Abstract
The role of nuclear factor (NF)-кBp65 pathway in the pathogenesis of follicular thyroid carcinoma (FTC) has not been fully investigated. We retrieved 10 cases of FTC from our file. Tissue microarrays (TMAs) were constructed using 2.0 mm cores from formalin-fixed, paraffin-embedded tissue blocks. TMA sections were immunohistochemically stained for phosphorylated (p)-NF-кBp65 (Ser 536), cyclooxygenase-2 (COX-2), IL-8, and glutathione S-transferase (GST)-pi. Staining intensity (0-3+), extensiveness (0-100%) and subcellular compartmentalization were evaluated. Both nuclear and cytoplasmic immunoreactivities with p-NF-кBp65 (Ser 536) antibodies were observed in all 10 cases, including moderate to strong nuclear staining intensity with a range of extensiveness in 20% - 100% of tumor cells. Moderate (2+) or strong (3+) cytoplasmic expressions of COX-2 and IL-8 were present in 60-100% and 50- 100% of tumor cells, respectively, in all cases. GST-pi was diffusely (70-100%) and moderately or strongly staining the tumor cytoplasm in all cases (except one case with insufficient tissue) with three of them demonstrating nuclear positivity as well. Morphoproteomic analysis reveals the constitutive activation of the NF-кBp65 pathway in follicular thyroid carcinomas as evidenced by phosphorylation at Ser 536 with nuclear translocation and with correlative expression of transcriptionally activated gene products (COX-2, IL-8, and GST-pi). This observation may provide a molecular basis for the tumor biology and targeted therapies for follicular thyroid carcinoma.
Keywords: COX-2, follicular thyroid carcinoma (FTC), GST-pi, IL-8, NF-kappaB, morphoproteomics
Introduction
Follicular thyroid carcinoma (FTC) is the second most common thyroid cancer. Distant metastases have been reported in up to 20% of patients at presentation [1], which is one of the main factors negatively affecting survival of patients with FTCs [2]. 131I therapy is the choice of the treatment for the distant metastases while the chemotherapeutic agents presently available are of limited benefit and cause significant morbidity [3]. However, sometimes metastases persist despite administration of radioiodine because of the decrease in iodine uptake by the tumors. Therefore, effective therapeutic modalities are being continuously sought. Combination therapy including targeted therapies may be an effective strategy against FTC. However, signaling pathways involved in the tumorigenesis and progression of this carcinoma have not been fully investigated.
Among cancer signaling pathways, the nuclear factor-κB (NF-κB) pathway has been increasingly studied and its activation has been reported in a variety of malignant neoplasms [4-6]. Moreover, some studies have indicated that the increased NF-κB activity at least partly reinforces the intrinsic chemo- and/or radio-resistance [7]. Therefore, the inhibition of NF-κB pathway could be a potential novel therapeutic approach. However, to date, there are only few studies of NF- κB pathway in FTC published in the literature. These studies were limited to a few genes and their products and most of them were in vitro experiments or using animal models [8-11].
Now, it becomes more desirable than ever to define the role of NF-κB pathway in FTC because several inhibitors for the NF-κB pathway, as potential pharmaceutical agents, have recently been identified and utilized in clinical or preclinical studies including thyroid carcinomas using cultured cells and animal models [9,10].
The activation of NF-κB signaling results in antiapoptosis, proliferation, angiogenesis and inflammation by inducing the production of inflammatory factors in neoplasms [12,13]. The inflammation further amplifies NF-κB signaling through autocrine signaling [14,15]. Among the inflammatory factors induced by NF-κB are cyclooxygenase-2 (COX-2) [16], interleukin (IL)-8 [13,17], and glutathione S-transferase (GST)-pi [18].
COX-2 is clearly at the center of inflammatory process. It is an enzyme that catalyzes the production of a series of prostaglandins from arachidonic acid, involving tumorigenesis, tumor progression and chemoradioresistence. COX-2 is a common and key target of non-steroidal anti-inflammatory drugs (NSAIDs). It has been demonstrated that NSAIDs block tumor promotion and tumor progression [4]. Because therapeutic agents that inhibit COX-2 are currently available and could play a role in treatment, extensive studies in this area have been conducted. In addition, some of COX-2 inhibitors (e.g., celecoxib) have demonstrated dural inhibition effect on both COX-2 and NF-κB pathway [19,20]. COX-2 overexpression has been observed in numerous neoplasms including FTCs [21,22]. However, to our knowledge, studies linking NF-κB and COX-2 in FTCs have not been reported.
