Abstract
Aim:
Neutropenic enterocolitis is a life threatening complication occurring most frequently after intensive chemotherapy in acute leukemia and solid tumors. This review aims to explore the pathogenesis of the condition and appraise the option and outcome of conservative and surgical management based on the literature review.
Material and Methods:
A Medline search was carried out and most of the relevant papers in English literature from 1973 onwards on neutropenic enterocolitis were reviewed
Results:
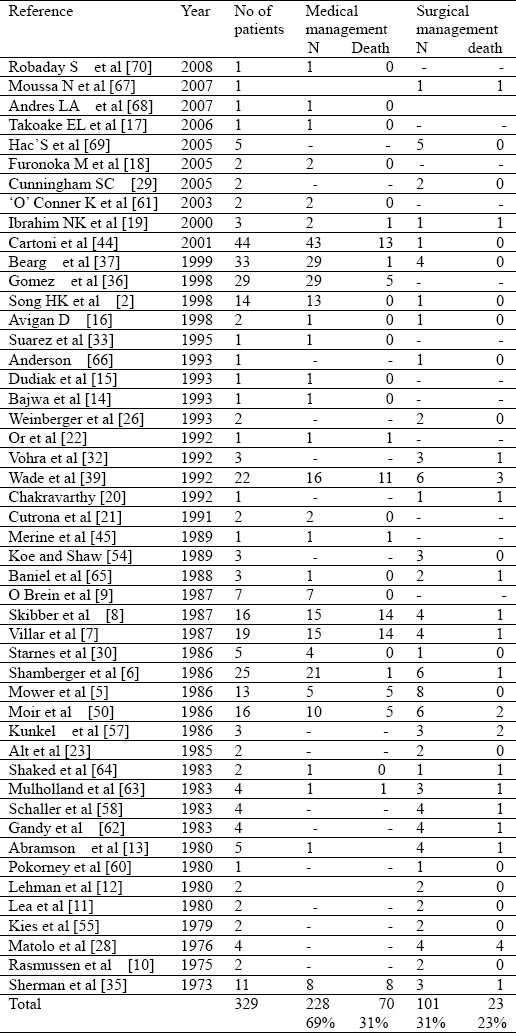
Twelve reports of single cases, 21 reports of 2 to 4 cases and 15 reports of 5 or more cases were identified. There were no prospective trials or case control studies on therapy of neutropenic enterocolitis. Among the total of 329 cases identified 69% were treated medically and 31% required surgical intervention . Even though a formal comparison of these 2 groups will not be appropriate, the mortality rate of 31% in the medically managed group was higher than those that required surgical intervention (23%)
Conclusion:
With the increasing use of multiple, new and aggressive chemotherapy for hematological and solid tumors there may be an increased frequency of neutropenic enterocolitis encountered in clinical practice. Clinicians should be acutely aware of the association of neutropenic enterocolitis with chemotherapy for the outcome would depend significantly on an early and appropriate treatment either conservative or surgical .
Keywords: Neutropenic enterocolitis, hemicolectomy, septicemia
Introduction
Neutropenic enterocolitis (NE) or typhilitis (from the Greek word Typhlon meaning cecum) is one of the serious complications of neutropenia characterized by segmental ulceration and inflammation with necrosis of ileum, caecum and ascending colon which may progress to perforation and septicemia[1–4]. NE has evolved from a complication of patients with leukemia[5–16] to a disease of patients who are neutropenic following high dose chemotherapy for many malignancies including hematological and solid tumors[17–20]. Neutropenia could also be associated with multiple myeloma, medications induced neutropenia, cyclical neutropenia, agranulocytosis, HIV disease and immunosuppression post transplant patients[21–26]. Risk factors include neutroapenia (absolute neutrophil count <500/mm3) associated with sepsis and is characterized by temperature greater than 38.5 along with right sided abdominal pain[1–4,25–32]. It may be confused with appendicitis, intussussception or intestinal obstruction or other gastrointestinal complications related to chemotherapy[2,5,6,24,25]. Computerized tomography and ultrasonography are useful adjuncts in diagnosing NE. Timely conservative treatment frequently allows resolution of NE without operation. Surgical intervention is however recommended in presence of bleeding, perforation or deterioration[1–4,12,14,23–25,28,29].
Incidence
The reported incidence in the literature of NE vary considerably from 0.8% to 26% in patients receiving intensive chemotherapy for leukemia or solid tumors[1–32]. However in a report of systematic analysis of 21 studies the incidence reported was 5.3% (266/5058 cases) in patients hospitalized for hematological malignancies for high dose chemotherapy[3]. It is possible that the incidence of NE is increasing in incidence[4,25]. A major reason is the greater use of multiagent aggressive therapeutic regimen for the treatment of neoplastic disease and the consequent neutropenia that may occur following such treatment[1–32]. While the majority of the cases occur in patients with acute leukemia and other hematological malignancies who undergo treatment with antineoplastic chemotherapy rarely it has been reported following chemotherapy for malignancies of the colon, breast, lung, testis, pancreas and bone[1–4,16–19,24,29,31,33]. Despite the condition being increasingly recognized and reported the etiology, pathogenesis and optimal clinical management of NE remains unclear.
