Abstract
Background
Neuroendocrine tumors (NET) are known for an overexpression of somatostatin receptors (SSTR). In light of very few and partially contradictory publications, the present study aims to achieve a definite immunohistochemical (IHC) quantification and assessment of the distribution of all five SSTR-subtypes on NET and to evaluate an implementable scoring system, comparing the immunoreactive score of Remmele and Stegner (IRS) to the Her2-score. In 21 patients 40 different tumor tissues were IHC analysed using polyclonal antibodies for SSTR1 and 3-5 and the monoclonal antibody UMB-1 for SSTR2A. SSTR expression was quantitatively evaluated according to HER2-score and IRS, correlated among each other and to the maximum standardized uptake value (SUVmax) in tumor lesions as measured by PET/CT using 68Ga-DOTA-NOC.
Results
According to the IRS, the expression of SSTR2A and 3 predominated equally with 84%, followed by SSTR4 (44%) and SSTR1 and 5 (32%). With the Her2-scoring system the most frequent subtype was found to be SSTR2A (68%), followed by SSTR3 (64%), SSTR1 (44%), SSTR5 (40%), and SSTR4 (36%). The IRS-classification and the Her2-score were found to be statistically comparable, and their correlation is highly significant for each SSTR assessment (p<0.01).
Conclusion
The results of the analyses revealed heterogeneous expression patterns. SSTR2A and 3 were highly expressed, demonstrating the importance of SSTR for diagnostics and therapy. Relatively high frequency of SSTR3 and 4 on NET give reasons to try pansomatostatin analogues for therapy rather than concentrating only on the SSTR2A. Statistically, none of the immunohistochemical scores was superior. However, due the heterogeneity of the cytoplasmic staining justice we propose the IRS as a uniform scoring scheme for IHC NET diagnostic.
Keywords: Gastroenteropancreatic neuroendocrine tumors, somatostatin receptors, UMB-1 antibody, immunohistochemistry, IRS, Her2-score
Introduction
Gastroenteropancreatic neuroendocrine tumor (GEP-NET) cells are known for displaying an overexpression of somatostatin receptors. This fact already serves as a molecular basis for various methods in diagnostics and therapies and realizes a prognostic estimation of the neoplastic disease [1,2]. So far, 6 subtypes of somatostatin receptors (SSTR) have been identified: SSTR1, 2A, 2B, 3, 4 and 5 [3]. The subtypes 2A and 2B are so called splice variants, only the 2A -variant has been found in human tissues. In order to estimate the prognosis of GEP-NET in a patient with an SSTR-positive tumor, the density, the distribution and the subtype-profile of the SSTR have to be determined. Well-differentiated NET express SSTR more frequently than poorly differentiated ones [4,5]. SSTR2A is expressed most frequently, followed by SSTR1 and 5, and rarely also by SSTR3 and 4 [6]. SSTR2A is a membrane-bound receptor, whereas SSTR1, 3 and 5 are located intracellularly [7,8]. Some studies have proven a strong significant correlation of IHC SSTR-subtype expression (SSTR2A, SSTR5) and the maximum standardized uptake value (SUVmax) as measured by somatostatin-receptor PET/CT [9,10]. Currently different methods have been established to visualize somatostatin receptor (SSTR) positive parenchymal cells. SSTR scintigraphy has shown practicable findings in these last few years, but for the diagnosis of NET the 68- Gallium SSTR PET/CT is the new “golden standard” [11,12]. The findings of the initial SSTR-PET/ CT are decisive for mode of the patient’s treatment. If the tumor is curativly resectable, surgery is of course the treatment of choice. In an advanced stage, however systemic therapeutic options have to be chosen, such as peptide-related radionuclide therapy or the new targeted medical treatments (e.g. “Everolimus”, “Sunitinib”) particularly in patients with well-differentiated NET [13,14]. Patients with a poorly differentiated NET can be treated by means of chemotherapy - the key to the proven medical treatment of choice is the IHC analysis of KI-67 of a biopsy specimen or of the samples from surgically removed NET. Currently, the IHC technique is a fairly standardized and a usually reproducible method.