Interleukin-8 (IL-8), a member of the CXC chemokine family, was initially identified as a neutrophil chemoattractant. Growing evidence suggests that IL-8 plays critical roles in the pathogenesis of a variety of cancers, including enhancing angiogenesis and promoting tumor growth and metastasis [23-25]. However, to our knowledge, the study of IL-8 on FTC tissue has not been reported in the literature.
GST-pi is one of the cytosolic isoforms of GST enzymes. Its overexpression is correlated to carcinogenesis and chemoresistance in cancer cells. Overexpression of GST-pi has been found in many human tumors, such as carcinoma cells of prostate, lung, colon, breast, stomach, ovary and head and neck, cholangiocarcinoma, CNS tumors, hematopoietic malignancies and sarcoma while this protein in the corresponding normal tissues is either absent or expressed at very low levels, which indicates the role of GSTpi in carcinogenesis [26-35]. Moreover, GST-pi plays an important role in detoxification of xenobiotics; however, it also allows the development of resistance to chemotherapy [32,35-37]. Although GST-pi has been extensively studied on a variety of other malignant neoplasms, to our knowledge based on a review of the Medline data base, the expression of GST-pi in FTC has not been reported.
Morphoproteomics utilizes immunohistochemical staining methods to identify the expression of protein analytes in signal transduction pathways in lesional tissue. It relies on the use of phosphospecific probes directed against putative sites of activation of molecules, subcellular compartmentalization, and/or correlative expressions of protein analytes to assess the constitutive activation of cell signaling pathways [38].
In this study, we investigated the role of NF-κBp65 and its downstream products including COX-2, IL-8 and GST-pi in FTC using morphoproteomics in an attempt to study the NF-κB signaling transduction pathway and to expose potential therapeutic targets.
Materials and methods
We searched our file and found 10 thyroidectomy specimens for FTC from 10 different patients. None of these patients had preoperative treatment for the thyroid tumors. This study protocol was approved by the Institutional Review Board of the University of Texas Health Science Center at Houston.
Immunohistochemical staining
Tissue microarrays (TMAs) were constructed using 2.0 mm cores from formalin-fixed, paraffin-embedded tissue blocks. Immunohistochemial stains were performed on unstained sections of 4 μm thickness. Four primary antibodies were employed: p-NF-κBp65 (rabbit monoclonal antibody) using a phosphospecific probe directed against Serine 536, a putative site of activation (dilution 1:50, Cell Signaling Technology, Beverly, MA), cyclooxygenase-2 (COX-2) (mouse monoclonal antibody, dilution 1:500, Leica Biosystems Newcastle Lld, Newcastle Upon Tyne, UK), IL-8 (mouse monoclonal antibody, dilution 1:50, R&D Systems, Inc., Minneapolis, MN), and glutathione S-transferase (GST)-pi (rabbit polyclonal antibody, dilution 1:800, BioGenex, San Ramon, CA).
We used a semiautomatic method for p-NF-κBp65. Paraffin-embedded tissues were sectioned and placed on glass slides, and dried at 60°C for 1-2 hours. Then, they were deparaffinized and underwent antigen retrieval. Slides were placed in a 1x solution of citric acid (DAKO S1699) and heated in a Pascal pressure cooker (125°C, 10 min, 25 psi) until the solution reaches boiling, followed by a 20 min cooling period outside the pressure cooker. The slides were then placed in 0.05 M Tris-HCl, 0.05% Tween-20 (TBST buffer) for 5 min (Tris-HCl in packs from Dako Cytomation, Tween-20 from EM Science). The tissue was then treated with 3% H2O2 (McKesson General Medical) for 5 min, and then rinsed with TBST buffer. A few drops of diluted normal blocking serum (Vectastain kit, Vector Laboratories) were placed on the tissue and incubated at room temperature for 1 hour. The serum was then carefully blotted off and the slides were incubated with primary antibody overnight at 4°C. The following day, the tissues were rinsed well with TBST buffer for a minimum of 5 min. The rest of the staining procedure took place on a DAKO Autostainer. The machine was programmed to treat each slide with diluted biotinylated secondary antibody solution (Vectastain Kit) for 30 min. The slides were rinsed with TBST buffer and incubated with Vectastain Elite Antigen Binding Complex Reagent (Vectastain Kit) for 30 min. The slides were rinsed with TBST buffer and incubated with DAB solution (3,3'-diaminobenzidine chromogen solution, DAKO K3468) for 10 min. The slides were then removed from the autostainer and rinsed with distilled water. They were then counterstained with Hematoxylin, dehydrated, treated with xylene and coverslipped.
