. Pathological classification of hepatic inflammatory pseudotumor with respect to IgG4-related disease. Mod Pathol. 2007 Aug;20(8):884–94. doi: 10.1038/modpathol.3800836. [DOI] [PubMed] [Google Scholar]
- 111.Kamisawa T, Shimosegawa T, Okazaki K, et al. Standard steroid treatment for autoimmune pancreatitis. Gut. 2009 Nov;58(11):1504–7. doi: 10.1136/gut.2008.172908. [DOI] [PubMed] [Google Scholar]
- 112.Hirano K, Tada M, Isayama H, et al. Long-term prognosis of autoimmune pancreatitis with and without corticosteroid treatment. Gut. 2007 Dec;56(12):1719–24. doi: 10.1136/gut.2006.115246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Karagiannidis A, Karavalaki M, Koulaouzidis A. Hepatic sarcoidosis. Ann Hepatol. 2006 Oct-Dec;5(4):251–6. [PubMed] [Google Scholar]
- 114.Mueller S, Boehme MW, Hofmann WJ, et al. Scand J Gastroenterol. 2000 Sep;35( 9):1003–8. doi: 10.1080/003655200750023110. [DOI] [PubMed] [Google Scholar]
- 115.Rybicki BA, Major M, Popovich J, Jr, et al. Racial differences in sarcoidosis incidence: A 5-year study in a health maintenance organization. Am J Epidemiol. 1997 Feb 1;45(3):234–41. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 116.Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001 Nov;164:1885–9. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 117.Irani SK, Dobbins WO., 3rd Hepatic granulomas: Review of 73 patients from one hospital and survey of the literature. J Clin Gastroenterol. 1979 Jun;1(2):131–43. [PubMed] [Google Scholar]
- 118.Cremers J, Drent M, Driessen A, et al. Liver-test abnormalities in sarcoidosis. Eur J Gastroenterol Hepatol. 2012 Jan;24(1):17–24. doi: 10.1097/MEG.0b013e32834c7b71. [DOI] [PubMed] [Google Scholar]
- 119.Lieberman J. Elevation of serum angiotensin-converting-enzyme (ACE) level in sarcoidosis. Am J Med. 1975 Sep;59(3):365–72. doi: 10.1016/0002-9343(75)90395-2. [DOI] [PubMed] [Google Scholar]
- 120.Farman J, Ramirez G, Brunetti J, et al. Abdominal manifestations of sarcoidosis. CT appearances. Clin Imaging. 1995 Jan-Mar;19(1):30–3. doi: 10.1016/0899-7071(94)00022-5. [DOI] [PubMed] [Google Scholar]
- 121.Kessler A, Mitchell DG, Israel HL, et al. Hepatic and splenic sarcoidosis: ultrasound and MR imaging. Abdom Imaging. 1993;18(2):159–63. doi: 10.1007/BF00198055. [DOI] [PubMed] [Google Scholar]
- 122.Devaney K, Goodman ZD, Epstein MS, et al. Hepatic sarcoidosis. Clinicopathologic features in 100 patients. Am J Surg Pathol. 1993 Dec;17(12):1272–80. [PubMed] [Google Scholar]
- 123.Geramizadeh B, Jahangiri R, Moradi E. Causes of hepatic granuloma: a 12-year single center experience from southern Iran. Arch Iran Med. 2011 Jul;14(4):288–9. [PubMed] [Google Scholar]
- 124.Kennedy PT, Zakaria N, Modawi SB, et al. Natural history of hepatic sarcoidosis and its response to treatment. Eur J Gastroenterol Hepatol. 2006 Jul;18(7):721–6. doi: 10.1097/01.meg.0000223911.85739.38. [DOI] [PubMed] [Google Scholar]
- 125.Ayyala US, Padilla ML. Diagnosis and treatment of hepatic sarcoidosis. Curr Treat Options Gastroenterol. 2006;9(6):475–83. doi: 10.1007/s11938-006-0004-9. [DOI] [PubMed] [Google Scholar]
- 126.Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: Results of a double blind, randomized trial. Sarcoid Vasc Diffuse Lung Dis. 2000 Mar;17(1):60–6. [PubMed] [Google Scholar]
- 127.Baratta L, Cascino A, Delfino M, et al. Ursodeoxycholic acid treatment in abdominal sarcoidosis. Dig Dis Sci. 2000 Aug;45(8):1559–62. doi: 10.1023/a:1005560927060. [DOI] [PubMed] [Google Scholar]
