Abstract
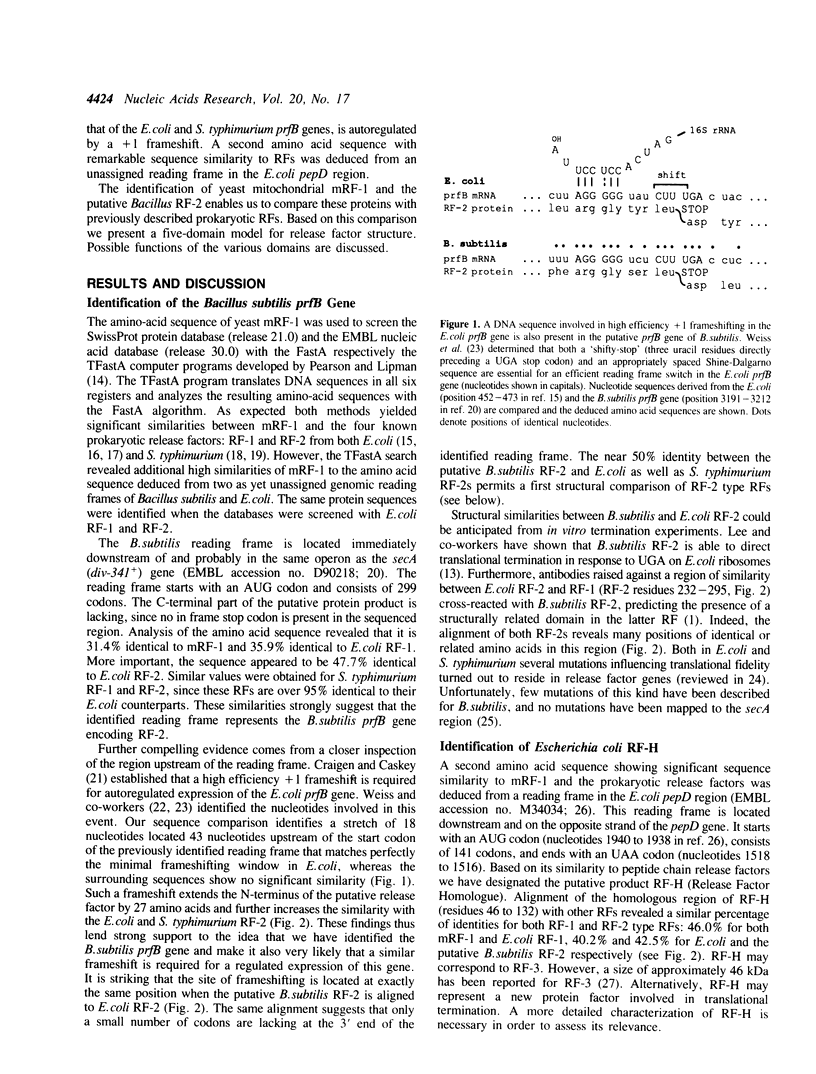
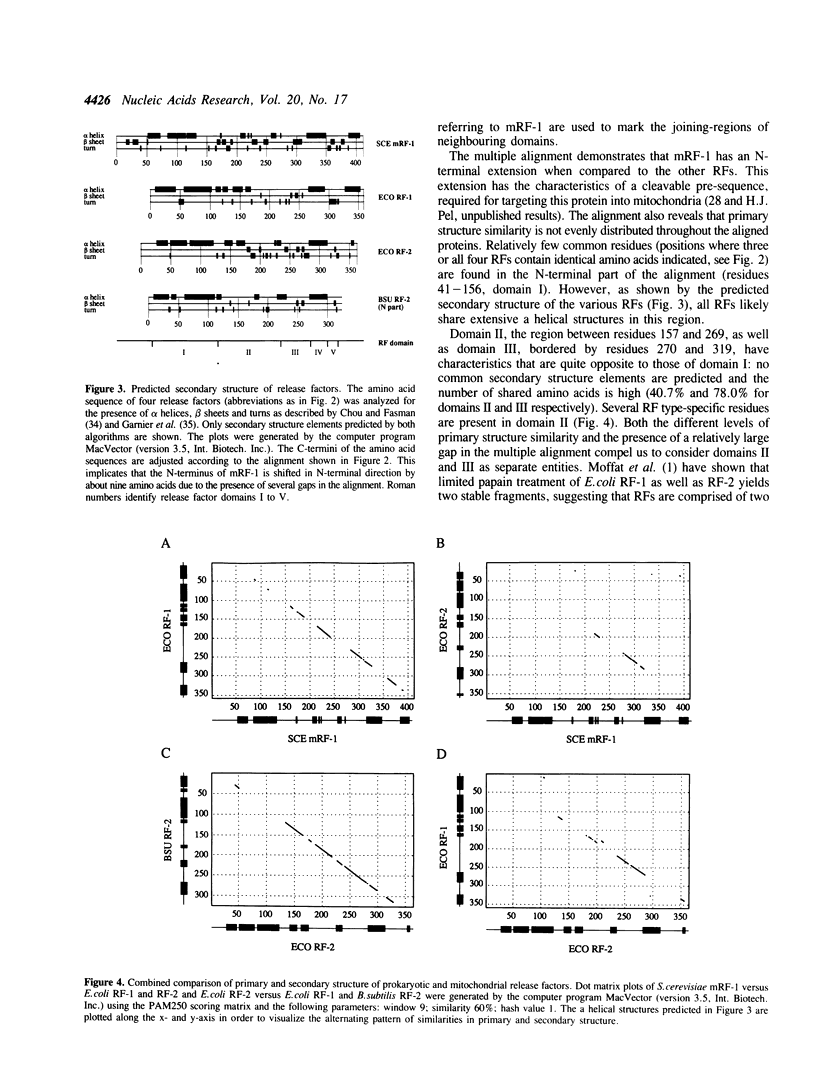
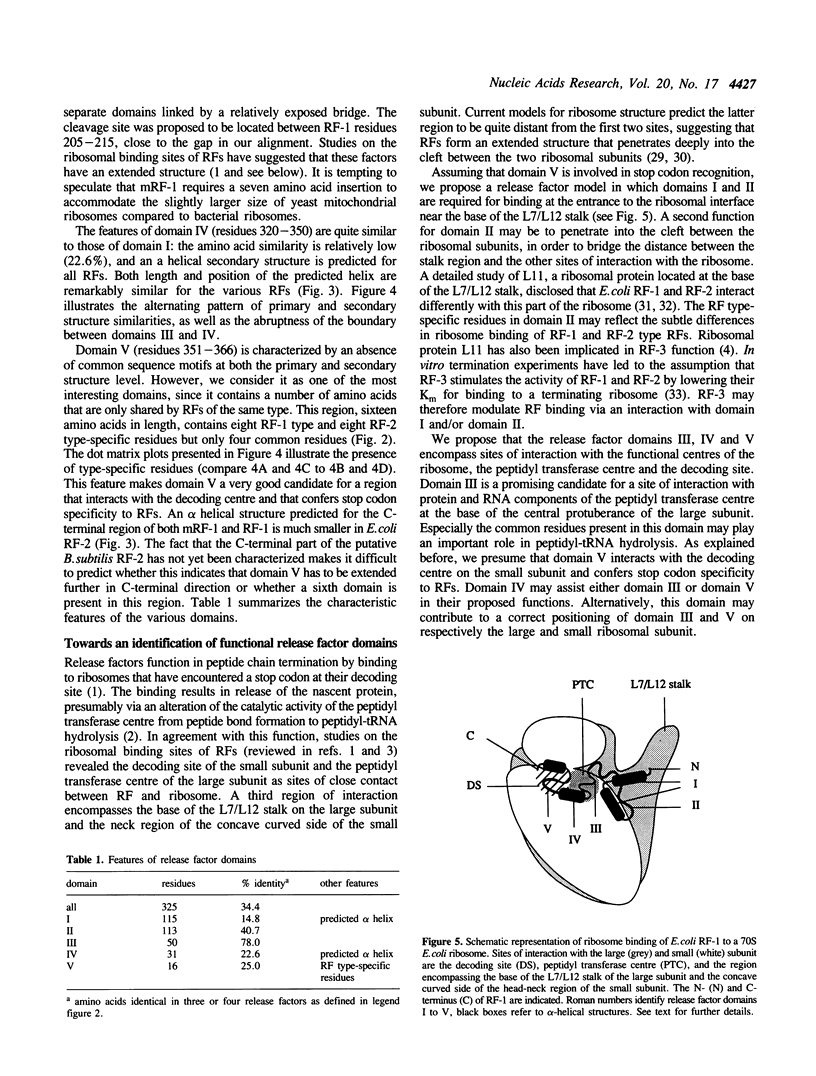
We have recently reported the cloning and sequencing of the gene for the mitochondrial release factor mRF-1. mRF-1 displays high sequence similarity to the bacterial release factors RF-1 and RF-2. A database search for proteins resembling these three factors revealed high similarities to two amino acid sequences deduced from unassigned genomic reading frames in Escherichia coli and Bacillus subtilis. The amino acid sequence derived from the Bacillus reading frame is 47% identical to E.coli and Salmonella typhimurium RF-2, strongly suggesting that it represents B.subtilis RF-2. Our comparison suggests that the expression of the B.subtilis gene is, like that of the E.coli and S. typhimurium RF-2 genes, autoregulated by a stop codon dependent +1 frameshift. A comparison of prokaryotic and mitochondrial release factor sequences, including the putative B.subtilis RF-2, leads us to propose a five-domain model for release factor structure. Possible functions of the various domains are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bange F. C., Flohr T., Buwitt U., Böttger E. C. An interferon-induced protein with release factor activity is a tryptophanyl-tRNA synthetase. FEBS Lett. 1992 Mar 30;300(2):162–166. doi: 10.1016/0014-5793(92)80187-l. [DOI] [PubMed] [Google Scholar]
- Buwitt U., Flohr T., Böttger E. C. Molecular cloning and characterization of an interferon induced human cDNA with sequence homology to a mammalian peptide chain release factor. EMBO J. 1992 Feb;11(2):489–496. doi: 10.1002/j.1460-2075.1992.tb05079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel M. S., Kjeldgaard M., Engelman D. M., Moore P. B. Positions of S2, S13, S16, S17, S19 and S21 in the 30 S ribosomal subunit of Escherichia coli. J Mol Biol. 1988 Mar 5;200(1):65–87. doi: 10.1016/0022-2836(88)90334-8. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Craigen W. J., Caskey C. T. Expression of peptide chain release factor 2 requires high-efficiency frameshift. Nature. 1986 Jul 17;322(6076):273–275. doi: 10.1038/322273a0. [DOI] [PubMed] [Google Scholar]
