Abstract
Angiogenesis is a fundamental process in tumor growth and metastasis. Expression of vascular endothelial growth factor (VEGF) as prognostic indicator has been documented in many types of human tumors. However, the mechanisms responsible for angiogenesis in urinary bladder carcinoma patients are not well defined. Certain carcinogens in tobacco cause DNA damage and may produce specific mutations. In order to investigate the relationship between tobacco smoking, altered patterns of VEGF expression and apoptosis, we have analyzed a group of 125 incident patients with transitional cell carcinoma and 100 cases of control with inflammatory lesions of the bladder. We assessed VEGF overexpression by the use of anti- VEGF antibody through immunohistochemistry, and apoptosis by TUNEL Assay. Expression of VEGF and apoptosis was noticed in 43.2% and 52.8% cases respectively. Both VEGF and apoptosis increased with increasing tumor grade. Apoptosis was seen to be significantly higher in both sexes in the age group of ≥ 50 years (p<0.05) but expression of VEGF was significantly higher among males in the age group of ≥ 50 years (p<0.05). We observed an insignificant association between cigarettes smoking and VEGF overexpression (p>0.05) and significant association with apoptosis. These data support the hypothesis that certain carcinogens derived from cigarette smoking may induce VEGF mutations and apoptosis which in turn are involved in early steps of bladder carcinogenesis.
Keywords: Bladder cancer, apoptosis and cigarette smoking, IHC, TUNEL Assay
Introduction
Urinary bladder carcinoma is approximately three times more common among men than women [1]. Cigarette smoking is the well established risk factor and contributes to more than 40% cancer of urinary bladder [2]. The effects of smoking duration, intensity, exposure to environmental tobacco smoke and changes in the composition of tobacco on risk of urinary bladder cancer are not clear [3].
Cancer causing chemicals in the cigarette smoke are absorbed into blood and filtered out by kidney and then as part of the urine, stored in the bladder. In the long term, this appears to cause damage to bladder lining and formation of DNA adducts resulting into transitional mutation [4]. Finding from recent prospective studies [5] suggest that cigarette smoking may act as an initiator of urinary bladder carcinogenesis. Earlier investigators described that p53/bcl2 overexpression/mutation and smoke are associated with bladder carcinoma [6,7]. VEGF is another protein that is a potent stimulator of angiogenesis, inducer of endothelial cell migration and vascular permeability [8-10]. VEGF is the key mediator of angiogenesis in cancer, where it is up-regulated by oncogenic expression and a variety of growth factors. VEGF was reported in previous studies, to contribute to high degree of vascularization in malignant tumor and promote tumor progression [11].
Apoptosis is one of the prerequisites to maintain the normal & healthy internal milieu. Disruption in this normal process of apoptosis may increase cell survival and facilitates the tumor development [12,13]. Regulation of apoptosis becomes very complicated in cancerous condition under certain tumor suppressor genes or the other oncogenes [14-16].
Earlier studies have found that cell death due to apoptosis is a significant process ultimately leading to bladder cancerous development [17]. In this study, we evaluated the possible role of VEGF and apoptosis in relation to cigarette smoking and urinary bladder cancer risk through immunohistochemistry and TUNEL assay respectively.
Materials and methods
Study population
Total numbers of 125 histopathologically confirmed cases of Transitional cell carcinoma (TCC) of urinary bladder and 100 cases of inflammatory lesions of the bladder as control were taken. The authors had prior approval from institutional ethics committee equivalent to Institutional Review Board (IRB).
Patients were asked to complete the questionnaire soliciting information on cigarette smoking. One hundred twenty five cases of Transitional cell carcinoma were classified into three Grades: Grade I, Grade II and Grade III according to the WHO grading system by experienced pathologists. Keeping the marker profile in view, the cases were further divided according to their age into two groups: Less than 50 years & ≥ 50 years.
Exposure
A complete historical background was compiled through the patient to find out a possible etiology of urinary bladder cancer.
Immunohistochemical analysis
Formalin fixed paraffin-embedded tissue blocks were cut in 5 microns thick serial sections. The sections were deparaffinized, rehydrated and rinsed with phosphate buffer saline (PBS). An Immunohistochemical assay for VEGF was performed on consecutive paraffin sections using straptavidine-biotin method. Monoclonal mouse antihuman antibody (G153-694, BD PharMingen) was taken as primary antibodies for VEGF. After antigen retrieval slides were incubated with primary antibody, followed by secondary biotinylated antibody. Sections were washed in PBS and then incubated with straptavadin peroxidase. Finally chromogen Diaminobenzedine (DAB) was used and sections were counterstained with haematoxylin.
