Abstract
There are few comprehensive studies of small intestinal malignancies. The author retrospectively reviewed 1,312 archival pathologic specimens of the small intestine in the last 10 years in our pathologic laboratory in search for malignant tumors of the small intestine. There were 22 cases (1.7%) of primary adenocarcinoma, 3 cases (0.2%) of primary squamous cell carcinoma, 6 cases (0.5%) of metastatic carcinoma, 6 cases (0.5%) of malignant lymphoma, 3 cases (0.2%) of carcinoid tumor, and 1 case (0.08%) of gastrointestinal stromal tumor (GIST). Of the 25 cases of primary adenocarcinoma and squamous cell carcinoma, 24 cases were located in the duodenum and 1 case in the ileum. The 22 cases of adenocarcinoma were classified into 7 well differentiated, 7 moderately differentiated, and 8 poorly differentiated adenocarcinomas. All the three squamous cell carcinomas were moderately differentiated ones with keratinization and intercellular bridges. In the 25 cases of carcinoma, immunoreactive p53 protein was present in 23 cases, and the Ki-67 labeling ranged from 40% to 95% with a mean of 76%. In the 6 cases of metastatic adenocarcinoma, the origin was ovary in 1 case, pancreas in 2 cases, gall bladder in 1 case, lung in 1 case, and colon in 1 case. In the 6 cases of lymphoma, 4 cases were diffuse large B-cell lymphomas and 2 cases were peripheral T-cell lymphomas. In the 3 cases of carcinoid tumor, all were typical carcinoids and immunohistochemically positive for at least one of neuroendocrine markers (chromogranin, synaptophysin, neuron specific enolase, and CD56). In the 1 case of GIST, the cell type is spindle and GIST cells were immunohistochemically positive for KIT and CD34. The histological risk was intermediate. Forty-one cases of small intestinal malignancies were reviewed histopathologically.
Keywords: Small intestine, carcinoma, adenocarcinoma, squamous cell carcinoma, histopathology
Introduction
Malignant tumors of the small intestine are very rare compared to other gastrointestinal organs [1]. There have been no comprehensive pathologic studies of small intestinal malignancies. According to epidemiologic studies, the most common malignant tumors were carcinoid and adenocarcinoma, followed in order by gastrointestinal stromal tumor and malignant lymphoma [2-6]. The incidence of small intestinal malignancies is increasing [2-6]. The overall 5-year survival is 54%; 25% for adenocarcinomas, 62% for lymphomas, 83% for carcinoids, 45% for sarcomas [2-6]. Therefore, the prognosis of patients with small intestinal malignancies is poor [2-6]. The author herein reports the histopathologies of 41 cases of malignant tumors of the small intestine among 1,312 consecutive pathologic specimens of the small intestine.
Materials and methods
The author retrospectively reviewed archival 1,312 consecutive pathologic specimens of the small intestine in the last 10 years in our pathologic laboratory in search for malignant tumors of small intestine. Clinical records were also reviewed.
In carcinoma cases, an immunohistochemical study was performed, using Dako Envision method (Dako Corp., Glostrup, Denmark), as previously described [7-10]. The antibodies employed were anti-cytokeratin (AE1/3, Dako), anti -cytokeratin (polyclonal wide, Dako), anti-p53 protein (DO-7, Dako) and anti-Ki-67 antigen (MIB-1, Dako). In cases of malignant lymphoma, the antibodies used were anti-cytokeratin (AE1/3, Dako), anti-cytokeratin (polyclonal wide, Dako), CD3 (M7193, Dako), CD10 (M0727, Dako), CD15 (M0733, Dako), CD30 (M0751, Dako), CD45 (M0855, DAKO), CD45RO (M0834, Dako), CD79α (M7050, Dako), CD56 (MOC-1, Dako), CD57 (HNK-1, Santa Cruz, CA, USA), kappa light chain (polyclonal, Dako), lambda light chain (polyclonal, Dako), p53 protein (DO-7, Dako), and Ki-67 antigen (MIB-1, Dako). In carcinoid cases, the antibodies were anti-cytokeratin (AE1/3, Dako), anti-cytokeratin (polyclonal wide, Dako), carcinoembryonic antigen (polyclonal, Dako), chromogranin (DAK-A3, Dako), synaptophysin (polyclonal, Dako), neuron -specific enolase (BBS/NC/VI-H14, Dako), CD56 (MOC-1, Dako), and glucagon (polyclonal, Dako). In gastrointestinal stromal tumor (GIST) cases, the antibodies were KIT (polyclonal Dako), PDGFRA (polyclonal, Santa Cruz, CA, USA), CD34 (QBEND10, Dako), vimentin (Vim 3B4, Dako), desmin (D33, Dako), α-smooth muscle actin (1A4, Dako), S100 protein (polyclonal, Dako), p53 protein (DO7, Dako), and Ki-67 antigen (MIB1, Dako). In lymphoma, WHO classification was adopted [9].