- Craigen W. J., Cook R. G., Tate W. P., Caskey C. T. Bacterial peptide chain release factors: conserved primary structure and possible frameshift regulation of release factor 2. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3616–3620. doi: 10.1073/pnas.82.11.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigen W. J., Lee C. C., Caskey C. T. Recent advances in peptide chain termination. Mol Microbiol. 1990 Jun;4(6):861–865. doi: 10.1111/j.1365-2958.1990.tb00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T. Cloning, genetic characterization, and nucleotide sequence of the hemA-prfA operon of Salmonella typhimurium. J Bacteriol. 1989 Jul;171(7):3948–3960. doi: 10.1128/jb.171.7.3948-3960.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckner J., Rasmussen H. H., Justesen J. Human interferon gamma potently induces the synthesis of a 55-kDa protein (gamma 2) highly homologous to rabbit peptide chain release factor and bovine tryptophanyl-tRNA synthetase. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11520–11524. doi: 10.1073/pnas.88.24.11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolova LYu, Sudomoina M. A., Grigorieva AYu, Zinovieva O. L., Kisselev L. L. Cloning and nucleotide sequence of the structural gene encoding for human tryptophanyl-tRNA synthetase. Gene. 1991 Dec 30;109(2):291–296. doi: 10.1016/0378-1119(91)90624-k. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Garret M., Pajot B., Trézéguet V., Labouesse J., Merle M., Gandar J. C., Benedetto J. P., Sallafranque M. L., Alterio J., Gueguen M. A mammalian tryptophanyl-tRNA synthetase shows little homology to prokaryotic synthetases but near identity with mammalian peptide chain release factor. Biochemistry. 1991 Aug 6;30(31):7809–7817. doi: 10.1021/bi00245a021. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Caskey C. T. Peptide chain termination: effect of protein S on ribosomal binding of release factors. Proc Natl Acad Sci U S A. 1970 Oct;67(2):537–543. doi: 10.1073/pnas.67.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl F. U., Pfanner N., Nicholson D. W., Neupert W. Mitochondrial protein import. Biochim Biophys Acta. 1989 Jan 18;988(1):1–45. doi: 10.1016/0304-4157(89)90002-6. [DOI] [PubMed] [Google Scholar]