Pathogenesis
NE is thought to be caused by damage to the gastrointestinal mucosa or immunosuppression[1–20,23–25,28]. Irrespective of the initial etiological factors the pathologic process appears to have a predisposition for the terminal ileum, appendix and cecum[23–25]. It may occur only in the cecum, in the cecum and ileum; in the cecum, ileum and ascending colon or in the cecum with occasional ulcers throughout the intestine [Figs. 1 & 2]. Immunohistochemical studies of the gut in patients with leukemia have demonstrated infiltration of the mucosa by leukemic and lymphoproliferative cells[34]. These deposits are more likely to result in ulceration following chemotherapy. By contrast metastasis from solid tumors are more likely to involve the serosal surface and may explain the relative rarity of the condition in solid tumors treated with antineoplastic chemotherapy[4]. The gastrointestinal mucosa are a subset of highly proliferative cells. Cytotoxic chemotherapy inhibits cellular replication and the mucosal proliferation may be insufficient to replace that which is lost by natural desquamation and so mucosal integrity may be lost. Other contributing factors include local bacterial or fungal infection with mucosal injury and necrosis of mural leukemic infiltrate[1,2,7]. Mucosal ischemia from sepsis induced hypotension may all contribute to initiating mucosal injury[1–4,22–25]. Agents most commonly associated with neutropenic enterocolitis include cytosine arabinoside (79%), etoposide (62%) and daunomycin (46%)[25,35]. Other implicated agents include doxorubicin, methotrexate, vincristine, nedaplatin, irinotecan, taxane based chemotherapeutic agents and prednisone[16–19,23–25,35–37].
Fig. 1.
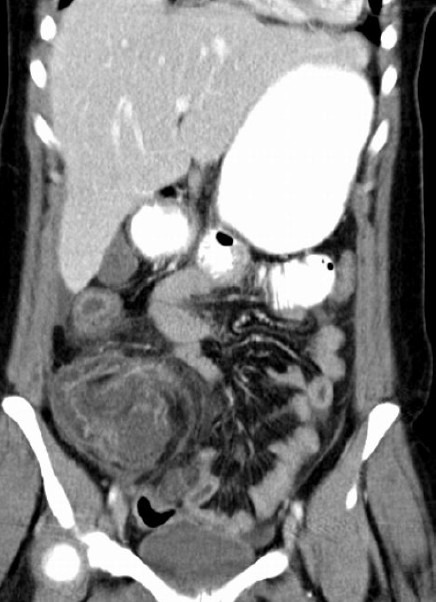
Coronal CT scan image. A coronal CT scan image of a patient with neutropenic enterocolitis 10 days following chemotherapy for breast cancer. The cecum and ileum is grossly thickened with obliteration of the lumen.
Fig. 2.
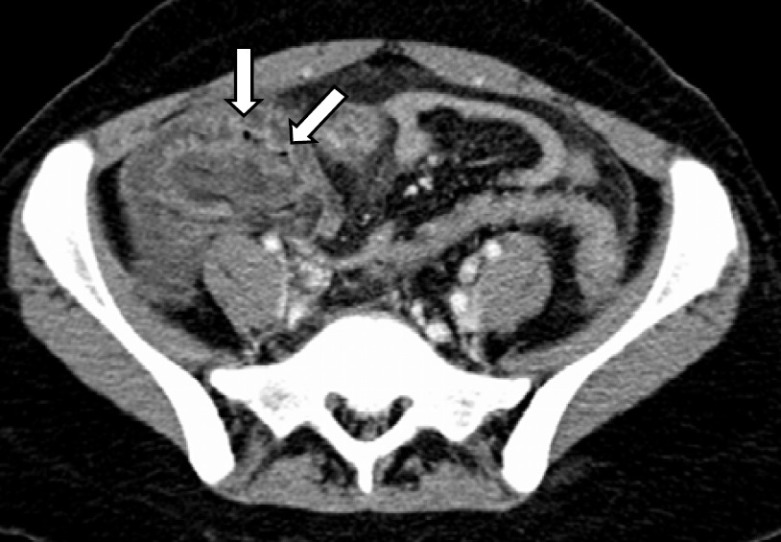
CT scan cross section. Contrast enhanced CT scan of the same patient showing thickened cecal wall with air luscencies consistent with pneumatosis intestinalis (arrows).
It is not entirely clear why cecal mucosa is predisposed to NE. However, the cecum appears to be at increased risk to develop NE because of its relatively decreased vascularity, increased stasis, tendency to be more distended than other regions of the colon and increased concentration of lymphatic tissue[4,23,25]. Typhilitis is thought to result from a combination of factors including neutropenia, chemotherapy or radiotherapy induced destruction of normal mucosa along with intramural hemorrhage caused by severe thrombocytopaenia[25,36,37]. Further the change in normal gastrointestinal flora caused by antibiotics and antifungal agents and colonization by certain flora contributes to the inflammatory process[23,25,36]. Certain chemotherapy regimen or medical conditions predispose the gastrointestinal tract to bacterial invasion either from the direct toxic effects of the agent(mucositis) or from agents causing distension and necrosis[1–4,23–25]. Neutropenia and steroids complicate the situation by reducing host defenses against infection[1,2,6,16,22,28]. Transmural necrosis and perforation may then develop in the presence of neutropenia. Several microbes have been found in affected patients including clostridium species, Pseudomonas species, Escherichia Coli, Klebsiella pneumoniae, Enterobacter species, Morganella Morganii, Stapylococcus aureus, Streptococcus viridians and Candida species[1–4,7,23–30,36–39]. In some series 80% of those with positive cultures revealed gram negative rods and gram positive cocci[23,25,36,27,39]. Of the Clostridium species, C septicum has been found to be the most common organism; when found the resultant fulminant enterocolitis often leads to death[23,25,39]. The pooled frequency of fungal neutropenic enterocolitis is reported to be 6.2% (calculated from 860 reported patients); Candida species was isolated in 94% of these patients and the pooled mortality rate in them was 81.8%[38].
