Abstract
Objective
Craniosynostosis is the premature fusion of the calvarial sutures and is associated with aesthetic impairment and secondary damage to brain growth. Associated neurological injuries can result from increased intracranial pressure (ICP) and abnormal cerebral blood flow (CBF). Arterial spin-labeling (ASL) MRI was used to assess regional CBF in developing rabbits with early-onset coronal suture synostosis (EOCS) and age-matched wild-type controls (WT).
Methods
Rabbits were subjected to ASL MRI at or near 10, 25, or 42 days of age. Differences in regional CBF were assessed using one-way ANOVA.
Conclusion
CBF was similar in WT and EOCS rabbits with the exception of the peridural surfaces in EOCS rabbits at 25 days of age. A twofold increase in peridural CBF at 25 days of age coincides with a transient increase in ICP. By 42 days of age, CBF in peridural surfaces had decreased.
Keywords: Craniosynostosis, Cerebral blood flow, Peridural surfaces, Rabbit
Introduction
Craniosynostosis is the premature fusion of one or more of the calvarial sutures and can be simple or can be associated with syndromes such as Crouzon and Apert syndrome. Simple craniosynostosis has been estimated to occur in 300–500 per 1,000,000 live births [6–8]. Craniosynostosis of the coronal sutures (CS) makes up approximately 24% of the total cases of simple craniosynostosis [6, 7, 20] and can lead to neurologic injury if not treated. Surgical intervention however can be risky in young children and selection of the best age at which to perform surgery may be critical.
Premature closure of the cranial suture has two main consequences: aesthetic deformity of the skull and the possibility of secondary damage of the growing brain. Neurologic injuries associated with craniosynostosis can result from increased intracranial pressure (ICP) [12–14, 22, 33] and abnormal cerebral blood flow (CBF) [9, 10, 29, 30]. For these reasons, surgery is commonly performed within the first year of life. However, the age at which cranial expansion procedures should be performed on patients with coronal suture synostosis is the subject of considerable debate. Views among surgeons vary widely with some preferring corrective surgery soon after birth and others deferring surgery until 12 months of age. This disagreement shows that there is no clear consensus on the relationship between brain growth and skull vault growth in patients with craniosynostosis [31].
Numerous studies have been published on neurological consequences in patients with craniosynostosis [1, 5–10, 14, 25, 28–31, 33]. However, there are significant limiting factors that need to be considered when evaluating these reports including small sample sizes and heterogenous samples, i.e., mixing syndromic and non-syndromic patients in one study [6]. Sgouros et al. [31] charge that a clearly defined view of the relationship between the brain and skull vault growth is lacking and that the mechanisms behind craniosynostosis are not clearly understood. A study of the natural progression of uncorrected craniosynostosis could provide valuable information regarding the brain and its ability to compensate for skull restriction when present.
Cranial morphological and ICP studies are well-documented in infants with craniosynostosis, but to date, little attention has been given to cerebral blood flow (CBF) changes and their possible relevance to the management of craniosynostosis. The majority of studies to date have focused on anomalous venous drainage that can accompany craniosynostosis [1, 25, 28].
The present study was undertaken to study age-related CBF differences in a homogeneous strain of rabbits with simple, familial coronal suture synostosis and to compare the CBF values found in this model of craniosynostosis with age-matched wild-type control rabbits. This model affords us the opportunity to monitor CBF in untreated craniosynostosis from the early-onset until a time when brain growth is complete. We quantify CBF non-invasively using continuous arterial spin-labeled perfusion MRI.
Materials and methods
Animal model
Thirty one rabbits were used in the present study. All rabbits were born and maintained in the vivarium at the Department of Anthropology, University of Pittsburgh. This is a breeding colony of rabbits with naturally occurring coronal suture synostosis [12, 13, 20–23]. Rabbits were housed in stainless steel cages. Food and water were given ad libitum. Up to the time of the experiment, rabbits were housed with mothers and littermates. This study was approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Synostotic rabbits were initially diagnosed at 9–10 days of age using previously described criteria [12, 13, 21–23] by assessing coronal suture morphology and the degree of coronal suture mobility. Rabbits with dysmorphic but patent coronal sutures and limited mobility were diagnosed with delayed onset synostosis and were omitted from the study. Rabbits with bilaterally fused coronal sutures with no mobility were diagnosed as early-onset coronal suture synostosis (EOCS) and were placed in the study.