- Henrich B., Monnerjahn U., Plapp R. Peptidase D gene (pepD) of Escherichia coli K-12: nucleotide sequence, transcript mapping, and comparison with other peptidase genes. J Bacteriol. 1990 Aug;172(8):4641–4651. doi: 10.1128/jb.172.8.4641-4651.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K., Nakamura Y. Autogenous suppression of an opal mutation in the gene encoding peptide chain release factor 2. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8432–8436. doi: 10.1073/pnas.87.21.8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. C., Craigen W. J., Muzny D. M., Harlow E., Caskey C. T. Cloning and expression of a mammalian peptide chain release factor with sequence similarity to tryptophanyl-tRNA synthetases. Proc Natl Acad Sci U S A. 1990 May;87(9):3508–3512. doi: 10.1073/pnas.87.9.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. C., Kohara Y., Akiyama K., Smith C. L., Craigen W. J., Caskey C. T. Rapid and precise mapping of the Escherichia coli release factor genes by two physical approaches. J Bacteriol. 1988 Oct;170(10):4537–4541. doi: 10.1128/jb.170.10.4537-4541.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. C., Timms K. M., Trotman C. N., Tate W. P. Isolation of a rat mitochondrial release factor. Accommodation of the changed genetic code for termination. J Biol Chem. 1987 Mar 15;262(8):3548–3552. [PubMed] [Google Scholar]
- Lovett P. S., Ambulos N. P., Jr, Mulbry W., Noguchi N., Rogers E. J. UGA can be decoded as tryptophan at low efficiency in Bacillus subtilis. J Bacteriol. 1991 Mar;173(5):1810–1812. doi: 10.1128/jb.173.5.1810-1812.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaughan K. K., Ward C. D., Trotman C. N., Tate W. P. The ribosomal binding domain for the bacterial release factors RF-1, RF-2 and RF-3. FEBS Lett. 1984 Sep 17;175(1):90–94. doi: 10.1016/0014-5793(84)80576-1. [DOI] [PubMed] [Google Scholar]
- Mikuni O., Kawakami K., Nakamura Y. Sequence and functional analysis of mutations in the gene encoding peptide-chain-release factor 2 of Escherichia coli. Biochimie. 1991 Dec;73(12):1509–1516. doi: 10.1016/0300-9084(91)90185-4. [DOI] [PubMed] [Google Scholar]
- Moffat J. G., Timms K. M., Trotman C. N., Tate W. P. Interaction of the release factors with the Escherichia coli ribosome: structurally and functionally-important domains. Biochimie. 1991 Jul-Aug;73(7-8):1113–1120. doi: 10.1016/0300-9084(91)90154-s. [DOI] [PubMed] [Google Scholar]