Clinical Presentation
The symptoms are non specific and include nausea, vomiting, abdominal discomfort and distension and diarrhea which may be bloody[1–32]. Invariably the episode will follow a course of antineoplastic chemotherapy and occur during a period of neutropenia classically beginning 7-10 days after treatment[4,16,19]. Pyrexia is seen in 90% of neutropenic patients in hospital and is nonspecific sign; however the combination of abdominal pain, pyrexia associated with right iliac fossa tenderness is seen in 60-80% of patients and may be more relevant in diagnosing NE[1,3,4,7,16,19,39]. In the later stages this may lead to localized peritonitis which may progress to generalized peritonitis. A right iliac fossa mass if felt represents a thickened dilated fluid filled cecum. The mass could also be a consequence of ileocaecal inflammatory mass or a localized collection around a perforated cecum or appendix. However even in the presence of severe sepsis, physical finding may be minimal and indeed rapid progression to fulminant septicaemia may precede the development of these abdominal signs. This aggressive presentation of the disease is usually rapidly fatal[24,26,29,36–39].
Diagnosis
NE continues to be a diagnostic challenge despite our increasing awareness of its occurrence in high risk groups. The occurrence of abdominal symptoms in the presence of progressive neutropenia should alert the clinician even in the presence of most minimal physical signs. Other gastrointestinal complications such as mucositis. psuedomemberanous colitis and invasive infection by opportunistic infection are common in patients receiving chemotherapy and may be important differential diagnosis[16,19,23,36,39]. In addition other conditions that may need to be considered include vincristine induced ileus, L-asparginase induced pancreatitis, drug induced cholestasis and cholecystitis, fungal infection and inflammation associated mesenteric lymphadenitis[23,25,39]. Patients with neutropenia localize poorly and may manifest sources of intra-abdominal sepsis in an atypical fashion. Hence investigations are required to exclude other causes requiring a different management approach. A full blood count may reveal thrombocytopenia as well as neutropenia. Blood cultures are positive in 28 to 84% of cases, with bowel organisms most frequently being isolated[24,28,39]. Endoscopic evaluation to rule out other causes of colitis is usually avoided for fear of inducing further hemorrhage or perforation and increasing the risk of bacterial translocation and exacerbating septicaemia as an aftermath of mechanically induced trauma to the mucosa[4]. If performed, findings would include mucosa which is diffusely friable and hemorrhagic with superficial ulceration associated with loss of haustration and loss of normal vascular pattern[19]. Peritoneal lavage may be helpful in confirming the diagnosis as gram staining of the recovered peritoneal fluid may reveal polymicrobial contamination[40].
Radiology
Plain films of the abdomen are both nonspecific and insensitive in detection of NE. However findings on plain X-ray may include right lower quadrant soft tissue density or mass, a fluid filled cecum with dilated small bowel and minimal or no large bowel gas and an associated ileus[4,25,36,39] Localized or diffuse thumb printing characteristic of mucosal edema may be noted and in patients with enteric perforation intraperitoneal free gas may be present[2,4,24,39]. Pneumatosis intestinalis of the cecum and ascending colon may be observed[4,22,23,41]. CT imaging and ultrasonography are more sensitive and specific than plain radiography or barium enema[42–48]. Findings in both US and CT include right lower quadrant inflammatory mass and pericecal fluid or inflammatory changes in the pericecal soft tissue including fat stranding along with gross thickening of ileal and cecal wall with intraluminal narrowing (Figures 1 & 2). US findings consistent with a diagnosis of NE are a rounded mass with dense central echoes and a wider hypoechoic periphery. US may also demonstrate psuedopolypoid changes of the cecal mucosa and pericolic fluid collection. It has been reported that it may be used to monitor the daily progression of the process and predict the outcome[42–45]. The symptoms of the patients with mural thickening were longer in duration (7.9 vs. 3.8 days) than those without mural thickness and their mortality rate was higher. Patient with bowel wall thickness greater than 10mm were found to have a higher mortality rate (60%) than those bowel walls that were less than 10mm (4.2%)[44]. CT imaging is often done for further evaluation of changes seen in US but it may be used as a first line study in neutropenic patient presenting with abdominal pain. CT scan may be more useful in determining the cecal wall thickness (Figs. 1 & 2). Angiography when performed in patients with NE who are bleeding demonstrates hypervascularity of the cecum, intense mucosal staining, arteriovenous shunting into mesenteric veins and opacification of superficial mucosal ulcers[48]. Barium studies were performed in earlier series and in the presence of NE may demonstrate cecal distension or rigidity and mucosal edema[49]. They are now avoided because of the theoretical risk of enteric perforation; moreover, they provide little additional information over that available on US or CT scan[4].
Following a systemic analysis of 21 studies of patients with NE, Gorschluter et al[3] proposed the following diagnostic criteria for NE. 1) presence of fever (axillary temperature >38°C or rectal temp >38.5°C; 2) abdominal pain(at least degree 3 determined by the patient using visual analogues scale pain score ranging from degree 1 to 10; 3)demonstration of bowel wall thickening of more than 4mm (transversal scan) over more than 30mm (longitudinal scan) in any segment by US or CT scan.
Histopathology
Histopathological confirmation of the diagnosis is not feasible in most of the patients unless the patient has undergone surgical resection. Gross appearance of the resected pathological lesion reveals the bowel to be dilated, edematous and often hemorrhagic[1,23,25]. The cecal wall is thickened with diffuse loss of mucosa, hemorrhage and necrotic surface (Figs. 3 & 4). Varying amounts of mucosal & submucosal necrosis, hemorrhage and ulceration may be noted. Microscopic features include loss of mucosa, significant edema of submucosa with deep mural and transmural necrosis[16,25].
Fig. 3.
