Summary
In this study, we investigated the mechanism(s) of altered expression of protooncogene SKP2 in metastatic melanoma and its clinical relevance in patients with metastatic melanoma. The genomic status of SKP2 was assessed in cell lines by sequencing, single nucleotide polymorphism array, and genomic PCR. Copy number status was then evaluated for concordance with SKP2 mRNA and protein expression. SKP2 protein was further evaluated by immunohistochemistry in 93 human metastatic tissues. No mutations were identified in SKP2. Increased copy number at the SKP2 locus was observed in 6/14 (43%) metastatic cell lines and in 9/22 (41%) human metastatic tissues which was associated with overexpression of SKP2 protein. Overexpression of SKP2 protein in human tissues was associated with worse survival in a multivariate model controlling for the site of metastasis. Copy number gain is a major contributing mechanism of SKP2 overexpression in metastatic melanoma. Results may have implications for the development of therapeutics that target SKP2.
Keywords: melanoma, metastasis, SKP2, SNP array, gene expression
Introduction
The acquisition of abnormalities at the G1/S-phase transition appears to be the most crucial step in the genesis and progression of melanoma (Hershko, 2008; Sauroja et al., 2000). F-box protein S-phase kinase-associated protein 2 (SKP2), the substrate-binding subunit of an SCF (Skp1, Cul1, F-box protein) ubiquitin ligase complex, regulates G1/S-phase transition via the degradation of the CDKN1B product (p27) (Sherr, 1996). Antisense oligonucleotides targeting the SKP2 transcript as well as the overexpression of a dominant-negative SKP2 mutant have been shown to stabilize p27 (Li et al., 2006). These findings suggest that SKP2 is specifically required for p27 ubiquitination and that it represents a rate-limiting component of the machinery that degrades phosphorylated p27.
Aberrations of the SKP2-p27 pathway have been demonstrated in several solid tumors including melanoma, and low expression of p27 has been linked to melanoma progression and poor outcome (Mouriaux et al., 2000; Alonso et al., 2004). Specifically, it has been shown that SKP2 and p27 levels exhibit a significant inverse relationship that correlates with melanoma progression (Li et al., 2004). Mutations in p27 are rare (Lee and Kim, 2009), suggesting that other molecular mechanisms might contribute to p27 loss such as regulation by microRNAs (Felicetti et al., 2008), altered cellular localization from the nucleus to the cytoplasm (Ahn et al., 2009; Garrett-Engele et al., 2007), and enhanced degradation by SKP2 (Calvisi et al., 2009; Bhatt et al., 2007). Other data suggest that certain oncogenic properties of SKP2 in melanoma are not directly related to the cellular status of p27 (Hu and Aplin, 2008; Katagiri et al., 2006; Yokoi et al., 2003; Sumimoto et al., 2006).
Although evidence supports a role for the SKP2-p27 network in the promotion of melanoma tumorigenesis, the underlying molecular alterations responsible for SKP2 overexpression have not been clearly defined. Previous studies of SKP2 in human melanoma tissue have focused mainly on assessing the correlation between SKP2 expression in the primary tumor and overall survival (Li et al., 2004; Woenckhaus et al., 2005). However, if SKP2 is to be further developed as a treatment target for melanoma, it will most likely be utilized in the setting of metastatic disease. Thus, it is important to specifically assess the mechanism of SKP2 alteration in metastatic tissue and its association with outcome in patients with recurrent/metastatic melanoma. Using melanoma cell lines and human metastatic melanoma tissue, we utilized a combination of assays to investigate possible SKP2 genomic alterations and their association with changes in mRNA and protein expression. We then evaluated the correlation between SKP2 protein expression and survival in a cohort of patients with metastatic melanoma.
Results
Increased DNA copy number at the SKP2 locus in melanoma cell lines
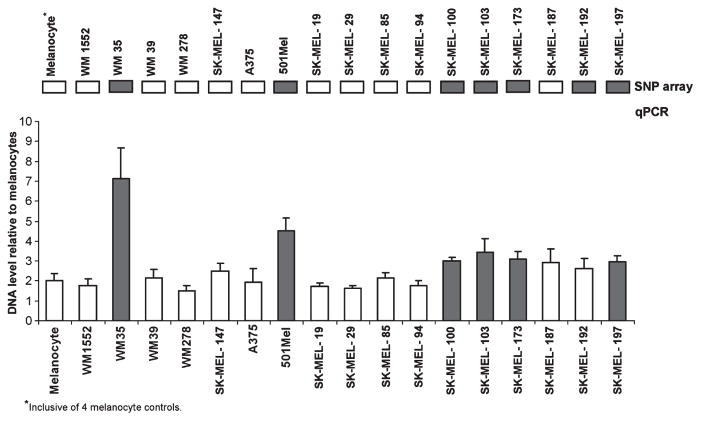
Single nucleotide polymorphism (SNP) array analysis of 18 melanoma cell lines and four melanocyte controls revealed increased DNA copy number at the SKP2 locus (5p13.2) in seven melanoma cell lines (one primary, six metastatic) (Figure S1A). The increased SKP2 copy number in the metastatic cell lines appeared to be part of a genomic alteration involving 5p rather than a global event resulting in an entire extra copy of chromosome 5 (aneuploidy) (Figure S1B). A summary of the segmentation report including the genomic coordinates and sizes of the regional 5p13 gains is provided in Table S1. To validate the SNP array results, we utilized quantitative PCR (qPCR) with primers specific for SKP2 and flanking genomic regions (Table S2A) in the same panel of melanoma cell lines. Using a threshold of DNA copy number ≥3 relative to diploid melanocyte controls, 6 of the 7 (86%) cell lines that demonstrated increased regional copy number gain at 5p13.2 on SNP array also showed increased SKP2 copy number as assessed by qPCR (Figure 1). Thus, only one of the regional amplifications identified by SNP array (SK-MEL-192) was not verified by qPCR, and no additional amplifications were detected by qPCR that were not identified by SNP array.
Figure 1.
