Abstract
P/midget ganglion cells mediate red-green color opponency in anthropoids. It has been proposed that these cells evolved as a specialization to subserve color vision in primates. If that is correct, they must have evolved about the same time as the long-wavelength (‘red’) and medium-wavelength (‘green’) pigment genes diverged, thirty million years ago. Strepsirhines are another group of primates that diverged from the ancestor of the anthropoids at least 55 million years ago. If P/midget ganglion cells evolved to subserve color vision, they should be absent in strepsirhines. We tested this hypothesis in a nocturnal strepsirhine, the greater bush baby Otolemur. The retinal ganglion cells were labeled with the lipophilic tracer DiI and the results show that bush babies have P/midget and M/parasol cells similar to those found in the peripheral retinas of anthropoids. A number of studies have shown that the P and M pathways of bush babies share many similarities with those of anthropoids, and our results show that the same is true for their retinal ganglion cells. These results support the hypothesis that the P system evolved prior to the emergence of red–green color opponency.
Keywords: Visual pathways, Color vision, Color opponency, Evolution, Primates
1. Introduction
Primates can be divided in two groups: the strepsirhines and the haplorhines. Bush babies are lemuriform primates that together with lemurs and lorises belong to the strepsirhine group. The haplorhines comprise the New and Old World anthropoids (or simians) plus the genus Tarsius (see ref. [1] for a review). Prosimian is another term used to describe lemuriform primates, but depending on the classification scheme, it may also include Tarsius [2]. According to fossil evidence, haplorhines and strepsirhines diverged at least 55 million years ago [1]. Nevertheless, several studies on the visual system in different species of primates have pointed out remarkable similarities that define a basic ‘primate pattern’ [3].
The similarity in the organization of the central visual pathways is contrasted with a remarkable diversity in color vision. Bush babies are nocturnal strepsirhines that have a single type of cone [4,5], with peak sensitivity at 545 nm [6]. It has been demonstrated in electroretinographic studies that they are monochromats [6]. Diurnal or crepuscular strepsirhines also exist however, and some of these are dichromats, with two classes of cones, one sensitive to short-wavelengths and the other to medium-to-long wavelengths [7]. A variety of additional color vision patterns have been found among anthropoids [8–11]. Old World anthropoids (humans included) have three different cone pigments [12,13] and trichromatic color vision. Most New World anthropoids have a polymorphic pattern of color vision, in which only two-thirds of the females are trichromats, but all males and the remaining females are dichromats [14,15]. Among dichromats and trichromats, six different phenotypes are possible due to polymorphism. Two genera of New World monkeys do not conform to this pattern, however. The howler monkey Alouatta is a trichromat similar to Old World anthropoids [16], and the nocturnal owl monkey Aotus is a monochromat like the bush baby [5,17]. This diversity in color vision among living primates provides an opportunity to investigate the evolution of the visual pathways.
It has been proposed that P/midget ganglion cells evolved as a specialization to subserve color vision in primates [18]. According to this hypothesis, foveal P cells and midget bipolar cells evolved small dendritic fields in order to mediate specific connections with long-wavelength (LWS or ‘red’) or medium-wavelength (MWS or ‘green’) sensitive cones. The blue–yellow color signal is conveyed by a different set of ganglion cells, the small-field bistratified cells [19,20]. If P cells evolved primarily to subserve red–green opponency, they must have evolved about the same time as the LWS and MWS pigment genes diverged, approximately 30 million years ago [13,21]. An alternative hypothesis is that P cells evolved to subserve spatial vision and only later became useful for color vision, after the mutations that gave rise to the LWS and MWS photopigments [8,22].
Retinal ganglion cells associated with the parvo- (P), magno- (M) and koniocellular (K) pathways have been studied extensively in Old World anthropoids [19,20,23–30] and more recently, also in New World monkeys [31–40]. Although not all aspects of the connectivity and receptive field properties have been investigated in New World monkeys, the evidence so far indicates that their M/parasol, P/midget and small-field bistratified cells are very similar to those found in Old World anthropoids. It is still not clear how similar strepsirhines are to anthropoids with respect to their retinal organization. There have been two studies of the ganglion cell distribution in bush babies [41,42], and the distribution of photoreceptors has also been described [4]. These studies reported features that are consistent with a nocturnal pattern: a lower ganglion cell density and a higher proportion of rods in comparison to diurnal anthropoids. Also, given that extant strepsirhines have only one pigment in the LWS/MWS range [6,7,9], it seems unlikely that they ever evolved red–green color opponency. If P ganglion cells are indeed a specialization for red–green color opponency, they should be absent in strepsirhines.
In this study, we labeled the ganglion cells in the bush baby retina with the lipophilic fluorescent tracer DiI. Based on dendritic branching pattern, soma and dendritic field size, we identified the retinal ganglion cells associated with the parvocellular and magnocellular pathways in the bush baby. We found that bush baby P and M cells share many similarities with those of anthropoids, supporting the idea that these cells were already present in their common ancestor.