For the analysis of the SSTR status of NET many commercially available polyclonal and monoclonal antibodies have been established in these last few years. The crux of the matter is the absence of a reliable semi-quantitative assessment procedure by the pathologist. Ongoing data actually do not exist concerning the quantification and interpretation of SSTR IHC findings, although scoring systems have been developed and applied for many years in the analysis of several other tumor cell receptors [15]. Most of the results of studies dealing with such IHC assessments of SSTR are based on data created by their own scoring systems or own modifications of already well-established ones [9,15-17]. Only few investigations have used the established scoring systems of Her2/neu or the IRS. The IRS was first developed for the IHC detection of estrogen receptors in mammary carcinoma [18], but has also gained importance in the evaluation of NET. The Her2-score was introduced by DakoCytomation in 1999 in context with the Hercep test, an IHC test which is used to detect the membrane bound receptor Her2 in mammary carcinoma. A reason for the implementation of the Her2-score in the evaluation of NET is found in the similarity between the membrane- bound staining of SSTR2A and the localization of the Her2-receptor’s immunoreactions, knowing that most IHC analyses are concentrated around SSTR2A, even though studies with SSTR antibodies show a high frequency of SSTR3 and 5, particularly in pancreatic NET [4,7]. These findings should give reason for a reassessment of the present practice in diagnostics and therapy of GEP-NET, which is currently primarily focused on the data obtained from IHC analyses of SSTR2A. Against the background of the diversity and the presence of a wide variety of different modifications of IHC scores in the diagnostics of NET, the aim of the present study was to try to reassess the reliability of the applied scores (IRS and Her2) with regard to the expression intensity of the five SSTR-subtypes in surgically removed GEP-NET tissue, evaluated by means of IHC analyse and compared to SSTR -PET/CT parameters.
Patients and methods
All 40 tumor samples, originating both from primary tumors and metastases from 21 patients with GEP-NET were analyzed retrospectively for SSTR1 to 5 (Figure 1). The samples were conventionally fixed in formalin and embedded in paraffin and sections with a thickness of 4 μm were prepared. The sections underwent IHC analysis as previously described [19]. The exact location of the tumor parechyma was defined by preoperative 68Gallium-DOTA-NOC-PET/CT-scans in combination with surgery and pathology protocols. SUVmax of tumor lesions were determined on PET/CT scans using commercially available software (E-soft Nuclear Medicine Workstation, Siemens, Illinois, USA).
Figure 1.
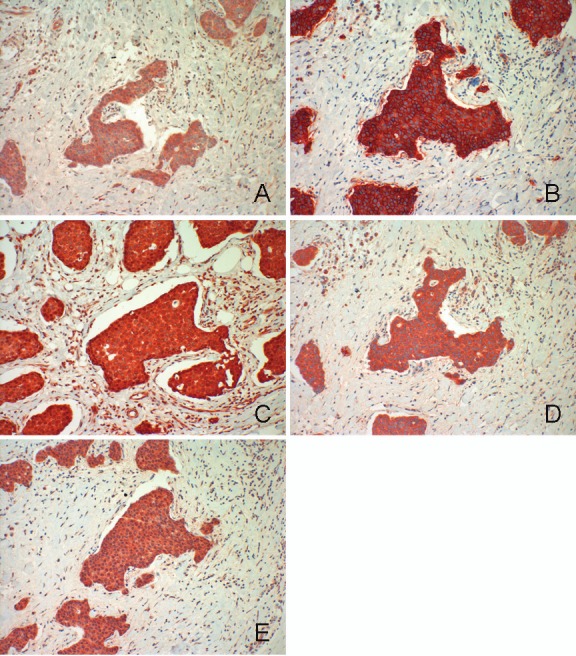
Liver metastasis of a well-differentiated neuroendocrine carcinoma. A: SSTR 1 B: SSTR 2A C: SSTR 3 D: SSTR 4 E: SSTR 5