In-situ apoptosis detection
Formalin fixed paraffin-embedded tissue were deparaffinized by xylene, ethanol and apoptoticaly fragmented cellular DNA was identified by TUNEL assay (Apoptosis Detection Kit CAT#QIA21-1EA Oncogen research products, USA). Apoptotic activity was quantified by the apoptotic index which represented the percentage of apoptotic epithelial cells in each tissue.
Scoring method
The slides were scored independently by one of the co-authors without reference to any clinical or pathological information. Tumors were classified as VEGF and apoptosis negative (i.e. low expression) if less than or equal to 10% of cells displayed positivity. If greater than 10% of cells were positive for VEGF and apoptosis (i.e. high expression) were considered as positive.
A total of 5 to 6 fields from each tissue section were chosen, and 100 cells from each field were counted at a final magnification of 400X. With every batch of staining a positive and negative control were used to verify the standard of staining.
Statistical analysis
Chi square test was performed to find out the possible correlation among VEGF, apoptosis and other clinical parameters. P<0.05 was considered as statistically significant. To examine aetiologic heterogeneity, odd ratios were calculated for the association between cigarette smoking, VEGF and apoptosis measures separately. Association between bladder tumors and cigarette smoking were measured using odds ratios and 95% confidence intervals.
Inclusion criteria
Histo-pathologically confirmed cases of Transitional cell carcinoma were included. The histopathologically confirmed cases of inflammatory lesions of urinary bladder were taken as a control.
Exclusion criteria
Patients with family history, Patients with addictions like alcoholism, drugs and Patients undergoing long term treatment for any other disease. Squamous cell carcinoma and Adenocarcinoma cases of the urinary bladder were also not included in the study.
Results
A total of 125 (104 males and 21 females) of histopathologically confirmed cases of Transitional cell carcinoma (TCC) and 100 cases of confirmed inflammatory lesions of urinary bladder as control comprising 75 males and 25 females were assessed from the biopsy samples.
The age of the patients ranged from 24 to 80 years with a mean age of 55 years, whereas in control group it was 14 to 55 years with mean being 38 ± 14 year. The incidence of carcinoma of urinary bladder was predominantly seen among males 83.2% patients as compared to the 16.8% females, giving the ratio 5:1 (male v/ s females). The maximum number of controls were in < 40 years age group (80%) followed by 40-55 years (20%).However when the control group was divided into males and females the mean age was 38 ± 15 years for males and 40 ± 10 year for females. The sex ratio of this group was 3:1. Of the 125 Transitional cell carcinoma (TCC) cases studied, a peak incidence was seen in the age group of 50-70 years. There were 76% (95) cases in the age group of 50-80 yrs, 24% (30) in the age group of 24-50 yrs. It was observed that out of the 125 total cases 38, 47 and 40 had grade I, grade II and grade III carcinoma respectively.
A detailed exposure history was taken in all subjects. In males, it was found that 71% (74 cases) smokers. Among females subject only 4.71% (1) were smokers.
VEGF expression and smoking in bladder cancer
Expression of VEGF was seen in 54 cases (43.2%). Out of the total 125 cases: 14 cases (36.8%) of grade I, 21 cases (44.6%) of grade II and 19 cases (47.4%) of grade III was positive for VEGF (Table 1 and Figure 1).
Table 1.
Expression pattern of VEGF and Apoptosis in TCC cases
| Clinical parameters of TCC | VEGF positive Cases | Apoptosis positive Cases | |||
|---|---|---|---|---|---|
| Total positive Cases | Percentage positivity | Total positive cases | Percentage positivity | ||
| Tumor Grades | Grade I | 14 | 36.8% | 18 | 47.3% |
| Grade II | 21 | 44.6% | 24 | 51.0% | |
| Grade III | 19 | 47.5% | 24 | 60.0% | |
| Total Cases | 54 | 43.2% | 66 | 52.8% | |
| Sex | Male | 45 | 43.2% | 60 | 57.6% |
| Female | 09 | 42.8% | 06 | 28.5% | |
| Age | <50 years | 12 | 28.5% | 13 | 30.9% |
| ≥50 years | 42 | 50.6% | 53 | 62.3% | |
Figure 1.