Rabbits were divided into the following experimental groups: wild-type control rabbits (WT) at or near 10 days of age (n=6); EOCS rabbits at or near 10 days of age (n=6);WT rabbits at or near 25 days of age (n=6); EOCS rabbits at or near 25 days of age (n=5);WT rabbits at or near 42 days of age (n=4); and EOCS rabbits at or near 42 days of age (n=4).
MR protocol
For magnetic resonance studies, at or near 10, 25, and 42 days of age, rabbits were transported to the Pittsburgh NMR Center for Biomedical Research at Carnegie Mellon University. Rabbits were preanesthetized with an intramuscular injection of ketamine (100 mg/ml) and xylazine (20 mg/ml) in a 9:1 solution at a dose of 0.6 mg/kg. All rabbits underwent tracheotomy and were ventilated (Harvard Apparatus, Holliston, MA) via an endotracheal tube with 1–1.5% isoflurane in a N2O:O2 (1:2) gas mixture. The rabbits were placed in dorsal recumbency and an indwelling catheter was inserted into the femoral artery. Arterial blood gases were collected immediately before and after MRI. Arterial CO2 tension (PaCO2) was maintained between 20 and 46 mmHg for the duration of the study. This has been reported as the normal PaCO2 for rabbits [38]. During MRI assessment animals were maintained at 36±0.5°C via a warm air heating system (SA Instruments, Stony Brook, NY).
MR image acquisition
MR studies were performed on a 4.7-T, 40-cm bore Bruker AVANCE-AV system, equipped with a 12-cm-diameter-shielded gradient insert and a 72-mm volume RF coil for 14 and 25 days of age. At 42 days of age, half-PCOS RF coil was used. T2-weighted spin-echo images were used to verify position and were acquired with the following parameters: field of view (FOV) = 6.4-cm, 2-mm slice thickness, TR/TE = 2,000/90 ms, two averages, five slices and a 128×70 matrix interpolated to 128×128. Perfusion studies were performed using continuous arterial spin-labeling [11] imaging technique (spin-echo, 128×70 matrix, TR=200 ms, summation of three echoes, TE = 10, 20, and 30 ms and two averages). The labeling and control continuous RF pulse for the inversion plane was positioned ±2 cm from the perfusion detection plane. The spin-lattice relaxation time of tissue water (T1obs) [16] was measured from a series of spin-echo images (TR=8,000, 4,300, 2,300, 1,200, 650, 350, 185, and 100 ms, TE=9 ms, two averages and a 128×70 matrix). Spin-labeling efficiency, α [36] was determined from intensities within the carotid arteries (gradient echo, 45° flip angle, eight averages, TR/TE=100/9.6 ms, 256×256 matrix and spin-labeling applied at ±6 mm).
Data analysis
All image processing was performed with the Bruker ParaVision 3.0.2 image analysis software. Pixel by pixel maps of (MC − ML) × MC−1 were generated from the perfusion data. MC and ML are the magnetization intensities from the control image and labeled image, respectively. T1obs maps were generated from the series of variable TR images by a three parameter non-linear fit. Regional CBF was then calculated according to Zhang et al. [37]. CBF=λ·(T1obs× 2α)−1 × (MC − ML) × MC−1, where λ is the blood–brain partition coefficient of water, with a spatially constant value of 0.9 mL/g assumed [17] and α is the spin-labeling efficiency measured in the carotids. T2-weighted images were used to define regions of interest (ROIs) in the left and right hemisphere, which were copied onto the CBF maps. Regions within each hemisphere including the cortex, thalamus, and the peridural surfaces were also defined, guided by assignments from a rabbit brain atlas [32].
Statistical analysis
Mean blood flow data were compared between groups at each age and significant differences were assessed using one-way ANOVA on SPSS software (v. 13, SPSS Incorporated, Chicago, IL). All differences were considered significant at p < 0.05.