We used an automatic stainer for COX-2, IL-8, and GST-pi. Citrate and EDTA were used for antigen retrievals for COX-2 and IL-8, respectively. No antigen retrieval was conducted for GST-pi. Incubation times for COX-2, IL-8 and GST-pi were 30 min, 30 min, and 120 min, respectively. PowerVisionTM (ImmunoVision Technologies Co., Daly City, VA, USA) detection system was used. The remaining procedures were similar to those for p-NF-κBp65 (Ser 536).
Assessment of immunohistochemical staining
Chromogenic signal and subcellular expression pattern were assessed by bright-field microscopy. Both staining intensity and extensiveness (percentage of positively stained tumor cells) were evaluated for p-NF-κBp65 (Ser536), COX-2, IL-8 and GST-pi. The staining intensity was graded as negative (0), weak (1+), moderate (2+), and strong (3+). The staining extensiveness was the percentage of tumor cells positively stained with a range from 0% to 100%. The subcellular expression pattern was evaluated and characterized as: nuclear, cytoplasmic, or plasmalemmal locations. The sections were semiquantitatively assessed by the authors.
Results
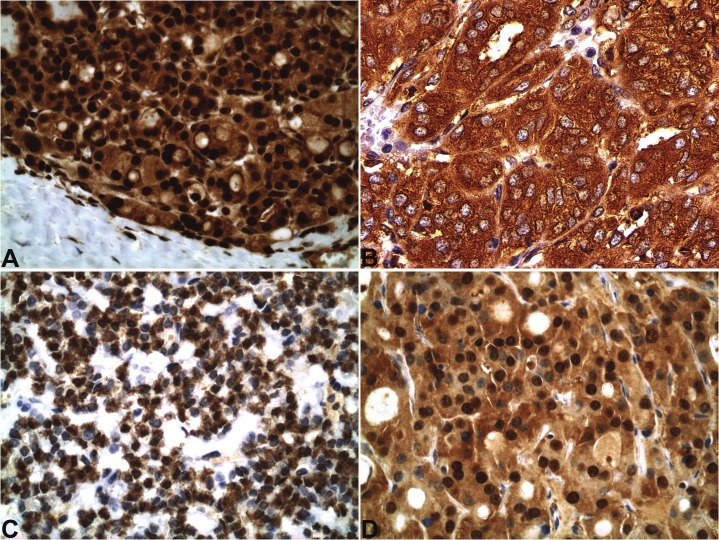
Both nuclear and cytoplasmic immunoreactivities with antibodies against p-NF-κBp65 (Ser 536) were observed in all 10 cases, including moderate to strong nuclear staining intensity with a range of extensiveness from 20% to 100% of tumor cells. Moderate or strong cytoplasmic expressions of COX-2 and IL-8 were present in 60% to 100% and 50% to 100% of tumor cells, respectively, in all cases. GST-pi diffusely (70% to 100%) and moderately or strongly stained the tumor cytoplasm in all cases (except one case with insufficient tissue) with 3 cases demonstrating nuclear positivity as well. The details of the staining results are demonstrated in Table 1. Examples of these cases are shown in Figure 1.
Table 1.
Immunohistochemial expressions of NF-κB, COX-2, IL-8 and GST-pi
| Cases | NF-κB | COX-2 | IL-8 | GST-pi | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytoplasm | Nucleus | Cytoplasm | Cytoplasm | Cytoplasm | Nucleus | |||||||
| Intensity | Extent (%) | Intensity | Extent (%) | Intensity | Extent (%) | Intensity | Extent (%) | Intensity | Extent (%) | Intensity | Extent (%) | |
| 1 | 1-2+ | 100 | 2-3+ | 60 | 3+ | 100 | 2+ | 50 | 3+ | 100 | 2+ | 100 |
| 2 | 2+ | 100 | 2-3+ | 100 | 2+ | 80 | 2-3+ | 80 | 2-3+ | 90 | - | - |
| 3 | 1-2+ | 100 | 2+ | 100 | 3+ | 100 | 3+ | 90 | 2-3+ | 100 | - | - |
| 4 | 1+ | 100 | 2+ | 20 | 3+ | 100 | 2+ | 100 | 2+ | 100 | - | - |
| 5 | 1+ | 100 | 2+ | 40 | 3+ | 100 | 2+ | 100 | 3+ | 100 | - | - |
| 6 | 1-2+ | 100 | 2-3+ | 100 | 2-3+ | 90 | 2+ | 100 | 2-3+ | 100 | - | - |
| 7 | 1-2+ | 100 | 2+ | 100 | 3+ | 100 | 2+ | 80 | 3+ | 100 | 3+ | 100 |
| 8 | 1+ | 100 | 2+ | 100 | 2+ | 60 | 2+ | 100 | 3+ | 100 | - | - |
| 9 | 1+ | 100 | 2+ | 20 | 2-3+ | 100 | 2+ | 100 | 2+ | 70 | 3+ | 70 |
| 10 | 1+ | 100 | 2+ | 100 | 2-3+ | 100 | 3+ | 100 | insufficient | insufficient | insufficient | insufficient |
-: negative; +: positive
Figure 1.