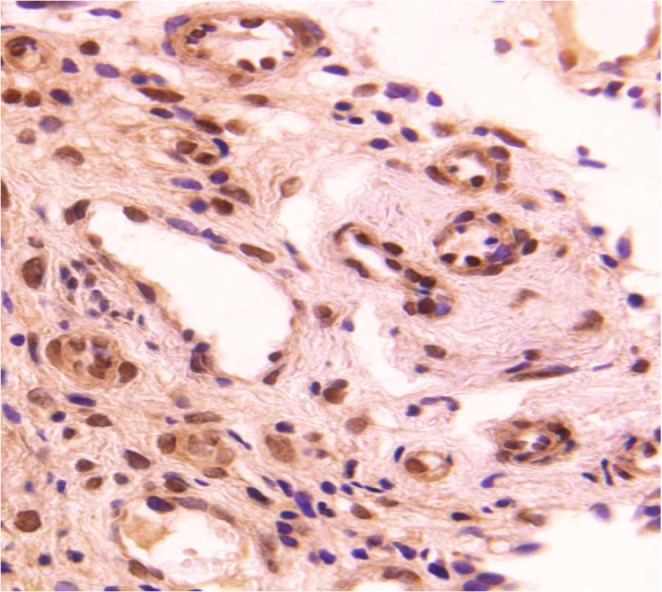
VEGF expression in Transitional cell carcinoma of urinary bladder. Typical immunostaining of VEGF in TCC (Orig. Magni. X400).
Expression of VEGF was found in tumor cell but not in the normal transitional epithelium. The result reveals the significant difference between the normal epithelium (0%) and cancerous tissue (43.2%) for the VEGF protein (p<0.05). With the progression in tumor grade, the rate of VEGF expression significantly increased (p<0.05). VEGF expression was further correlated on the basis of sex and age of the patients. The expression of VEGF was modified with gender and age. The patients based on the above, were divided into two groups < 50 years and ≥ 50 years (Table 2). Among females, there was no significant difference of VEGF expression between the two age groups (< 50 and ≥ 50 years). However; among males with the age ≥ 50 years the expression of VEGF was significantly higher (59.2%) as compared to the < 50 years of age group (28%) (p<0.05).
Table 2.
Relationship between VEGF expression, sex and age
| Sex | Age | VEGF Positivity | |||
|---|---|---|---|---|---|
| Total cases | Positive cases | Percentage | P- Value | ||
| < 50 | 32 | 09 | 28% | ||
| Male | ≥ 50 | 68 | 36 | 52.9% | P<0.05 |
| < 50 | 10 | 03 | 30% | P>0.05 | |
| Female | ≥ 50 | 15 | 06 | 40% | |
Cigarette smoking history was analyzed in VEGF positive cases. We found that bladder cancer patients who reported smoking exposure did not maintain VEGF expression; among smokers; 71% (50 cases) patients showed VEGF expression where as among non smocker it was 61% (34 cases) non-smokers (Table 4). The exposure pattern of VEGF with respect to cigarette smoking was therefore found to be insignificant.
Table 4.
VEGF overexpression, Apoptosis and smoking association
| Subject of study | Variable | No. of cases | Cases shown | Odd ratio | 95% CL | P-Value | |
|---|---|---|---|---|---|---|---|
| Positive No. (%) | Negative No. (%) | ||||||
| VEGF | Non smoking | 55 | 34(61) | 21(38) | 0.24 | 0.116- 0.523 | P>0.05 |
| Smoking | 70 | 50(71) | 20(29) | ||||
| Apoptosis | Non smoking | 55 | 22 (37) | 33 (60) | 2.25 | 1.03-4.94 | P<0.05 |
| Smoking | 70 | 42(60) | 28 (40) | ||||
The association between cigarette smoking and positivity of VEGF were analysed in odd ratio and were found to be 0.24 (95% CI 0.116- 0.523) (p trend P>0.05) for current smokers when compared with non smokers (Table 4).
Apoptosis in TCC of urinary bladder
Cells were evaluated for apoptosis by the TUNEL assay on the basis of staining and their specific cellular and morphological characteristics displayed during apoptosis [18]. The apoptotic staining revealed that the cells undergoing apoptotosis were randomly distributed in the cancerous transitional epithelium of bladder (Figure 2). Apoptosis was seen in 66 cases (52.8%) in all. Where as on grade wise analysis, 18 cases (47.3%) in grade I, 24 cases (51%) in +-grade II and 24 cases (60%) of grade III were positive for apoptosis (Table 1).