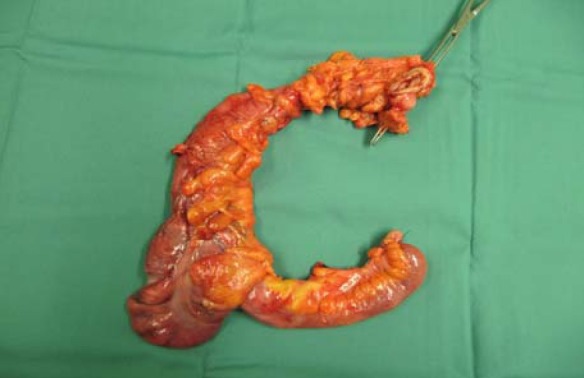
Right hemicolectomy specimen. Resected specimen showing edematous, dilated ischemic cecum, ileum and ascending colon of a patient with neutropaenic enterocolitis 10 days after chemotherapy for carcinoma of the breast.
Fig. 4.
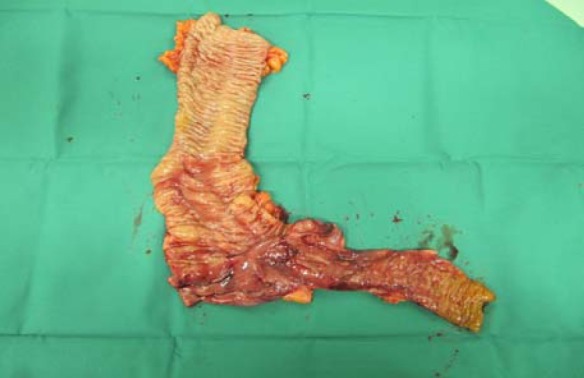
Right hemicolectomy specimen-mucosal surface. Mucosal surface of the resected specimen showing mucosal ischemia, hemorrhage and edema predominately in the cecum and terminal ileum with isolated lesion in the ascending colon.
Management
The optimal treatment of patients with NE remains controversial due to paucity of quality studies in the literature. In general terms patients with mild NE, normalization of leukocyte count allows containment of the process and eventual healing. Persistent bacterial invasion of the bowel mucosa, increasing size of the bowel lesion, and possible bowel wall perforation may result in failure of normalization of leukocyte count[1,4,25,37,39]. Patients treated conservatively have a higher mortality rates when leukocyte count do not return to levels greater than 1000cells/cu mm[25]. Younger patients tend to do better than older patients[50]. Conservative treatment is reasonable in patients without peritonitis. Patients receiving conservative treatment require close observation. The general consensus regarding conservative treatment is the use of broad spectrum antibiotics, bowel rest, abdominal decompression and nutritional support[2,4,6,9,23,24,25,35,37,39].
The recommended antibiotics include monotherapy with carbapenum or piperacillin-tazobactum or duotherapy with another antipsuedomonal b lactum antibiotic in combination with an aminoglycoside or duotherapy with cefepime or ceftazidime plus metronidazole[3,51]. The use of systemic antimycotics in NE is not well supported by either prospective or high quality retrospective studies. However most clinicians believe that amphotercin B therapy is recommended when the patients remain febrile and profoundly neutropenic for more than 5 days despite administration of broad spectrum antibiotics in adequate doses[3,52]. Neither prospective or high quality retrospective studies concerning administration of G-CSF (granulocyte colony stimulating factor), omeprazole, bowel rest, total parenteral nutrition (TPN) or nasogastric aspiration in the management of NE is available. The use G-CSF is usually recommended in high risk patients including those with profound neutropenia (absolute count <100/ml) , uncontrolled primary disease, pneumonia, hypotension, multiorgan dysfunction and invasive fungal infection; however the benefit in these patients have not been proven[3,52].
Bowel rest and TPN is recommended by many authors[1,2,4,6,24–26,37,39,57–65]. While in patients with milder NE responding to conservative treatment, oral nutrition may be considered; those with severe form may require bowel rest and TPN[1–4,6,9,25,52–57]. Routine use of omeprazole and nasogastric suction have been questioned by some due to potential risk of migration of gram negative bacteria from the bowel into the respiratory tract facilitated by weakened barrier (lower gastric acid level and incompetent oesophagastric sphincter) and the risk of pneumonia may then be increased[3]. Omeprazole may be justified in selected circumstances like those with epigastric pain, known gastritis, gastric ulcers or on corticosteroid therapy[3].
If the patients deteriorate while on conservative therapy then a surgical intervention is warranted[2,4,6,7,12,14,23–25,32,36–39,57,66–70]. This decision however would follow repeated clinical assessment and frequent radiographic evaluation to document the deterioration. The criteria for surgical intervention would include, 1) persistent gastrointestinal bleeding after resolution of neutropenia and thrombocytopenia and correction of clotting abnormality, 2)evidence of free intraperitoneal perforation, 3) suggestion of uncontrolled sepsis based on requirement for large volumes of fluid or vasopressors and 4) in the absence of neutropenia , development of intra-abdominal process that requires surgical intervention.