In follicular thyroid carcinoma, expressions of p-NF-κBp65 (Ser 536) in both nucleus and cytoplasm (A), COX- 2 in cytoplasm (B), IL-8 in perinuclear cytoplasm (C) and GST-pi in both nucleus and cytoplasm (D). (immunohistochemical stains, x400).
Discussion
This study demonstrates the constitutive activation of NF-κBp65 in follicular thyroid carcinomas (FTCs) as evidenced by phosphorylation at Ser 536, nuclear translocation and correlative expressions of its transcriptionally activated gene products (proteins) including COX-2, IL-8, and GST-pi.
NF-κB is a key regulator of genes involved in carcinogenesis via regulating gene expression. It is normally retained in the cytoplasm in an inactive state through association with IκBs, its inhibitory proteins. The pathway becomes active when, in response to NF-κB-activating agents, IκBs dissociate thereby allowing nuclear translocation of NF-κB. The phosphorylation of NF-κBp65 at serine 536 and its nuclear translocation create an opportunity for the tumor cells to form p-NF-κB65-DNA complexes leading to transcriptional activation. In the nucleus, NF-κB activates the expression of genes involved in inflammation, immune response, cell proliferation and resistance to apoptosis and promotes tumor metastasis and angiogenesis [12,39]. Pacifico and coworkers found that the increased nuclear localization of NF-κB correlated with the increased malignant phenotype of thyroid cancers whereas no nuclear staning was detected in normal thyroid follicular cells and that 50% follicular carcinoma cells showed their nuclei stained for NF-κB [7]. Therefore, the demonstration of NF-κBp65, phosphorylated on serine 536 and translocated to the nucleus in our present study confirms the activated state of NF-κBp65 in FTCs.
Because COX-2, IL-8 and GST-pi are induced by the activated NF-κB, the overexpressions of these markers can be considered as further indicators of NF-κB activation [16-18].
COX-2 expression in FTCs has been evaluated by previous studies using immunohistochemistry and Western blot assay. COX-2 overexpression was found in FTCs with a range from 26% to 100% while its expression was not seen or was at a lower level in normal thyroid tissue [40-43]. Additionally, the study by Haynik et al indicated that the immunohistochemical expression of COX-2 might correlate with increased tumor recurrence and death [22]. Elevated tumor COX- 2 gene has been found to be associated with increased angiogenesis, tumor invasion and anti-apoptosis [44], which are coincide with the effects of NF-κB activation and, therefore, support the concept of NF-κB activating COX-2 gene [16]. Because the COX-2 gene is strongly induced by NF-κB, one would speculate a link between NF-κB activation and COX-2 protein expression. Our current observation not only confirms the overexpression of COX-2 protein in FTCs as reported in the literature [21,22] but also demonstrates for the first time concomitant expressions of p-NF-κB65 and COX-2, supporting a link between the activated NF-κB and COX- 2 in FTCs.
The expression of IL-8 shown in the current study is the first observation reported in FTC tissue. Although there are no previous studies on FTC tissue as comparison, this finding is supported by the study of serum IL-8 in thyroid diseases. Kobawala et al found that serum IL-8 levels were not only significantly higher than healthy individuals but also significantly positively correlated with the stage in thyroid cancer patients [45]. The concomitant expression of phosphorylated NF-κBp65 at serine 536 in the nuclear location of FTC in our current study suggests the connection between the activated NF- κB and IL-8. This is in accordance with previous studies that showed IL-8 upregulated by NF-κB in several other malignancies [46,47]. Therefore, IL-8 overexpression may be another indicator for the activation of NF-κB pathway in FTC.