Figure 2.
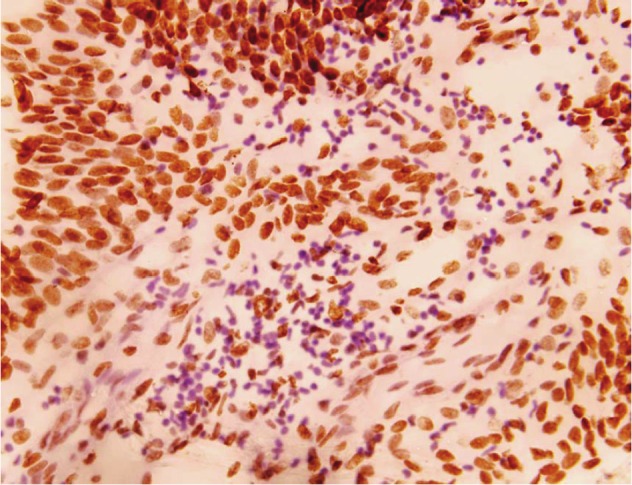
Typical apoptotic cells in transitional cell carcinoma of bladder (Original magnification X400).
Apoptosis index (i.e. mean percentage of apoptotic cells) were low in normal cases (0.1 ± 0.1) whereas apoptosis index was high in TCCs cases (>1.0). The average apoptosis index for grade III TCCs (1 ± 0.2) was the highest whereas the apoptotic index was lowest in grade I (0.2 ± 0.1), with that for grade II TCCs (0.8 ± 0.2). It was found that along the progression of tumor grade, apoptotic index significantly increased. Apoptosis positive cases were further compared evaluated on the basis of sex and age of the patients. It was found that the apoptosis were significantly higher in both sexes of ≥ 50 years age group (p< 0.05) (Table 3). Apoptosis was observed in 60% (42 cases) of smokers, 37% (22 cases) non-smokers. The association between cigarette smoking and apoptosis was analysed and found to be 2.25 (95% CI 1.03-4.94) (p trend P<0.05) as compared to non-smoker (Table 4).
Table 3.
Relationship between apoptosis, sex and age
| Sex | Age | Apoptosis Positivity | |||
|---|---|---|---|---|---|
| Total cases | Positive cases | Percentage | P- Value | ||
| < 50 | 32 | 12 | 37.5% | ||
| Male | ≥ 50 | 68 | 48 | 70.5 % | P<0.05 |
| < 50 | 10 | 01 | 10% | ||
| Female | ≥ 50 | 15 | 05 | 33% | P<0.05 |
Correlation between VEGF and apoptosis in bladder cancer
VEGF immunoreactivity and apoptosis in TCC of bladder were found to be increasing in the similar fashion from grade I, grade II to grade III of the carcinoma. Statistical analyses showed correlation among the variables like progression of tumor grade, the rate of VEGF expression and apoptotic index. VEGF expression and apoptosis were further evaluated on the basis of sex and age. Both apoptosis as well as VEGF expression was seen to be positively correlated to the grades of tumor in ≥ 50 years age group of both sexes. Interestingly, of the 125 Transitional Cell Carcinoma cases examined, 60% cases showed both apoptosis and VEGF expression, indicating that apoptosis and VEGF play an important role in the genesis of urinary bladder carcinoma.
Discussion
TCC is the most common cancer of urinary bladder and accounts for 90% of all bladder carcinoma [19]. Our findings also indicate similar type of pattern with more than 92% of the patients had TCC, whereas SCC and Adenocarcinoma accounted for 6% and 2% respectively. Though there are many studies on the etiology of cancer but the exact pathogenesis still remains uncertain. The various risk factors have been analysed and reported in the literature including cigarette smoking, age and sex. The incidence of bladder cancer has been reported to increase with the increase in age.
In our study the peak incidence of bladder cancer was observed in the age group of 50-70 years. The reasons for this remain unclear, but it might be due to the cumulative effects of long time exposures to carcinogens, the failure of DNA repair mechanisms and aging [20].