Results
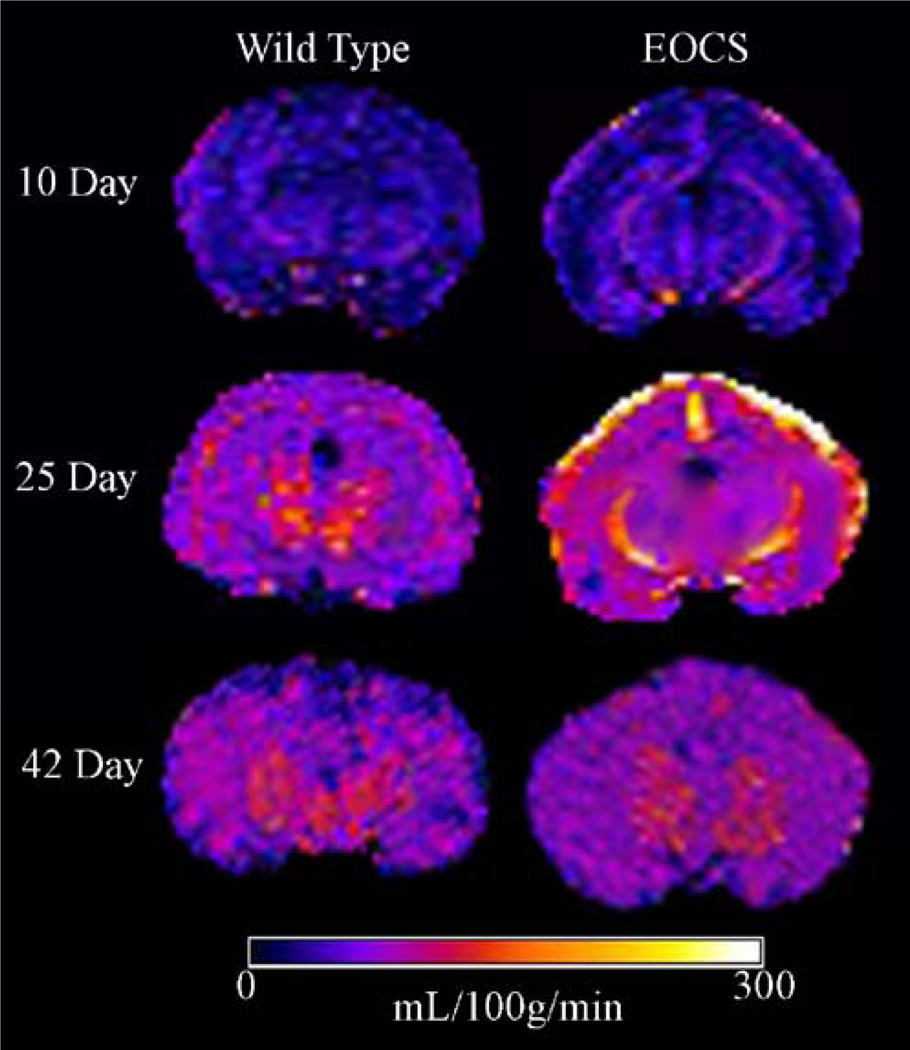
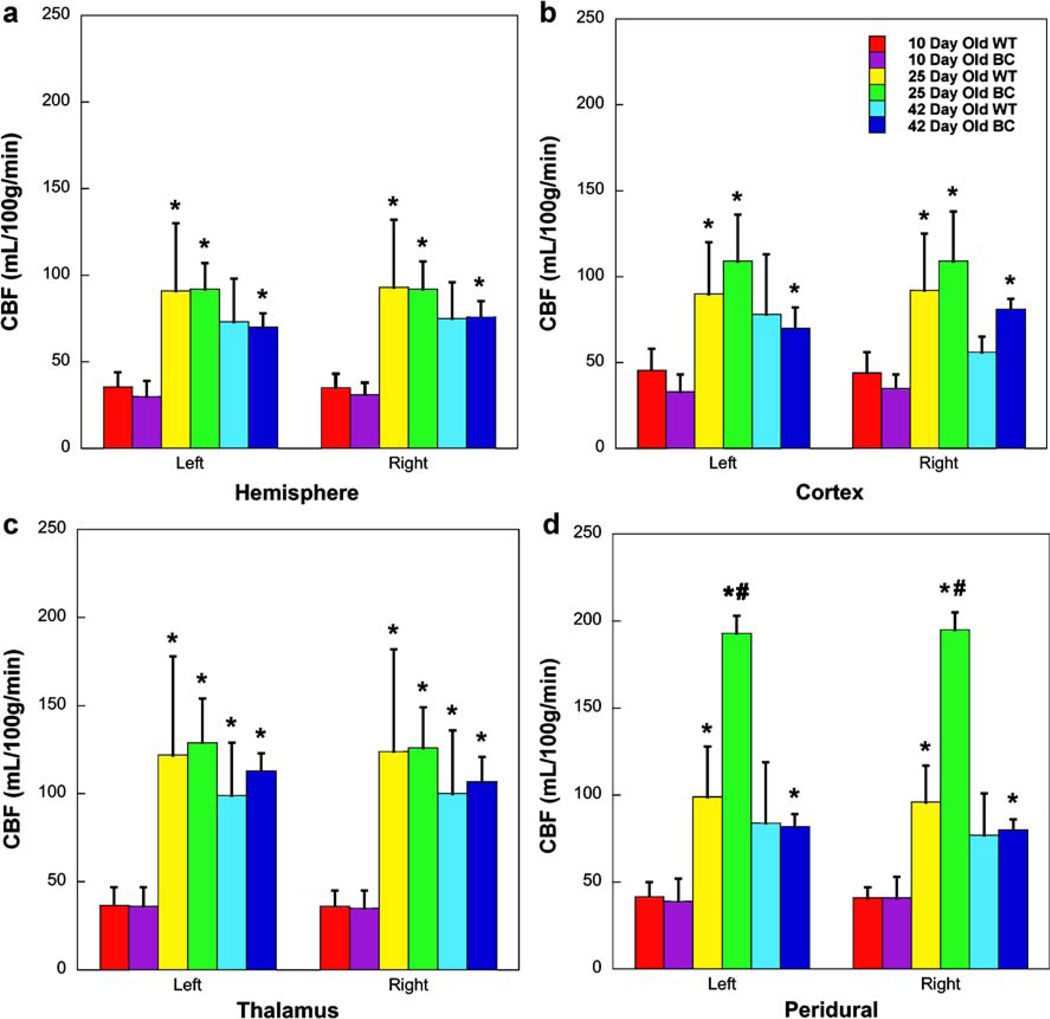
Representative CBF maps for WT and EOCS rabbits from each group are shown in Fig. 1. Mean regional CBF values for all studies are shown in Fig. 2 and Table 1. Comparing anatomical brain ROIs, there was a significant increase in CBF in all regions measured between 10 and 25 days of age in both WT rabbits and EOCS rabbits (Figs. 1 and 2). This increase averaged 35% in the left and right hemispheres, 38.5% in the left and right cortices, and 28% in the left and right thalamus. By 42 days of age, CBF was lower in both the WT and EOCS groups when compared to 25-day rabbits, albeit not significantly, and in most regions CBF was still significantly higher when compared to the 10-day-old groups.
Fig. 1.
Representative CBF maps from wild-type and EOCS rabbits at or near 10, 25, and 42 days of age
Fig. 2.
CBF in wild-type and craniosynostotic rabbits at or near 10, 25, and 42 days of age. *p < 0.05 compared to a 10-day-old group, #p < 0.05 compared to WT
Table 1.
Regional CBF (mL·100 g−1 min−1)
| Hemisphere | Cortex | Thalamus | Peridural | |||||
|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | |
| Wild-type | ||||||||