The detection of SSTR-subtypes was performed using the labeled streptavidin-biotin-method (LSAB). Primary antibodies for SSTR-subtype detection had been developed and proven to identify their targeted receptor highly specific by Schulz et al. [7,19-21].The antibodies used for detection of SSTR2A were produced by Epitomics, Burlingame, CA (USA) and by Gramsch Laboratories, Schwabhausen, Germany, for detection of SSTR1, 3, 4 and 5. The semi-quantitative analysis of the stained sections was done by light-microscopy according to the immunoreactive score (IRS) by Remmele and Stegner (Table 1) and in analogy to the Her2-scoring system. The IRS-evaluation was based on a modification, which does not only evaluate the visualized grade of color intensity (staining), but also adds the fraction of cells in each intensity category [22]. As suggested by Remmele and Stegner, the predominant grade of intensity was used and the IRS with points from 0 to 12 were adapted to an additional 4-point- IRS-classification derived from the latter score to make it comparable to the HER2-score. Also, according to the scheme used by Volante et al., cytoplasmatic staining in the detection of SSTR2A was generally graded lower than membranebound staining [15].
Table 1.
IRS, Her2-and IRS-classification scoring systems
| Percentage of positive cells | X Intensity of Staining | = IRS (0 – 12) |
|---|---|---|
| 0 = no positive cells | 0 = no colour reaction | 0 – 1 = negative |
| 1 = < 10% of positive cells | 1 = mild reaction | 2 – 3 = mild |
| 2 = 10-50% positive cells | 2 = moderate reaction | 4 – 8 = moderate |
| 3 = 51-80% positive cells | 3 = intense reaction | 9 – 12 = strongly positive |
| 4 = > 80% positive cells | ||
| IRS – points | IRS – classification | |
| 0 – 1 | 0 = negative | |
| 2 – 3 | 1 = positive, weak expression | |
| 4 – 8 | 2 = positive, mild expression | |
| 9 – 12 | 3 = positive, strong expression | |
| Her2/neu Score | Reaction Format | Evaluation |
| 0 | No or less than 10% cells | Negative |
| 1+ | > 10% cells with minimal staining intensity | Negative |
| 2+ | > 10% cells with moderate staining intensity | Mildly positive |
| 3+ | > 10% cells with strong staining intensity | Strongly positive |
Patients data
21 patients were included in the study from which in total 40 different tumor samples were processed as paraffin embedded blocks. The median age of the patients at the time of surgery was 56 ± 11 years (range 33 to 76 years).
Tumor localization
The most frequent organ localization of tumor tissue were the liver metastases with 30% (n=12) and the ileum, meso specimen, and others with each 12.5% (n=5), followed by peritoneal specimen10% (n=4) and pancreas and appendix with each 7.5% (n=3) and appendix and gastric each 5% (n=2).
Statistics
Data were analyzed using SPSS for Windows 19.0. The following parameters were normally distributed according to the Kolmogorov- Smirnov test: IRS, SSTR1, 3 and 5. All other variables were not normally distributed. After assessment of normal distribution, the following tests were used: Kendall’s Tau correlation analysis and Spearman’s rank correlation analysis.
Results
Ki-67 Index
In 40 specimens the Ki-67 index was assigned. In 28% (n = 11) the Ki-67 index was ≤ 2%, in 40% (n = 17) between 2 and 12%, and in 20% (n = 12) > 10%.