Results
A total of 41 malignant tumors (3%) were found among the 1,312 specimens. There were 22 cases (1.7%) of primary adenocarcinoma, 3 cases (0.2%) of primary squamous cell carcinoma, 6 cases (0.5%) of metastatic carcinoma, 6 cases (0.5%) of malignant lymphoma, 3 cases (0.2%) of carcinoid tumor, and 1 case (0.08%) of gastrointestinal stromal tumor (GIST).
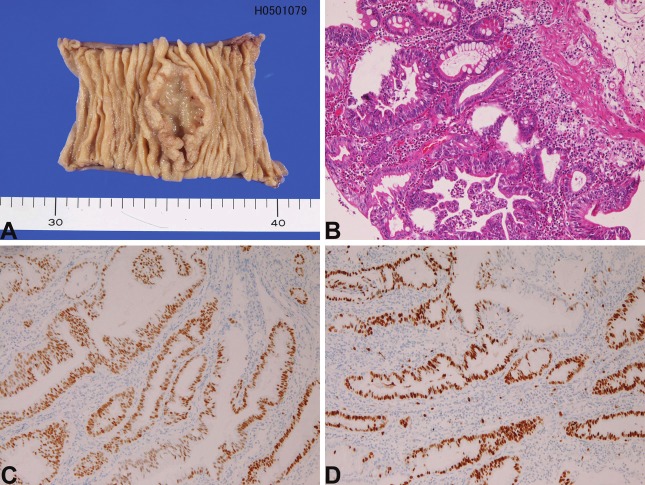
In the 22 cases of primary adenocarcinoma, the age ranged from 45 years to 85 years with a mean of 62 years. The male to female ratio was 14: 8. Of the 22 cases, 21 cases were biopsies and one case is tumor resection. The location was duodenum in 21 cases and jejunum in 1 case. In the present study, carcinomas of papilla Vater were excluded from the study. In the 21 cases of duodenal carcinoma, the location was first portion in 2 cases, second portion in 17 cases, and third portion in 2 cases; therefore the small intestinal carcinoma is most frequent in the second portion near the papilla of Vater. Grossly, 16 cases showed ulcerated tumors (Figure 1A), and 6 cases elevated tumors. Histologically, the 22 cases of adenocarcinoma were classified into 7 well differentiated, 7 moderately differentiated, and 8 poorly differentiated adenocarcinomas (Figure 1B). Immunohistochemically, cytokeratin (CK) was present in all cases, and p53 protein was recognized in 21 cases (Figure 1C). The Ki-67 labeling ranged from 40% to 95% with a mean of 71% (Figure 1D).
Figure 1.
Adenocarcinoma of the small intestine. A: Macroscopic picture in the ileum. An ulcerated tumor is present. B: Histology of well differentiated adenocarcinoma of the duodenum. HE, x200 C: p53 expression in adenocarcinoma of the duodenum. D: Ki-67 expression in adenocarcinoma of the duodenum. The labeling is 90%, x100.
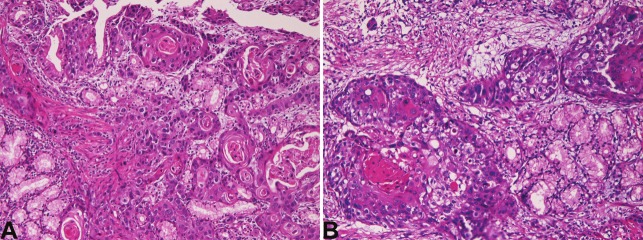
In the three cases of squamous cell carcinoma, the age of the patients was 75, 58, and 54 years, and male to female ratio was 2:1. All cases were biopsies. All the three cases were located in the second portion near the papilla Vater of the duodenum. Grossly, all the 3 cases showed ulcerated tumor. Histologically, all the three squamous cell carcinomas were moderately differentiated squamous cell carcinomas with keratinization and intercellular bridges (Figure 2A and 2B). Immunohistochemically, all cases expressed CK and p53 protein. The Ki-67 labeling ranged from 50% to 76% with a mean of 62%.