Quantitative PCR (qPCR) verification of single nucleotide polymorphism (SNP) array data showing increased DNA copy number at the SKP2 locus (5p13.2) in melanoma cell lines. Only one cell line (SK-MEL-192) showing increased DNA copy number in the region of 5p13.2 on SNP array did not show increased DNA copy number at the SKP2 locus by qPCR. Solid boxes indicate cell lines with increased copy number at the 5p13 locus identified by SNP array (top). Represented qPCR data are the average fold change using six primer sets covering the SKP2 gene and flanking sequences. Data were normalized using controls GAPDH, GUSB, and B2M and values normalized to diploid melanocytes (copy number 2). Copy number ≥3 was regarded as increased SKP2 copy number (solid bars). Metastatic cell line SK-MEL-87 (not shown) was assessed only by SNP array and showed no evidence of increased SKP2 copy number.
Relationship between SKP2 copy number and SKP2 gene expression
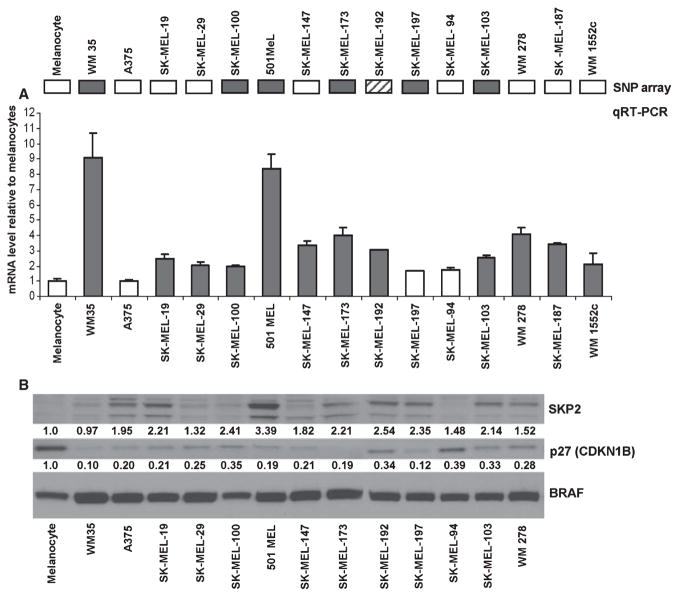
To determine whether copy number gains at the 5p13 locus result in increased expression of SKP2, we examined mRNA levels in 15 of the same melanoma cell lines using qRT-PCR. SKP2 was overexpressed (≥2 fold increase relative to melanocytes) in 12 (80%) of the total melanoma cell lines assessed (Figure 2A). Of the six cell lines with increased SKP2 copy number at the genomic level, 5 (83%) also showed increased expression of SKP2 mRNA (Figure 2A).
Figure 2.
(A) Gene expression of SKP2 assessed using qRT-PCR in a panel of melanoma cell lines that were also evaluated for copy number alterations using single nucleotide polymorphism (SNP) array. Triplicate reactions were run for all samples/primer sets. Data were analyzed by the Pfaffl method with normalization to three internal controls and fold change determined relative to melanocyte or Hermes controls. mRNA level ≥2 times the level in the melanocyte control was considered overexpression (red bars). Twelve of 15 (80%) melanoma cell lines show evidence of increased SKP2 expression. Of the six cell lines with increased SKP2 DNA copy number validated by genomic PCR (solid boxes, top), 5 (83%) also show evidence of increased gene expression. SK-MEL-192, which showed evidence of increased SKP2 DNA copy number by SNP array only (striped box), also shows evidence of increased SKP2 gene expression. (B) Western blot analysis of SKP2 and p27 shows increased SKP2 expression (≥2× melanocyte control) in seven melanoma cell lines: SK-MEL-19, SK-MEL-100, 501 MEL, SK-MEL-173, SK-MEL-192, SK-MEL-197, and SK-MEL-103. Expression of p27 was lower than the melanocyte control in all melanoma cell lines assessed. Levels of BRAF protein served as loading control. Densitometric values for SKP2 and p27 protein levels were normalized to BRAF levels and compared relative to cultured melanocytes.
Evaluation of SKP2 protein by western blot in 13 melanoma cell lines revealed evidence of increased SKP2 expression (≥2× melanocyte control) in 7 (54%) (Figure 2B). Of these seven cell lines, 4 (57%) demonstrated both increased SKP2 DNA copy number and elevated SKP2 mRNA expression (Figure 2B). All melanoma cell lines demonstrated reduced expression of p27 relative to melanocyte control. The inverse correlation of SKP2 and p27 expression is as expected; however, depletion of SKP2 by siRNA in several of these cell lines did not result in appreciable p27 stabilization suggesting an uncoupling of the two (not shown).
No evidence of FZR or SKP2 coding nucleotide changes in melanoma cell lines
Recognizing that mutations in SKP2 or FZR [which encodes Cdh1, an activator of the anaphase promoting complex (APC) ubiquitin ligase that targets SKP2 for degradation] might also contribute to SKP2 overexpression by making the protein more stable, we performed sequencing analyses of both genes. Sequencing of all 16 FZR exons in a cohort of 18 melanoma cell lines revealed only one previously reported SNP (NCBI ref# 10155) in the 3′-untranslated region (3′-UTR) characterized by a G to A polymorphism at position 1524, 7 bp downstream of the coding sequence. Six of 18 cell lines were heterozygous for the G to A polymorphism, and two cell lines were homozygous. Mutational analysis of SKP2 cDNA (10 exons) in the same panel of 18 melanoma cell lines revealed only a single amino acid mismatch in one melanoma cell line, SK-MEL-187. The nucleotide change identified corresponds to a heterozygous mutation (A/G) on exon 7 that has been previously reported in other tumor types as a SNP that produces a silent TCA to TCG codon change (ref #61755301, SNP database NCBI). The relatively low incidence of FZR and SKP2 mutations in our panel of 18 melanoma cell lines suggests that they are rare events that did not justify further SKP2 sequencing analyses in human melanoma tissues.
