Abstract
Primary small cell carcinoma of the nose and paranasal sinuses is very rare; only a few reports are present in the English literature. The author herein reports a very rare case of primary small cell carcinoma of the maxillary sinus with an emphasis on immunohistochemistry and on KIT and PDGFRA. A 64-year-old man was admitted to our hospital because of left nasal obstruction. Endoscopy revealed three nasal polyps, and imaging modalities revealed an infiltrative tumor (45 x 45 mm) in the left maxillary sinus with invasion into nasal cavity. Multiple biopsies are taken from the nasal lesions. Histologically, the tumor consists of proliferation of malignant small epithelioid cells with hyperchromatic nuclei, fine chromatin, scant cytoplasm, molded nuclei, and absent nucleoli. Immunohistochemically, the malignant cells were positive for cytokeratin (CK) 18, synaptophysin, CD56, p53, Ki-67 (labeling=95%), bcl-2, KIT, and PDGFRA. However, they were negative for pancytokeratins, high molecular weight CK, CK5/6, CK7, CK 14, CK 19, CK20, vimentin, neuron-specific enolase, chromogranin, CD15, CD45, S100 protein, CEA, CA19-9, glial fibrillary acidic protein, neurofilaments, neuroblastoma, CD99, surfactant apoprotein A, melanosome, and TTF-1. The pathologic diagnosis was small cell carcinoma. A molecular genetic analysis using PCR-direct sequencing was performed using paraffin sections, and it showed no mutations of KIT (exons 9, 11, 13, and 17) and PDGFRA (exons 12 and 18) genes. Imaging modalities including CT, MRI and PET did not reveal any tumors, including the lung, other than the maxillary sinus tumor. The present case is the first of small cell carcinoma of the maxillary sinus with a comprehensive immunohistochemical examination and a gene analysis of KIT and PDGFRA.
Keywords: Small cell carcinoma, maxillary sinus, immunohistochemistry, KIT, PDGFRA
Introduction
KIT and platelet-derived growth factor-α (PDGFRA) genes are mapped to 4q12, and encode receptor tyrosine kinase oncoproteins called KIT (CD117) and PDGFRA, respectively [1,2]. Both molecules are transmembranous oncoproteins involved in the tumorigenesis of some cancers, particularly in gastrointestinal stromal tumor [1,2]. Primary small cell carcinoma of the nasal cavity and paranasal sinuses is very rare; only a few reports are recorded in the literature [3-5].
Recently, it has been identified that small cell lung carcinoma (SCLC) expresses KIT [6-13]. Small cell carcinoma can occur in any organ. Extrapulmonary small cell carcinoma also may express KIT and PDGFRA [14-16]. However, protein expressions of KIT and PDGFRA have not been investigated in small cell carcinoma of the nasal cavity and paranasal sinuses. In addition, mutations of KIT and PDGFRA have not been investigated in small cell carcinoma of nasal cavity and paranasal sinuses. The author reports herein a case of small cell carcinoma of the left maxillary sinuses with an emphasis on immunohistochemistry and KIT and PDGFRA.
Case report
Clinical summary
A 64-year-old man was admitted to our hospital because of left nasal obstruction. Nasal endoscopy revealed three polypoid tumors in the left nasal cavity. Imaging modalities revealed a tumor (45 x 45 mm) in the left maxillary sinus Figure 1). The tumor was seen to invade the surrounding tissue including the left nasal cavity (Figure 1). Multiple biopsies were performed from the nasal tumor. After the pathological diagnosis, imaging modalities including X-P, CT, MRI, and PET were performed. They did not reveal any tumors other than the maxillary sinus tumor. The lungs were free from tumors.
Figure 1.
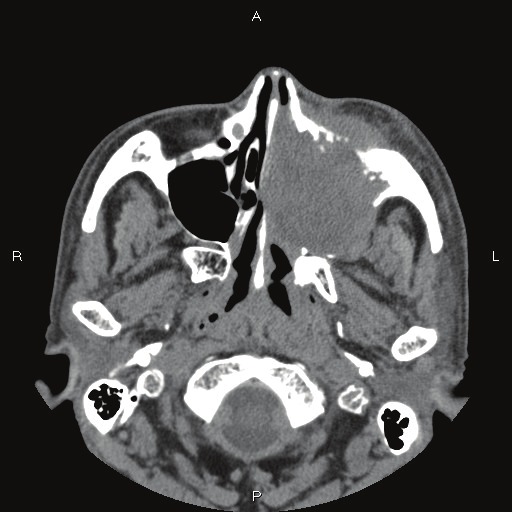
CT findings. The left maxillary sinus shows infiltrative tumor. Nasal cavity is also involved.