2. Materials and methods
2.1. DiI labeling
Five retinas from four adult bush babies Otolemur garnettii (previously known as Galago crassicaudatus or Galago garnettii) were used in this study. The eyes were obtained at the conclusion of unrelated anatomical experiments. The animals were euthanized with an overdose of pentobarbital sodium (Nembutal) and then enucleated. All procedures followed NIH Committee guidelines. The eyes were hemisected, the posterior eye cup was cut into temporal and nasal halves, and the vitreous was removed. We placed a fluorescent lipophilic tracer DiI (Molecular Probes) into living or lightly-fixed retinas to label the retinal ganglion cells. For DiI labeling, small crystals were typically inserted into the retina from the vitread side using minuten pins glued to insect pins. For experiments with living retinas, the pieces were maintained in carboxygenated (CO2:O2 5:95 v/v) AMES medium (Sigma) for 2–3 h at room temperature after application of DiI and were then fixed in 2% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB) pH 7.4 overnight at 20°C. For fixed retina experiments, DiI crystals were applied after the tissue was fixed in 2 or 4% PFA in PB for 30 min at 20°C. The pieces were usually transferred to a more dilute solution (0.2%) of PFA in the same buffer, where they remained for 1–3 weeks before they were flat-mounted. Otherwise, they were maintained in 2% PFA in PB.
2.2. Analysis
DiI-labeled cells were studied in retinal whole-mount preparations using a Zeiss confocal laser scanning microscope with a krypton–argon laser. Excitation was at 568 nm and the emission filter was high pass 590 nm. VoxelView 2.5.2 (Vital Images, Fairfield, IO) was used for dendritic field and soma measurements on confocal images. The dendritic field was defined as the area within a convex polygon circumscribing the tips of the distal dendrites. Using Zeiss LSM software, images of the entire dendritic tree were obtained by reconstructing stacks of ten to 20 0.5–0.7 µm optical sections. For soma size measurements, we took the outline of the largest profile. Eccentricity is the distance in mm from the center of the fovea or area centralis. Retinal ganglion cells were classified based on their dendritic morphology and soma size (see Section 3). Photoshop 3.0 (Adobe Systems, Mountain View, CA) was used to process images for publication.
3. Results and discussion
3.1. Retinal ganglion cell morphology
Bush baby retinal ganglion cells were labeled at several eccentricities. Except for the three most peripheral P cells that were located in the nasal quadrant, all other cells included in our sample were from either the temporal or dorsal quadrants. Based on comparisons with intracellularly-injected ganglion cells from anthropoids, the cells appeared to be labeled completely. Other types of retinal neurons were also labeled, including HI horizontal cells, AII and other types of amacrine cells and several types of bipolar cells, but these will not be discussed further here.
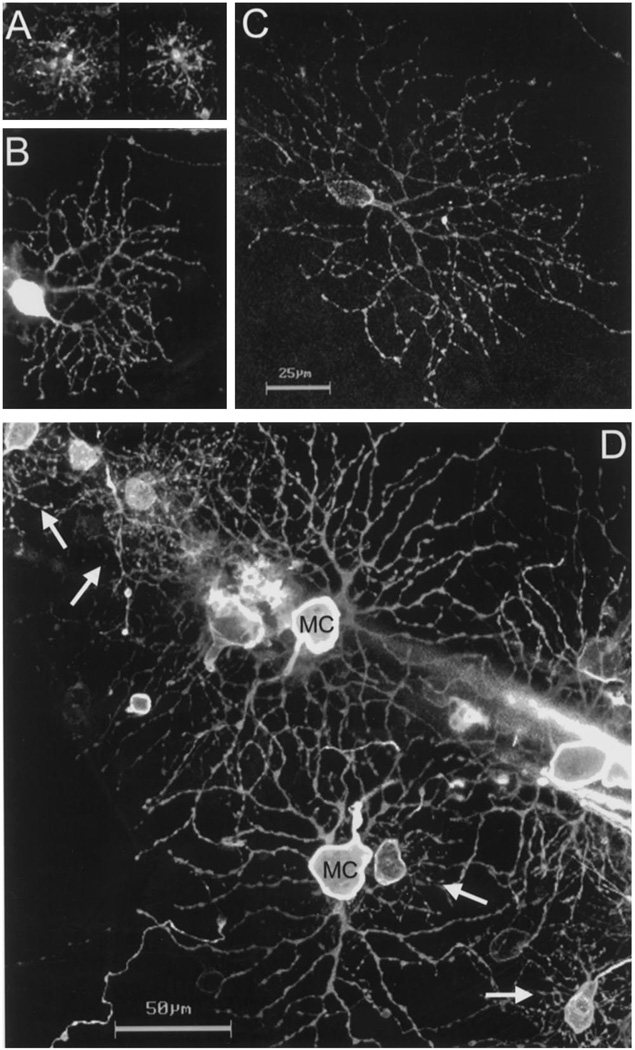
Fig. 1 shows examples of DiI-labeled P and M ganglion cells of the bush baby retina. The P cells had small- to medium-sized somas, one to two primary dendrites and small, densely-branched dendritic trees composed of tortuous dendrites (Fig. 1A–C). In contrast, M cells had larger somas with two to four thick primary dendrites that branched in a more radiate manner, giving rise to much larger dendritic trees (Fig. 1D). According to the depth of stratification of their dendrites in the inner plexiform layer (IPL), both P and M cells could be subdivided into inner or outer varieties. Based on electrophysiological results in macaque, M and P cells ramifying in the inner half of the IPL are expected to have ON responses to light and those branching in the outer half, OFF responses [19]. In the central retina, P cells had one primary dendrite that ascended through the IPL before it ramified. In the periphery, P cells with either one or two primary dendrites were observed (Fig. 1B,C). Somas of some P cells were found at the centers of the dendritic fields, whereas others were eccentric, at the edges of the dendritic fields. Dendritic trees of peripheral P cells most closely resembled those described as tightly branched in the marmoset, a diurnal New World monkey [33,35,39]. P cells with less uniform, clustered dendrites, as reported in the peripheral retinas of macaques and humans [25,27,28], were never encountered in the bush baby. We did not observe dendritic overlap between homologous subclasses of P cells in the central region, but we did observe some overlap in the periphery. We did not quantify this overlap since our sample was not large enough to draw a definite conclusion.