- 128.Ebert EC, Kierson M, Hagspiel KD. Gastrointestinal and hepatic manifestations of sarcoidosis. Am J Gastroenterol. 2008 Dec;103(12):3184–92. doi: 10.1111/j.1572-0241.2008.02202.x. [DOI] [PubMed] [Google Scholar]
- 129.Moreno-Merlo F, Wanless IR, Shimamatsu K, et al. The role of granulomatous phlebitis and thrombosis in the pathogenesis of cirrhosis and portal hypertension in sarcoidosis. Hepatology. 1997 Sep;26(3):554–60. doi: 10.1002/hep.510260304. [DOI] [PubMed] [Google Scholar]
- 130.Casavilla FA, Gordon R, Wright HI, et al. Clinical course after liver transplantation in patients with sarcoidosis. Ann Intern Med. 1993 Jun 1;118(11):865–6. doi: 10.7326/0003-4819-118-11-199306010-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Quigley EM, Marsh MN, Shaffer JL. Hepatobiliary complications of total parenteral nutrition. Gastroenterology. 1993 Jan;104(1):286–301. doi: 10.1016/0016-5085(93)90864-9. [DOI] [PubMed] [Google Scholar]
- 132.Grant JP, Cox CE, Kleinman LM, et al. Serum hepatic enzyme and bilirubin elevations during parenteral nutrition. Surg Gynecol Obstet. 1977 Oct;145(4):573–80. [PubMed] [Google Scholar]
- 133.Fisher RL. Hepatobiliary abnormalities associated with total parenteral nutrition. Gastroenterol Clin North Am. 1989 Sep;18(3):645–66. [PubMed] [Google Scholar]
- 134.Chan S, McCowen KC, Bistrian BR, et al. Incidence, prognosis, and etiology of end-stage liver disease in patients receiving home parenteral nutrition. Surgery. 1999 Jul;126(1):28–34. doi: 10.1067/msy.1999.98925. [DOI] [PubMed] [Google Scholar]
- 135.Touloukian RJ, Seashore JH. Hepatic secretory obstruction with total parenteral nutrition in the infant. J Pediatr Surg. 1975 Jun;10(3):353–60. doi: 10.1016/0022-3468(75)90098-6. [DOI] [PubMed] [Google Scholar]
- 136.Thunberg BJ, Golub L, et al. Decreased cholestasis with enteral instead of intravenous protein in the very low-birth-weight infant. J Pediatr Gastroenterol Nutr. 1989 Jul;9(1):21–7. [PubMed] [Google Scholar]
- 137.Meguid MM, Akahoshi MP, Jeffers S, Hayashi RJ, Hammond WG. Amelioration of metabolic complications of conventional total parenteral nutrition. Arch Surg. 1984 Nov;119(11):1294–8. doi: 10.1001/archsurg.1984.01390230060014. [DOI] [PubMed] [Google Scholar]
- 138.Beau P, Labat-Labourdette J, Ingrand P, et al. Is ursodeoxycholic acid an effective therapy for total parenteral nutrition-related liver disease? J Hepatol. 1994 Feb;20(2):240–4. doi: 10.1016/s0168-8278(05)80064-9. [DOI] [PubMed] [Google Scholar]
- 139.Capron J-P, Herve M-A, Gineston J-L, et al. Metronidazole in prevention of cholestasis associated with total parenteral nutrition. Lancet. 1983 Feb 26;1(8322):446–7. doi: 10.1016/s0140-6736(83)91442-3. [DOI] [PubMed] [Google Scholar]
- 140.Grant D. Current results of intestinal transplantation. The International Intestinal Transplant Registry. Lancet. 1996 Jun 29;347(9018):1801–3. doi: 10.1016/s0140-6736(96)91619-0. [DOI] [PubMed] [Google Scholar]
- 141.Roger D, Vaillant L, Fignon A, et al. Specific pruritic diseases of pregnancy. A prospective study of 3192 pregnant women. Arch Dermatol. 1994 Jun;130(6):734–9. [PubMed] [Google Scholar]
- 142.Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009 May 7;15(17):2049–66. doi: 10.3748/wjg.15.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Reyes H, Radrigan ME, Gonzalez MC, et al. Steatorrhea in patients with intrahepatic cholestasis of pregnancy. Gastroenterology. 1987 Sep;93(3):584–90. doi: 10.1016/0016-5085(87)90922-x. [DOI] [PubMed] [Google Scholar]