Materials and methods
The biopsy samples were fixed in 10% formalin and were embedded in paraffin wax. Several 3- μm sections were cut, and one of them were stained with hematoxylin and eosin. The remaining sections were immunohistochemically examined using Dako's Envision method, as previously reported [17,18], for pancytokeratins (AE1/3 and polyclonal wide spectrum, Dako Corp, Glostrup, Denmark, and KL-1, Immunotech, Marseille, France), high molecular weight cytokeratin (CK) (34βE12, Dako), CK 5/6 (D5/16 B4, Dako), CK 7 (N1626, Dako), CK 14 (LL002, Novocastra, Newcastle upon Tyne, UK), CK 18 (DC10, Dako), CK 19 (RCK 108, Progen, Heidelberg, Germany), CK 20 (K20.8, Dako), vimentin (Vim 3B4, Dako), neuron-specific enolase (BBS/NC/H14, Dako), chromogranin (DAK-A3, Dako), synaptophysin (polyclonal, Dako), CD56 (UJ13A, Dako), p53 (DO-7, Dako), CD15 (C3D, Dako), CD45 (PD7/16+2B11, Dako), S100 (polyclonal, Dako), CEA (polyclonal, Kyowa, Tokyo, Japan), CA19-9 (TFB Lab., Tokyo, Japan), glial fibrillary acidic protein (GFAP) (polyclonal, Dako), neurofilaments (2F11, Dako), neuroblastoma (NB84a, Dako), CD99 (12E7, Dako), Ki-67 (MIB-I, Dako), melanosome (HMB-45, Dako), surfactant apoprotein A (PE10, Dako), bcl-2 (124, Dako), thyroid-transcriptional factor-1 (TTF-1) (8G7G3/1, Dako), KIT (polyclonal, Dako), and PDGFRA (polyclonal, Santa Cruz, CA, USA).
A molecular genetic analysis for KIT (exons 9, 11, 13, and 17) and PDGFRA (exons 12 and 18) genes was performed, using paraffin sections, by employing PCR-direct sequencing method, as previously described [19-27]. In brief, genomic DNA was extracted from paraffin blocks with proteinase K digestion and phenol/chloroform extraction, and subjected to PCR for 40 cycles (94°C for one minute, 52°C for one minute, 72°C for one minute), using a thermal cycler (GeneAmp PCR system 9700, Applied Biosystems, ABI, CA). The primers are shown in Table 1. The annealing temperature was 53°C. PCR products were extracted and subjected to a computed automatic DNA sequencer (ABI PRISM 3100 Genetic Analyzer, Applied Biosystems, ABI, CA). Two cases of gastric GISTs were used as positive controls, and two uterine leiomyomas as negative controls.
Table 1.
Primer sequence
| Forward | Reverse | |
|---|---|---|
| KIT exon 9 | 5’-TCC TAG AGT AAG CCA GGG CTT-3’ | 5’-TGG TAG ACA GAG CCT AAA CAT CC-3’ |
| KIT exon11 | 5’-GAT CTA TTT TTC CCT TTC TC-3’ | 5’AGC CCC TGT TTC ATA CTG AC-3’ |
| KIT exon 13 | 5’-GCT TGA CAT CAG TTT GCC AG -3’ | 5’-AAA GGC AGC TTG GAC ACG GCT TTA-3’ |
| KIT exon 17 | 5’-CTC CTC CAA CCT AAT AGT GT-3’ | 5’-GTC AAG CAG AGA ATG GGT AC-3’ |
| PDGFRA exon12 | 5’-TTG GAT ATT CAC CAG TTA CCT GTC-3’ | 5’-CAA GGG AAA AGC TCT TGG-3’ |
| PDGFRA exon 18 | 5’-ACC ATG GAT CAG CCA GTC TT-3’ | 5’-TGA AGG AGG ATG AGC CTG ACC-3’ |
Results
No tumor formations were recognized by imaging modalities including X-P, CT, MRI, and PET other than the maxillary tumor. The lungs were free from tumors. Therefore, the maxillary sinus tumor is primary in the present case.