Distribution of the SSTR-subtypes
The presence of a SSTR-subtype was defined positive, if the IRS-classification and/or Her2- score were ≥ 2. All tumors showing a value < 2 were defined as negative for the specific SSTR. According to the IRS-classification, the most common subtype was found to be SSTR2A (84%, 21/25), as much as SSTR3 (84%, 21/25) followed by SSTR4 (44%, 11/25), SSTR1 (32%, 8/25) as much as SSTR5 (32%, 8/25).
Using the Her2-score, the most common subtype was found to be SSTR2A (68%, 17/25), followed by SSTR3 (64%, 16/25), SSTR1 (44%, 11/25), SSTR5 (40%, 10/25) and SSTR4 (36%, 9/25). The distributions of every SSTR subtype and their scores are shown in Table 2.
Table 2.
Somatostatin receptor distribution
| IRS | IRS-classification | Her2/neu | |||
|---|---|---|---|---|---|
| SSTR1 | |||||
| Score Frequency (%) | Score Frequency (%) | Score Frequency (%) | |||
| 0 | 5 (20) | 0 | 5 (20) | 0+ | 5(20) |
| 2 - 3 | 11 (44) | 1 | 12 (48) | 1+ | 9(36) |
| 4 - 8 | 9 (36) | 2 | 8 (32) | 2+ | 10(40) |
| 9 - 12 | 0 (0) | 3 | 0 (0) | 3+ | 1 (4) |
| SSTR1 positiv total | 8 (32%) | 11 (44%) | |||
| SSTR 2A | |||||
| 0 | 0 (0) | 0 | 0 (0) | 0+ | 0(0) |
| 2 - 3 | 4 (16) | 1 | 4 (16) | 1+ | 8(32) |
| 4 - 8 | 14 (56) | 2 | 14 (56) | 2+ | 2(8) |
| 9 - 12 | 7 (28) | 3 | 7 (28) | 3+ | 15 (60) |
| SSTR2A positiv total | 21 (84%) | 17 (68.0%) | |||
| SSTR 3 | |||||
| 0 | 2 (8) | 0 | 2 (8) | 0+ | 2(8) |
| 2 - 3 | 2 (8) | 1 | 2 (8) | 1+ | 7(28) |
| 4 - 8 | 17 (68) | 2 | 17 (68) | 2+ | 6(24) |
| 9 -12 | 4 (16) | 3 | 4 (16) | 3+ | 10 (40) |
| SSTR3 positiv total | 21 (84%) | 16 (64.%) | |||
| SSTR4 | |||||
| 0 | 1 (4) | 0 | 1 (4) | 0+ | 1 (4) |
| 2 - 3 | 11 (44) | 1 | 13 (52) | 1+ | 15 (60) |
| 4 - 8 | 13 (52) | 2 | 11 (44) | 2+ | 9 (36) |
| 9 - 12 | 0 (0) | 3 | 0 (0) | 3+ | 0 (0) |
| SSTR4 positiv total | 11 (44)% | 9 (36%) | |||
| SSTR5 | |||||
| 0 | 5 (20) | 0 | 7 (28) | 0+ | 9 (36) |
| 2 - 3 | 12 (48) | 1 | 10 (40) | 1+ | 6(24) |
| 4 - 8 | 8 (32) | 2 | 8 (32) | 2+ | 8 (32) |
| 9 - 12 | 0 (0) | 3 | 0 (0) | 3+ | 2(8) |
| SSTR5 positiv total | 8 (32%) | 10 (40%) | |||
IRS-classification versus Her2-Score
The IRS-classification and Her2-score were found to be comparable and their correlation highly significant for each SSTR assessment [SSTR1 0.58 (p<0.001), SSTR2A 0.53 (p<0.001), SSTR3 0.42 (p<0.001), SSTR4 0.45 (p<0.001), SSTR5 0.81 (p<0.001)].
IRS/Her2-Score versus SUVmax
Analysis of each score with the corresponding SUVmax revealed a significant correlation of 0.33 (p=0.05) between IRS of SSTR2A and SUVmax in PET/CT. Correlation with the Her2-score showed the same almost significant correlation to SSTR2A (0.33; p=0.08). The other SSTR subtypes did not correlate significantly to the PET/ CT parameter.