Expression of SKP2 during the cell cycle progression
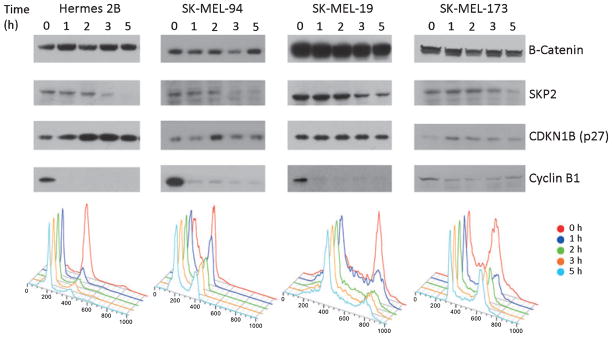
To assess SKP2 overexpression as it relates to cell cycle progression, we examined SKP2 levels by western blot in two metastatic melanoma cell lines with normal SKP2 copy number status (SK-MEL-19 and SK-MEL-94) and one cell line showing increased copy number status by SNP array and qPCR (SK-MEL-173). We also assessed p27 expression in the same cell lines. Cells were synchronized in the M phase using nocodazole with Cyclin B expression serving as a synchronization control. Expression of SKP2 in the primary immortal melanocyte control (Hermes 2B) was high in the M phase (0 h) and gradually decreased in the transition from M to G1 phases (5 h) (Figure 3). This decrease in SKP2 expression was inversely related to p27 levels in the melanocyte control that showed an increase in expression from M to G1 phase. In the 3 melanoma cell lines studied, SKP2 expression remained cell cycle dependent and appeared to be uncoupled from p27 expression, a finding that was independent of the SKP2 copy number status.
Figure 3.
Expression of SKP2 and CDKN1B (p27) during cell cycle progression (M phase, 0 h; G1 phase, 5 h) as assessed by Western blot in three melanoma cell lines (SK-MEL-94, SK-MEL-19, SK-MEL-173) and primary immortalized melanocytes (Hermes 2B). Cells were synchronized in the M phase with nocodazole and harvested at indicated times after release from nocodazole block. Cyclin B1 serves as control for synchronization and B-catenin as loading control.
SKP2 copy number status and protein expression in metastatic melanoma tissue
We next sought to ascertain whether our finding of increased SKP2 DNA copy number and SKP2 overexpression in melanoma cell lines was also observed in human tissues. Twenty-two metastatic melanoma tissues were evaluated for SKP2 genomic amplification and SKP2 protein overexpression. Increased SKP2 copy number (≥3 copies) as assessed by genomic qPCR was observed in 9 of 22 (41%) metastatic tumors. Overexpression of SKP2 protein by IHC was observed in 5 of 22 (23%) metastatic tissues, of which all but one also showed increased SKP2 copy number. Overall, expression of SKP2 protein by IHC was concordant with SKP2 copy number status (i.e, increased copy number and overexpression or diploid copy number and normal expression) in the majority (N = 16, 73%) of metastatic tissues studied.
SKP2 expression differs by site of melanoma metastasis and is an independent predictor of worse post-recurrence survival
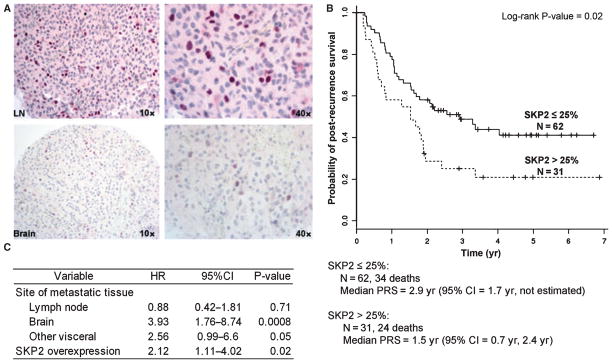
SKP2 expression was assessed in 93 metastatic tissues from 77 patients with melanoma using IHC. Based on previous reports and the distribution of the expression values, a cutoff point of >25% was utilized to dichotomize the data into SKP2 overexpression versus normal/low expression. The SKP2 antibody showed a generally intense nuclear staining of the tumor cells (Figure 4A). There were significant differences noted in mean SKP2 expression by metastatic site (P < 0.001, ANOVA): soft tissue (6%, SD 10), brain (16%, SD 31), lymph node (35%, SD 40), and other visceral (88%, SD28). As expected, there were also significant differences in post-recurrence survival by site of metastasis. Patients with brain and visceral metastases had a worse post-recurrence survival relative to patients with soft tissue metastasis [HR 3.76 (95% CI 1.70–8.34), P = 0.001 and HR 4.11 (95% CI 1.77–9.60), P = 0.001, respectively]. Patients with tumors showing >25% SKP2 staining had a significantly worse post-recurrence survival than those with ≤25% staining [HR 1.89 (95% CI 1.12–3.19), P = 0.02]. The median post-recurrence survival for patients with SKP2 staining ≤25% was 2.9 years (95% CI 1.7–not estimated) versus 1.5 years (95% CI 0.70–2.4 years) for patients with SKP2 staining >25% (P = 0.02, Figure 4B). The association between SKP2 overexpression and worse post-recurrence survival remained statistically significant after controlling for the site of metastasis in a multivariate model [HR 2.12 (95% CI 1.11–4.02), P = 0.02] (Figure 4C).
Figure 4.
Assessment of SKP2 protein expression in human metastatic melanoma tissues and its association with post-recurrence survival in patients with metastatic melanoma. (A) Representative IHC images of strong nuclear SKP2 expression in a metastatic lymph node (top) and weak SKP2 expression in a melanoma brain metastasis (bottom) (counterstaining FastRed). (B) Kaplan–Meier survival curves for patients with high (>25%) versus low/normal (≤25%) SKP2 expression (based on data for 93 specimens). (C) Multivariate model of the association between site of metastasis (soft tissue being the reference group), SKP2 expression (low/normal expression being the reference group), and post-recurrence survival in metastatic melanoma.
Discussion
We report that SKP2 DNA copy number gain contributes to altered SKP2 expression in metastatic melanoma and that SKP2 expression in metastatic tissue is associated with worse survival. Although overexpression of SKP2 mRNA has been reported in several cancers including melanoma, the molecular mechanism driving this overexpression in metastatic melanoma has not been reported. SNP array analysis of metastatic melanoma cell lines revealed copy number gain in 43%, with all but one cell line showing concordant overexpression of SKP2 mRNA. Sequencing data suggest that mutations in SKP2 are not responsible for the aberrant SKP2 expression observed in our melanoma cell lines. These results are consistent with previous studies in lung cancer reporting SKP2 copy number gains in squamous cell carcinomas that were also associated with increased expression at the gene and protein level (Zhu et al., 2004).