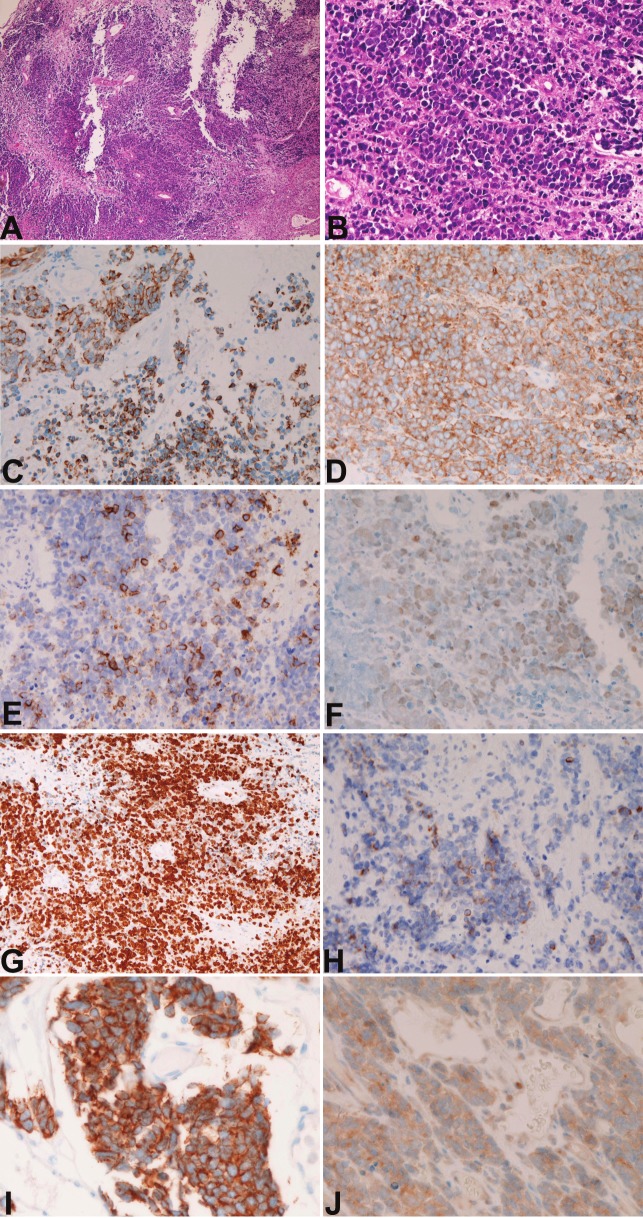
The biopsy specimens were composed of aggregates of small carcinomatous cells with hyperchromatic nuclei, fine granular chromatin, molded nuclei, absent or inconspicuous nucleoli, and scant cytoplasm (Figures 2A and 2B). Many mitotic figures were recognized. Necrotic areas are present. No differentiation was recognized. No fibrillar elements suggestive of neuroblastoma were recognized. The histological diagnosis was small cell carcinoma, but olfactory neuroblastoma could not be denied completely.
Figure 2.
A. Low-power view of small cell carcinoma. HE, x200; B. High-power view of small cell carcinoma. HE, x200; C. Cytokeratin 18 is positive. Immunostaining, x200; D. Synaptophysin is positive. Immunostaining, x200; E. CD56 is positive. Immunostaining, x200; F. P53 protein is positive. Immunostaining, x200; G. K-67 antigen labeling is about 95%, Immunostaining, x200; H. Bcl-2 is positive. Immunostaining, x200; I. KIT is positive. Immunostaining, x200; J. PDGFRA is positive. Immunostaining, x400.
The immunohistochemical study showed positive reactions for CK 18 (Figure 2C), synaptophysin (Figure 2D), CD56 (Figure 2E), p53 (Figure 2F), KI-67 (labeling=95%) (Figure 2G), bcl-2 (Figure 2H), KIT (Figure 2I) and PDGFRA (Figure 2J). However, it showed negative reactions for any types of pancytokeratins, high molecular weight CK, CK5/6, CK7, CK 14, CK 19, CK20, vimentin, neuron-specific enolase, chromogranin, CD15, CD45, S100 protein, CEA, CA19-9, GFAP, neurofilaments, neuroblastoma, CD99, surfactant apoprotein A, melanosome, and TTF-1. The pathologic diagnosis was small cell carcinoma.