| 10 days of age | 36±8 | 35±8 | 46±12 | 44±12 | 37±10 | 36±9 | 42±8 | 41±6 |
| 25 days of age | 91±39 | 93±39 | 90±30 | 92±33 | 112±56 | 124±58 | 99±29 | 96±21 |
| 42 days of age | 73±25 | 75±21 | 78±35 | 56±9 | 99±30 | 100±36 | 84±35 | 77±24 |
| EOCS | ||||||||
| 10 days of age | 30±9 | 31±7 | 33±10 | 35±8 | 36±11 | 35±10 | 39±13 | 41±12 |
| 25 days of age | 92±15 | 92±16 | 109±27 | 109±29 | 129±25 | 126±23 | 193±10 | 195±10 |
| 42 days of age | 70±8 | 76±9 | 70±12 | 81±6 | 113±10 | 107±14 | 82±7 | 80±6 |
Data are mean±SD; SD in CBF represents the intragroup variability in the (MC − ML) × MC−1
CBF at the peridural surfaces was significantly increased at 25 days of age in both the WT rabbits and EOCS rabbits (Figs. 1 and 2) when compared with the 10-day-old groups. At 25 days of age there was a dramatic and significant increase in the CBF of the EOCS rabbits when compared to the WT. The CBF in EOCS rabbits was twice as high as that in WT rabbits. By 42 days of age, there was a significant decrease in CBF in EOCS rabbits when compared to WT and EOCS rabbits at 25 days of age. There were no significant differences in CBF in the peridural surfaces of either the WT or EOCS groups between the 10- and 42-day-old rabbits (Figs. 1 and 2).