Figure 2.
Histology of squamous cell carcinoma of the duodenum. A: Squamous cell carcinoma with keratinization is seen in a patient. HE, x200 B: Squamous cell carcinoma with keratinization and intercellular bridges is seen in another patient. x200.
In the 6 cases of metastatic adenocarcinoma, the origin was ovary in 1 case, pancreas in 2 cases, gall bladder in 1 case, lung in 1 case, and colon in 1 case.
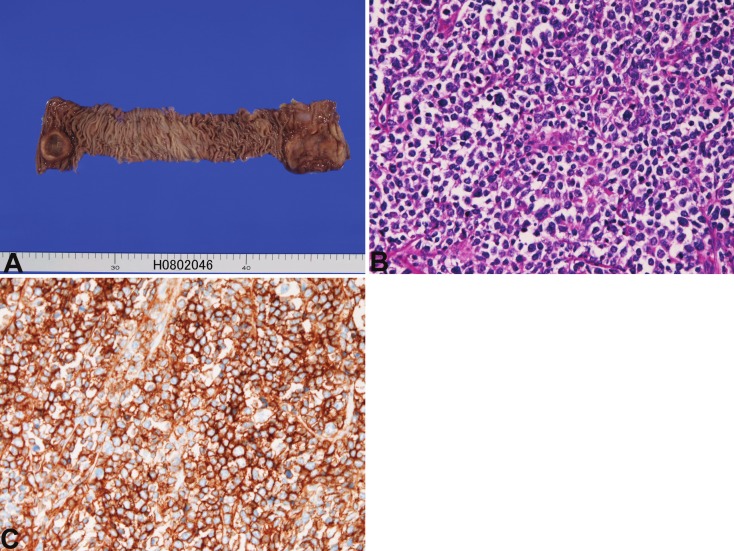
In the 6 cases of lymphoma, all cases showed ulcerated tumors (Figure 3A). All cases were partial intestinal resection. All cases were located in the ileum. One case showed multiple tumors, and one case coexisted with colon carcinoma, and one case is associated gastric MALT lymphoma. The age of patients ranged from 69 years to 72 years with a mean of 74 years. Male to female ratio was 2:4. The clinical diagnosis was carcinoma in 2 cases, tumor in 2 cases, and suspected lymphoma in two cases. Of the 6 cases, 4 cases were diffuse large B-cell lymphomas (Figure 3B and 3C) positive for CD15, CD20, CD45 and CD79α and negative for CK, CD3, CD30, CD45RO, CD46 and CD47. The remaining 2 cases were peripheral T-cell lymphomas positive for CD3, CD45 and CD45RO and negative for CD15, CD20, CD30, CD46, CD47, and CD79α.
Figure 3.
Malignant lymphoma of the small intestine. A: Gross findings. Two ulcerated tumors (both end) are recognized in the ileum. B: Histology of diffuse large B-cell lymphoma. x200. CD20 stain of B. CD20 is strongly positive. x200.
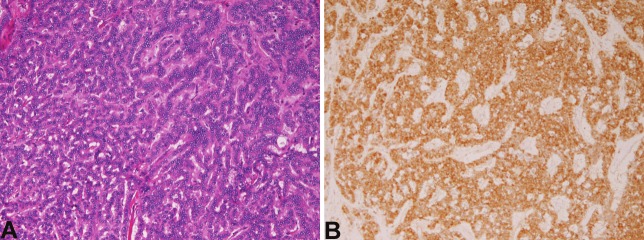
In the 3 cases of carcinoid tumor, the age of the patients was 56, 34, and 81 years, and male to female ratio was 2:1. All cases were endoscopic mucosal resections. The size of the carcinoids was 8mm, 12mm, and 16 mm in diameter. Histologically, all cases were typical carcinoids (Figure 4A). The cells were arranged in trabecular and ribbon patterns. Immunohistochemically all the 3 carcinoids were positive for at least one of neuroendocrine markers (chromogranin, synaptophysin, neuron-specific enolase, and CD56) (Figure 4B).
Figure 4.
Carcinoid tumor of small intestine. A: Histologies. Carcinoid tumor with trabecular cell arrangement is seen. HE, x200. B: synaptophysin is strongly positive. x200.