- Parker J. Errors and alternatives in reading the universal genetic code. Microbiol Rev. 1989 Sep;53(3):273–298. doi: 10.1128/mr.53.3.273-298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin B. Y., Anderson S. L., Xing L., Powell R. J., Tate W. P. Interferon induces tryptophanyl-tRNA synthetase expression in human fibroblasts. J Biol Chem. 1991 Dec 25;266(36):24245–24248. [PubMed] [Google Scholar]
- Sadaie Y., Takamatsu H., Nakamura K., Yamane K. Sequencing reveals similarity of the wild-type div+ gene of Bacillus subtilis to the Escherichia coli secA gene. Gene. 1991 Feb 1;98(1):101–105. doi: 10.1016/0378-1119(91)90110-w. [DOI] [PubMed] [Google Scholar]
- Tate W. P., McCaughan K. K., Ward C. D., Sumpter V. G., Trotman C. N., Stoffler-Meilicke M., Maly P., Brimacombe R. The ribosomal binding domain of the Escherichia coli release factors. Modification of tyrosine in the N-terminal domain of ribosomal protein L11 affects release factors 1 and 2 differentially. J Biol Chem. 1986 Feb 15;261(5):2289–2293. [PubMed] [Google Scholar]
- Tate W. P., Schulze H., Nierhaus K. H. The Escherichia coli ribosomal protein L11 suppresses release factor 2 but promotes the release factor 1 activities in peptide chain termination. J Biol Chem. 1983 Nov 10;258(21):12816–12820. [PubMed] [Google Scholar]
- Walleczek J., Schüler D., Stöffler-Meilicke M., Brimacombe R., Stöffler G. A model for the spatial arrangement of the proteins in the large subunit of the Escherichia coli ribosome. EMBO J. 1988 Nov;7(11):3571–3576. doi: 10.1002/j.1460-2075.1988.tb03234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. B., Dunn D. M., Atkins J. F., Gesteland R. F. Slippery runs, shifty stops, backward steps, and forward hops: -2, -1, +1, +2, +5, and +6 ribosomal frameshifting. Cold Spring Harb Symp Quant Biol. 1987;52:687–693. doi: 10.1101/sqb.1987.052.01.078. [DOI] [PubMed] [Google Scholar]
- Weiss R. B., Dunn D. M., Dahlberg A. E., Atkins J. F., Gesteland R. F. Reading frame switch caused by base-pair formation between the 3' end of 16S rRNA and the mRNA during elongation of protein synthesis in Escherichia coli. EMBO J. 1988 May;7(5):1503–1507. doi: 10.1002/j.1460-2075.1988.tb02969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