Discussion
Limitations
One limitation is seen in the semi-quantitative IHC analysis of the tissue sections; it was subjective. Thereby, it limits the comparability.
Frequency of the SSTR subtypes
The predominant SSTR subtypes in the IHC analysis according to the IRS-classification were found to be SSTR2A with 84% as much as SSTR3 (84%), followed by SSTR4 (44%) and SSTR1 (32%) as much as SSTR5 (32%). Using the Her2-score, the most common subtype observed was SSTR2A (68%) followed by SSTR3 (64%), SSTR1 (44%), SSTR5 (40%) and SSTR4 (36%) (Table 2). As already described, SSTR1 and SSTR3-5 showed a cytoplasmatic staining, whereas the immunoreaction of SSTR2A was membrane bound [7,8]. The result of a predominant expression of SSTR2A is supported by the findings of Reubi et al., who have already been reporting SSTR expressions between 80% and 100% [23,24]. Also in an IHC analysis in 94 patients with GEP-NET of the five SSTR subtypes, SSTR2A predominated with an incidence of over 80% [8]. In another study by Kulaksiz et al. SSTR2A was found to be present in 86% similar to our study [7]. The surprising part in our survey was the finding of a high incidence of SSTR3. Whereas Papotti et al. showed a comparable incidence of 60% in tumor samples from 15 patients with GEP-NET and Kulaksiz et al. showed a commonness of 71%, Zamora et al. reported about 26% and Reubi and Waser described a much lesser incidence of SSTR3 with 15% as a result of a study restricted to patients with ileum NET only [4,7,8,25]. However, in the study of Zamora et al. there was a noticeably high presences of SSTR3 among the pancreatic NET. The high proportion of pancreatic NET in our investigation (35.3%) could, therefore, be an explanation for the high incidence of SSTR3. With a presence of 44% in the IRS-classification and 36% using the Her2-Score, respectively, SSTR4 was also surprisingly high in our evaluation. Its incidence has been described as marginal (<15%) in previous studies [3,24]. The frequency of SSTR1 (IRS: 32%; Her2: 44%) and SSTR5 (IRS: 32%; Her2: 40%) in our study are on the other hand comparable to the findings of Reubi and Waser and Zamora et al. Papotti et al. and Kulaksiz et al. described a much higher frequency of SSTR5 (up to 83%) than in our investigation [4,7,8,25]. While discussing the findings of our project in comparison with those of other researchers, it is essential to be aware of divergent methods (e.g. autoradiography vs. IHC) and the application of different antibodies for analysing expression of SSTR, which makes it difficult to compare results. Furthermore, and as discussed below, the use of a uniform score for the IHC evaluation of SSTR is not yet available. When looking at the score distribution of the IRS for SSTR3, there was an accumulation in the middle domain. The domain 3 (positive, high expression) was reached only by a small fraction (14%) compared to the SSTR2A (41%), which would be supported by the findings of Reubi and Waser and Zamora et al. [8,25]. The findings in our study may indicate that the applied antibody is more sensitive towards expression of the middle IRS domain of SSTR3. Such a result would certainly have an impact on future diagnostic and therapeutic strategies. Another reason for the accumulation in the middle IRS domain for SSTR3 could be a high false-positive rate or an incorrect definition of the value, which recognizes a tumor as positive. However, the antibodies have been repeatedly tested both by our group as well as by other investigators in various tumor tissues, so that a high false-positive rate seems rather unlikely [19,20].