Discussion
Cerebral blood flow in the immature rabbit
To the best of our knowledge, this is the first report of changes in CBF with age in craniosynostotic rabbits using ASL-MRI. Our results demonstrate that cortical, hemispheric, and thalamic CBF were not different in rabbits with early-onset craniosynostosis of the coronal sutures when compared to age-matched wild-type rabbits. The increase in both groups seen between 10- and 25-days of age is the normal pattern of CBF in the immature brain [2, 26, 34]. Tuor [34] demonstrated low cerebral blood flow in the early postnatal development in the rabbit (between 1 and 8 days of age) and a marked increase in CBF by 17 days of age. One of the most important factors determining the level of CBF is the local energy demand of the tissue for cell maintenance, growth, differentiation, and myelination. The marked increase in CBF observed between 8 and 17 days of age in rabbits occurs at a time of marked maturational advances in anatomy, electrophysiology, and behavior.
Peridural blood flow
Remarkably, EOCS rabbits at 25 days of age displayed areas of high CBF on the peridural surfaces of the brain. This coincides with the time of increased intracranial pressure previously reported in this colony of rabbits [12]. During intracranial hypertension pial arteries are not constricted but rather respond by dilation [3]. According to Auer et al. [3] during elevation of ICP, arteries not only remain open but even dilate because smooth muscle cells relax. The results of the present study agree with this finding.
By 42 days of age, CBF in EOCS rabbits had decreased to normal range previously reported in adult rabbits [24] and pial vasculature no longer demonstrated high CBF. We know from previous studies that ICP also returns to normal in CS rabbits between 25 and 42 days of age [12].
Autoregulation of CBF
CBF is maintained at a constant level in normal brain in the face of the usual fluctuations in blood pressure by the process of autoregulation [35]. Autoregulation of CBF is confounded due to the brain’s encasement in the skull. This circumstance becomes especially apparent during periods of increased intracranial pressure (ICP). Earlier work by Grubb et al. [15] found that pial vessels dilated as the ICP increased and that the dilation of cerebral vessels accompanies an increase in ICP when CBF autoregulation is intact. Autoregulation is a quantitative phenomenon that becomes more defective with increasing insult to the brain [19]. At 42 days of age, ICP in EOCS rabbits had returned to normal. This, paired with the decrease in pial flow, may suggest that CBF autoregulation is no longer intact, or, more likely, that CBF, as a result of decreased ICP, has returned to a more normal pattern of flow.
Variability among groups
We acknowledge that there is a large degree of variability in the present study, most notably in both WT and EOCS rabbits at 25 days of age. This may be due to small sample size. Caution should be used in extrapolating findings from this rabbit model to the clinical setting. We would argue, however, that the degree of variability in WT and EOCS rabbits at 10 days of age was well within normal limits and the sample size was identical to that of 25 day rabbits. We propose that the high degree of variability present in both WT and EOCS rabbits at 25 days of age may be due, at least in part, to the fact that this is the period of significant increases in CBF and rapid development in the brain [3, 34].
Rabbit model
This rabbit model was adopted by investigators because of the similarity between its cerebrovascular architecture and that of the human [4, 18, 27, 34]. The rabbit model of craniosynostosis is well-established [12–14, 20–23] and appears to be an appropriate model of simple synostosis of the coronal sutures [13]. However, other factors need to be considered when extrapolating findings from this rabbit model to the clinical setting. These include species-specific differences in morphology of the brain, in neurocapsular growth patterns and in timing seen between humans and rabbits. Ninety percent of brain growth is completed between 35 and 42 days of age in rabbits compared with 4–6 years of age in humans [23].
Conclusion
Both wild-type rabbits and rabbits with early-onset bilateral coronal suture craniosynostosis demonstrate normal cerebral blood flow patterns for their age. EOCS rabbits had significantly increased CBF in the peridural surfaces at 25 days of age when compared to WT controls. This CBF pattern was absent at 42 days of age in EOCS rabbits suggesting that CBF had returned to normal.