Fig. 1.
A–C: DiI-labeled P ganglion cells of the bush baby retina in a whole-mount preparation; scale bar = 25 µm. A: dendritic trees of two ON-P cells at 0.6 mm temporal. B: an ON-P cell at 4 mm dorsal. C: an ON-P cell at 8 mm nasal. D: Patch of bush baby retina at 2.5 mm of eccentricity (temporal retina) with neighboring P (arrows) and M cells (MC); scale bar = 50 µm. All images are reconstructed stacks of confocal optical sections.
M cells in diurnal simians have numerous small branches arising from the larger primary and secondary dendrites [25,38]. M cells in the bush baby (Fig. 1D) seemed to be less extensively branched, and in this respect, they resembled M cells found in the owl monkey Aotus [32,39]. Fig. 1D shows neighboring M and P cells in the bush baby retina located 2.5 mm from the area centralis and serve to illustrate the differences between the two types in their morphology.
3.2. Dendritic field and soma sizes
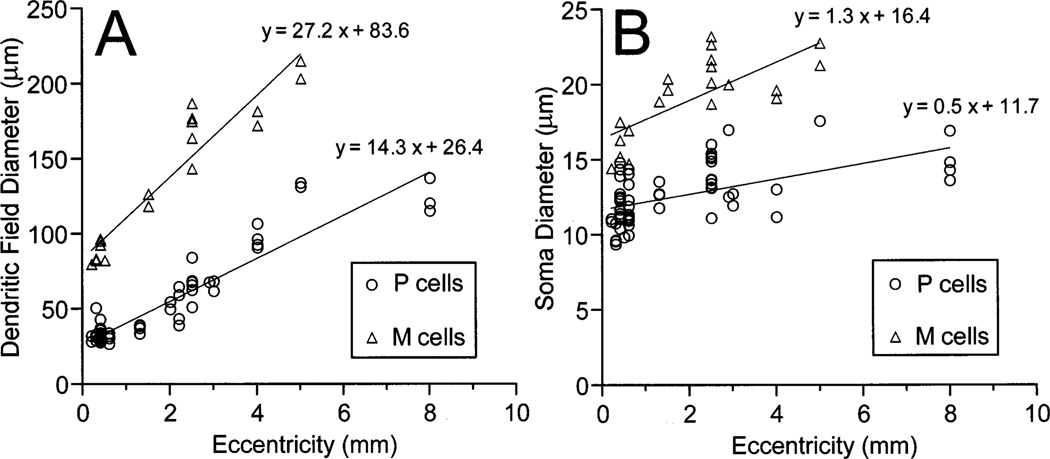
In order to quantitatively compare the P and M cell classes identified here, we measured the sizes of their dendritic fields and somas at different eccentricities. The results are shown in Fig. 2. P cell dendritic field diameters (n = 52) ranged from 28 µm at 0.2 mm from the area centralis to 136 mm in the far periphery. Dendritic fields of M cells (n = 19) were two to three times larger than P cells, with dendritic field diameters ranging from 79 µm in central retina to 214 µm at 5 mm of eccentricity. M cells had, on average, 1.5-fold larger somas than P cells. The soma diameters ranged from nine to 17 µm (n = 61) for P cells and from 14 to 23 µm (n = 20) for M cells. In a previous study, Casagrande and DeBruyn [43] described ganglion cells after HRP injections into LGN. Although they obtained only partial filling of the dendritic trees, it is clear that the DiI-labeled P cells of the present study correspond to their type 2 cells which project to the parvocellular layers, and the DiI-labeled M cells correspond to their type 1 cells which project to the magnocellular layers. In another study, Itoh et al. [44] reported the soma sizes of the retinal ganglion cells projecting to the parvocellular and magnocellular layers after localized injections of horseradish peroxidase (HRP) into the LGN. They also found that parvocellular-projecting ganglion cells had smaller somas than the magnocellular-projecting cells. Fig. 2B indicates that DiI-labeled P and M cells could be distinguished by the sizes of their somas. Like Itoh et al. [44], we found only a small amount of overlap between the soma sizes of P and M cells. We are thus confident that our P cells correspond to the parvocellular-projecting cells and the M cells to the magnocellular-projecting cells in these two previous studies.
Fig. 2.
Dendritic field (A) and soma (B) diameters of bush baby P cells (circles) and M cells (triangles) as a function of retinal eccentricity.