The male to female ratio of the urinary bladder cancer is reported to be 3:1 in USA, and 2.2:1 in Italy [21]. In our study made on Indian patients it was found to be 5:1 a different observation made by earlier investigators from other metropolis of the country [22] and different from other part of the world [23]. A much higher incidence of tumors was noted in males compared with females. The variation of incidence of bladder cancer in the two genders may be due to the variation in the environmental, dietary exposures, innate sexual characteristics such as anatomic differences, urination habits, or hormonal factors [24]. It is also important to note that in Indian scenario tobacco chewing and smoking are much higher among men than women, further due to social factors, the men acquire these habits earlier than women.
Cigarette smokers as reported have four times higher incidence of bladder cancer [25]. The risk of cigarette smoking was observed in both the sexes. Earlier investigators reported that p53 mutation was high in bladder cancer of smokers as compared to non smokers [26]. This suggests that cigarette smoking might increase the mutations in urothelial cells.
In present study, among male patients, 71% were smokers, where as the previous study showed that there were 50% smokers among male and 31% among female [26]. In accordance with earlier findings, our data shows that the incidence of smoking was much higher among male cases if compared with female cases (71% versus 4.7%).
Angiogenesis is an independent prognostic tumor marker in several types of tumors and VEGF is major factor in this process [27]. VEGF expression is related to systemic metastasis via angiogenesis. It promotes invasion and migration of endothelial cells and by increasing vascular permeability. Multiple studies have suggested that the expression of VEGF correlated with stages and grades of bladder TCC [27,28]. Our study made on Indian patients, for the expression of VEGF in urinary bladder carcinoma hereby, reports 43.2% VEGF expression was seen in high grade TCC of urinary bladder.
VEGF expression is reported to be more prevalent in advanced and progressing bladder carcinoma [11]. Our finding indicates there is a strong positive correlation between VEGF expressions and tumor grade (Grade I- 36.8%, Grade II - 44.6% and Grade III - 47.5%).
The overexpression of VEGF and angiogenesis in transitional cell carcinoma remained largely unknown. It might be due to the reason that the smoking exposure impairs VEGF-induced endothelial cell migration and tube formation [29]. VEGF regulates neovascularization in malignant cells and alter cancer cell formation and tumor progression. It promotes invasion, migration of endothelial cells and facilitates the entry of tumor cells into the circulation allowing them to metastasize.
VEGF expression was significantly high among male patients with age ≥ 50 years as compared to < 50 age group. The exact reason of this difference is difficult to explain. However, the possible explanation of this difference may lie behind the fact that the habit of tobacco chewing and cigarette smoking is much higher among men than women and the men acquire these habits earlier in their life than the women. There further seems to be a longer latency period required for carcinogenic induction. VEGF also appears to yield significant prognostic information in addition to the tumor grade and may be of much value for the clinical management of bladder carcinoma. The pattern of VEGF immunostaining and its association with tumor progression makes it a candidate for antigrowth factor therapy.
Apoptosis was analysed in the present study by in situ terminal transferarse-mediated dUTP nick end labeling (TUNEL) and was semiquantified by apoptotic index [18]. It was found that average apoptotic index and apoptotic cells were more frequently seen in high grade tumors (0.2 ± 0.1 in grade I, 0.8 ± 0.2 in grade II and 1 ± 0.2 in grade III) this was statistically significant. It was also found that 47.30%, 51.60% and 60.0% of TCCs cases were positive for apoptosis in grade I, grade II and III respectively.
This analysis showed that apoptotic cell death is increased in high grade tumors as compared to the tumors of low grade malignancy. Earlier investigator reported similar observations that apoptosis was higher in higher grade of tumors [11], suggesting that the tumor show high proliferative activity, leading to cellular turnover in these tumors. What triggers apoptosis during tumor development is still a debated topic [14,15]. A variety of signals play an important role in the development of tumor. Earlier studied showed that cigarette smoke can produce single stranded DNA [30,31] and alter regulation of the c-fos, jun at the transcriptional level [32]. Previous studies showed that cigarette smoking play a role in stimulation of iNOS and c-fos via regulation of protein tyrosine phosphorylation and MAP kinase under oxidative stress, which in turn, may promote lung pathogenesis [32,33].