Finally, literature data have consistently shown that NF-κB is one of the key regulators for GST-pi gene expression via binding on the GST-pi promoter, i.e. the NF-κB binding activates GSTpi gene expression [18]. This conception is further suggested by the finding that NF-κB inhibition reduces GST-pi expression [48]. Therefore, the concomitant expression of p-NF-κBp65 and GST-pi in the present study supports the activation of NF-κB pathway in FTC. In addition to be an indicator of NF-κB pathway activation, the expression of GST-pi, especially nuclear expression suggests the chemoresistence in cancer cells [18,49,50]. Accordingly, in the current study, the expression of GST-pi, especially the nuclear localization, may be a potential evaluation factor for drug resistance in FTC.
In conclusion, this study demonstrates the constitutive activation of NF-κBp65 and concomitant expressions of its downstream effectors including COX-2, IL-8 and GST-pi in FTC histology tissue sections. Therefore, the results provide correlates to confirm the activation of the NF-κB pathway. This is the first time demonstrating the expressions of IL-8 and GST-pi and concomitant expressions of COX-2 and p-NF-κBp65 (Ser 536) in FTC. The findings may provide insight into the tumor biology and molecular basis for chemoresistence and potential therapeutic targets in follicular thyroid carcinoma.
Acknowledgement
The authors would like to thank Ms. Pamela K. Johnston, HT (ASCP) for her technical assistance and expertise and Ms. Bheravi Patel for her secretarial and graphic design expertise.
References
- 1.Sobrinho Simoes M, Asa SL, Kroll TG, Nikforov Y, DeLellis R, Farid P, Kitamura Y, Noguchi SU, Eng C, Harach HR, Williams ED, Schneider AB, Fagin JA, Ghossein RA, Mazzaferri EL, Lloyd RV, Livolsi V, Chan JKC, Baloch Z, Clark OH. Papillary carcinoma. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. WHO classification of tumors - pathology and genetics of tumours of endocrine organs. Lyon, France: IARC press; 2004. pp. 67–72. [Google Scholar]
- 2.Nikiforov YE, Ohori NP. Follicular carcinoma. In: Nikiforov YE, Biddinger PW, Thompson LDR, editors. Diagnostic pathology and molecular genetics of the thyroid. Philadelphia, Pennsylvania: Wolters Kluwer/Lippincott William & Wilkins; 2000. pp. 132–159. [Google Scholar]
- 3.Haq M, Harmer C. Medical management of thyroid cancer. In: Arora A, Tolley NS, Tuttle RM, editors. A practical manual of thyroid and parathyroid disease. West Sussex, UK: Wiley-Blackwell; 2010. pp. 125–141. [Google Scholar]
- 4.Courtois G, Gilmore TD. Mutations in the NF-κB signaling pathway: implications for human disease. Oncogene. 2006;25:6831–6843. doi: 10.1038/sj.onc.1209939. [DOI] [PubMed] [Google Scholar]
- 5.Bargou RC, Emmerich F, Krappmann D, Bomert K, Mapara MY, Arnold W, Royer HD, Grinstein E, Greiner A, Scheidereit C, Dörken B. Constitutive nuclear factor-κB-RelA activation is required for proliferation and survival of Hodgkin’s disease tumor cells. J Clin Investig. 1997;100:2961–2969. doi: 10.1172/JCI119849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-κB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188–4197. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 7.Pacifico F, Mauro C, Barone C, Crescenzi E, Mellone S, Monaco M, Chiappetta G, Terrazzano G, Liguoro D, Vito P, Consiglio E, Formisano S, Leonardi A. Oncogenic and antiapoptotic activity of NF-κB in human thyroid carcinomas. J Biol Chem. 2004;279:54610–54619. doi: 10.1074/jbc.M403492200. [DOI] [PubMed] [Google Scholar]
- 8.Starenki DV, Namba H, Saenko VA, Ohtsuru A, Maeda S. Induction of thyroid cancer cell apoptosis by a novel nuclear factor κB inhibitor, dehydroxymethylepoxyquinomicin. Clin Cancer Res. 2004;10:6821–6829. doi: 10.1158/1078-0432.CCR-04-0463. [DOI] [PubMed] [Google Scholar]