Acknowledgments
Funding This project was funded by The Walter L. Copeland Fund of the Pittsburgh Foundation. The Pittsburgh NMR Center for Biomedical Research is supported by a grant from the National Institutes of Health (P41EB-001977).
Footnotes
This study was approved by the University of Pittsburgh Institutional Animal Care and Use Committee. This study was presented in part at the 14th Scientific Meeting of the International Society for Magnetic Resonance in Medicine in Seattle Washington on May 6–12, 2006.
Contributor Information
Lesley M. Foley, Pittsburgh NMR Center for Biomedical Research, Carnegie Mellon University, Pittsburgh, PA, USA
Wendy Fellows-Mayle, Email: fellowsmaylew@upmc.edu, Department of Neurological Surgery, B 400 PUH, 230 Lothrop Street, Pittsburgh, PA 15213, USA.
T. Kevin Hitchens, Pittsburgh NMR Center for Biomedical Research, Carnegie Mellon University, Pittsburgh, PA, USA.
Joseph E. Losee, Division of Pediatric Plastic Surgery, Pittsburgh Cleft-Craniofacial Center, Children’s Hospital of Pittsburgh, Pittsburgh, PA, USA
Timothy Barbano, Department of Anthropology, University of Pittsburgh, Pittsburgh, PA, USA.
Michael I. Siegel, Department of Anthropology and Orthodontics, University of Pittsburgh, Pittsburgh, PA, USA
Mark P. Mooney, Department of Anthropology, University of Pittsburgh, Pittsburgh, PA, USA Department of Anthropology and Orthodontics, University of Pittsburgh, Pittsburgh, PA, USA; Department of Oral Biology, Cleft Palate-Craniofacial Center, University of Pittsburgh, Pittsburgh, PA, USA; Department of Plastic and Reconstructive Surgery, Cleft Palate-Craniofacial Center, University of Pittsburgh, Pittsburgh, PA, USA.
References
- 1.Al-Otibi M, Jea A, Kalkarni AV. Detection of important venous collaterals by computed tomography venogram in multisutural synostosis. Case report and review of the literature. J Neurosur. 2007;107(6 Suppl):508–510. doi: 10.3171/PED-07/12/508. [DOI] [PubMed] [Google Scholar]
- 2.Armstead WM. Age and cerebral circulation. Pathophysiology. 2005;12:5–15. doi: 10.1016/j.pathophys.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Auer LM, Ishiyama N, Pucher R. Cerebrovascular response to intracranial hypertension. Acta Neurochir. 1987;84:124–128. doi: 10.1007/BF01418837. [DOI] [PubMed] [Google Scholar]
- 4.Beiner JM, Olgivy CS, DuBois AB. Cerebral blood flow changes in response to elevated intracranial pressure in rabbits and bluefish: a comparative study. Comp Biochem Physiol. 1997;116A(3):245–252. doi: 10.1016/s0300-9629(96)00206-x. [DOI] [PubMed] [Google Scholar]
- 5.Camfield P, Camfield C, Cohen MM. Neurological aspects of craniosynostosis. New York: Oxford University Press; 2000. [Google Scholar]
- 6.Cohen MM., Jr . The etiology of craniosynostosis. In: Cohen MM Jr, editor. Craniosynostosis: diagnosis, evaluation, and management. New York: Raven; 1986. pp. 59–80. [Google Scholar]
- 7.Cohen MM, Jr, MacLean RE. Craniosynostosis: diagnosis, evaluation, and management. New York: Oxford University Press; 2000. [Google Scholar]
- 8.Cohen MM, Jr, Kreiborg S. Birth prevalence studies of Crouzon syndrome: comparison of direct and indirect methods. Clin Genet. 1992;41:12–15. doi: 10.1111/j.1399-0004.1992.tb03620.x. [DOI] [PubMed] [Google Scholar]