Once a decision to operate has been made the question of which procedure to adopt is a source of debate. Most authors agree that right hemicolectomy is the operation of choice[4,5,23,28,29,53,54,57]. Less extensive surgical intervention such as appendicectomy, caecostomy and limited resection may be inadequate in the management of NE as the extent of mucosal necrosis may often be greater than that which is obvious by inspection of the serosa[4,9,12,36,37,39]. At operation the surgeons must also decide whether to perform end to end anastomosis or to exteriorize the bowel. While there are reports of successful anastomosis following resection[4,5,24,25,50,58,66], end ileostomy and mucous fistula is most appropriate in the presence of extensive sepsis, significant peritoneal soiling and hypotension[4,24,25,37,39] Another area of debate is in the management of those patients who respond to conservative treatment but require additional chemotherapy. Some have recommended elective hemicolectomy given the potential risk of recurrence with subsequent cycles of chemotherapy[59,60] .Although the incidence of recurrence varies from 12-67% there is sufficient data to warrant genuine concern[16,18,59,60]. Elective hemicolectomy, prophylactic antibiotics are reasonable methods to prevent recurrence but there are no controlled studies to support these measures[25,59,60]. Moreover elective surgery may delay subsequent chemotherapy for several weeks. The only documented method to decrease the incidence of recurrence is dose reduction for subsequent cycles of chemotherapy[18,25].
Outcome and Prognosis
The reported results in the literature review are outlined in Table 1. Three hundred and twenty nine cases were reviewed; among these 12 were single case reports, 21 studies comprised of 2 to 4 cases and 15 studies had 5 or more cases. Among these 69% were managed conservatively[2,5,6–9,13–19,21,22,30–33,35–37,39,44,45,50,61,63–65,68–70] and 31% were subjected to surgical intervention[2,5–8,10–13,16,19,20,23,26,28–30,32,35,37,39,44,50,54,55,57,58,60,62–66,69]. The mortality rate in the patients managed conservatively was 31% in comparison to 23% in those managed surgically. Even though those treated surgically appeared to have better outcome caution must be exercised as the 2 groups are almost certainly not comparable. It is possible that medically treated patients were generally more debilitated to begin with and may have been deemed unfit for surgery. Although earlier surgical intervention may potentially be advantageous in terms of reducing the complication due to perforation or obstruction including death there are no randomized trials or case control studies comparing conservative therapy versus surgical intervention. However randomized controlled study comparing conservative management versus surgical resection may be difficult to perform in terms of patient safety or patient recruitment. Although untreated neutropenic enterocolitis carries a 50% to 100% mortality rate[25,36] with appropriate medical or surgical management it could be reduced to 23 to 31% (Table 1).
Table 1.
Literature Review-Management and outcome of patients with neutropenic enterocolitis
Conclusions
Neutropenic enterocolitis remains a major clinical challenge both in terms of diagnosis and management. With increasing use of new, multiple and aggressive chemotherapeutic regimen in treatment of various malignancies it becomes increasingly important to be alert to the life threatening complications of these medications. Fever, abdominal pain and bowel wall thickening in neutropenic patient should alert a clinician. US and CT scan are useful adjuncts for early diagnosis. Resolution of NE is based on the return of neutrophil count to normal, provision of broad spectrum antibiotics and bowel rest. Although conservative therapy is often employed with success, surgical intervention may be required in patients with perforation, bleeding or failure to respond to conservative measures. While the literature review would suggest a better outcome in patients managed surgically this issue could be resolved only by properly conducted randomized controlled trials. Even with appropriate therapy the mortality rate remains significant. High index of clinical suspicion and prompt appropriate treatment is essential to achieve a lower mortality rate.
References
- 1.Quigley MM, Bethel K, Nowacki M, Millard F, Sharpe R, et al. Neutropenic enterocolitis: a rare presentation complication of acute leukemia. Am J Haematol. 2001;66:213–219. doi: 10.1002/1096-8652(200103)66:3<213::aid-ajh1047>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.Song HK, Kreisel D, Canter R, Krupnick AS, Stadtmauer EA, Buzby G. Changing presentation and management of neutropenic enterocolitis. Arch Surg. 1998;133(9):979–982. doi: 10.1001/archsurg.133.9.979. [DOI] [PubMed] [Google Scholar]