- 9.Mitsiades CS, McMillin D, Kotoula V, Poulaki V, McMullan C, Negri J, Fanourakis G, Tseleni- Balafouta S, Ain KB, Mitsiades N. Antitumor effects of the proteasome inhibitor bortezomib in medullary and anaplastic thyroid carcinoma cells in Vitro . J Clin Endocrinol Metab. 2006;91:4013–4021. doi: 10.1210/jc.2005-2472. [DOI] [PubMed] [Google Scholar]
- 10.Visconti R, Cerutti J, Battista S, Fedele M, Trapasso F, Zeki K, Woitowitz HJ. Expression of the neoplastic phenotype by human thyroid carcinoma cell lines requires NFκB p65 protein expression. Oncogene. 1997;15:1987–1994. doi: 10.1038/sj.onc.1201373. [DOI] [PubMed] [Google Scholar]
- 11.Kato Y, Ying H, Zhao L, Furuya F, Araki O, Willingham MC, Cheng SY. PPARgamma insufficiency promotes follicular thyroid carcinogenesis via activation of the nuclear factor-kappaB signaling pathway. Oncogene. 2006;25:2736–2747. doi: 10.1038/sj.onc.1209299. [DOI] [PubMed] [Google Scholar]
- 12.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-κB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foo SY, Nolan GP. NF-kappaB to the rescue: RELs, apoptosis and cellular transformation. Trends Genet. 1999;15:229–235. doi: 10.1016/s0168-9525(99)01719-9. [DOI] [PubMed] [Google Scholar]
- 14.Hwang JH, Hwang JH, Chung HK, Kim DW, Hwang ES, Suh JM, Kim H, You KH, Kwon OY, Ro HK, Jo DY, Shong M. CXC chemokine receptor 4 expression and function in human anaplastic thyroid cancer cells. J Clin Endocrinol Metab. 2003;88:408–416. doi: 10.1210/jc.2002-021381. [DOI] [PubMed] [Google Scholar]
- 15.Russell JP, Shinohara S, Melillo RM, Castellone MD, Santoro M, Rothstein JL. Tyrosine kinase oncoprotein, RET/PTC3, induces the secretion of myeloid growth and chemotactic factors. Oncogene. 2003;22:4569–4577. doi: 10.1038/sj.onc.1206759. [DOI] [PubMed] [Google Scholar]
- 16.Weinberg RA. Multistep tumorigenesis. In: Weinberg RA, editor. The Biology of Cancer. New York: Garland Science, Taylor and Francis Group, LLC; 2007. pp. 399–462. [Google Scholar]
- 17.Jenkins GJS, Mikhail J, Alhamdani A, Brown T, Caplin S, Manson J, Bowden R, Toffazal N, Griffiths AP, Parry JM, Baxter JN. Immunohistochemical study of NF-κB activity and IL-8 abundance in oesophageal adenocarcinoma; a useful strategy for monitoring these biomarkers. J Clin Pathol. 2007;60:1232–1237. doi: 10.1136/jcp.2006.043976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morceau F, Duvoix A, Delhalle S, Schnekenburger M, Dicato M, Diederich M. Regulation of glutathione S-transferase Pi-1 gene expression by NF-kappaB in tumor necrosis factor alpha-treated K562 leukemia cells. Biochem Pharmacol. 2004;67:1227–1238. doi: 10.1016/j.bcp.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 19.Sareddy GR, Geeviman K, Ramulu C, Babu PP. The nonsteroidal anti-inflammatory drug celecoxib suppresses the growth and induces apoptosis of human glioblastoma cells via the NF-κB pathway. J Neurooncol. 2012;106:99–109. doi: 10.1007/s11060-011-0662-x. [DOI] [PubMed] [Google Scholar]
- 20.Vaish V, Sanyal SN. Chemopreventive effects of NSAIDs on cytokines and transcription factors during the early stages of colorectal cancer. Pharmacol Rep. 2011;63:1210–1221. doi: 10.1016/s1734-1140(11)70641-7. [DOI] [PubMed] [Google Scholar]
- 21.Ito Y, Yoshida H, Nakano K, Takamura Y, Miya A, Kobayashi K, Yokozawa T, Matsuzuka F, Matsuura N, Kuma K, Miyauchi A. Cyclooxygenase- 2 expression in thyroid neoplasms. Histopathology. 2003;42:492–497. doi: 10.1046/j.1365-2559.2003.01624.x. [DOI] [PubMed] [Google Scholar]