The concordance noted between increased SKP2 copy number and elevated SKP2 protein levels in our metastatic tissues was not as strong as that observed between copy number status and gene expression in melanoma cell lines. This may be attributable to tumor heterogeneity, the sensitivity of IHC in detecting SKP2 overexpression, or to differences in proliferation and cell cycle distribution. We also recognize that the 5p13 locus is commonly amplified in several solid malignancies including melanoma. Thus, we cannot exclude the possibility that a different gene, such as the recently described GOLPH3, is a possible additional target of the regional gains observed at the DNA level (Scott et al., 2009). Although the regions identified using SNP array are broad, the primers utilized for the genomic qPCR validation studies target a narrow genomic region containing SKP2 and flanking genes. Therefore, even if other 5p13 genes are targeted by the regional 5p13 gain, it does not make it less plausible that increased copy number contributes to the observed SKP2 overexpression.
Our synchronization studies showed that SKP2 protein expression remained partially cell cycle dependent, whereas p27 expression appeared to be cell cycle independent. These findings are consistent with a recent study of human retinoblastoma cells showing that although SKP2 overexpression was common, it did not correlate with levels of p27 (Wang et al., 2010). The same study also demonstrated that Rb1-deficient cells are dependent upon SKP2 for survival. This finding led the authors to propose a model of tumorigenesis in which pRb loss leads not only to deregulation of its immediate downstream target E2F but also to deregulation of SKP2 (Wang et al., 2010). Another potential mechanism of p27 downregulation in melanoma includes transcriptional regulation by the FOXO (Fork-head) transcription factors, some of which (FOXO1) are also controlled by SKP2-mediated proteasomal degradation following AKT phosphorylation (Huang et al., 2005). In this regard, we recently reported that microRNA-182 represses FOXO3 expression in melanoma (Segura et al., 2009), and other groups have shown that microRNA-182 and microRNA-96 regulate FOXO1 expression in breast cancer (Guttilla and White, 2009).
SKP2 overexpression was significantly associated with worse post-recurrence survival independent of the site of metastasis. As reflected in the tumor, lymph node, metastasis (TNM) staging system, the site of melanoma metastasis is the predominant determinant of the M stage, with primary tumor characteristics such as thickness and ulceration no longer playing a role. Increasing evidence suggests that markers present in metastatic tissues are relevant and may be useful determinants of survival for recurrent/metastatic patients. Recent studies by our group demonstrated that a specific gene expression profile present in metastatic tissue significantly enhances clinical staging in predicting outcome for recurrent/metastatic patients (Bogunovic et al., 2009). Our results demonstrating differential expression of SKP2 by the site of metastasis are consistent with emerging reports that identify molecular mediators of site-specific metastasis in melanoma and other malignancies (Zhang et al., 2009; Padua et al., 2008; Bos et al., 2009). Thus, it is possible that SKP2 expression is not merely a read-out of aggressive disease but rather might be reflective of unique molecular alterations that guide site-specific tropism. Our results show that SKP2 overexpression does not need to be accompanied by low p27 expression to be a negative prognostic marker. Several studies have shown that the composite variable of high SKP2/low p27 is associated with worse outcome in many solid cancers (Traub et al., 2006; Osoegawa et al., 2004). We found that this was true in our data (not shown), but we also show that SKP2 overexpression alone is an independent prognostic variable in metastatic melanoma. This suggests that SKP2 may have oncogenic effects in melanoma that are not related to p27 loss. To this end, it has been shown that SKP2 plays a role in the degradation of other critical cell cycle regulators such as E2F1 (Marti et al., 1999), cyclin E (Yeh et al., 2001), p21 (Bornstein et al., 2003), and p57 (Kamura et al., 2003). Previous studies have also shown that SKP2 expression is inversely correlated with PTEN expression in human gastric cancer and that mouse fibroblasts with PTEN deletions have both increased SKP2 expression and reduced p27 (Ma et al., 2005; Mamillapalli et al., 2001). And finally, evidence in non-small cell lung cancer (NSCLC) suggests that SKP2 may promote tumorigenesis via cooperation with ras (Zhu et al., 2004), which is known to be mutated in approximately 15% of melanomas.
New insight into the structure of the SKP1–SKP2 complex suggests that it may be possible to target SKP2 using small molecule inhibitors (Schulman et al., 2000; Zheng et al., 2002). Previous functional studies suggest, however, that successful reduction in cellular proliferation via SKP2 knockdown is dependent upon the presence or absence of other molecular alterations in key cell cycle regulators (Lin et al., 2010). SKP2 knockdown has been shown to result in tetraploid cell cycle arrest only in p53 wild-type melanoma cells in spite of upregulation of p27 observed in p53 mutants (Hu and Aplin, 2008). Similarly, another study revealed that the combined suppression of BRAF (V599E) and SKP2 inhibited cell growth and attenuated the invasive potential of melanoma cell lines in vitro prompting speculation regarding the possibility of combination therapy targeting BRAF and SKP2 (Sumimoto et al., 2006). Only three melanoma cell lines utilized in the study, however, showed overexpression of SKP2, and only two of these three lines also had BRAF mutations. Thus, further studies are needed to determine whether melanomas with high SKP2 and low p27 expression are also commonly enriched for BRAF mutations. Our data in melanoma cell lines did not reveal an association between SKP2 copy number status and BRAF mutational status – only two cell lines with increased SKP2 copy number (WM35 and SK-100) are BRAF mutant. Similarly, the aforementioned study of Skp2 in Rb-1-deficient cells demonstrated that although there was no difference in survival between Skp2+/+ and Skp2−− mice treated with a tumorigenic protocol, Skp2 knockdown in human retinoblastoma cells induced apoptosis (Wang et al., 2010). The effects of Skp2 knockdown were blocked by restoration of pRb function, consistent with a hypothesis that two major molecular defects are needed in order for SKP2 knockdown to be effective. Although Rb deletions are not frequently detected in melanoma, it is known that Rb defects can be recapitulated by p16INK4a inactivation which is common in melanoma (Lin et al., 2008; Berger et al., 2010).