- 3.Gorschluter M, Mey U, Strehl J, Zinske C, Schepke M, Schmid F, Wolf IG, Sauerbruch T, Glasmacher A, et al. Neutropenic enterocolitis in adults: systematic analysis of evidence quality. Eur J Haematol. 2005;75(1):1–13. doi: 10.1111/j.1600-0609.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 4.Williams N, Scott AD. Neutropaenic enterocolitis : a continuing surgical challenge. Br J Surg. 1997;84(9):1200–1205. [PubMed] [Google Scholar]
- 5.Mower WJ, Hawkins JA, Nelson EW. Neutropenic enterocolitis in adults with acute leukemia. Arch Surg. 1986;121(5):571–574. doi: 10.1001/archsurg.1986.01400050089012. [DOI] [PubMed] [Google Scholar]
- 6.Shamberger RC, Weinstein HJ, Delorey MJ, Levey RH. The medical and surgical management of typhilitis in children with acute nonlymphocytic (myelogenous) leukemia. Cancer. 1986;57(3):603–609. doi: 10.1002/1097-0142(19860201)57:3<603::aid-cncr2820570335>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 7.Villar HV, Warneke JA, Peck MD, Durie B, Bjelland JC, Hunter TB. Role of surgical treatment in the management of complications of the gastrointestinal tract in patients with leukemia. Surg Gynecol Obstet. 1987;165(3):217–222. [PubMed] [Google Scholar]
- 8.Skibber JM, Matter GJ, Pizzo PA, Lotze MT. Right lower quadrant pain in young patients with leukemia.A surgical perspective. Ann Surg. 1987;206(6):711–716. doi: 10.1097/00000658-198712000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien S, Kantarjian HM, Anaissie E, Dodd G, Bodey GP. Successful medical management of neutropenic enterocolitis in adults with acute leukemia. South Med J. 1987;80(10):1233–1235. doi: 10.1097/00007611-198710000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen BL, Freeman JS. Major surgery in leukemia. Am J Surg. 1975;130(6):647–651. doi: 10.1016/0002-9610(75)90414-6. [DOI] [PubMed] [Google Scholar]
- 11.Lea JW, Jr, Masys DR, Shackford SR. Typhilitis: a treatable complication of acute leukemia therapy. Cancer clinic Trials. 1980;3(4):355–362. [PubMed] [Google Scholar]
- 12.Lehman JA, Armitage JO. Surgical intervention in complications of acute leukemia. Postgrad Med. 1980;68(5):89–92. doi: 10.1080/00325481.1980.11715591. [DOI] [PubMed] [Google Scholar]
- 13.Abramson SJ, Berdon WE, Baker DH. Childhood typhilitis: its increasing association with acute myelogenous leukaemia.Report of five cases. Radiology. 1983;146(1):61–64. doi: 10.1148/radiology.146.1.6571759. [DOI] [PubMed] [Google Scholar]
- 14.Bajwa RP, Marwaha RK, Garewal G. Neutropenic enterocolitis and cecal perforation in acute lymphatic leukemia. Indian J Cancer. 1993;30(1):31–33. [PubMed] [Google Scholar]
- 15.Dudiak KM. Abdominal case of the day.Neutropenic enterocolitis associated with leukemia. AJR. Am J Roentgenol. 1993;160(6):1323–1324. doi: 10.2214/ajr.160.6.8498247. [DOI] [PubMed] [Google Scholar]
- 16.Avigan D, Richardson P, Elias A, Demetri G, Shapiro M, Schnipper L, Wheeler C. Neutropenic enterocolitis as a complication of high dose chemotherapy with stem cell rescue in patients with solid tumours; a case series with review of literature. Cancer. 1998;83(3):409–414. [PubMed] [Google Scholar]
- 17.Takaoka E, Kawai K, Ando S, Simazui T, Akaza H. Neutropenic colitis during standard dose combination chemotherapy with nedaplatin and Irinotecan for testicular tumour. Jpn J Clin Oncol. 2006;36(1):60–63. doi: 10.1093/jjco/hyi219. [DOI] [PubMed] [Google Scholar]
- 18.Furonaka M, Miyazaki M, Nakajima M, Hirata S, Fujitaka K, Kondo K, et al. Neutropenic enterocolitis in lung cancer: a report of 2 cases and review of the literature. Intern Med. 2005;44(5):467–470. doi: 10.2169/internalmedicine.44.467. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim NK, Sahin AA, Dubrow RA, Lynch P PM, Boehnke-Michaud L, Valero V, et al. Colitis associated with docitexal based chemotherapy in patients with metastatic breast cancer. Lancet. 2000;355(9200):281–283. doi: 10.1016/S0140-6736(99)06195-4. [DOI] [PubMed] [Google Scholar]
- 20.Chakravarty K, Scott DG, McCann BG. Fatal neutropenic enterocolitis associated with sulphasalazine therapy for rheumatoid arthritis. Br J Rheumatol. 1992;31(5):351–353. doi: 10.1093/rheumatology/31.5.351. [DOI] [PubMed] [Google Scholar]
- 21.Cutrona AF, Blinkhorn RJ, Crass J, Spagnuolo PJ. Probable neutropenic enterocolitis in patients with AIDS. Rev of infect Dis. 1991;13(5):828–831. doi: 10.1093/clinids/13.5.828. [DOI] [PubMed] [Google Scholar]
- 22.Or R, Mehta J, Nagler A, Craciun I. Neutropenic enterocolitis associated with autologus bone marrow transplantation. Bone Marrow Transplant. 1992;9(5):383–385. [PubMed] [Google Scholar]
- 23.Alt B, Glass NR, Sollinger H. Neutropenic enterocolitis in adults: Review of the literature and assessment of surgical intervention. Am J Surg. 1985;149(3):405–408. doi: 10.1016/s0002-9610(85)80119-7. [DOI] [PubMed] [Google Scholar]
- 24.Katz JA, Wagner ML, Gresik MV, Mahoney DH, Jr, Fernbach DJ, et al. Typhilitis: An 18 year experience and post mortum review. Cancer. 1990;65(4):1041–1047. doi: 10.1002/1097-0142(19900215)65:4<1041::aid-cncr2820650433>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 25.Bravaro MF. Neutropenic enterocolitis. Curr Gastroenterol Rep. 2002;4(2):297–301. doi: 10.1007/s11894-002-0079-y. [DOI] [PubMed] [Google Scholar]