When apoptosis was analyzed on the basis of age and sex, it was found that apoptosis was significantly higher among ≥ 50 age group of either sex as compared to the patients with age group < 50 years (p<0.05). The reason for this difference may be due to the high endogenous level of oxidative adducts, cigarette smoking, dietary imbalances, infections, hormones, occupational exposures and agin [25]. Carcinogenesis/ tumorigenesis may further be associated with molecular alterations of genes involved in control of cellular proliferation and other physiological function. The cumulative exposure of such carcinogens might be the reason of mutation or silencing in some crucial tumor suppressor gens or in the apoptotic regulatory genes thereby leading to development or progression of such carcinomas [37,38]. To investigate the association between apoptosis, VEGF, smoking in urinary bladder carcinoma, we analyzed tumor specimens for, VEGF and categorized them according to patients smoking exposure.
To estimate the individual relative risk of apoptosis positive versus apoptosis negative, and VEGF positive versus negative cases, a control was used for comparative analysis. The tumors showing positivity for apoptosis significantly were associated with smoking status whereas VEGF was not.
In present study there was a positive correlation between apoptosis and VEGF suggesting that overexpression of VEGF may be important for apoptosis to occur during urinary bladder carcinogenesis. To our knowledge, this is the first study regarding the relationship between Vascular Endothelial Growth Factor, Apoptosis, smoking and the risk of bladder carcinoma.
Our data support that apoptosis is associated with smoking and the risk of urinary bladder carcinoma whereas VEGF was not. The molecular mechanism behind this is not properly understood. Cigarette smoking has been known to be associated with high risk of bladder cancer [34]. There are earlier reports showing interaction of genetic factor and effect of smoking on the genesis of bladder cancer [35,36].
The data obtained in the present study led us to conclude that (1) Cigarette smoke causes carcinogenesis through the forming DNA adducts that result in transitional mutation. (2) Cigarette smoke impairs VEGF function via VEGF -induced endothelial cell migration and tube formation. (3) Cigarette smoke induces apoptosis through certain undefined mechanism involving the release of apoptosis related protein(s).
References
- 1.Madeb R, Meesing EM. Gender, racial and age differences in bladder cancer incidence and mortality. Urol Oncol. 2004;22:86–92. doi: 10.1016/S1078-1439(03)00139-X. [DOI] [PubMed] [Google Scholar]
- 2.Strope SA, Montie JE. The Causal Role of Cigarette Smoking in Bladder Cancer Initiation and Progression, and the Role of Urologists in Smoking Cessation. J Urol. 2008;180:31–37. doi: 10.1016/j.juro.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 3.Baris D, Karagas MR, Verrill C, Johnson A, Andrew AS, Marsit CJ, Schwenn M, Colt JS, Cherala S, Samanic C, Waddell R, Cantor KP, Schned A, Rothman N, Lubin J, Fraumeni JF Jr, Hoover RN, Kelsey KT, Silverman DT. A case-control study of smoking and bladder cancer risk: emergent patterns over time. Journal of the national cancer institute. J Natl Cancer Inst. 2009;101:1553–1561. doi: 10.1093/jnci/djp361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hemelt M, Yamamoto H, Cheng KK, Zeegers MP. The effect of smoking on the male excess of bladder cancer: a meta-analysis and geographical analyses. Int J Cancer. 2009;124:412–419. doi: 10.1002/ijc.23856. [DOI] [PubMed] [Google Scholar]
- 5.Zhang ZF, Sarkis AS, Carlos CC, Dalbagani D, Melamed J, Aprikian A, Pollack D, Shaienfold j, Herr HW, Fair WR, Reuter VE, Begg C. Tobacco Smoking, Occupation, and p53 Nuclear Overexpression in Early Stage Bladder Cancer. Cancer Epidemiol Biomarkers Prev. 1994;3:19–24. [PubMed] [Google Scholar]