- 144.Lunzer MR. Jaundice in pregnancy. Baillieres Clin Gastroenterol. 1989 Apr;3(2):467–83. doi: 10.1016/0950-3528(89)90011-0. [DOI] [PubMed] [Google Scholar]
- 145.Leslie KK, Reznikov L, Simon FR, et al. Estrogens in intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2000 Mar;95(3):372–6. doi: 10.1016/s0029-7844(99)00533-5. [DOI] [PubMed] [Google Scholar]
- 146.Pauli-Magnus C, Meier PJ, Stieger B. Genetic determinants of drug-induced cholestasis and intrahepatic cholestasis of pregnancy. Semin Liver Dis. 2010 May;30(2):147–59. doi: 10.1055/s-0030-1253224. [DOI] [PubMed] [Google Scholar]
- 147.Shaw D, Frohlich J, Wittmann BA, Willms M. A prospective study of 18 patients with cholestasis of pregnancy. Am J Obstet Gynecol. 1982 Mar 15;142(6 Pt 1):621–5. doi: 10.1016/s0002-9378(16)32430-9. [DOI] [PubMed] [Google Scholar]
- 148.Heikkinen J. Serum bile acids in the early diagnosis of intrahepatic cholestasis of pregnancy. Obstet Gynecol. 1983 May;61(5):581–7. [PubMed] [Google Scholar]
- 149.Walker IA, Nelson-Piercy C, Williamson C. Role of bile acid measurement in pregnancy. Ann Clin Biochem. 2002 Mar;39(Pt 2):105–13. doi: 10.1258/0004563021901856. [DOI] [PubMed] [Google Scholar]
- 150.Azzaroli F, Mennone A, Feletti V, et al. Clinical trial: modulation of human placental multidrug resistance proteins in cholestasis of pregnancy by ursodeoxycholic acid. Aliment Pharmacol Ther. 2007 Oct;26(8):1139–46. doi: 10.1111/j.1365-2036.2007.03462.x. [DOI] [PubMed] [Google Scholar]
- 151.Hirvioja ML, Tuimala R, Vuori J. The treatment of intrahepatic cholestasis of pregnancy by dexamethasone. Br J Obstet Gynaecol. 1992 Feb;99(2):109–11. doi: 10.1111/j.1471-0528.1992.tb14465.x. [DOI] [PubMed] [Google Scholar]
- 152.Moseley RH. Sepsis and cholestasis. Clin Liver Dis. 1999 Aug;3(3):465– 75. doi: 10.1016/s1089-3261(05)70080-5. [DOI] [PubMed] [Google Scholar]
- 153.Tung CB, Tung CF, Yang DY. Hepatogastroenterology. 2005 Sep-Oct;52(65):1347–50. [Google Scholar]
- 154.Szabo G, Romics L, Jr, Frendl G. Liver in sepsis and systemic inflammatory response syndrome. Clin Liver Dis. 2002 Nov;6(4):1045–66. doi: 10.1016/s1089-3261(02)00058-2. [DOI] [PubMed] [Google Scholar]
- 155.Brandorg L, Goldman I. Hepatology, A Textbook of the Liver. Philadelphia, PA: W.B. Saunders; 1990. Bacterial and miscellaneous infections of the liver; pp. 1086–1098. [Google Scholar]
- 156.Lazaridis KN, Gores GJ, Lindor KD. Ursodeoxycholic acid ‘mechanisms of action and clinical use in hepatobiliary disorders’. J Hepatol. 2001 Jul;35(1):134–146. doi: 10.1016/s0168-8278(01)00092-7. [DOI] [PubMed] [Google Scholar]
- 157.Zimmerman HJ. Drug-induced liver disease. Clin Liver Dis. 2000 Feb;4(1):73–96. doi: 10.1016/s1089-3261(05)70097-0. [DOI] [PubMed] [Google Scholar]
- 158.Larrey D. Drug-induced liver diseases. J Hepatol. 2000;32(1 Suppl):77–88. doi: 10.1016/s0168-8278(00)80417-1. [DOI] [PubMed] [Google Scholar]
- 159.Geubel AP, Sempoux CL. Drug and toxin-induced bile duct disorders. J Gastroenterol Hepatol. 2000 Nov;15(11):1232–8. [PubMed] [Google Scholar]
- 160.Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005 Jun;4(6):489–99. doi: 10.1038/nrd1750. [DOI] [PubMed] [Google Scholar]