Our estimates of dendritic field diameters of P and M cells are also very consistent with receptive field center measurements reported elsewhere. According to the data of Fig. 3 of Irvin et al. [45], the mean receptive field diameter for LGN parvocellular neurons is 0.38 ± 0.1° (n = 12) for the central 20° of eccentricity (≈ 2.8 mm; see also legend of Fig. 3). Our estimates for P cell dendritic field diameter at comparable eccentricity is 0.29 ± 0.1° (n = 40). For magnocellular neurons, the mean receptive field diameter is 0.85 ± 0.45° (n = 20), while the M ganglion cell mean dendritic field diameter is 0.89 ± 0.29° (n = 15). This correlation between dendritic field and receptive field center sizes gives an additional support for the view that the P cells described here indeed project to the parvocellular layers, and the M cells to the magnocellular layers.
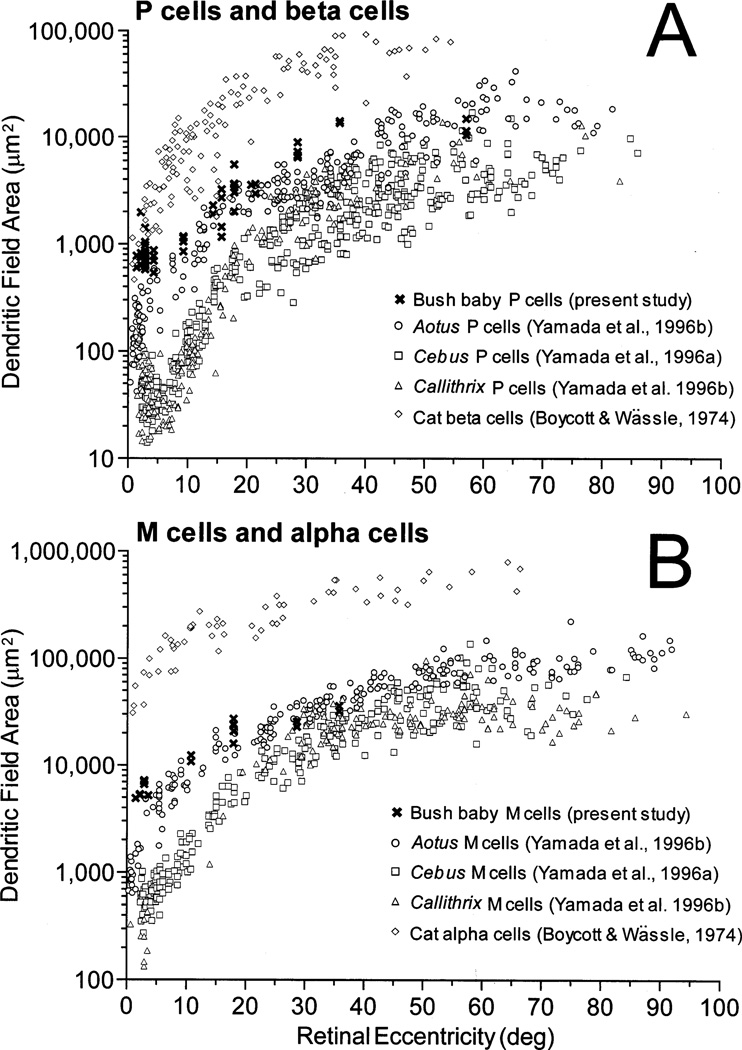
Fig. 3.
Comparison between bush baby, owl monkey (Aotus), capuchin monkey (Cebus), marmoset (Callithrix) and cat retinal ganglion cells. A: Dendritic field area for primate P cells and cat β cells. B: Dendritic field area for primate M cells and cat α cells. Data for Cebus were replotted from Yamada et al. [38] and those for Aotus and Callithrix, from Yamada et al. [39]. Data for cat were from Boycott and Wässle [49] as replotted by Rodieck et al. [60]. Eccentricity is expressed in degrees of visual angle in order to normalize the retinal extent. For bush baby eccentricities, we used 140 µm/° as the conversion factor from linear to angular distance [41].
3.3. Comparison to other species
The P and M ganglion cells of bush babies are very similar to those found in the peripheral retinas of anthropoids. The major difference is that the very small cells found in central retinas of anthropoids are absent in bush babies. Since bush babies are nocturnal and most anthropoids are diurnal, it is essential to determine whether the larger dendritic field sizes represent an adaptation to nocturnal life. For this purpose we compared bush babies to a more distantly related nocturnal species. The cat is particularly appropriate for this comparison for several reasons. Bush babies and cats have similar ganglion cell densities [41,46]. Like primates, cats have frontal eyes and a specialized central area at the intersection of the horizontal and vertical meridians, rather than a horizontal visual streak as found in species with more lateral eyes [47]. The owl monkey, Aotus, is the only living nocturnal anthropoid and the lack of typical nocturnal features such as a tapetum indicates that Aotus may have become nocturnal more recently than bush babies and cats [48]. Nevertheless, a comparison with this species is also essential.