- 9.David L, Wilson J, Watson NE, Argenta LC. Cerebral perfusion defects secondary to simple craniosynostosis. J Craniofacial Surg. 1996;7(3):177–185. doi: 10.1097/00001665-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 10.David LR, Genecov DG, Camastra AA, Wilson JA, Argenta LC. Positron emission tomography studies confirm the need for early surgican intervention in patients with single-suture craniosynostosis. J Craniofacial Surg. 1999;10(1):38–42. doi: 10.1097/00001665-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Detre JA, Leigh JS, Williams DS, Korestsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- 12.Fellows-Mayle W, Mooney MP, Losken HW, Dechant J, Cooper GM, Burrows AM, Smith TD, Pollack IF, Siegel MI. Age-related changes in intracranial pressure in rabbits with uncorrected familial coronal suture synostosis. Cleft Palate-Craniofacial J. 2000;37(4):370–378. doi: 10.1597/1545-1569_2000_037_0370_arciip_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 13.Fellows-Mayle W, Hitchens TK, Simplaceanu E, Horner J, Barbano T, Nakaya K, Losee JE, Losken HW, Siegel MI, Mooney MP. Age-related changes in lateral ventricle morphology in craniosynostotic rabbits using magnetic resonance imaging. Childs Nerv Sys. 2004;21:385–391. doi: 10.1007/s00381-004-1107-z. [DOI] [PubMed] [Google Scholar]
- 14.Gosain AK, McCarthy JG, Wisoff JH. Morbidity associated with increased intracranial pressure in Apert and Pfeiffer syndromes: the need for long-term evaluation. Plast Reconstru Surg. 1996;97:292–301. doi: 10.1097/00006534-199602000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Grubb RL, Raichle ME, Phelps ME, Ratcheson RA. Effects of increased intracranial pressure on cerebral blood volume, blood flow, and oxygen utilization in monkeys. J Neurosurg. 1975;43:385–398. doi: 10.3171/jns.1975.43.4.0385. [DOI] [PubMed] [Google Scholar]
- 16.Hendrich KS, Kochanek PM, Williams DS, Schinding JK, Marion DW, Ho C. Early perfusion after controlled cortical impact in rats: quantification by arterial spin-labeled MRI and the influences of spin-lattice relaxation time heterogeneity. Magn Reson Med. 1999;42(4):673–681. doi: 10.1002/(sici)1522-2594(199910)42:4<673::aid-mrm8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Herscovitch P, Racichle ME. What is the correct value for the brain-blood partition coefficient for water? J Cereb Blood Flow Metab. 1985;5:65–69. doi: 10.1038/jcbfm.1985.9. [DOI] [PubMed] [Google Scholar]
- 18.Littleton-Kearney MT, Agnew DM, Traystman RJ, Hurn PD. Effects of estrogen on cerebral blood flow and pial microvasculature in rabbits. Am J Physiol Heart Circ Physiol. 2000;279:H1208–H1214. doi: 10.1152/ajpheart.2000.279.3.H1208. [DOI] [PubMed] [Google Scholar]
- 19.Miller JD, Stanek A, Langfitt TW. Concepts of cerebral perfusion pressure and vascular compression during intracranial hypertension. Prog Brain Res. 1972;35:411–430. doi: 10.1016/S0079-6123(08)60102-8. [DOI] [PubMed] [Google Scholar]
- 20.Mooney MP, Losken HW, Siegel MI, Lalikos JF, Losken A, Burrows AM, Smith TD. Development of a strain of rabbits with congenital, simple, nonsyndromic coronal suture synostosis: part II. Somatic and craniofacial growth patterns. Cleft Palate Craniofacial J. 1994;31:8–16. doi: 10.1597/1545-1569_1994_031_0008_doasor_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 21.Mooney MP, Siegel MI, Burrows AM, Smith TD, Losken HW, Dechant J, Cooper G, Fellows-Mayle W, Kapucu MR, Kapucu LO. A rabbit model of human familial, nonsyndromic unicoronal suture synostosis: part II. intracranial contents, intracranial volume, and intracranial pressure. Childs Nervous System. 1998;14:247–255. doi: 10.1007/s003810050220. [DOI] [PubMed] [Google Scholar]
- 22.Mooney MP, Fellows-Mayle W, Losken HW, Dechant J, Burrows AM, Smith TD, Cooper GM, Pollack I, Siegel MI. Increased intracranial pressure after coronal suturectomy in craniosynostotic rabbits. J Craniofacial Surg. 1999;10:104–110. doi: 10.1097/00001665-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Mooney MP, Siegel MI, Opperman LM. Animal models of craniosynostosis: experimental, congenital, and transgenic models. In: Mooney MP, Siegel MI, editors. Understanding craniofacial anomalies: the etiopathogenesis of craniosynostosis and facial clefting. New York: Wiley-Liss; 2002. pp. 209–250. [Google Scholar]