- 161.Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008 May;134( 6):1641–54. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yoshikado T, Takada T, Yamamoto T, et al. Itraconazole-induced cholestasis: involvement of the inhibition of bile canalicular phospholipid translocator MDR3/ABCB4. Mol Pharmacol. 2011 Feb;79(2):241–50. doi: 10.1124/mol.110.067256. [DOI] [PubMed] [Google Scholar]
- 163.Vermillion SE, Gregg JA, Baggenstoss AH, et al. Jaundice associated with bacteremia. Arch Intern Med. 1969 Nov;(12495):611–8. [PubMed] [Google Scholar]
- 164.Ramachandran R, Kakar S. Histological patterns in drug-induced liver disease. J Clin Pathol. 2009 Jun;62(6):481–92. doi: 10.1136/jcp.2008.058248. [DOI] [PubMed] [Google Scholar]
- 165.Papaseit E, Farré M, López MJ, et al. A case of acute valproic acid poisoning treated successfully with L-carnitine. Eur J Emerg Med. 2012 Feb;19(1):57–8. doi: 10.1097/MEJ.0b013e328345d67e. [DOI] [PubMed] [Google Scholar]
- 166.Wree A, Dechêne A, Herzer K, et al. Steroid and ursodeoxycholic acid combination in severe drug-induced liver injury. Digestion. 2011;84(1):54–9. doi: 10.1159/000322298. [DOI] [PubMed] [Google Scholar]
- 167.Kratzer W, Mason RA, Kächele V. Prevalence of gallstones in sonographic surveys worldwide. J Clin Ultrasound. 1999 Jan;27(1):1–7. doi: 10.1002/(sici)1097-0096(199901)27:1<1::aid-jcu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 168.Lee JG. Diagnosis and management of acute cholangitis. Nat Rev Gastroenterol Hepatol. 2009 Aug 4;Sep 4;6(9):533–41. doi: 10.1038/nrgastro.2009.126. [DOI] [PubMed] [Google Scholar]
- 169.Rosseland AR, Glomsaker TB. Asymptomatic common bile duct stones. Eur J Gastroenterol Hepatol. 2000 Nov;12(11):1171–3. doi: 10.1097/00042737-200012110-00001. [DOI] [PubMed] [Google Scholar]
- 170.Wada K, Takada T, Kawarada Y, et al. Diagnostic criteria and severity assessment of acute cholangitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg. 2007;14(1):52–8. doi: 10.1007/s00534-006-1156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sahu MK, Chacko A, Dutta AK, et al. Microbial profile and antibiotic sensitivity pattern in acute bacterial cholangitis. Indian J Gastroenterol. 2011 Sep;30(5):204–8. doi: 10.1007/s12664-011-0135-3. [DOI] [PubMed] [Google Scholar]
- 172.Lindsell DR. The diagnostic accuracy of magnetic resonance cholangiopancreatography (MRCP) and ultrasound compared with direct cholangiography in the detection of choledocholithiasis. Clin Radiol. 2000 Jul;55(7):579. doi: 10.1053/crad.1999.0426. [DOI] [PubMed] [Google Scholar]
- 173.Sugiyama M, Atomi Y. Endoscopic ultrasonography for diagnosing choledocholithiasis: a prospective comparative study with ultrasonography and computed tomography. Gastrointest Endosc. 1997 Mar;45( 3):143–6. doi: 10.1016/s0016-5107(97)70237-2. [DOI] [PubMed] [Google Scholar]
- 174.Gigot J, Nagorney D, Farnell M, et al. Bile duct cysts: a changing spectrum of disease. J Hepatobiliary Pancreat Surg. 1996;3:405–11. [Google Scholar]
- 175.Yu ZL, Zhang LJ, Fu JZ, Li J, Zhang QY, Chen FL. Anomalous pancreaticobiliary junction: image analysis and treatment principles. Hepatobiliary Pancreat Dis Int. 2004 Feb;3(1):136–9. [PubMed] [Google Scholar]
- 176.Shah OJ, Shera AH, Zargar SA, et al. Choledochal cysts in children and adults with contrasting profiles: 11-year experience at a tertiary care center in Kashmir. World J Surg. 2009 Nov;33(11):2403–11. doi: 10.1007/s00268-009-0184-2. [DOI] [PubMed] [Google Scholar]