- 6.Zhang ZF, Shu XM, Cordon-Cardo C, Orlow I, Lu ML, Millon TV, Cao PQ, Connolly-Jenks C, Dalbagni G, Lianes P, Lacombe L, Reuter VE, Scher H. Cigarette smoking and chromosome 9 alterations in bladder cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:321–326. [PubMed] [Google Scholar]
- 7.Ferrara N, Davis-Smyth T. The biology of vascular endothelial vascular factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 8.Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, Hicklin DJ, Zhu Z, Witte L, Crystal RG, Moore MA, Rafii S. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. 2001;193:1005–1014. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 10.Izawa JI, Slaton JW, Kedar D, Karashima T, Perrotte P, Czerniak B, Grossman HB, Dinney CP. Differential expression of progression-related genes in the evolution of superficial to invasive transitional cell carcinoma of the bladder. Oncol Rep. 2001;8:9–15. doi: 10.3892/or.8.1.9. [DOI] [PubMed] [Google Scholar]
- 11.Huovinen R, Warri A, Collan Y. Mitotic activity, apoptosis and TRPM-2 mRNA expression in DMBA-induced rat mammary carcinoma treated with anti-estrogen toremifene. Int J Cancer. 1993;55:685–691. doi: 10.1002/ijc.2910550429. [DOI] [PubMed] [Google Scholar]
- 12.Hollowood K, Macartney JC. Reduced apoptotic cell death in follicular lymphoma. J Pathol. 1991;163:337–342. doi: 10.1002/path.1711630411. [DOI] [PubMed] [Google Scholar]
- 13.Sachs L, Lotem J. Control of programmed cell death in normal and leucemic cells: new implication for therapy. Blood. 1993;82:15–21. [PubMed] [Google Scholar]
- 14.Nisreen SA, Mehdi SJ, Alam MS, Ali A, Mandal AK, Gupta S, Singh I, Rizvi MM. PTEN mediated AKT activation contributes to the reduced apoptosis among Indian oral squamous cell carcinoma patients. J Cancer Res Clin Oncol. 2012;138:103–109. doi: 10.1007/s00432-011-1077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehdi SJ, Alam MS, Batra S, Rizvi MM. Allellic loss at 6q25-27, the Parkin tumor suppressor gene locus in cervical carcinoma. Med Oncol. 2011;28:1520–1526. doi: 10.1007/s12032-010-9633-x. [DOI] [PubMed] [Google Scholar]
- 16.Szende B, Juhasaz E, Lapis K, Schally AV. Inhibition of two-step urinary bladder carcinogenesis by the somatostalin analogue. RC-160. Urol Res. 1992;20:383–386. doi: 10.1007/BF00294492. [DOI] [PubMed] [Google Scholar]
- 17.Chan WY, Yew DT. Apoptosis and bcl-2 oncoprotein expression in the human fetal central nervous system. Anat Rec. 1998;252:165–175. doi: 10.1002/(SICI)1097-0185(199810)252:2<165::AID-AR2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Gupta P, Jain M, Kapoor R, Muruganandham K, Srivastava A, Mandhani A. Impact of age and gender on the clinicopathological characteristics of bladder cancer. Indian J urol. 2009;25:207–210. doi: 10.4103/0970-1591.52916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung I, Messing E. Molecular Mechanisms and Pathways in Bladder Cancer Development and Progression. Cancer Control. 2000;7:325–334. doi: 10.1177/107327480000700401. [DOI] [PubMed] [Google Scholar]
- 20.Horstmann M, Witthuhn R, Falk M, Stenzl A. Gender-specific differences in bladder cancer: A retrospective analysis. Gend Med. 2008;5:385–394. doi: 10.1016/j.genm.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Gangwar R, Mandhani A, Mittal RD. Caspase 9 and Caspase 8 Gene Polymorphisms and Susceptibility to Bladder Cancer in North Indian Population. Ann Surg Oncol. 2009;16:2028–2034. doi: 10.1245/s10434-009-0488-3. [DOI] [PubMed] [Google Scholar]
- 22.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. CA. Cancer statistics, 2005. Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 23.Horn EP, Tucker MA, Lambert G, Silverman D, Zametkin D, Sinha R, Hartge T, Landi MT, Caporaso NE. A study of gender-based cytochrome P4501A2 variability: a possible mechanism for the male excess of bladder cancer. Cancer Epidemiol Biomarkers Prev. 1995;4:529–533. [PubMed] [Google Scholar]
- 24.Burch JD, Rohan TE, Howe GR, Risch HA, Hill GB, Steele R, Miller AB. Risk of bladder cancer by source and type of tobacco exposure: a case-control study. Int J Cancer. 1989;44:622–628. doi: 10.1002/ijc.2910440411. [DOI] [PubMed] [Google Scholar]