The molecular genetic analysis using PCR-direct sequencing showed no mutations of KIT (exons 9, 11, 13, and 17) and PDGFRA (exons 12 and 18) genes. The positive control of gastric GISTs showed a point mutation of KIT, and negative control of uterine leiomyomas showed no mutations of KIT and PDGFRA.
Discussion
According to WHO criteria [29], small cell carcinoma is defined as a malignant epithelial tumor consisting of small cells with scant cytoplasm, ill-defined cell borders, finely granular nuclear chromatin, and absent or inconspicuous nucleoli. The cells are round, oval and spindle-shaped, nuclear molding is prominent. Necrosis is typically extensive and mitotic count is high. The present case fulfills these criteria, and is compatible with small cell carcinoma. Olfactory neuroblastoma and small round cell tumor such as PNET/Ewing tumor are unlikely in the histology as well as negative immunoreactions to GFAP, neurofilaments, neuroblastoma, and CD99.
More than 90 % of small cell carcinoma has neuroendocrine features [29]. The present case also showed neuroendocrine features (positive synaptophysin and CD56). Small cell carcinoma is positive for CK [30]. The present case was positive for only CK18 and negative for vimentin. This indicates that the present case is epithelial malignancy. CK profile is not well known in small cell carcinoma. More studies on CK profile are required in small cell carcinoma.
The present case was positive for bcl-2. Bcl-2 is expressed in 100% in KIT-positive SCLC [31]. The present case was negative for TTF-1. TTF-1 is expressed in 44% in extrapulmonary small cell carcinoma [11].
Small cell carcinoma can occur in any organ, although the vast majority occurs in the lung. In general, small cell carcinoma is a very aggressive tumor with a poor prognosis. In the present case, the patient now is treated with cisplatin-based chemotherapy and radiation. However, the prognosis seems poor.
KIT is expressed in various tumors including gastrointestinal stromal tumor (GIST), mast cell neoplasm, melanoma, germ cell tumor, hematopoietic malignancies and SCLC [1]. Results of protein expression in SCLC vary among researchers; it is reportedly 30%-100% [7-11]. KIT expression without KIT gene mutations is thought to be due to KIT gene amplification [9]. The prognostic implications of positive KIT protein in SCLC are controversial and no definite conclusions have been made [7-11]. If activating KIT mutations are present, treatment with imatinib mesylate may be effective [1].
KIT mutations are frequent in GIST, acute myeloid leukemia, and mast cell neoplasms [1]. KIT mutations have been reported to be none or few [5,8] in SCLC. In extrapulmonary small cell carcinoma, KIT mutations have been reported to be negative [14-16].
There is only one study on PDGFRA mutation in small cell carcinoma. Sihto et al. [9] found no PDGFRA mutations in 31 cases of SCLC. The present case also showed no PDGFRA mutations. In extrapulmonary small cell carcinoma, PDGFRA mutations have not been reported [14-16].
PDGFRA protein expression has not been reported in SCLC. The present case showed a weak expression of PDGFRA, suggesting that a small amount of PDGFRA is present in small cell carcinoma. In extrapulmonary small cell carcinoma, weak expression of PDGFRA has been reported [14-16]. Many more studies are necessary regarding PDGFRA expression and PDFGRA gene mutation.