- 177.Huang CT, Lee HC, Chen WT, et al. Usefulness of magnetic resonance cholangiopancreatography in pancreatobiliary abnormalities in pediatric patients. Pediatr Neonatol. 2011 Dec;52(6):332–6. doi: 10.1016/j.pedneo.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 178.Dinsmore JE, Murphy JJ, Jamieson D. Pediatric surgical images: MRCP evaluation of choledochal cysts. J Pediatr Surg. 2001 May;36(5):829–30. doi: 10.1053/jpsu.2001.22971. [DOI] [PubMed] [Google Scholar]
- 179.de Vries JS, de Vries S, Aronson DC, et al. Choledochal cysts: Age of presentation, symptoms, and late complications related to Todani’s classification. J Pediatr Surg. 2002;37:1568–73. doi: 10.1053/jpsu.2002.36186. [DOI] [PubMed] [Google Scholar]
- 180.Edil BH, Cameron JL, Reddy S, et al. Type III is intraduodenal (choledochocele). Choledochal cyst disease in children and adults: a 30-year single-institution experience. J Am Coll Surg. 2008 May;206(5):1000–5. doi: 10.1016/j.jamcollsurg.2007.12.045. discussion 1005–8. [DOI] [PubMed] [Google Scholar]
- 181.Richardson MC, Bell G, Fullarton GM Richardson MC, Bell G, Fullarton GM. Incidence and nature of bile duct injuries following laparoscopic cholecystectomy: an audit of 5913 cases. West of Scotland Laparoscopic Cholecystectomy Audit Group. Br J Surg. 1996 Oct;83(10):1356–60. doi: 10.1002/bjs.1800831009. [DOI] [PubMed] [Google Scholar]
- 182.Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 2008 Jun;14(6):759–69. doi: 10.1002/lt.21509. [DOI] [PubMed] [Google Scholar]
- 183.Rohrman CA, Baron RL. Biliary complications of pancreatitis. Radiol Clin North Am. 1989 Jan;27(1):93–104. [PubMed] [Google Scholar]
- 184.Deviere J, Cremer M, Baize M, et al. Management of common bile duct stricture caused by chronic pancreatitis with metal mesh self expandable stents. Gut. 1994 Jan;35(1):122–6. doi: 10.1136/gut.35.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis. An unusual tumor with distinctive clinical and pathological features. Am J Med. 1965 Feb;38:241–56. doi: 10.1016/0002-9343(65)90178-6. [DOI] [PubMed] [Google Scholar]
- 185.Dixon JM, Armstrong CP, Duffy SW, et al. Factors affecting morbidity and mortality after surgery for obstructive jaundice: a review of 373 patients. Gut. 1983 Sep;24(9):845–52. doi: 10.1136/gut.24.9.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Patel AH, Harnois DM, Klee GG, et al. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000 Jan;95(1):204–7. doi: 10.1111/j.1572-0241.2000.01685.x. [DOI] [PubMed] [Google Scholar]
- 187.Kipp BR, Stadheim LM, Halling SA, et al. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. Am J Gastroenterol. 2004 Sep;99(9):1675–168. doi: 10.1111/j.1572-0241.2004.30281.x. [DOI] [PubMed] [Google Scholar]
- 188.Datta DV, Sherlock S. Treatment of pruritus of obstructive jaundice with cholestyramine. Br Med J. 1963 Jan 26;1(5325):216–9. doi: 10.1136/bmj.1.5325.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Carey JB, Jr, Williams G. Relief of the pruritus of jaundice with a bile-acid sequestering resin. JAMA. 1961 May 6;176:432–5. doi: 10.1001/jama.1961.03040180034008. [DOI] [PubMed] [Google Scholar]
- 190.Podesta A, Lopez P, Terg R, et al. Treatment of pruritus of primary biliary cirrhosis with rifampin. Dig Dis Sci. 1991 Feb;36(2):216–20. doi: 10.1007/BF01300759. [DOI] [PubMed] [Google Scholar]