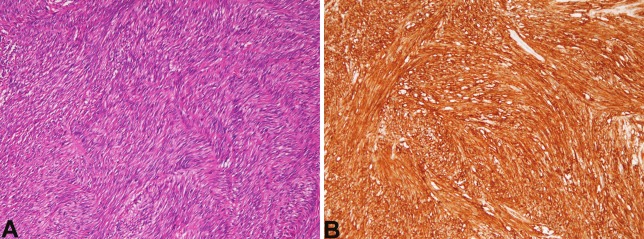
In the 1 case of GIST, the patient was 67-year old man. The case was tumor resection. Grossly, the tumor was solid and measured 6 x 6 x 5 cm. Histologically, cellular spindle cell proliferation was recognized (Figure 5A). Mitotic figures were seen in 4 per 50 high power fields. Immunohistochemically, the GIST cells were positive for KIT (Figure 5B) and CD34, but negative for other mesenchymal markers. The Ki-67 labeling was 10%. The histological malignant risk was intermediate.
Figure 5.
Gastrointestinal stromal tumor of the small intestine. A: spindle cell tumor is seen. HE, x100. B: KIT is strongly positive. x100.
Discussion
A large study of adenocarcinoma of the small intestine was performed by Dabaja et al. [12]. They examined 217 case of small intestine adenocarcinoma, and found that the location is duodenum in 52%, jejunum in 25%, ileum in 13%, and not clear in 10% [12]. The locations of the present 22 cases of primary adenocarcinoma and 3 cases of squamous cell carcinoma was mostly duodenum. In the duodenum, the second portion near the ampulla of Vater was the most common site. Similar results are described in WHO text book [1]. The preferential location may be because the periampullary sites are areas irritated by pancreatic juice and bile, putative carcinogens. The age of patient with primary small intestinal carcinoma ranged from 45 years to 85 years with a mean of 63 years. The male to female ratio was 15:10. Thus, the primary small intestinal carcinoma is frequent in middle or old aged persons. This is in accordance with previous epidemiologic studies [1-6]. The male preponderance is also compatible with previous epidemiologic study [1-6].
The 3 squamous cell carcinomas are very interesting. A review of the English literature revealed only two cases of squamous cell carcinoma of the duodenum [13,14]. One case of adenosquamous carcinoma was also reported [15]. Therefore, the present three cases of squamous cell carcinoma of the duodenum were extremely rare. The pathogenesis of squamous cell carcinoma of the duodenum is only speculative. Barnhill et al. [16] reported an interesting tumor of the duodenum. The tumor showed tripartite differentiations, i.e. adenocarcinoma, squamous cell carcinoma, and neuroendocrine carcinoma [16]. He speculated that their case had arisen from duodenal pluripotential stem cells capable of differentiating into multiple cell types [16]. The present cases of squamous cell carcinoma might have arisen from such pluripotential stem cells.
In the present study, immunoreactive p53 protein was present in most carcinoma cases, suggesting that p53 mutations are present in most of the primary carcinoma of the small intestine. The Ki-67 labeling ranged from 40% to 95%, suggesting active proliferation in the present small intestinal carcinomas. In the present series, 6 cases of metastatic carcinoma were recognized. In the 6 cases of metastatic adenocarcinoma, the origin was ovary in 1 case, pancreas in 2 cases, gall bladder in 1 case, lung in 1 case, and colon in 1 case. The findings indicate that the small intestine may be the site of metastases of other malignancies.
In the present series, 6 cases of malignant lymphoma were identified. Four of them were diffuse large B-cell lymphomas and two were peripheral T-cell lymphomas. In general, most of lymphomas of the gastrointestinal cells are of B-cell type. For example, Grody et al. [17] stated that B-cell lymphomas accounted for 84% (21/25) in gastrointestinal lymphomas. In lymphoma of small intestine, B-cell lymphoma predominates over T-cell lymphoma [18,19]). Mantle cell lymphoma, Burkitt lymphoma, and Hodgkin disease [18,19] were not observed in the present study.
In the present study, 3 cases of typical carcinoids were demonstrated. Carcinoid tumors, also called neuroendocrine tumors (NET), are relatively rare in the digestive organs [20-27]. The incidence is reported to be less that 0.1% of all digestive organ tumors [20-22]. Carcinoid tumors are potentially malignant tumors, but the malignant potential depends on tumor size and morphologies [3]. In general, carcinoid tumors less than 20mm have low grade malignant potential, and carcinoids more than 20 mm have high malignant potential [22]. In the present study, the 3 cases of carcinoid were less than 20 mm, suggesting a low malignant potential.