References
- 1.Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissue, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13:205–220. doi: 10.1097/01.pai.0000173054.83414.22. [DOI] [PubMed] [Google Scholar]
- 2.Hirota S, Isozaki K. Pathology of gastrointestinal stromal tumor. Pathol Int. 2006;56:1–9. doi: 10.1111/j.1440-1827.2006.01924.x. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Ordonez B, Caruana SM, Huvos AG, Shah JP. Small cell neuroendocrine carcinoma of the nasal cavity and paranasal sinuses. Hum Pathol. 1998;29:826–832. doi: 10.1016/s0046-8177(98)90452-x. [DOI] [PubMed] [Google Scholar]
- 4.Barin E, Rouleau V, Vedrine PO, Toussaint B, de Raucourt D, Malard O, Cosmidis A, Makaeieff M, Dehesdin D. Small cell neuroendocrine carcinoma of the nasal cavity and paranasal sinuses. J Raryngol Otol. 2006;120:289–297. doi: 10.1017/S0022215106000594. [DOI] [PubMed] [Google Scholar]
- 5.Tarozzi M, Demarosi F, Lodi G, Sardella A, Carrassi A. Primary small cell carcinoma of the nasal cavity with an unusual oral manifestation. J Oral Pathol Med. 2007;36:252–254. doi: 10.1111/j.1600-0714.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 6.Terada T. Primary small cell carcinoma of the pleura: A case report with immunohistochemical and molecular genetic analyses of KIT and PDGFRA genes. Med Oncol. 2010;27:1119–1122. doi: 10.1007/s12032-009-9345-2. [DOI] [PubMed] [Google Scholar]
- 7.Mojica WD, Saxena R, Starostik P, Cheney RT. CD117 + small cell lung cancer lacks the asp 816 val point mutation in exon 17. Histopathology. 2005;47:517–522. doi: 10.1111/j.1365-2559.2005.02259.x. [DOI] [PubMed] [Google Scholar]
- 8.Boldrini L, Ursino S, Gisfredi S, Faviana P, Donati V, Camcci T, Lucchi M, Mussi A, Basolo F, Pingitore R, Fontanini G. Expression and mutational status of c-kit in small-cell lung cancer: prognostic relevance. Clin Cancer Res. 2004;15:4101–4108. doi: 10.1158/1078-0432.CCR-03-0664. [DOI] [PubMed] [Google Scholar]
- 9.Burger H, Den Bakker MA, Stoter G, Verweij J, Nooter K. Lack of c-kit exon 11 activating mutations in c-kit/CD117-positive SCLC tumor specimens. Eur J Cancer. 2003;39:793–799. doi: 10.1016/s0959-8049(03)00026-1. [DOI] [PubMed] [Google Scholar]
- 10.Sihto H, Sarlomo-Rikara M, Tynnienen O, Tanner M, Andersson LC, Franssila K, Nupponen NN, Joensuu H. KIT and platelet-derived growth factor receptor alpha tyrosine kinase gene mutations and KIT amplifications in human solid tumors. J Clin Oncol. 2005;23:49–57. doi: 10.1200/JCO.2005.02.093. [DOI] [PubMed] [Google Scholar]
- 11.LaPoint RJ, Bourne PA, Wang HL, Xu H. Coexpression of c-kit and bcl-2 in small cell carcinoma and large cell neuroendocrine carcinoma of the lung. Appl Immunohistochem Mol Morphol. 2007;15:401–406. doi: 10.1097/01.pai.0000213153.41440.7d. [DOI] [PubMed] [Google Scholar]
- 12.Lopes-Martin A, Ballenstin C, Garcia-Carbonero R, Castano A, Lopez-Rios F, Lopes-Encuentra A, Sanchez-Cespedes M, Castellano D, Bartolomes A, Cortes Funes H, Paz-Ares L. Prognostic value of KIT expression in small cell lung carcinoma. Lung Cancer. 2007;56:405–413. doi: 10.1016/j.lungcan.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 13.Micke P, Basral M, Faldum A, Bittinger F, Ronnstrand L, Blaukat A, Beeh KM, Oesch F, Fischer B, Buhl R, Hengstler JG. Characterization of c-kit expression in small cell lung carcinoma: prognostic and therapeutic implications. Clin Cancer Res. 2003;9:188–194. [PubMed] [Google Scholar]
- 14.Terada T. Primary small cell carcinoma of the mediastinum: A case report with immunohistochemical and molecular genetic analyses of KIT and PDGFRA genes. Med Oncol. 2009;26:247–250. doi: 10.1007/s12032-008-9116-5. [DOI] [PubMed] [Google Scholar]
- 15.Terada T. Primary small cell carcinoma of the ureter: A case report involving immunohistochemical and molecular genetic analyses of KIT and PDGFRA genes. Pathology. 2010;42:101–102. doi: 10.3109/00313020903443018. [DOI] [PubMed] [Google Scholar]