- 22.Haynik DM, Prayson RA. Immunohistochemical expression of cyclooxygenase 2 in follicular carcinomas of the thyroid. Arch Pathol Lab Med. 2005;129:736–741. doi: 10.5858/2005-129-736-IEOCIF. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto J, Aoki I, Khatun S, Toyoki H, Tamaya T. Clinical implications of expression of interleukin- 8 related to myometrial invasion with angiogenesis in uterine endometrial cancers. Ann Oncol. 2002;13:430–434. doi: 10.1093/annonc/mdf078. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Yang J, Gao Y, Du Y, Bao L, Niu W, Yao Z. Regulatory effect of e2, IL-6 and IL-8 on the growth of epithelial ovarian cancer cells. Cell Mol Immunol. 2005;2:365–372. [PubMed] [Google Scholar]
- 25.Itoh Y, Joh T, Tanida S, Sasaki M, Kataoka H, Itoh K, Oshima T, Ogasawara N, Togawa S, Wada T, Kubota H, Mori Y, Ohara H, Nomura T, Higashiyama S, Itoh M. IL-8 promotes cell proliferation and migration through metalloproteinase- cleavage proHB-EGF in human colon carcinoma cells. Cytokine. 2005;29:275–282. doi: 10.1016/j.cyto.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Toffoli G, Viel A, Tumiotto L, Giannini F, Volpe R, Quaia M, Boiocchi M. Expression of glutathione- S-transferase-pi in human tumours. Eur J Cancer. 1992;28A:1441–1446. doi: 10.1016/0959-8049(92)90540-i. [DOI] [PubMed] [Google Scholar]
- 27.Cookson MS, Reuter VE, Linkov I, Fair WR. Blutathione S-transferase PI (GST-pi) class expression by immunohistochemistry in benign and malignant prostate tissue. J Urol. 1997;157:673–676. [PubMed] [Google Scholar]
- 28.Carmichael J, Forrester LM, Lewis AD, Hayes JD, Hayes PC, Wolf CR. Glutathione S-transferase isoenzymes and glutathione peroxidase activity in normal and tumour samples from human lung. Carcinogenesis. 1988;9:1617–1621. doi: 10.1093/carcin/9.9.1617. [DOI] [PubMed] [Google Scholar]
- 29.Peters WH, Nagengast FM, Wobbes T. Glutathione S-transferases in normal and cancerous human colon tissue. Carcinogenesis. 1989;10:2371–2374. doi: 10.1093/carcin/10.12.2371. [DOI] [PubMed] [Google Scholar]
- 30.Moscow JA, Townsend AJ, Cowan KH. Elevation of pi class glutathione S-transferase activity in human breast cancer cells by transfection of the GST pi gene and its effect on sensitivity to toxins. Mol Pharmacol. 1989;36:22–28. [PubMed] [Google Scholar]
- 31.Shi H, Lu D, Shu Y, Shi W, Lu S, Wang K. Expression of multidrug resistance-related proteins p-glycoprotein, glutathione-s-transferases, topoisomerase-II and lung resistance protein in primary gastric cardiac adenocarcinoma. Hepatogastoenterology. 2008;55:1530–1536. [PubMed] [Google Scholar]
- 32.Nakajima T, Takayama T, Miyanishi K, Nobuoka A, Hayashi T, Abe T, Kato J, Sakon K, Naniwa Y, Tanabe H, Niitsu Y. Reversal of multiple drug resistance in cholangiocarcinoma by the glutathione S-transferase-pi-specific inhibitor O1- hexadecyl-gamma-glutamyl-S-benzylcysteinyl-D-phenylglycine ethylester. J Pharmacol Exp Ther. 2003;306:861–869. doi: 10.1124/jpet.103.052696. [DOI] [PubMed] [Google Scholar]
- 33.Calatozzolo C, Gelati M, Ciusani E, Sciacca FL, Pollo B, Cajola L, Marras C, Silvani A, Vitellaro- Zuccarello L, Croci D, Boiardi A, Salmaggi A. Expression of drug resistance proteins Pgp, MRP1, MRP3, MRP5 and GST-pi in human glioma. J Neurooncol. 2005;74:113–121. doi: 10.1007/s11060-004-6152-7. [DOI] [PubMed] [Google Scholar]
- 34.Sauerbrey A, Zintl F, Volm M. P-glycoprotein and glutathione S-Transferase pi in childhood acute lymphoblastic leukaemia. Br J Cancer. 1994;70:1144–1149. doi: 10.1038/bjc.1994.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toffoli G, Frustaci S, Tumiotto L, Talamini T, Gherlinzoni F, Picci P, Boiocchi M. Expression of MDR1 and GST-pi in human soft tissue sarcomas: relation to drug resistance and biological aggressiveness. Ann Oncol. 1992;3:63–69. doi: 10.1093/oxfordjournals.annonc.a058073. [DOI] [PubMed] [Google Scholar]