Comparison of the scores
In order to allow for a correlation between the IRS and Her2-score systems, we chose to use the IRS-classification system, because of its similarity to the Her2-score system (Table 1). In both scoring systems the SSTR expression rate of stained tumor tissue is assessed and divided into four groups (0=no expression to 3=strong expression). Among each other the scores correlated differently, from 0.42 for SSTR3 to 0.81 for SSTR5. Besides, we did not find a statistical way to show a superiority of one of the scores. We applied the comparison to SUVmax, a SSTR-PET/ CT parameter which measures the tracer uptake, for that purpose as it has been proven by different studies that SUVmax shows a valid correlation to the IHC expression pattern of SSTR2A and 5 [9,10]. In our investigation the IRS of SSTR2A noted a significant positive correlation to SUVmax (p<0.05) and the Her2-score of SSTR2 noted a positive correlation with a strong trend (p=0.08) but without significance. Both correlation factors were of equal value (0.33). Although, from the statistical point of view, both scoring systems seem to be on a par, in our opinion the IRS system was found to be more adequate in the evaluation of GEP-NET, because of the additional breakdown of the percentage of positive cells in the stained tissue, compared to the Her2-scoring system. The consequence is a more accurate analysis of the heterogenous GEP-NET-tissue and a relativization of false-positive results. When analyzing tumor samples with the Her2-system, it was often more difficult to specify tumor-criteria and to relate the stained tissue to a specific group.
As accounted for above the IRS according to Remmele and Stegner was firstly developed for the IHC detection of estrogen receptors in mammary carcinoma, but it has also gained importance in the evaluation of NET. Many IHC analyses of NET are also based on the Her2-scoring system because of the similarity between the membrane-bound staining of SSTR2A and the localization of the immunoreactions of the Her2- receptor, knowing that most IHC analyses are concentrated around SSTR2A. As also mentioned above most of the studies are often creating their own scoring systems or modify well-established ones. Actually, the application of a uniform scoring system in the evaluation of NET is not yet available. Table 3 demonstrates the confusing use of different scores throughout the literature. Score results from 0+ to 3+ without further subdivision may be adequate for a positive membrane-bound staining of SSTR2A. However, the use of the previous scoring systems in the assessment of heterogenous cytoplasmatic staining of the other SSTR subtypes is insufficient. As shown in Table 3 the scoring system of Papotti et al. offers a relatively accurate classification of the SSTR-expression in different NET [4]. When looking at the scoring system of Volante et al., it is evident that a realistic estimation of the expression of SSTR3 and SSTR5 through a single-factor quantitative description of the cytoplasmatic staining is difficult [15]. In future, it is therefore recommended that at least the heterogeneity of SSTR1 and SSTR3 to SSTR5 should be analyzed with a more detailed scoring scheme like the IRS system. From our point of view the Her2-score and other scores, which are currently used for IHC analyse of SSTR, should be substituted by the IRS.
Table 3.
Immunohistochemical scores by different authors
| Author and score | Expression pattern and evaluation |
|---|---|
| Papotti et al. (2002) | |
| - | negative |
| + | < 25% of tumor cells positive |
| ++ | 25%-50% of tumor cells positive |
| +++ | > 50% of tumor cells positive |
| Volante et al. (2007) | – only for SSTR2A |
| 0 | no immunoreactivity |
| 1 | only cytoplasmatic immunoreaction, focal or diffuse |
| 2 | membrane immunoreaction < 50% |
| 3 | membrane immunoreaction > 50% |
| Volante et al. (2007) | - for SSTR 3 and 5 |
| positive | > 10% cytoplasmatic staining |
| Corleto et al. (2009) | |
| 0 | no staining |
| + | mildly positive |
| ++ | moderately positive |
| +++ | intensely positive |
| Miederer et al. (2009) | - for SSTR2A |
| 0 | negative |
| 1+ | mild incomplete membrane reaction |
| 2+ | moderate complete membrane reaction |
| 3+ | strong complete membrane reaction |
| Zamora et al. (2010) | |
| Negative | no receptor expression |
| focally positive | < 25% of positive cells with heterogenous pattern |
| positive | > 25% of positive cells with moderate to strong density |
Conclusion
Our IHC observations revealed heterogeneous expression patterns with high incidences for all SSTR subtypes. SSTR2A and 3 showed the highest expression with up to 84%, demonstrating the importance of these SSTR for diagnostics and therapy. Relatively high frequencies of SSTR3 and 4 in GEP-NET give reasons to try pansomatostatin analogs for therapy rather than concentrating only on the SSTR2A.