In the present series, 1 case of GIST was found. The locations of GIST were highest in the stomach, followed in order by colorectum and small intestine [28,29]. Positive reaction for KIT and/ or CD34 is a hallmark of GIST [28-30]. According to the consensus report of GIST by Fletcher et al. [31], the malignant potential of GIST depends on tumor size and mitotic counts. In very low malignant risk group, tumor size is less than 2 cm and mitotic counts are less than 5 per 50 high power field (HPF). In low malignant rick group, tumor size is 2cm < 5cm, and mitotic counts are < 5/50 HPF. In intermediate rick group, tumor size is 5cm < 10cm, and mitotic counts are < 5/50 HPF. In high rick group, tumor size is > 10 cm, and mitotic counts are > 10/50 HPF [31]. According to mitotic counts and tumor size, the malignant risk of the present case was intermediate.
In summary, the author reported the histopathologies of 41 malignant tumors of the small intestine.
References
- 1.Wright NH, Pennazio M, Howe JR, Sobin LH, Rossini FP, Carr NJ, Shepherd NA, Talbot I. Carcinoma of the small intestine . In: Hamilton SR, Aaltonen LA, editors. WHO Classification of tumours. Pathology and genetics, Tumor of the digestive system. Lyon: IARC press; 2000. pp. 71–76. [Google Scholar]
- 2.Haselkorn T, Whittemore AS, Lilienfeld DE. Incidence of small bowel cancer in the United States and worldwide: geographic, temporal and racial difference. Cancer Causes Control. 2005;16:781–787. doi: 10.1007/s10552-005-3635-6. [DOI] [PubMed] [Google Scholar]
- 3.Billimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the Unites States: changes in epidermiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63–71. doi: 10.1097/SLA.0b013e31818e4641. [DOI] [PubMed] [Google Scholar]
- 4.Chow JS, Chen CC, Ahsan H, Neugut AI. A population- bases study of the incidence of malignant small bowel tumours: SEER, 1973-1990. Int J Epidemiol. 1996;25:722–728. doi: 10.1093/ije/25.4.722. [DOI] [PubMed] [Google Scholar]
- 5.DiSario JA, Burt RW, Vargas H, McWhorter WP. Small bowel cancer: epidermiological and clinical characteristics from a population-based registry. Am J Gastroenterol. 1994;89:699–701. [PubMed] [Google Scholar]
- 6.Hatzaras I, Palesty JA, Abir F, Sullivan P, Kozeol RA, Dudrick SJ, Longo WE. Small-bowel tumours: epidemiologic and clinical characteristics of 1260 cases from the Connecticut tumor registry. Arch Surg. 2007;142:229–235. doi: 10.1001/archsurg.142.3.229. [DOI] [PubMed] [Google Scholar]
- 7.Terada T, Kawaguchi M. Primary clear cell adenocarcinoma of the peritoneum. Tohoku J Exp Med. 2005;206:271–275. doi: 10.1620/tjem.206.271. [DOI] [PubMed] [Google Scholar]
- 8.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740–746. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 9.Terada T. Ductal adenoma of the breast: Immunohistochemistry of two cases. Pathol Int. 2008;58:801–805. doi: 10.1111/j.1440-1827.2008.02315.x. [DOI] [PubMed] [Google Scholar]
- 10.Terada T, Tanigichi M. Intraductal oncocytic papillary neoplasm of the liver. Pathol Int. 2004;54:116–123. doi: 10.1111/j.1440-1827.2004.01594.x. [DOI] [PubMed] [Google Scholar]
- 11.Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. WHO classification of tumours. Pathology and genetics of tumours of hematopoietic and lymphoid tissues. Ryon: IARC press; 2001. [Google Scholar]
- 12.Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. Adenocarcinoma of the small bowel: presentation, prognostic factor and outcome of 217 patients. Cancer. 2004;101:518–526. doi: 10.1002/cncr.20404. [DOI] [PubMed] [Google Scholar]
- 13.Friedman E, Kwan MR, Cummins L. Squamous cell carcinoma of the transverse duodenum. Gastrointest Endosc. 1986;32:99–101. doi: 10.1016/s0016-5107(86)71766-5. [DOI] [PubMed] [Google Scholar]