In Fig. 3A, we compared dendritic field sizes of bush baby P cells, cat β cells [49] and P cells from three different species of New World monkeys: two diurnal, the capuchin monkey Cebus [38] and the marmoset Callithrix [39], and one nocturnal monkey, the owl monkey Aotus [39]. Previous studies have shown that P and M ganglion cells of Cebus and Callithrix are very similar to those of macaque monkeys and humans, and are thus representative of the diurnal anthropoid pattern [33,35,38,39]. In the central 10°, bush baby P cells (Fig. 3A, crosses) tend to have larger dendritic fields than all monkeys, including the nocturnal Aotus (empty circles), although the discrepancy with Aotus is less pronounced. On the other hand, bush baby P cells fall within the same range as cat β cells (empty diamonds) for the centralmost eccentricities. Towards the periphery, bush baby P cells tend to have smaller dendritic trees than cat β cells [49,50], but a larger sample is needed to confirm this trend. In Fig. 3B we show a similar comparison for primate M cells [38,39] and cat α cells [49]. Primate M cells tend to form a cluster remaining highly segregated from cat α cells (empty diamonds), the latter exhibiting relatively larger dendritic trees. When only primates are compared, bush baby M cells (crosses) have larger dendritic fields than the three monkeys at the centralmost eccentricities, but they fall within the same size range for eccentricities > 10°. Since all three nocturnal species have ganglion cells with larger dendritic trees than the diurnal species, we conclude that this must be an adaptation to nocturnal life. Presumably, larger dendritic fields provide greater absolute sensitivity by increasing the area for summation of photons.
3.4. Cone convergence
One feature that is characteristic of the anthropoid retina is the low convergence from cones to central P cells. It has been shown by light microscopy and directly, by electron microscopy, that macaque foveal P cells receive input from only one cone through a single midget bipolar cell [51–54]. The similarity in the sizes of dendritic fields of P cells and axon terminals of midget bipolar cells in New World monkeys suggests that a similar one-to-one relation is also present in this group of primates [55]. We believe that P cells in bush babies also receive input from midget bipolar cells since we have observed bipolar cells morphologically similar to the midget bipolar cells found in the periphery of macaque and human retina in our DiI-labeled retinal preparations (Yamada, Marshak and Casagrande, unpublished observations). To determine whether bush babies conform to this anthropoid pattern, we estimated the numerical cone convergence for its central P cells. This feature has been shown to clearly separate P cells from M cells independently of dendritic field size [32,35]. Our results show that cone convergence to P cells in bush baby central retina is relatively low, as in other primates, but it is higher than one. Cone convergence was estimated by taking a mean dendritic field area of 853 µm2 (n = 19, S.D. = 332 µm2, eccentricities from 0 to 0.5 mm) and a cone density of 6000/mm2 for the central area [4]. This gives a numerical convergence of five cones per P cell dendritic field area, a ratio equivalent to that reported for anthropoids between 10 to 15° of eccentricity [35]. A similar calculation for cat central β cells gives a much higher value, ≈ 30 cones per β cell (Fig. 5 of [35]), a ratio comparable to the value found directly by electron microscopy [56]. Thus, despite having relatively large dendritic trees, bush baby P cells show a much lower cone convergence than cat β cells. This suggests that a low cone convergence for central P cells is a characteristic feature of primate retinal organization.
We have also estimated numerical cone convergence for bush baby M cells. Taking a mean dendritic field area of 5931 µm2 (n = 7, S.D. = 976 µm2, eccentricities from 0 to 0.5 mm) and the same cone density as above, we obtained 35 cones per M cell dendritic field area. This value is in close agreement with the ones reported for M cells in diurnal anthropoids and for cat β cells [35]. In contrast, cat α cells have a much higher cone convergence, ≈ 800 cones per α cells in the central retina [32,35]. The similarity between bush baby M cells and those of anthropoids provides further support for the idea that the M pathways are organized similarly in all primates.
3.5. Rod convergence
The bush baby retina has a much higher ratio of rods to cones than diurnal anthropoids and does not have a rod-free central area [4]. Numerical rod convergence to bush baby P and M cells was estimated by taking the same values of dendritic field area as above and a rod density of 360000/mm2 [4]. This gives a rod convergence of 307 rods per P cells and 2135 rods per M cell in the central retina. These values are comparable to those of diurnal anthropoids at ≈ 25° of eccentricity [35]. A similar calculation for cat β cells taking a dendritic field area of 908 µm2 [56] and a rod density of 220000/mm2 [57] gives 200 rods per β cell in the area centralis. Numerical rod convergence to central α cells was reported previously as 4600 rods per α cell [32]. On the other hand, Goodchild et al. [35] obtained numerical convergence values that were considerably higher outside the central 10° for both types of cells in cats. The rod convergence they reported between 20 and 30° was ≈ 10000 rods per β cell and 100000 per α cells [35]. In comparison, bush baby has 1000 rods per P cell and 10000 rods per M cell for the corresponding retinal regions. Towards the periphery, therefore, P and M cells are more similar to those of other primates than to β and α cells in the cat. We conclude that bush babies have approximately the same number of rods converging on their P cells in the central retina as cats do on their β cells. The same is true for central M cells and α cells. Our results predict that bush baby P and M cells have a larger amount of rod input than P and M cells of diurnal anthropoids and that this difference is most pronounced in the central retina. Thus, bush baby central retina exhibits a pattern that is compatible with a nocturnal lifestyle, but the rest of the retina more closely resembles that of other primates.