In conclusion, we demonstrated that increased DNA copy number at the SKP2 locus is a common event in metastatic melanoma cell lines and is concordant with gene expression in most cases. These results suggest that increased SKP2 copy number may contribute to the observed SKP2 overexpression in melanoma. The majority of human tissues that overexpressed SKP2 also showed evidence of increased SKP2 copy number, and overexpression was associated with worse post-recurrence survival independent of the metastatic site. Further studies are warranted to determine the role of targeting SKP2 in melanoma therapy.
Methods
Melanoma cell lines and metastatic tissues
The panel of cell lines utilized for the SNP array included two normal primary melanocytes cultured from human skin (NHM), two primary immortalized melanocytes (Hermes), four melanoma cell lines derived from primary tumors (WM1552c, WM35, WM278, WM39), and 14 melanoma cell lines derived from metastatic tumors (SK-MEL series, A375, 501Mel, and Lu451). SK-MEL metastatic cell lines were kindly provided by Dr Alan Houghton of Memorial Sloan-Kettering Cancer Center (New York, NY, USA). Lu451 and the WM-series were purchased from the Wistar Melanoma Institute (Philadelphia, PA, USA). Cell lines were not further tested or authenticated.
Metastatic melanoma tissues were obtained from 77 patients (45 men, 32 women; median age 54 years) presenting for treatment at the NYU Langone Medical Center who were prospectively enrolled in the NYU Interdisciplinary Melanoma Cooperative Group (IMCG) from December 2002–December 2007 as previously described (Wich et al., 2009). Of the 93 metastatic tissues from 77 patients that were utilized for immunohistochemistry (IHC), 40 were lymph node metastases, 29 were skin/soft tissue metastases, 13 were brain metastases, and 11 were metastases to visceral organs other than the brain. The median follow-up time based on survivors from the date of primary melanoma diagnosis to date of last follow-up was 4.12 years (range: 0.3–16.8 years). Informed consent for the use of clinical data and tissue was obtained from each patient under a protocol approved by the NYU Internal Review Board. Clinicopathologic and survival data were recorded prospectively for all patients.
SNP array analysis
Melanoma and melanocyte DNA was extracted using the Qiagen QIAamp DNA Mini kit (Qiagen, Valencia, CA, USA) and hybridized to the Affymetrix Genome-Wide Human SNP 6.0 Array as per the manufacturer protocol (Affymetrix, Santa Clara, CA, USA). The array contains 1.8 million SNP and copy number probes with an average resolution of 696 base pairs. DNA quantity was assessed by UV spectrophotometry and quality assayed using agarose gel electrophoresis. CEL files were generated using the GeneChip Command Console software (Affymetrix), and the Birdseed v2 algorithm (Korn et al., 2008) was used to make the genotype calls and summarize the probe-set intensity values for ensuing copy number analysis. A single normal reference genome profile assayed in the same batch was created from the two normal melanocytes cultured from human skin. Genomic segments were associated with overlapping genes and annotated according to the University of California Santa Cruz Genome Browser, Build 36.1, hg18 (Kent et al., 2002). The complete MIAME-compliant GeneChip data set has been deposited in the NCBI Gene Expression Omnibus under accession number GSE22305.
Validation of SNP array data using quantitative PCR
qPCR was used to validate SKP2 copy number alterations identified in melanoma cell lines by SNP array and to assess SKP2 DNA copy number status in a cohort of 22 metastatic melanoma tissues. DNA was extracted from melanoma cell lines and paraffin-embedded tissues using the Qiagen QIAmp DNA Mini kit following manufacturer’s instructions (Qiagen). Six primer pairs were designed to cross the common application region in 5p13 as identified using the SNP array with three primers located within the SKP2 gene. The primer sequences and the genomic location of the genes analyzed are summarized in Table S2A. PCRs were performed using Applied Biosystems (Carlsbad, CA, USA) HT7900 in 384-well plates with reaction mixture (10 μl) containing 20 ng of DNA and 5 μl of Applied Biosystems Power SYBR Green PCR Master Mix. Triplicate reactions were run for all samples/primer sets. After a 10-min hot start to initialize the Taq polymerase, two-step cycling of 15 s at 95°C followed by 60 s at 60°C was performed for 40 cycles, followed by melt curve analysis to ensure amplification specificity. Data were exported using SDS 2.3 and analyzed using the methods of Pfaffl (Tichopad et al., 2010) assuming 100% efficiency of amplification with normalization to three control endogenous genomic loci: GAPDH, GUSB, and B2M. Fold change was determined relative to melanocyte or Hermes immortalized melanocyte controls. Expression values were normalized to diploid melanocytes (copy number 2), with copy number ≥3 indicative of increased copy number.
Sequencing of FZR and SKP2
All 10 exons of SKP2 were sequenced bi-directionally in 18 melanoma cell lines via direct sequencing of reverse transcription (RT)-PCR-amplified SKP2 transcripts. We also sequenced all 16 exons of FZR – encoding Cdh1, an activator of the APC ubiquitin ligase, which negatively controls SKP2 levels. Sequences of the primer pairs for FZR and SKP2 are provided in Table S2B. PCR amplification was performed with the Fast Start High Fidelity PCR system, dNTPPack (Roche 04 738 284 001 Basel, Switzerland) following the manufacturer’s instructions, with annealing at 58°C. Screening of identified human FZR and SKP2 mutations was performed using http://www.genewiz.com, and data were analyzed by Sequencher in http://www.genecodes.com.
Assessment of SKP2 gene expression using quantitative RT-PCR
qRT-PCR was used to evaluate SKP2 gene expression in 15 melanoma cell lines. Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA, USA) and the RNeasy mini kit (Qiagen). The quantity and quality of the isolated RNA were measured by UV spectrophotometer. The cDNA was synthesized from the RNA with the Oligo (dT)12–18 primer using Taqman reverse transcription kit (Applied Biosystems) according to the manufacturer’s instructions. cDNA was diluted with primers, 10 nM fluorescence (reference dye), and Fast-Start 2× SYBR Green Master mix (Roche). PCRs were performed in 96-well format using a BioRad MyIQ qRT-PCR system. Triplicate reactions were run for all samples/primer sets. Data were analyzed by the Pfaffl method with normalization to GAPDH and fold change determined relative to melanocyte or Hermes controls. SKP2 expression ≥2 times the level observed in the melanocyte control was considered overexpression.