- 36.Yu DS, Hsieh DS, Chang SY. Increasing expression of GST-pi MIF, and ID1 genes in chemoresistant prostate cancer cells. Arch Androl. 2006;52:275–281. doi: 10.1080/01485010600630124. [DOI] [PubMed] [Google Scholar]
- 37.Schipper D, Wagenmans M, Wagener D, Peters W. Glutathione S-transferases and cancer (Review) Int J Oncol. 1997;10:1261–1264. doi: 10.3892/ijo.10.6.1261. [DOI] [PubMed] [Google Scholar]
- 38.Brown RE. Morphoproteomics: exposing protein circuitries in tumors to identify potential therapeutic targets in cancer patients. Expert Rev Proteomics. 2005;2:337–348. doi: 10.1586/14789450.2.3.337. [DOI] [PubMed] [Google Scholar]
- 39.Baldwin AS Jr. Series introduction: the transcription factor NF-κB and human disease. J Clin Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SJ, Lee JH, Yoon JS, Mok JO, Kim YJ, Park HK, Kim CH, Byun DW, Suh KI, Yoo MH. Immunohistochemical expression of COX-2 in thyroid nodules. Korean J Intern Med. 2003;18:225–229. doi: 10.3904/kjim.2003.18.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casey MB, Zhang S, Jin L, Kajita S, Lloyd RV. Expression of cyclooxygenase-2 and thromboxane synthase in non-neoplastic and neoplastic thyroid lesions. Endocr Pathol. 2004;15:107–116. doi: 10.1385/ep:15:2:107. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Gonzalez M, Abdulkader I, Boquete AV, Neo XM, Forteza J, Cameselle-Teijeiro J. Cyclooxygenase-2 in normal, hyperplastic and neoplastic follicular cells of the human thyroid gland. Virchows Arch. 2005;447:12–17. doi: 10.1007/s00428-005-1235-1. [DOI] [PubMed] [Google Scholar]
- 43.Lee KJ, Jung YS, Kim WH, Yoon IT, Joo HJ, Soh EY. Cyclooxygenase-2 expression in human thyroid disease. J Endocrinol Invest. 2008;31:111–118. doi: 10.1007/BF03345576. [DOI] [PubMed] [Google Scholar]
- 44.Khan Z, Khan N, Tiwari RP, Sah NK, Prasad GB, Bisen PS. Biology of Cox-2: an application in cancer therapeutics. Curr Drug Targets. 2011;12:1082–1093. doi: 10.2174/138945011795677764. [DOI] [PubMed] [Google Scholar]
- 45.Kobawala TP, Patel GH, Gajjar DR, Patel KN, Thakor PB, Parekh UB, Patel KM, Shukla SN, Shah PM. Clinical utility of serum interleukin-8 and interferon-alpha in thyroid diseases. J Thyroid Res. 2011;2011:270149. doi: 10.4061/2011/270149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang S, Liu Z, Wang L, Zhang X. NF-κB signaling pathway, inflammation and colorectal cancer. Cell Mol Immunol. 2009;6:327–334. doi: 10.1038/cmi.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang S, DeGuzman A, Bucana CD, Fidler IJ. Nuclear factor-kappaB activity correlates with growth, angiogenesis, and metastasis of human melanoma cells in nude mice. Clin Cancer Res. 2000;6:2573–2581. [PubMed] [Google Scholar]
- 48.Duvoix A, Morceau F, Delhalle S, Schmitz M, Schnekenburger M, Galteau MM, Dicato M, Diederich M. Induction of apoptosis by curcumin: mediation by glutathione S-transferase P1- 1 inhibition. Biochem Pharmacol. 2003;66:1475–1483. doi: 10.1016/s0006-2952(03)00501-x. [DOI] [PubMed] [Google Scholar]
- 49.Kawakatsu M, Goto S, Yoshida T, Urata Y, Li TS. Nuclear translocation of glutathione S-transferase π is mediated by a non-classical localization signal. Biochem Biophys Res Commun. 2011;411:745–750. doi: 10.1016/j.bbrc.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 50.Rolland D, Raharijaona M, Barbarat A, Houlgatte R, Thieblemont C. Inhibition of GST-pi nuclear transfer increases mantle cell lymphoma sensitivity to cisplatin, cytarabine, gemcitabine, bortezomib and doxorubicin. Anticancer Res. 2010;30:3951–3957. [PubMed] [Google Scholar]