3.6. A basic primate pattern
A number of other studies have also found similarities between the P and M pathways in bush babies and anthropoids. LGN P cells in both bush babies and anthropoids have higher acuity, lower luminance contrast sensitivity and lower temporal frequency sensitivity than M cells (see ref. [58] for a review). The pattern of their retino-thalamo-cortical projections and cortical connectivity also share many similarities ([3] for a review). Taken with the results presented here, these findings indicate that the P and M pathways in bush babies fit the basic primate pattern, and therefore that the basic organization was almost certainly present in the common ancestor of haplorhines and strepsirhines at least 55 million years ago [48,1]. This is long before the divergence of the LWS and MWS pigment genes [13,21,59], a finding which supports the hypothesis that the P pathway evolved long before the emergence of red–green color opponency. The M pathway has also remained highly conserved, not only in the retina, but also in regard to the cortical connectivity through extra-striate areas [3]. This is consistent with the idea that the M pathway was also present in the earliest primates. In summary, our results indicate that bush baby central P cells resemble midperipheral P cells of diurnal anthropoids in many respects. Bush babies do not have a single cone connected to a single midget bipolar cell and then to a single P cell, as in the central retina of anthropoids, but the cone and rod convergence to P and M cells in the bush baby is closer to the values estimated for other primates than those estimated for cat β and α cells. This evidence strongly supports the idea of a basic primate pattern of retinal ganglion cell morphology.
Acknowledgements
This work was supported by Grants EY06472 and EY01778 from the National Eye Institute. E.S. Yamada receives support from CNPq (scholarship no. 200647/96-2, Brazil) and the Pew Latin American Fellows Program. L.C.L. Silveira is a CNPq Research Fellow. We thank Drs Jon Kaas and Jaime Olavarria for providing some of the eyes used in this work. We also thank Andrzej Zych, Julie Mavity-Hudson and Dr Jamie Boyd for their technical assistance.
References
- 1.Kay RF, Ross C, Williams BA. Anthropoid origins. Science. 1997;272:797–804. doi: 10.1126/science.275.5301.797. [DOI] [PubMed] [Google Scholar]
- 2.Fleague JG. Primate Adaptation and Evolution. San Diego: Academic Press; 1988. [Google Scholar]
- 3.Casagrande VA, Kaas JH. The afferent, intrinsic, and efferent connections of primary visual cortex in primates. In: Peters A, Rockland KS, Jones EG, editors. Cerebral Cortex, vol. 10, Primary Visual Cortex in Primates. New York: Plenum Press; 1994. pp. 201–259. series editors. [Google Scholar]
- 4.Wikler KC, Rakic P. Distribution of photoreceptor subtypes in the retina of diurnal and nocturnal primates. J Neurosci. 1990;10:3390–3401. doi: 10.1523/JNEUROSCI.10-10-03390.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs GH, Neitz M, Neitz J. Mutations in S-cone pigment genes and the absence of colour vision in two species of nocturnal primate. Proc R Soc B (Lond) 1996;263:705–710. doi: 10.1098/rspb.1996.0105. [DOI] [PubMed] [Google Scholar]
- 6.Deegan JF, II, Jacobs GH. Spectral sensitivity and photopigments of a nocturnal prosimian, the bushbaby (Otolemur crassicaudatus) Am J Primatol. 1996;40:55–66. doi: 10.1002/(SICI)1098-2345(1996)40:1<55::AID-AJP4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs GH, Deegan JF., II Photopigments underlying color vision in ringtail lemurs (Lemur catta) and brown lemurs (Eulemur fulvus) Am J Primatol. 1993;30:243–356. doi: 10.1002/ajp.1350300307. [DOI] [PubMed] [Google Scholar]
- 8.Mollon JD. Uses and evolutionary origins of primate colour vision. In: Cronly-Dillon JR, Gregory RL, editors. Vision and Visual Dysfunction, vol. 2, Evolution of the Eye and Visual System. London: MacMillan; 1991. pp. 306–319. series editor. [Google Scholar]
- 9.Jacobs GH. The distribution and nature of colour vision among the mammals. Biol Rev. 1993;68:413–471. doi: 10.1111/j.1469-185x.1993.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs GH. Primate photopigments and primate color vision. Proc Natl Acad Sci USA. 1996;93:577–581. doi: 10.1073/pnas.93.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tovée MJ. The molecular genetics and evolution of primate colour vision. Trends Neurosci. 1994;17:30–37. doi: 10.1016/0166-2236(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 12.Bowmaker JK, Dartnall HJA, Mollon JD. Microspectrophotometric determination of four classes of photoreceptors in an Old World primate, Macaca fascicularis. J Physiol. 1980;298:131–143. doi: 10.1113/jphysiol.1980.sp013071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathans J, Thomas D, Hogness DS. Molecular genetics of human color vision: the genes encoding blue, green and red pigment. Science. 1986;232:193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs GH. Within-species variations in visual capacity among squirrel monkeys (Saimiri sciureus): color vision. Vis Res. 1984;24:1267–1277. doi: 10.1016/0042-6989(84)90181-0. [DOI] [PubMed] [Google Scholar]