The IRS was interpreted as reliable in the analysis of NET sample. Thus, it is proposed as a uniform score in the routine diagnostics, because it does not only include the different localizations of the immunoreactions, but it also is able to assess the heterogeneity of the cytoplasmatic reactions of the SSTR-subtypes.
Acknowledgement
The authors of the manuscript attest that we have nothing to disclose or any financial or other relationships that could be construed as conflict of interest regarding to this study.
References
- 1.Corleto VD, Falconi M, Panzuto F, Milione M, De Luca O, Perri P, Cannizzaro R, Bordi C, Pederzoli P, Scarpa A, Delle Fave G. Somatostatin receptor subtypes 2 and 5 are associated with better survival in well-differentiated endocrine carcinomas. Neuroendocrinology. 2009;89:223–230. doi: 10.1159/000167796. [DOI] [PubMed] [Google Scholar]
- 2.Reubi JC. Somatostatin and other Peptide receptors as tools for tumor diagnosis and treatment. Neuroendocrinology. 2004;80(Suppl 1):51–56. doi: 10.1159/000080742. [DOI] [PubMed] [Google Scholar]
- 3.Taniyama Y, Suzuki T, Mikami Y, Moriya T, Satomi S, Sasano H. Systemic distribution of somatostatin receptor subtypes in human: an immunohistochemical study. Endocr J. 2005;52:605–611. doi: 10.1507/endocrj.52.605. [DOI] [PubMed] [Google Scholar]
- 4.Papotti M, Bongiovanni M, Volante M, Allia E, Landolfi S, Helboe L, Schindler M, Cole SL, Bussolati G. Expression of somatostatin receptor types 1-5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. A correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis. Virchows Arch. 2002;440:461–475. doi: 10.1007/s00428-002-0609-x. [DOI] [PubMed] [Google Scholar]
- 5.Reubi JC, Laissue J, Waser B, Horisberger U, Schaer JC. Expression of somatostatin receptors in normal, inflamed, and neoplastic human gastrointestinal tissues. Ann N Y Acad Sci. 1994;733:122–137. doi: 10.1111/j.1749-6632.1994.tb17262.x. [DOI] [PubMed] [Google Scholar]
- 6.Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev. 2003;24:389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- 7.Kulaksiz H, Eissele R, Rossler D, Schulz S, Hollt V, Cetin Y, Arnold R. Identification of somatostatin receptor subtypes 1, 2A, 3, and 5 in neuroendocrine tumours with subtype specific antibodies. Gut. 2002;50:52–60. doi: 10.1136/gut.50.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zamora V, Cabanne A, Salanova R, Bestani C, Domenichini E, Marmissolle F, Giacomi N, O'Connor J, Mendez G, Roca E. Buenos Aires and La Plata Argentina Argentum Working Group. Immunohistochemical expression of somatostatin receptors in digestive endocrine tumours. Dig Liver Dis. 2010;42:220–225. doi: 10.1016/j.dld.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Miederer M, Seidl S, Buck A, Scheidhauer K, Wester HJ, Schwaiger M, Perren A. Correlation of immunohistopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur J Nucl Med Mol Imaging. 2009;36:48–52. doi: 10.1007/s00259-008-0944-5. [DOI] [PubMed] [Google Scholar]
- 10.Kaemmerer D, Peter L, Lupp A, Schulz S, Sanger J, Prasad V, Kulkarni H, Haugvik SP, Hommann M, Baum RP. Molecular imaging with Ga-SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011;38:1659–1668. doi: 10.1007/s00259-011-1846-5. [DOI] [PubMed] [Google Scholar]