- 16.Terada T. Autopsy case of primary small cell carcinoma of the urinary bladder: KIT and PDGFRA expression and mutations. Pathol Int. 2009;59:247–250. doi: 10.1111/j.1440-1827.2009.02358.x. [DOI] [PubMed] [Google Scholar]
- 17.Terada T, Kawaguchi M. Primary clear cell adenocarcinoma of the peritoneum. Tohoku J Exp Med. 2005;206:271–275. doi: 10.1620/tjem.206.271. [DOI] [PubMed] [Google Scholar]
- 18.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740–746. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 19.Terada T. Gastrointestinal stromal tumor of the uterus: a case report with genetic analyses of c-kit and PDGFRA genes. Int J Gynecol Pathol. 2009;28:29–34. doi: 10.1097/PGP.0b013e3181808000. [DOI] [PubMed] [Google Scholar]
- 20.Terada T. Primary extragastrointestinal stromal tumors of the transverse mesocolon without ckit mutations but with PDGFRA mutations. Med Oncol. 2009;26:233–237. doi: 10.1007/s12032-008-9092-9. [DOI] [PubMed] [Google Scholar]
- 21.Terada T. Primary multiple extragastrointestinal stromal tumors of the omentum with different mutations of c-kit gene. World J Gastroenterol. 2008;14:7256–7259. doi: 10.3748/wjg.14.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terada T. Mediastinal seminoma with multiple KIT gene mutations. Pathology. 2009;41:695–697. doi: 10.3109/00313020903305852. [DOI] [PubMed] [Google Scholar]
- 23.Terada T. KIT-positive primary small cell carcinoma of the endometrium: A case report with immunohistochemical and molecular genetic analyses of KIT and PDGFRA genes. Arch Gynecol Obstet. 2010;282:413–416. doi: 10.1007/s00404-009-1324-5. [DOI] [PubMed] [Google Scholar]
- 24.Terada T. Large cell neuroendocrine carcinoma with sarcomatous changes of the endometrium: a case report with immunohistochemical studies and molecular genetic study of KIT and PDGFRA. Pathol Res Pract. 2010;206:420–425. doi: 10.1016/j.prp.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Terada T. Low incidence of KIT gene mutations and no PDGFRA gene mutations in primary cutaneous melanoma: an immunohistochemical and molecular genetic study of Japanese cases. Int J Clin Oncol. 2010;15:453–456. doi: 10.1007/s10147-010-0087-0. [DOI] [PubMed] [Google Scholar]
- 26.Terada T. Neuroendocrine carcinoma of the esophagus: a case report with immunohistochemical and molecular genetic analysis of KIT and PDGFRA. Med Oncol. 2011;28:509–512. doi: 10.1007/s12032-010-9499-y. [DOI] [PubMed] [Google Scholar]
- 27.Terada T. Mutations and protein expression of KIT and PDGFRA genes in ipsilateral testicular seminomas: an immunohistochemical and molecular genetic study. Appl Immunohistochem Mol Morphol. 2011;19:450–453. doi: 10.1097/PAI.0b013e31820d2872. [DOI] [PubMed] [Google Scholar]
- 28.Terada T. Protein expression and gene mutation status of KIT and PDGFRA in renal cell carcinoma. Histol Histopathol. 2012;27:297–302. doi: 10.14670/HH-27.297. [DOI] [PubMed] [Google Scholar]
- 29.Travis W, Petersen I, Nicholson S, Meyerson M, Hisrch FR, Hanash SM, Pugatch B, Jen J, Geisinger K, Takahashi K, Brambillia E, Fernandez EA, Gazdar A, Capron F. Small cell carcinoma. In: Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, editors. WHO Classification of tumous. Pathology and genetics, Tumours of the lung, pleura, thymus and hear. Ryon: IARC Press; pp. 31–34. [Google Scholar]
- 30.Van Muijen GN, Ruiter DJ, van Leeuwen C, Prins FA, Rietsema K, Warnaar SO. Cytokeratin and neurofilament in lung carcinomas. Am J Pathol. 1984;116:363–369. [PMC free article] [PubMed] [Google Scholar]
- 31.Agoff SN, Lamps LW, Philip AT, Amin MB, Schmidt RA, True LD, Folpe AL. Thyroid transcriptional factor-1 is expressed in extrapulmonary small cell carcinomas but not in other extrapulmonary neuroendocrine tumors. Mod Pathol. 2000;13:238–242. doi: 10.1038/modpathol.3880044. [DOI] [PubMed] [Google Scholar]