- 15.Mollon JD, Bowmaker JK, Jacobs GH. Variations of colour vision in a New World primate can be explained by polymorphism of retinal photopigments. Proc R Soc B (Lond) 1984;222:373–399. doi: 10.1098/rspb.1984.0071. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs GH, Neitz M, Deegan JF, II, Neitz J. Trichromatic colour vision in New World monkeys. Nature. 1996;382:156–158. doi: 10.1038/382156a0. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs GH, Neitz M, Deegan JF, II, Neitz J, Crognale MA, Neitz M. Photopigments and color vision in the nocturnal monkey, Aotus. Vis Res. 1993;33:1773–1783. doi: 10.1016/0042-6989(93)90168-v. [DOI] [PubMed] [Google Scholar]
- 18.Shapley R, Perry VH. Cat and monkey ganglion cells and their visual functional roles. Trends Neurosci. 1986;9:229–235. [Google Scholar]
- 19.Dacey DM, Lee BB. The ‘blue-on’ opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367:731–735. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- 20.Lee BB. Receptive field structure in the primate retina. Vis Res. 1996;36:631–644. doi: 10.1016/0042-6989(95)00167-0. [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama S, Yokoyama R. Molecular evolution of human visual pigment genes. Mol Biol Evol. 1989;6:186–197. doi: 10.1093/oxfordjournals.molbev.a040537. [DOI] [PubMed] [Google Scholar]
- 22.Wässle H, Boycott BB. Functional architecture of the mammalian retina. Physiol Rev. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- 23.Perry VH, Oehler R, Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984;12:1101–1123. doi: 10.1016/0306-4522(84)90006-x. [DOI] [PubMed] [Google Scholar]
- 24.Rodieck RW, Binmoeller KF, Dineen J. Parasol and midget ganglion cells of the human retina. J Comp Neurol. 1985;233:115–132. doi: 10.1002/cne.902330107. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe M, Rodieck RW. Parasol and midget ganglion cells. J Comp Neurol. 1989;289:434–454. doi: 10.1002/cne.902890308. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan E, Lee BB, Shapley RM. New views of primate retinal function. Progr Retinal Res. 1990;9:272–336. [Google Scholar]
- 27.Kolb H, Lindberg K, Fisher SK. Neurons of the human retina: a Golgi study. J Comp Neurol. 1992;318:147–187. doi: 10.1002/cne.903180204. [DOI] [PubMed] [Google Scholar]
- 28.Dacey DM. The mosaic of midget ganglion cells in the human retina. J Neurosci. 1993;13:5334–5355. doi: 10.1523/JNEUROSCI.13-12-05334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dacey DM. Morphology of a small-field bistratified ganglion cell type in the macaque and human retina. Vis Neurosci. 1993;10:1081–1098. doi: 10.1017/s0952523800010191. [DOI] [PubMed] [Google Scholar]
- 30.Dacey DM. Circuitry for color coding in the primate retina. Proc Natl Acad Sci USA. 1996;93:582–588. doi: 10.1073/pnas.93.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leventhal AG, Thompson KG, Liu D. Retinal ganglion cells within the foveola of New World (Saimiri sciureus) and Old World (Macaca fascicularis) monkeys. J Comp Neurol. 1993;338:242–254. doi: 10.1002/cne.903380208. [DOI] [PubMed] [Google Scholar]
- 32.Silveira LCL, Yamada ES, Perry VH, Picanço-Diniz CW. M and P retinal ganglion cells of diurnal and nocturnal New World monkeys. NeuroReport. 1994;5:2077–2081. doi: 10.1097/00001756-199410270-00022. [DOI] [PubMed] [Google Scholar]
- 33.Ghosh KK, Goodchild AK, Sefton AE, Martin P. Morphology of retinal ganglion cells in a New World monkey, the marmoset Callithrix jacchus. J Comp Neurol. 1996;366:76–92. doi: 10.1002/(SICI)1096-9861(19960226)366:1<76::AID-CNE6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 34.Ghosh KK, Martin P, Grünert U. Morphological analysis of the blue cone pathway in the retina of a New World monkey, the marmoset Callithrix jacchus. J Comp Neurol. 1997;379:211–225. [PubMed] [Google Scholar]
- 35.Goodchild AK, Ghosh KK, Martin P. A comparison of photoreceptor spatial density and ganglion cell morphology in the retina of human, macaque monkey, cat, the marmoset Callithrix jacchus. J Comp Neurol. 1996;366:55–75. doi: 10.1002/(SICI)1096-9861(19960226)366:1<55::AID-CNE5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 36.Lee BB, Silveira LCL, Yamada ES, Kremers J. Parallel pathways in the retina of Old and New World primates. Proc Int Symp Neurosci, Belém, Pará, Brazil, Revista Brasileira de Biologia. 1996;56(Suppl 1):323–338. [PubMed] [Google Scholar]
- 37.Lima SMA, Silveira LCL, Perry VH. The distribution of M retinal ganglion cells in diurnal and nocturnal New World monkeys. J Comp Neurol. 1996;368:538–552. doi: 10.1002/(SICI)1096-9861(19960513)368:4<538::AID-CNE6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 38.Yamada ES, Silveira LCL, Perry VH. Morphology, dendritic field size, somal size, density and coverage of M and P retinal ganglion cells of dichromatic Cebus monkeys. Vis Neurosci. 1996;13:1011–1029. doi: 10.1017/s0952523800007677. [DOI] [PubMed] [Google Scholar]