- 191.Tandon P, Rowe BH, Vandermeer B, et al. The efficacy and safety of bile Acid binding agents, opioid antagonists, or rifampin in the treatment of cholestasis-associated pruritus. Am J Gastroenterol. 2007 Jul;102( 7):1528–36. doi: 10.1111/j.1572-0241.2007.01200.x. [DOI] [PubMed] [Google Scholar]
- 192.Bloomer JR, Boyer JL. Phenobarbital effects in cholestatic liver diseases. Ann Intern Med. 1975 Mar;82( 3):310–7. doi: 10.7326/0003-4819-82-3-310. [DOI] [PubMed] [Google Scholar]
- 193.Mayo MJ, Handem I, Saldana S, et al. Sertraline as a first-line treatment for cholestatic pruritus. Hepatology. 2007 Mar;45(3):666–74. doi: 10.1002/hep.21553. [DOI] [PubMed] [Google Scholar]
- 194.Zylicz Z, Krajnik M, Sorge AA, et al. Paroxetine in the treatment of severe non-dermatological pruritus: a randomized, controlled trial. J Pain Symptom Manage. 2003 Dec;26( 6):1105–12. doi: 10.1016/j.jpainsymman.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 195.Bergasa NV, Talbot TL, Alling DW, et al. A controlled trial of naloxone infusions for the pruritus of chronic cholestasis. Gastroenterology. 1992 Feb;102(2):544–9. doi: 10.1016/0016-5085(92)90102-5. [DOI] [PubMed] [Google Scholar]
- 196.Jones EA, Neuberger J, Bergasa NV. Opiate antagonist therapy for the pruritus of cholestasis: the avoidance of opioid withdrawal-like reactions. QJM. 2002 Aug;95(8):547–52. doi: 10.1093/qjmed/95.8.547. [DOI] [PubMed] [Google Scholar]
- 197.Heathcote EJ, Cauch-Dudek K, Walker V, et al. The Canadian multicenter double blind randomized controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology. 1994 May;19(5):1149–56. [PubMed] [Google Scholar]
- 198.Gross CR, Malinchoc M, Kim WR, et al. Quality of life before and after liver transplantation for cholestatic liver disease. Hepatology. 1999 Feb;29(2):356–64. doi: 10.1002/hep.510290229. [DOI] [PubMed] [Google Scholar]
- 199.Vuoristo M, Farkilla M, Kamonen AL, et al. A placebo controlled trial of primary biliary cirrhosis with colchicines and ursodeoxycholic acid. Gastroenterology. 1995 May;108( 5):1470–8. doi: 10.1016/0016-5085(95)90696-7. [DOI] [PubMed] [Google Scholar]
- 200.Jones DE, Newton JL. An open study of modafinil for the treatment of daytime somnolence and fatigue in primary biliary cirrhosis. Aliment Pharmacol Ther. 2007 Feb 15;25(4):471–6. doi: 10.1111/j.1365-2036.2006.03223.x. [DOI] [PubMed] [Google Scholar]
- 201.Guanabens N, Pares A, Ros I, et al. Alendronate is more effective than etidronate for increasing bone mass in osteopenic patients with primary biliary cirrhosis. Am J Gasteroenterol. 2003 Oct;98(10):2268–74. doi: 10.1111/j.1572-0241.2003.07639.x. [DOI] [PubMed] [Google Scholar]
- 202.Balan V, Dickson ER, Jorgensen RA, et al. Effect of ursodeoxycholic acid on serum lipids of patients with primary biliary cirrhosis. Mayo Clin Proc. 1994 Oct;69(10):923–9. doi: 10.1016/s0025-6196(12)61815-1. [DOI] [PubMed] [Google Scholar]
- 203.Schaffner F. Paradoxical elevation of serum cholesterol by clofibrate in patients with primary biliary cirrhosis. Gastroenterology. 1969 Sep;57( 3):253–6. [PubMed] [Google Scholar]
- 204.Stojakovic T, Putz-Bankuti C, Fauler G, et al. Atorvastatin in patients with primary biliary cirrhosis and incomplete biochemical response to ursodeoxycholic acid. Hepatology. 2007 Sep;46(3):776–84. doi: 10.1002/hep.21741. [DOI] [PubMed] [Google Scholar]
- 205.Cohen LB, Ambinder EP, Wolke AM, et al. Role of plasmapheresis in primary biliary cirrhosis. Gut. 1985 Mar;26(3):291–4. doi: 10.1136/gut.26.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]