Immunohistochemical evaluation of SKP2 expression in human metastatic melanoma tissues
Tissue microarrays were created from samples obtained from the biospecimen repository of the NYU-IMCG. IHC was performed on 93 formalin-fixed, paraffin-embedded metastatic tissues from 77 patients using a mouse anti-human SKP2 antibody (clone 2C8D9; Zymed Laboratorie, Carlsbad, CA, USA). In brief, after deparaffinized and rehydrated, sections were pretreated with 1.0 mM EDTA buffer (pH 8.0) in a microwave oven at 1200 W at 90% power for 20 min, following cool for 30 min and then rinsed in distilled water. Antibody incubations and detection were carried out at 37°C on a NEXes instrument (Ventana Medical Systems, Tucson, AZ, USA) using Ventana’s reagent buffer and detection kits. SKP2 (diluted 1:150) was applied overnight at room temperature. The following day, tissue sections were incubated with Ventana’s biotinylated goat anti-mouse secondary antibody and a streptavidin-horseradish-peroxidase conjugate. The complex was visualized with Naphthol-AS-MX phosphatase and Fast Red complex and nuclei counterstained with hematoxylin. Appropriate positive and negative controls were included with each run.
Blinded to patients’ clinical data, an attending pathologist (FD) scored the proportion of cells staining positive for SKP2 using the nuclear-labeling index (LI) per 100 melanoma cells on a continuous scale of 0–100%. The representative mean and median of positive melanoma cells were recorded in tumors with focal regions of positivity. Patients were categorized into two subgroups: expression of SKP2 with a LI of >25% staining of tumor cells (referred to as overexpression) and SKP2 expression with a LI of ≤25% staining of tumor cells (reference level). Previous studies in melanoma (Li et al., 2004) and other solid cancers (Hashimoto et al., 2009) utilizing SKP2 IHC to stratify patients by outcome have defined overexpression as LI > 25%. Thus, our chosen threshold of 25% is consistent with contemporary published reports.
Cell culture and cell cycle analysis
Three human metastatic melanoma cell lines (SK-MEL-19, SK-MEL-94, and SK-MEL-173) were used to assess the expression of SKP2 and p27 at different points in the cell cycle. Primary immortal melanocyte (Hermes 2B) served as a control. Cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum and 2 mM L-glutamine and 100 units/ml penicillin–streptomycin (all reagents from Invitrogen) and incubated at 37°C in 5% CO2. Cells were synchronized in G1/S by incubation in medium containing 2.5 mM thymidine (Sigma Chemical Co., St. Louis, MO, USA) for 24 h. After washing three times with phosphate-buffered saline, cells were synchronized in G2/M by incubation in medium containing 50 ng/ml of nocodazole (Sigma) for 16 h (Zieve et al., 1980). Cells were then shaken-off, released from the mitotic block by being plated in fresh medium, and harvested at different time points up to 5 h after cell release. The synchronization and progression of the cell cycle were monitored by flow cytometry (propidium iodide, PI staining) and Western blot using Cyclin B antibody.
Protein extraction and western blot analysis
Cells were harvested and lysed using the RadioImmuno Precipitation Assay buffer (Pierce/Thermo Fisher Scientific, Rockford, IL, USA) and protease inhibitor cocktail tablets (Roche) for 20 min on ice. Protein concentration was determined using the bicinchoninic acid method (Pierce); 20 μg of protein was subjected to SDS-PAGE and transferred to polyvinylidene fluoride membrane. Membranes were blocked with 5% milk and probed with anti-SKP2 polyclonal antibody (32-3300; Zymed Laboratories, San Francisco, CA, USA); anti-p27 polyclonal antibody (610242; BD Biosciences, San Jose, CA, USA), anti cyclin B, and Ran polyclonal antibody (sc-25764 and sc-1156; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at dilutions of 1:500, 1:1000, 1:1000, and 1:200, respectively. We used anti-rabbit or anti-mouse Ig (Santa Cruz Biotechnology) diluted 1:2500 as secondary antibodies. Membranes were developed with the ECL Plus Western blotting detection kit (Thermo Scientific). Densitometry for western blot protein levels was performed using Scion Image software using the Gelplot2 macro. SKP2 and p27 bands were normalized to BRAF (as a loading control). Normalized values were compared to cultured melanocyte control to yield relative protein expression levels.
Statistical analysis
Post-recurrence survival (from date of recurrence to date of last follow- up or death) was studied with Cox proportional hazards models. The effects that the site of metastasis and SKP2 expression each have on the hazard function as well as their combined contribution in a multivariable model were considered. Soft tissue was chosen as the reference group for the metastatic site and underexpression for the SKP2 expression. Hazard ratios and their 95% confidence intervals were estimated and were reported together with two-sided p-values based on log-rank tests. An analysis of variance (ANOVA) was carried out to assess differences in SKP2 expression means among groups defined by their metastatic site. All analyses were performed using R and the library survival (http://www.r-project.org/).
Supplementary Material
Significance.
Previous melanoma studies investigating protooncogene SKP2 have focused on primary tumors. Therapeutics targeting SKP2 are currently under development and will likely be utilized to treat metastatic patients. Thus, it is important to define the mechanism(s) of altered SKP2 in metastatic melanoma. We report that increased DNA copy number at the SKP2 locus is common in metastatic melanoma cell lines and human tissues and is associated with increased mRNA and protein. SKP2 protein overexpression in human metastatic melanoma was associated with worse survival independent of the site of metastasis, supporting further investigation of SKP2 as a therapeutic target in metastatic melanoma.
Acknowledgments
This work was funded by the NYU Cancer Center Core Grant (5 P30 CA 016087-27 Osman), the Marc Jacobs Campaign for Melanoma Research and grants from the National Institutes of Health (R01-GM057587, R37-CA076584, and R21-AG032560) to MP. We thank Connie Zhao of the Genomics Resource Center of Rockefeller University for technical assistance in performing the SNP arrays. MP is an Investigator with the Howard Hughes Medical Institute.