- 14.von Delius S, Lersch C, Neu B, Huber B, Eckel F, Pitzl H, Fend F, Gaa J, Schmid RM. Squamous - cell carcinoma of the duodenum as a rare cause of upper gastrointestinal bleeding. Endoscopy. 2006;38:956. doi: 10.1055/s-2006-925161. [DOI] [PubMed] [Google Scholar]
- 15.de la Cruz A, de la Cruz E, Sanchez MJ, Ortiz S, Lobato A, Merino E. Adenosquamous carcinoma of the duodenum: an immunohistochemical study. Pathol Res Pract. 1993;189:481–485. doi: 10.1016/S0344-0338(11)80345-6. [DOI] [PubMed] [Google Scholar]
- 16.Barnhill M, Hess E, Guccion JG, Nam LH, Bass BL, Patterson RH. Tripartite differentiation in a carcinoma of the duodenum. Cancer. 1994;73:266–272. doi: 10.1002/1097-0142(19940115)73:2<266::aid-cncr2820730206>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Grody WW, Magidson JG, Weiss LM, Hu E, Warnke HA, Lewin KJ. Gastrointestinal lymphomas: Immunohistochemical studies on the cell of origin. Am J Surg Pathol. 1985;9:328–337. [PubMed] [Google Scholar]
- 18.Gascoyne RD, Muller-Hermelink HK, Chott A, Wotherspoon A. B-cell lymphoma of the small intestine. In: Hamilton SR, Asltonen , editors. WHO Classification of tumours. Pathology and genetics of tumours of the digestive system. Ryon: IARC press; 2000. pp. 83–86. [Google Scholar]
- 19.Gascoyne RD, Muller-Hermelink HK, Chott A, Wotherspoon A. Intestinal T-cell lymphoma. In: Hamilton SR, Asltonen , editors. WHO Classification of tumours. Pathology and genetics of tumours of the digestive system. Ryon: IARC press; 2000. pp. 87–89. [Google Scholar]
- 20.Capella C, Solcia E, Sobin LH, Arnoid R. Endocrine tumours of the small intestine. In: Hamilton SR, Asltonen , editors. WHO Classification of tumours. Pathology and genetics of tumours of the digestive system. Ryon: IARC press; 2000. pp. 77–82. [Google Scholar]
- 21.Capella C, Solcia E, Sobin LH, Arnoid R. Endocrine tumours of the colon and rectum. In: Hamilton SR, Asltonen , editors. WHO Classification of tumours. Pathology and genetics of tumours of the digestive system. Ryon: IARC press; 2000. pp. 137–141. [Google Scholar]
- 22.Modlin IM, Kidd M, Latich I, Zikusoka ME, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology. 2005;128:1717–1751. doi: 10.1053/j.gastro.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 23.Pinchot SN, Holen K, Sippel RS, Chen H. Carcinoid tumors. Oncologist. 2008;13:1255–1269. doi: 10.1634/theoncologist.2008-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burke AP, Federspiel BH, Sobin LH, Shekitka KM, Helxig EB. Carcinoids of the duodenum: a histologic and immunohistochemical study of 65 tumors. Am J Surg Pathol. 1989;13:828–837. doi: 10.1097/00000478-198910000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Al-Khafaji B, Noffsinger AE, Miller MA, Devoe G, Stemmermann GN, Fenoglio-Preiser C. Immunohistochemical analysis of gastrointestinal and pulmonary carcinoid tumors. Hum Pathol. 1998;29:992–999. doi: 10.1016/s0046-8177(98)90206-4. [DOI] [PubMed] [Google Scholar]
- 26.Van Eeden S, Quaedvlieg PF, Taal BG, Offerhaus GJ, Lamers CB, Van-Velthuysen ML. Classification of low grade neuroendocrine tumors of midgut and unknown origin. Hum Pathol. 2002;33:1126–1132. doi: 10.1053/hupa.2002.129204. [DOI] [PubMed] [Google Scholar]
- 27.Modlin IM, Oberrg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP, Moss SF, Nilsson O, Rindi G, Salazar R, Ruszniewski P, Sundin A. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 28.Hirota S, Isozaki K. Pathology of gastrointestinal stromal tumor. Pathol Int. 2006;56:1–9. doi: 10.1111/j.1440-1827.2006.01924.x. [DOI] [PubMed] [Google Scholar]
- 29.Miettinen M, Lasota J. Gastrointestinal strumal tumors: Review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–1478. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 30.Terada T. Gastrointestinal stromal tumor of the digestive organs: a histopathologic study of 31 cases in a single Japanese institute. Int J Clin Exp Pathol. 2010;3:162–168. [PMC free article] [PubMed] [Google Scholar]
- 31.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]