- 26.Weinberger M, Hollingsworth H, Feuerstein IM, Young NS, Pizzo PA. Successful surgical management of neutropenic enterocolitis in two patients with severe plastic anaemia.Case reports and review of the literature. Arch Intern Med. 1993;53(1):107–113. [PubMed] [Google Scholar]
- 27.Dosik GM, Luna M, Valdivieso M, McCredie KB, Gehan EA, Gil-Extremera B, et al. Necrotising colitis in patients with cancer. Am J Med. 1979;67(4):646–656. doi: 10.1016/0002-9343(79)90248-1. [DOI] [PubMed] [Google Scholar]
- 28.Mateo NM, Garfunkel SE, Wolman EF., Jr Intestinal necrosis and perforation in patients receiving immunosuppressive drugs. Am J Surg. 1976;132(6):753–754. doi: 10.1016/0002-9610(76)90451-7. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham SC, Fakery K, Bass BL, Napolitano LM. Neutropenic enterocolitis in adults: case series and review of literature. Dig Dis ScI. 2005;50(2):215–220. doi: 10.1007/s10620-005-1585-1. [DOI] [PubMed] [Google Scholar]
- 30.Starnes HF, Jr, Moore FD, Jr, Mentzer S, Osteen RT, Steele GD, Jr, Wilson RE. Abdominal pain in neutropenic cancer patients. Cancer. 1986;57(3):616–621. doi: 10.1002/1097-0142(19860201)57:3<616::aid-cncr2820570337>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 31.Colebunders R, Bultinck J, Servais J, Denis L. A patient with testis seminoma, sarcoidosis and neutropenic enterocolitis. Hum Pathol. 1984;15(4):394–396. doi: 10.1016/s0046-8177(84)80041-6. [DOI] [PubMed] [Google Scholar]
- 32.Vohra R, Prescott RJ, Banerjee SS, Wilkinson PM, Schofield PF. Management of neutropenic colitis. Surg Oncol. 1992;1(1):11–15. doi: 10.1016/0960-7404(92)90051-l. [DOI] [PubMed] [Google Scholar]
- 33.Suarez B, Kalifa G, Adamsbaum C, Saint- Martin C, Barbotin-Larrieu F. Sonographic diagnosis and follow up of diffuse neutropenic colitis: a case report of child treated for osteogenic sarcoma. Paediatr Radiol. 1995;25(5):373–374. doi: 10.1007/BF02021707. [DOI] [PubMed] [Google Scholar]
- 34.Smith G, Crocker J, Nar P, Chesner I, Leyland MJ. Immunogold-silver technique applied to showing malignant B cell infiltration of the gastrointestinal tract in patients with chronic lymphocytic leukemia and non Hodgkins lymphoma. J Clin Pathol. 1987;40(7):756–759. doi: 10.1136/jcp.40.7.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherman NJ, Woolley MM. The ileocecal syndrome in acute childhood leukemia. Arch Surg. 1973;107(1):39–42. doi: 10.1001/archsurg.1973.01350190029008. [DOI] [PubMed] [Google Scholar]
- 36.Gomez L, Martino R, Rolston KV. Neutropenic enterocolitis: spectrum of the disease and comparison of definite and possible cases. Clin Infect Dis. 1998;27:695–699. doi: 10.1086/514946. [DOI] [PubMed] [Google Scholar]
- 37.Baerg J, Murphy JJ, Anderson R, Magee JP. Neutropenic enteropathy: a 10 year review. J Pediatr Surg. 1999;34(7):1068–1071. doi: 10.1016/s0022-3468(99)90566-3. [DOI] [PubMed] [Google Scholar]
- 38.Gorschluter M, Mey U, Strehl J, Schmitz V, Rabe C, Pauls K, Ziske C, et al. Invasive fungal infections in neutropenic enterocolitis. A systematic analysis of pathogens, incidence, treatment and mortality in adult patients. BMC Infect Dis. 2006;6:35. doi: 10.1186/1471-2334-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wade DS, Nava HR, Douglass HO., Jr Neutropenic enterocolitis: clinical diagnosis and treatment. Cancer. 1992;69(1):17–23. doi: 10.1002/1097-0142(19920101)69:1<17::aid-cncr2820690106>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 40.Sauter ER, Vauthey JN, Bolton JS, Sardi A. Selective management of patients with neutropenic enterocolitis using peritoneal lavage. J Surg Oncol. 1990;45(1):63–67. doi: 10.1002/jso.2930450115. [DOI] [PubMed] [Google Scholar]
- 41.Jaffe N, Carlson DH, Vawter GF. Pneumatosis cystoides intestinalis in acute leukemia. Cancer. 1972;30(1):239–243. doi: 10.1002/1097-0142(197207)30:1<239::aid-cncr2820300134>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 42.Teefey SA, Montana MA, Goldfogel GA, Shuman WP. Sonographic diagnosis of neutropenic typhilitis. AJR. Am J Roentgenol. 1987;149(4):731–733. doi: 10.2214/ajr.149.4.731. [DOI] [PubMed] [Google Scholar]
- 43.Glass- Royal MC, Choyke PL, Gootenberg JE, Grant EG. Sonography in the diagnosis of neutropenic colitis. J Ultrasound Med. 1987;6(11):671–673. doi: 10.7863/jum.1987.6.11.671. [DOI] [PubMed] [Google Scholar]
- 44.Cartoni C, Dragoni F, Micozzi A, Pescarmona E, Mecarocci S, Chirletti P, et al. Neutropenic enterocolitis in patients with acute leukemia.Prognostic significance of bowel wall thickening detected by ultrasonography. J Clin Oncol. 2001;19(3):756–761. doi: 10.1200/JCO.2001.19.3.756. [DOI] [PubMed] [Google Scholar]
- 45.Merine D, Nussbaum AR, Fishman EK, Sanders RC. Sonographic observation in patients with typhilitis. Clin Pediatr. 1989;28(8):377–379. doi: 10.1177/000992288902800809. [DOI] [PubMed] [Google Scholar]