- 11.Baum RP, Prasad V, Hommann M, Horsch D. Receptor PET/CT imaging of neuroendocrine tumors. Recent Results Cancer Res. 2008;170:225–242. doi: 10.1007/978-3-540-31203-1_18. [DOI] [PubMed] [Google Scholar]
- 12.Prasad V, Fetscher S, Baum RP. Changing role of somatostatin receptor targeted drugs in NET: Nuclear Medicine's view. J Pharm Pharm Sci. 2007;10:321s–337s. [PubMed] [Google Scholar]
- 13.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Horsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 14.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Oberg K. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volante M, Brizzi MP, Faggiano A, La Rosa S, Rapa I, Ferrero A, Mansueto G, Righi L, Garancini S, Capella C, De Rosa G, Dogliotti L, Colao A, Papotti M. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod Pathol. 2007;20:1172–1182. doi: 10.1038/modpathol.3800954. [DOI] [PubMed] [Google Scholar]
- 16.Pisarek H, Pawlikowski M, Kunert-Radek J, Winczyk K. Does the response of GH-secreting pituitary adenomas to octreotide depend on the cellular localization of the somatostatin receptor subtypes SSTR2 and SSTR5? Endokrynol Pol. 2010;61:178–181. [PubMed] [Google Scholar]
- 17.Thodou E, Kontogeorgos G, Theodossiou D, Pateraki M. Mapping of somatostatin receptor types in GH or/and PRL producing pituitary adenomas. J Clin Pathol. 2006;59:274–279. doi: 10.1136/jcp.2005.026914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 19.Fischer T, Doll C, Jacobs S, Kolodziej A, Stumm R, Schulz S. Reassessment of sst2 somatostatin receptor expression in human normal and neoplastic tissues using the novel rabbit monoclonal antibody UMB-1. J Clin Endocrinol Metab. 2008;93:4519–4524. doi: 10.1210/jc.2008-1063. [DOI] [PubMed] [Google Scholar]
- 20.Schulz S, Schreff M, Schmidt H, Handel M, Przewlocki R, Hollt V. Immunocytochemical localization of somatostatin receptor sst2A in the rat spinal cord and dorsal root ganglia. Eur J Neurosci. 1998;10:3700–3708. doi: 10.1046/j.1460-9568.1998.00386.x. [DOI] [PubMed] [Google Scholar]
- 21.Schulz S, Schulz S, Schmitt J, Wiborny D, Schmidt H, Olbricht S, Weise W, Roessner A, Gramsch C, Hollt V. Immunocytochemical detection of somatostatin receptors sst1, sst2A, sst2B, and sst3 in paraffin-embedded breast cancer tissue using subtype-specific antibodies. Clin Cancer Res. 1998;4:2047–2052. [PubMed] [Google Scholar]
- 22.McCarty KS Jr, Miller LS, Cox EB, Konrath J, McCarty KS Sr. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;109:716–721. [PubMed] [Google Scholar]
- 23.Reubi JC, Schaer JC, Waser B, Mengod G. Expression and localization of somatostatin receptor SSTR1, SSTR2, and SSTR3 messenger RNAs in primary human tumors using in situ hybridization. Cancer Res. 1994;54:3455–3459. [PubMed] [Google Scholar]
- 24.Reubi JC, Waser B, Khosla S, Kvols L, Goellner JR, Krenning E, Lamberts S. In vitro and in vivo detection of somatostatin receptors in pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 1992;74:1082–1089. doi: 10.1210/jcem.74.5.1349024. [DOI] [PubMed] [Google Scholar]
- 25.Reubi JC, Waser B. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging. 2003;30:781–793. doi: 10.1007/s00259-003-1184-3. [DOI] [PubMed] [Google Scholar]