- 39.Yamada ES, Silveira LCL, Gomes FL, Lee BB. The retinal ganglion cell classes of New World primates. Proc Int Symp Neurosci, Belém, Pará, Brazil, Revista Brasileira de Biologia. 1996;56(Supl 1):381–396. [PubMed] [Google Scholar]
- 40.Martin PR, White ARJ, Goodchild AK, Wilder HD, Sefton AE. Evidence that blue-ON cells are part of the third geniculocortical pathway in primates. Eur J Neurosci. 1997;9:1536–1541. doi: 10.1111/j.1460-9568.1997.tb01509.x. [DOI] [PubMed] [Google Scholar]
- 41.DeBruyn EJ, Wise VL, Casagrande VA. The size and topographic arrangement of retinal ganglion cells in the Galago. Vis Res. 1980;20:315–327. doi: 10.1016/0042-6989(80)90018-8. [DOI] [PubMed] [Google Scholar]
- 42.Stone J, Johnston E. The topography of primate retina: a study of the human, bush baby, and new- and old-world monkeys. J Comp Neurol. 1981;196:205–233. doi: 10.1002/cne.901960204. [DOI] [PubMed] [Google Scholar]
- 43.Casagrande VA, DeBruyn EJ. The galago visual system: aspects of normal organization and developmental plasticity. In: Haines DE, editor. The Lesser Bushbaby (Galago) as an Animal Model: Selected Topics. Boca Raton, FL: CRC Press; 1982. pp. 137–168. [Google Scholar]
- 44.Itoh K, Conley M, Diamond IT. Retinal ganglion cell projections to individual layers of the lateral geniculate body in Galago crassicaudatus. J Comp Neurol. 1982;205:282–290. doi: 10.1002/cne.902050308. [DOI] [PubMed] [Google Scholar]
- 45.Irvin GE, Casagrande VA, Norton TT. Center/surround relationships of magnocellular, parvocellular, and koniocellular relay cells in primate lateral geniculate nucleus. Vis Neurosci. 1993;10:363–373. doi: 10.1017/s0952523800003758. [DOI] [PubMed] [Google Scholar]
- 46.Hughes A. Population magnitudes and distribution of the major modal classes of cat retinal ganglion cell as estimated from HRP filling and a systematic survey of the soma diameter spectra for classical neurones. J Comp Neurol. 1981;197:303–339. doi: 10.1002/cne.901970209. [DOI] [PubMed] [Google Scholar]
- 47.Stone J. Parallel Processing in the Visual System: the Classification of Retinal Ganglion Cells and its Impact on the Neurobiology of Vision. New York: Plenum; 1983. [Google Scholar]
- 48.Martin RD. Primate origins: plugging the gaps. Nature. 1993;363:223–234. doi: 10.1038/363223a0. [DOI] [PubMed] [Google Scholar]
- 49.Boycott BB, Wässle H. The morphological types of ganglion cells in the domestic cat’s retina. J Physiol. 1974;240:397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stein JJ, Johnson SA, Berson DM. Distribution and coverage of β cells in the cat retina. J Comp Neurol. 1996;372:597–617. doi: 10.1002/(SICI)1096-9861(19960902)372:4<597::AID-CNE8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 51.Polyak SL. The Vertebrate Retina. Chicago, IL: University of Chicago Press; 1941. [Google Scholar]
- 52.Boycott BB, Dowling JE. Organization of the primate retina: Light microscopy. Philos Trans R Soc B (Lond) 1969;255:109–184. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- 53.Kolb H, DeKorver L. Midget ganglion cells of parafovea of the human retina: a study by electron microscopy and serial-section reconstruction. J Comp Neurol. 1991;303:617–636. doi: 10.1002/cne.903030408. [DOI] [PubMed] [Google Scholar]
- 54.Calkins DJ, Schein SJ, Tsukamoto Y, Sterling P. M and L cones in macaque fovea connect to midget ganglion cells by different numbers of excitatory sinapses. Nature. 1994;371:70–72. doi: 10.1038/371070a0. [DOI] [PubMed] [Google Scholar]
- 55.Silveira LCL, Lee BB, Yamada ES, Kremers J, Hunt DM. Post-receptoral mechanisms of colour vision in new world primates. Vision Research. 1998;38:3329–3337. doi: 10.1016/s0042-6989(97)00335-0. [DOI] [PubMed] [Google Scholar]
- 56.Cohen E, Sterling P. Parallel circuits from cones to the on-β ganglion cell. Eur J Neurosci. 1992;4:506–520. doi: 10.1111/j.1460-9568.1992.tb00901.x. [DOI] [PubMed] [Google Scholar]
- 57.Williams RW, Cavada C, Reinoso-Suarez F. Rapid evolution of the visual system: a cellular assay of the retina and dorsal lateral geniculate nucleus of the spanish wildcat and the domestic cat. J Neurosci. 1993;13:208–228. doi: 10.1523/JNEUROSCI.13-01-00208.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Casagrande VA, Norton TT. Lateral geniculate nucleus: a review of its physiology and function. In: Leventhal A, Cronly-Dillon JR, editors. Vision and Visual Dysfunction, vol. 4, The Neural Basis of Visual Function. London: MacMillan Press; 1991. pp. 41–84. series editor. [Google Scholar]
- 59.Shyue S-K, Hewett-Emmett D, Sperling HG, Hunt DM, Bowmaker JK, Mollon JD, Li W-H. Adaptive evolution of color vision genes in higher primates. Science. 1995;269:1265–1276. doi: 10.1126/science.7652574. [DOI] [PubMed] [Google Scholar]
- 60.Rodieck RW, Brening RK, Watanabe M. The origin of parallel visual pathways. In: Shapley R, Lam DM-K, editors. Proceedings of the Retina Research Foundation Symposia, vol. 5, Contrast Sensitivity. London: MIT Press; 1993. pp. 115–144. [Google Scholar]