- 46.Vas WG, SeelIg R, Mahanta B, Patel B, Salimi Z, Markivee C, et al. Neutropenic colitis: Evaluation with computed tomography. J Comput Tomogr. 1988;12(3):211–215. doi: 10.1016/0149-936x(88)90011-2. [DOI] [PubMed] [Google Scholar]
- 47.Adams WG, Rauch RF, Kelvin FM, Silverman PM, Korobkin M. CT detection of typhilitis. J Comput Assist Tomogr. 1985;9(2):363–365. doi: 10.1097/00004728-198503000-00026. [DOI] [PubMed] [Google Scholar]
- 48.Meyerovitz MF, Fellows KE. Typhilitis: a cause of gastrointestinal hemorrhage in children.AJR. Am J Roengenol. 1984;143(4):833–835. doi: 10.2214/ajr.143.4.833. [DOI] [PubMed] [Google Scholar]
- 49.Del Fava RL, Cronin TG., Jr Typhilitis complicating leukemia in an adult.Barium enema findings. AJR. Am J Roentgenol. 1977;129(2):347–348. doi: 10.2214/ajr.129.2.347. [DOI] [PubMed] [Google Scholar]
- 50.Moir CR, Scudamore CH, Benny WB. Typhilitis.Selective surgical management. Am J Surg. 1986;151(5):563–566. doi: 10.1016/0002-9610(86)90547-7. [DOI] [PubMed] [Google Scholar]
- 51.Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34(6):730–751. doi: 10.1086/339215. [DOI] [PubMed] [Google Scholar]
- 52.Greil R, Psenak O, Roila F ESMO Guidelines Working Group. Hematopoietic growth factors: ESMO recommendations for the applications. Ann Oncol. 2008;19(suppl 2:ii):116–1118. doi: 10.1093/annonc/mdn107. [DOI] [PubMed] [Google Scholar]
- 53.Hsu TF, Huang HH, Yen DH, Kao WF, Chen JD, Wong LM, et al. ED presentation of neutropenic enterocolitis in adult patients with acute leukemia. Am J Emerg Med. 2004;22(4):276–279. doi: 10.1016/j.ajem.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 54.Koea JB, Shaw JH. Surgical management of neutropenic enterocolitis. Br J Surg. 1989;76(8):821–824. doi: 10.1002/bjs.1800760821. [DOI] [PubMed] [Google Scholar]
- 55.Kies MS, Luedke DW, Boyd JF, McCue MJ. Neutropenic enterocolitis; two case reports of long term survival following surgery. Cancer. 1979;43(2):730–734. doi: 10.1002/1097-0142(197902)43:2<730::aid-cncr2820430249>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 56.Blijlevan NM. Neutropenic enterocolitis: Challenges in diagnosis and treatment. Clin Adv Hematol Oncol. 2009;7(8):528–530. [PubMed] [Google Scholar]
- 57.Kunkel JM, Rosenthal D. Management of the ileocecal syndrome.Neutropenic enterocolitis. Dis Colon Rectum. 1986;29(3):196–199. doi: 10.1007/BF02555024. [DOI] [PubMed] [Google Scholar]
- 58.Schaller RT, Jr, Schaller JF. The acute abdomen in the immunologically compromised child. J Paediatr Surg. 1983;18(4):937–944. doi: 10.1016/s0022-3468(83)80050-5. [DOI] [PubMed] [Google Scholar]
- 59.Keidan RD, Fanning J, Gatenby RA, Weese Jl. Recurrent typhilitis: A disease resulting from aggressive chemotherapy. Dis Colon Rectum. 1989;32(3):206–209. doi: 10.1007/BF02554529. [DOI] [PubMed] [Google Scholar]
- 60.Pokorney BH, Jones JM, Shaikh BS, Aber RC. Typhilitis: A treatable cause of recurrent septicemia. JAMA. 1980;243(7):682–683. doi: 10.1001/jama.243.7.682. [DOI] [PubMed] [Google Scholar]
- 61.‘O’ Conner K, Dijkstra B, Kelly L, Mc Dermott EW, Hill AD, ‘O’ Higgins N. ANZ J Surg. 2003;73(6):463–465. doi: 10.1046/j.1445-2197.2003.02660.x. [DOI] [PubMed] [Google Scholar]
- 62.Gandy W, Greenberg BR. Successful medical management of neutropenic enterocolitis. Cancer. 1983;51(8):1551–1555. doi: 10.1002/1097-0142(19830415)51:8<1551::aid-cncr2820510833>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 63.Mullholland MW, Delaney JP. Neutropenic colitis and aplastic anemia: a new association. Ann Surg. 1983;197(1):84–90. [PMC free article] [PubMed] [Google Scholar]
- 64.Shaked A, Shinar F, Freund H. Neutropenic typhilitis.A plea for conservatism. Dis colon Rectum. 1983;26(5):351–352. doi: 10.1007/BF02561717. [DOI] [PubMed] [Google Scholar]
- 65.Baniel J, Lombrozo R, Ziv Y, Wolloch Y. Neutropenic enterocolitis.Case report. Acta Chir Scand. 1988;154(1):71–73. [PubMed] [Google Scholar]
- 66.Anderson PE. Neutropenic enterocolitis treated by primary resection with anastomosis in a leukemic patient receiving chemotherapy. Aust N Z J Surg. 1993;63(1):74–76. doi: 10.1111/j.1445-2197.1993.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 67.Moussa N, Decre D, Baudel JL, Offenstadt G, Maury E. A fulminant enterocolitis. Dig Dis Sci. 2007;52(6):1479–1480. doi: 10.1007/s10620-006-9417-5. [DOI] [PubMed] [Google Scholar]
- 68.Andres LA, Ford RD, Wilcox RM. Necrotising colitis caused by systemic aspergillosis in a burn patient. J Burn Care Res. 2007;28(6):918–921. doi: 10.1097/BCR.0b013e318159a3d8. [DOI] [PubMed] [Google Scholar]
- 69.Hac’ S, Stachera-Grzenkowicz M, Mital A, SledzInski Z. Necrotising enterocolitis in acute lymphoblastic leukemic patients: department experience. Int J Haematol. 2005;82(4):319–323. doi: 10.1532/IJH97.E0426. [DOI] [PubMed] [Google Scholar]
- 70.Robaday S, Kerleau JM, Tapon E, Levesque H, Marie I. Typhilitis: report of a case and review of literature. Rev Med Interne. 2008;29(3):224–227. doi: 10.1016/j.revmed.2007.08.018. [DOI] [PubMed] [Google Scholar]