- 24.Murphy BD, Chen X, Lee T-Y. Serial changes in CT cerebral blood volume and flow after 4 hours of middle cerebral occlusion in an animal model of embolic cerebral ischemia. Am J Neuroradiol. 2007;28:743–749. [PMC free article] [PubMed] [Google Scholar]
- 25.Mursch K, Enk T, Christen HJ, Markakis E, Benke-Mursch J. Venous intracranial haemodynamics in children undergoing operative treatment for the repair of craniosynostosis. A prospective study using transcranial colour-coded duplex sonography. Childs Nervous System. 1999;15:110–116. doi: 10.1007/s003810050344. [DOI] [PubMed] [Google Scholar]
- 26.Nehlig A, Pereira de Vasconcelos A, Boyet S. Postnatal changes in local cerebral blood flow measured by the quantitative autoradiographic [14C] iodoantipyrine technique in freely moving rats. J Cereb Blood Flow Metab. 1989;9(5):579–588. doi: 10.1038/jcbfm.1989.83. [DOI] [PubMed] [Google Scholar]
- 27.Roatta S, Roncari S, Micieli G, Bosone D, Passatore M. Doppler sonography to monitor flow in different cerebral arteries in the rabbit. Exp Physiol. 2000;85(4):432–438. doi: 10.1111/j.1469-445x.2000.02038.x. [DOI] [PubMed] [Google Scholar]
- 28.Rollins N, Booth T, Shapiro K. MR venography in children with complex craniosynostosis. Pediatr Neurosurg. 2000;32(6):308–315. doi: 10.1159/000028959. [DOI] [PubMed] [Google Scholar]
- 29.Sainte-Rose C, LaCombe J, Pierre-Kahn A, Renier D, Hirsch J-F. Intracranial venous sinus hypertension: cause or consequence of hydrocephalus in infants? J Neurosurg. 1984;60:727–736. doi: 10.3171/jns.1984.60.4.0727. [DOI] [PubMed] [Google Scholar]
- 30.Sen A, Dougal P, Padhy AK, Bhaltachaya A, Kumar R, Bal C, Saspai M, Bharadwaj M, Mitra DK, Basu AK. Technetium-99 m-HMPAO SPECT cerebral blood flow study in children with craniosynostosis. J Nucl Med. 1995;36:394–398. [PubMed] [Google Scholar]
- 31.Sgouros S, Hockley A, Goldin JH, Wake MJC, Matarajan K. Intracranial volume changes in craniosynostosis. J Neurosurg. 1999;91:617–625. doi: 10.3171/jns.1999.91.4.0617. [DOI] [PubMed] [Google Scholar]
- 32.Shek JW, Wen GY, Wisniewski HM. Atlas of the rabbit brain and spinal cord. Switzerland: Karger, Basel; 1986. [Google Scholar]
- 33.Stavrou P, Sgouros S, Willshaw HE, Goldin JH, Hockley AD, Wake MJC. Visual failure caused by raised intracranial pressure in craniosynostosis. Childs Nervous System. 1997;13(2):64–67. doi: 10.1007/s003810050043. [DOI] [PubMed] [Google Scholar]
- 34.Tuor UI. Local cerebral blood flow in the newborn rabbit: an autoradiographic study of changes during development. Pediatr Res. 1991;29(5):517–523. doi: 10.1203/00006450-199105010-00020. [DOI] [PubMed] [Google Scholar]
- 35.Walters FJM. Intracranial pressure and cerebral blood flow. Physiology. 1998;8(article 4):1–4. [Google Scholar]
- 36.Zhang W, Williams DW, Koretsky AP. Measurement of rat brain perfusion by NMR using spin labeling of arterial water: in vivo determination of the degree of spin labeling. Magn Reson Med. 1993;29:416–421. doi: 10.1002/mrm.1910290323. [DOI] [PubMed] [Google Scholar]
- 37.Zhang W, Silva AC, Williams DS, Koretsky AP. NMR measurements of perfusion using arterial spin labeling without saturation of macromolecular spins. Magn Reson Med. 1995;33:370–376. doi: 10.1002/mrm.1910330310. [DOI] [PubMed] [Google Scholar]
- 38.Zhang F, Xie J, Han H. MRI reveals changes in intracellular calcium in ischaemic areas of rabbit brain. Neuroradiology. 2003;45(11):773–779. doi: 10.1007/s00234-003-1001-5. [DOI] [PubMed] [Google Scholar]