Footnotes
Additional Supporting Information may be found in the online version of this article:
Figure S1. (A) Evidence of increased DNA copy number at the SKP2 locus (5p13.2) in 7 melanoma cell lines (of 18 assessed) identified using SNP array. (B) Cytoband view showing the location of the amplifications in the metastatic melanoma cell lines.
Table S1. Segmentation analysis of copy number alterations (N = 7) identified at the 5p13.2 locus using SNP array in melanoma cell lines (N = 18) and melanocyte controls (n = 4).
Table S2. Primers utilized for genomic qPCR of SKP2 (A) and sequencing of FZR and SKP2 (B).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Ahn J, Hong S, Lee SE, Kim J, Oh Y, Park S, Chung Y. Cytoplasmic localization of Jab1 and p27 Kip1 might be associated with invasiveness of papillary thyroid carcinoma. Endocr J. 2009;56:707–713. doi: 10.1507/endocrj.k08e-372. [DOI] [PubMed] [Google Scholar]
- Alonso SR, Ortiz P, Pollan M, et al. Progression in cutaneous malignant melanoma is associated with distinct expression profiles: a tissue microarray-based study. Am J Pathol. 2004;164:193–203. doi: 10.1016/s0002-9440(10)63110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger MF, Levin JZ, Vijayendran K, et al. Integrative analysis of the melanoma transcriptome. Genome Res. 2010;20:413–427. doi: 10.1101/gr.103697.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt KV, Hu R, Spofford LS, Aplin AE. Mutant B-RAF signaling and cyclin D1 regulate Cks1/S-phase kinase-associated protein 2-mediated degradation of p27Kip1 in human melanoma cells. Oncogene. 2007;26:1056–1066. doi: 10.1038/sj.onc.1209861. [DOI] [PubMed] [Google Scholar]
- Bogunovic D, O’neill DW, Belitskaya-Levy I, et al. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci USA. 2009;106:20429–20434. doi: 10.1073/pnas.0905139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J Biol Chem. 2003;278:25752–25757. doi: 10.1074/jbc.M301774200. [DOI] [PubMed] [Google Scholar]
- Bos PD, Zhang XH, Nadal C, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvisi D, Ladu S, Pinna F, et al. SKP2 and CKS1 promote degradation of cell cycle regulators and are associated with hepatocellular carcinoma prognosis. Gastroenterology. 2009;137:1816–1826. doi: 10.1053/j.gastro.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Felicetti F, Errico MC, Bottero L, et al. The promyelocytic leukemia zinc finger-microRNA-221/-222 pathway controls melanoma progression through multiple oncogenic mechanisms. Cancer Res. 2008;68:2745–2754. doi: 10.1158/0008-5472.CAN-07-2538. [DOI] [PubMed] [Google Scholar]
- Garrett-Engele C, Tasch M, Hwang H, Fero M, Perlmutter R, Clurman B, Roberts J. A mechanism misregulating p27 in tumors discovered in a functional genomic screen. PLoS Genet. 2007;3:e219. doi: 10.1371/journal.pgen.0030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttilla I, White B. Coordinate regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast cancer cells. J Biol Chem. 2009;284:23204–23216. doi: 10.1074/jbc.M109.031427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N, Yachida S, Okano K, et al. Immunohistochemically detected expression of p27(Kip1) and Skp2 predicts survival in patients with intrahepatic cholangiocarcinomas. Ann Surg Oncol. 2009;16:395–403. doi: 10.1245/s10434-008-0236-0. [DOI] [PubMed] [Google Scholar]
- Hershko DD. Oncogenic properties and prognostic implications of the ubiquitin ligase Skp2 in cancer. Cancer. 2008;112:1415–1424. doi: 10.1002/cncr.23317. [DOI] [PubMed] [Google Scholar]
- Hu R, Aplin A. Skp2 regulates G2/M progression in a p53-dependent manner. Mol Biol Cell. 2008;19:4602–4610. doi: 10.1091/mbc.E07-11-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Regan KM, Wang F, Wang D, Smith DI, Van Deursen JM, Tindall DJ. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci USA. 2005;102:1649–1654. doi: 10.1073/pnas.0406789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamura T, Hara T, Kotoshiba S, Yada M, Ishida N, Imaki H, Hatakeyama S, Nakayama K, Nakayama K. Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc Natl Acad Sci USA. 2003;100:10231–10236. doi: 10.1073/pnas.1831009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri Y, Hozumi Y, Kondo S. Knockdown of Skp2 by siRNA inhibits melanoma cell growth in vitro and in vivo. J Dermatol Sci. 2006;42:215–224. doi: 10.1016/j.jdermsci.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn JM, Kuruvilla FG, Mccarroll SA, et al. Integrated genotype calling and association analysis of SNPs, common copy number polymorphisms and rare CNVs. Nat Genet. 2008;40:1253–1260. doi: 10.1038/ng.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kim S. The function of p27 KIP1 during tumor development. Exp Mol Med. 2009;41:765–771. doi: 10.3858/emm.2009.41.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Murphy M, Ross J, Sheehan C, Carlson JA. Skp2 and p27kip1 expression in melanocytic nevi and melanoma: an inverse relationship. J Cutan Pathol. 2004;31:633–642. doi: 10.1111/j.0303-6987.2004.00243.x. [DOI] [PubMed] [Google Scholar]
- Li W, Sanki A, Karim RZ, Thompson JF, Soon Lee C, Zhuang L, Mccarthy SW, Scolyer RA. The role of cell cycle regulatory proteins in the pathogenesis of melanoma. Pathology. 2006;38:287–301. doi: 10.1080/00313020600817951. [DOI] [PubMed] [Google Scholar]