- 25.Spruck CH rd, Rideout WM rd, Olumi AF, Ohneseit PF, Yang AS, Tsai YC, Nichols PW, Horn T, Hermann GG, Steven K, Ross RK, Yu MC, Jones PA. Distinct pattern of p53 mutations in bladder cancer: Relationship to tobacco usage. Cancer Res. 1993;53:1162–1166. [PubMed] [Google Scholar]
- 26.Crew JP, O'Brien T, Bradburn M, Fuggle S, Bicknell R, Cranston D, Harris AL. Vascular Endothelial Growth Factor Is a Predictor of Relapse and Stage Progression in Superficial Bladder Cancer. Cancer Res. 1997;57:5281–5285. [PubMed] [Google Scholar]
- 27.O'Brien T, Cranston D, Fuggle S, Bicknell R, Harris AL. Different Angiogenic Pathways Characterize Superficial and Invasive Bladder Cancer. Cancer Res. 1995;55:510–513. [PubMed] [Google Scholar]
- 28.Michaud SE, Dussault S, Groleau J, Haddad P, Rivard A. Cigarette smoke exposure impairs VEGF-induced endothelial cell migration: Role of NO and reactive oxygen species. J Mol Cell Cardiol. 2006;41:275–284. doi: 10.1016/j.yjmcc.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Fielding S, Short C, Davies K, Wald N, Bridges BA, Waters R. Studies on the ability of smoke from different types of cigarettes to induce DNA single-strand breaks in cultured human cells. Mutat Res. 1989;214:147–151. doi: 10.1016/0027-5107(89)90208-x. [DOI] [PubMed] [Google Scholar]
- 30.Leanderson P, Tagesson C. Cigarette smoke-induced DNA damage in cultured human lung cells: role of hydroxy radicals and endonuclease activation. Chem Biol Interact. 1992;81:197–208. doi: 10.1016/0009-2797(92)90034-i. [DOI] [PubMed] [Google Scholar]
- 31.Muller T. Expression of c-fos in quiescent Swiss 3T3 cells exposed to aqueous cigarette smoke fractions. Cancer Res. 1995;55:1927–1932. [PubMed] [Google Scholar]
- 32.Chang WC, Lee YC, Liu CL, Hsu JD, Wang HC, Chen CC, Wang CJ. Increased expression of iNOS and c-fos via regulation of protein tyrosine phosphorylation and MEK1/ERK2 proteins in terminal bronchiole lesions in the lungs of rats exposed to cigarette smoke. Arch Toxicol. 2001;75:28–35. doi: 10.1007/s002040000168. [DOI] [PubMed] [Google Scholar]
- 33.Yu MC, Skipper PL, Tannenbaum SR, Chan KK, Ross RK. Arylamine exposures and bladder cancer risk. Mutat Res. 2002;506-507:21–28. doi: 10.1016/s0027-5107(02)00148-3. [DOI] [PubMed] [Google Scholar]
- 34.Closas MG, Malats N, Silverman D, Dosemeci M, Kogevinas M, Hein DW, Tardón A, Serra C, Carrato A, Closas RG, Lloreta J, Vinyals GC, Yeager M, Welch R, Chanock S, Chatterjee N, Wacholder S, Samanic C, Torà M, Fernández F, Real FX, Rothman N. NAT2 slow acetylation, GSTM1 null genotype, and risk of bladder cancer: results from the Spanish Bladder Cancer Study and meta-analyses. Lancet. 2005;366:649–659. doi: 10.1016/S0140-6736(05)67137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matullo G, Guarrera S, Sacerdote C, Polidoro S, Davico L, Gamberini S, Karagas M, Casetta G, Rolle L, Piazza A, Vineis P. Polymorphisms/ haplotypes in DNA repair genes and smoking: a bladder cancer case-control study. Cancer Epidemiol Biomarkers Prev. 2005;14:2569–2567. doi: 10.1158/1055-9965.EPI-05-0189. [DOI] [PubMed] [Google Scholar]
- 36.Berman DM, Wang Y, Liu Z, Dong Q, Burke LA, Liotta LA, Fisher R, Wu X. A functional polymorphism in RGS6 modulates the risk of bladder cancer. Cancer Res. 2004;64:6820–6826. doi: 10.1158/0008-5472.CAN-04-1916. [DOI] [PubMed] [Google Scholar]
- 37.Rizvi MMA, Alam MS, Ali A, Mehdi SJ, Batra S, Mandal AK. Aberrant promoter methylation and inacivation of PTEN gene in cervical carcinoma from northern Indian population. J Cancer Res Clin Oncol. 2011;137:1255–1262. doi: 10.1007/s00432-011-0994-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizvi MMA, Alam MS, Mehdi SJ, Ali A, Batra S. Allelic loss at 10q23.3, the PTEN gene locus in cervical carcinoma from northern Indian population. Pathol Oncol Res. 2011 doi: 10.1007/s12253-011-9446-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