- Lin HK, Chen Z, Wang G, et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 2010;464:374–379. doi: 10.1038/nature08815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WM, Baker AC, Beroukhim R, et al. Modeling genomic diversity and tumor dependency in malignant melanoma. Cancer Res. 2008;68:664–673. doi: 10.1158/0008-5472.CAN-07-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Liu Y, Guo JW, Liu JH, Zuo LF. Relation of overexpression of S phase kinase-associated protein 2 with reduced expression of p27 and PTEN in human gastric carcinoma. World J Gastroenterol. 2005;11:6716–6721. doi: 10.3748/wjg.v11.i42.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamillapalli R, Gavrilova N, Mihaylova VT, Tsvetkov LM, Wu H, Zhang H, Sun H. PTEN regulates the ubiquitindependent degradation of the CDK inhibitor p27(KIP1) through the ubiquitin E3 ligase SCF(SKP2) Curr Biol. 2001;11:263–267. doi: 10.1016/s0960-9822(01)00065-3. [DOI] [PubMed] [Google Scholar]
- Marti A, Wirbelauer C, Scheffner M, Krek W. Interaction between ubiquitin-protein ligase SCFSKP2 and E2F-1 underlies the regulation of E2F-1 degradation. Nat Cell Biol. 1999;1:14–19. doi: 10.1038/8984. [DOI] [PubMed] [Google Scholar]
- Mouriaux F, Maurage CA, Labalette P, Sablonniere B, Malecaze F, Darbon JM. Cyclin-dependent kinase inhibitory protein expression in human choroidal melanoma tumors. Invest Ophthalmol Vis Sci. 2000;41:2837–2843. [PubMed] [Google Scholar]
- Osoegawa A, Yoshino I, Tanaka S, Sugio K, Kameyama T, Yamaguchi M, Maehara Y. Regulation of p27 by S-phase kinase-associated protein 2 is associated with aggressiveness in non-small-cell lung cancer. J Clin Oncol. 2004;22:4165– 4173. doi: 10.1200/JCO.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massague J. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauroja I, Smeds J, Vlaykova T, Kumar R, Talve L, Hahka-Kemppinen M, Punnonen K, Jansen CT, Hemminki K, Pyrhonen S. Analysis of G(1)/S checkpoint regulators in metastatic melanoma. Genes Chromosomes Cancer. 2000;28:404–414. doi: 10.1002/1098-2264(200008)28:4<404::aid-gcc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Schulman BA, Carrano AC, Jeffrey PD, Bowen Z, Kinnucan ER, Finnin MS, Elledge SJ, Harper JW, Pagano M, Pavletich NP. Insights into SCF ubiquitin ligases from the structure of the Skp1-Skp2 complex. Nature. 2000;408:381–386. doi: 10.1038/35042620. [DOI] [PubMed] [Google Scholar]
- Scott KL, Kabbarah O, Liang MC, et al. GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature. 2009;459:1085–1090. doi: 10.1038/nature08109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura MF, Hanniford D, Menendez S, et al. Aberrant miR-182 expression promotes melanoma metastasis by repressing FOXO3 and microphthalmia-associated transcription factor. Proc Natl Acad Sci USA. 2009;106:1814–1819. doi: 10.1073/pnas.0808263106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Sumimoto H, Hirata K, Yamagata S, Miyoshi H, Miyagishi M, Taira K, Kawakami Y. Effective inhibition of cell growth and invasion of melanoma by combined suppression of BRAF (V599E) and Skp2 with lentiviral RNAi. Int J Cancer. 2006;118:472–476. doi: 10.1002/ijc.21286. [DOI] [PubMed] [Google Scholar]
- Tichopad A, Bar T, Pecen L, Kitchen R, Kubista M, Pfaffl M. Quality control for quantitative PCR based on amplification compatibility test. Methods. 2010;50:308–312. doi: 10.1016/j.ymeth.2010.01.028. [DOI] [PubMed] [Google Scholar]
- Traub F, Mengel M, Luck HJ, Kreipe HH, Von Wasielewski R. Prognostic impact of Skp2 and p27 in human breast cancer. Breast Cancer Res Treat. 2006;99:185–191. doi: 10.1007/s10549-006-9202-3. [DOI] [PubMed] [Google Scholar]
- Wang H, Bauzon F, Ji P, et al. Skp2 is required for survival of aberrantly proliferating Rb1-deficient cells and for tumorigenesis in Rb1 +/− mice. Nat Genet. 2010;42:83–88. doi: 10.1038/ng.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wich LG, Hamilton HK, Shapiro RL, et al. Developing a multidisciplinary prospective melanoma biospecimen repository to advance translational research. Am J Transl Res. 2009;1:35–43. [PMC free article] [PubMed] [Google Scholar]
- Woenckhaus C, Maile S, Uffmann S, Bansemir M, Dittberner T, Poetsch M, Giebel J. Expression of Skp2 and p27KIP1 in naevi and malignant melanoma of the skin and its relation to clinical outcome. Histol Histopathol. 2005;20:501–508. doi: 10.14670/HH-20.501. [DOI] [PubMed] [Google Scholar]
- Yeh KH, Kondo T, Zheng J, Tsvetkov LM, Blair J, Zhang H. The F-box protein SKP2 binds to the phosphorylated threonine 380 in cyclin E and regulates ubiquitin-dependent degradation of cyclin E. Biochem Biophys Res Commun. 2001;281:884–890. doi: 10.1006/bbrc.2001.4442. [DOI] [PubMed] [Google Scholar]
- Yokoi S, Yasui K, Iizasa T, Takahashi T, Fujisawa T, Inazawa J. Down-regulation of SKP2 induces apoptosis in lung-cancer cells. Cancer Sci. 2003;94:344–349. doi: 10.1111/j.1349-7006.2003.tb01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhang F, Tsan R, Fidler I. Transforming growth factor-beta2 is a molecular determinant for site-specific melanoma metastasis in the brain. Cancer Res. 2009;69:828–835. doi: 10.1158/0008-5472.CAN-08-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N, Schulman BA, Song L, et al. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature. 2002;416:703–709. doi: 10.1038/416703a. [DOI] [PubMed] [Google Scholar]
- Zhu CQ, Blackhall FH, Pintilie M, et al. Skp2 gene copy number aberrations are common in non-small cell lung carcinoma, and its overexpression in tumors with ras mutation is a poor prognostic marker. Clin Cancer Res. 2004;10:1984–1991. doi: 10.1158/1078-0432.ccr-03-0470. [DOI] [PubMed] [Google Scholar]
- Zieve GW, Turnbull D, Mullins JM, McIntosh JR. Production of large numbers of mitotic mammalian cells by use of the reversible microtubule inhibitor nocodazole. Nocodazole accumulated mitotic cells. Exp Cell Res. 1980;126:397–405. doi: 10.1016/0014-4827(80)90279-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.