Abstract
Bilateral primary angiosarcoma of breast is an extremely rare disease. Only 4 cases had been described in the literature. Hypoxia inducible factor- 1 α (HIF-1α) is a transcription factor that binds to hypoxia response elements in the promoters of target genes. Vascular endothelial growth factor (VEGF) is an important signaling protein involved in angiogenesis. Wilms tumor -1 protein (WT-1) is a transcription factor that plays an important role in angiogenesis. We present a 29-year old female with bilateral primary angiosarcoma of breast. Five-μm sections were stained with CD31, FLI-1, HIF-1α, WT-1, VEGF, VEGF-R, D2-40, estrogen receptor, and progesterone receptor. The neoplastic cells show diffuse immunoreactivity to CD31, FLI-1, HIF- 1α, VEGF, VEGFR, and WT-1 protein. The neoplastic cells show no immunoreactivity to estrogen receptor, progesterone receptor and D2-40. In conclusion, HIF- 1α, WT-1 and VEGF are possible protagonists in the development of bilateral primary angiosarcoma of breast. The neoplastic process involves endothelial cell of blood vessels lineage rather than lymphatic lineage. Painless breast tumors in young women that are highly vascular at the time of biopsy should be considered as malignant until proven otherwise. Tissue biopsy is the gold standard in the diagnosis of primary angiosarcoma of breast.
Keywords: Breast, angiosarcoma, HIF-1α, VEGF, WT-1
Introduction
Breast sarcomas are rare neoplasms that account for less than 1% of all breast malignancies [1]. Breast angiosarcoma (BAS) is a rare neoplasm. They can arise de novo (primary) or as a consequence of treatment of breast carcinoma (secondary) [1].
Bilateral primary BAS is an extremely rare disease. Only 4 cases had been described in the literature [3-6]. Vascular endothelial growth factor (VEGF) is an important signaling protein involved in angiogenesis and stimulates cellular responses by binding to tyrosine kinase receptors (VEGFRs) on the cell surface, causing them to dimerize and become activated through transphosphorylation. The VEGFRs have an extracellular portion consisting of 7 immunoglobulin-like domains, a single transmembrane spanning region and an intracellular portion containing a split tyrosine-kinase domain [7]. Hypoxia inducible factor 1 (HIF-1) is a heterodimeric transcription factors composed of HIF-1α which dimerize with a constitutively expressed β subunit and subsequently bind to hypoxia response elements in the promoters of target genes [8]. HIF-1α protein expression in cells is regulated by a variety of stimuli, including changes in cellular oxygen concentration, growth factors, oncogenic activation, or loss of tumor suppressor function [8]. Wilms tumor-1 (WT-1) protein is a transcription factor that plays an important role in cellular development, cell survival and angiogenesis [9].
We report HIF-1α, VEGF and WT-1 as possible protagonists in bilateral primary BAS which to the best of our knowledge, has not been previously reported in the literature.
Case report
A 29- year old Para 2 gravida 3 woman noticed a painless lump in the left breast during the 24th week of pregnancy. The mass was growing rapidly and increasing in size during lactation. She had regular menses, menarche at the 12th year and never uses oral contraceptive pills at any time. She has a family history of a 2nd degree cousin with breast carcinoma. On examination, a 6x5 cm non- tender, firm, fixed mass at the left upper outer quadrant was noticed. Ultrasound showed a well defined mass. Fine needle aspiration cytology was done twice and was negative for malignancy. After delivery, left breast lumpectomy was performed and the histopathology revealed angiosarcoma. Computerized tomography (CT) was negative for metastasis. The patient was referred to our breast care center, for further management. A Mammogram showed post-operative hematoma on the left side and a normal right breast. Magnetic Resonance Image (MRI) revealed postoperative anomalies visualized in the left side at the level of inner quadrant with probable unclear margins mainly seen at the level of the posterior aspect of the cavity, four masses visualized in the right side mainly situated in the lower quadrants and suspected malignant appearance of a lymph node seen in the left axilla. Core needle biopsies were taken from left axillary lymph nodes and were consistent with metastatic angiosarcoma. Core needle biopsy from right breast masses revealed angiosarcoma. The patient underwent bilateral skin sparing mastectomy, left axillary clearance and right axillary sampling with immediate reconstruction with tissue expander.
The patient then underwent local radiation therapy to both breast areas and received a dose of 5040 cGy/ 28 fraction/ 6 weeks to each area. The patient complained of vaginal discharge with lower abdominal discomfort for 10 days after radiation therapy.
Six months after bilateral subcutaneous mastectomy a follow up Abdominal Ultrasound showed an abdominal mass and a follow up CT scan for chest, abdomen and pelvis revealed multiple tiny lesions in the right lung, a homogeneously attenuated well defined mass in the midline in the abdomino-pelvic region just below the aortic bifurcation, measures 7.7x6.9 cm and a small left ovarian cyst. Colonoscopy was unremarkable. Exploratory laprotomy revealed a cystic mass in the right lower abdomen measuring 10x10 cm and arising from right ovary. Right Oopherectomy and left partial Oopherectomy for a 2x2 cm cystic lesion in the left ovary were performed and revealed metastatic angiosarcoma to both ovaries. The patient then was restaged as stage 4, hence the oncologist decided to give her chemotherapy. The first line of chemotherapy consisted of 7 cycles. Each cycle consisted of taxol 80 mg/m2 every week for 3 weeks + avastin 10 mg/Kg every 2 weeks intravenously. The patient, then received the 2nd line of chemotherapy which consisted of sutent (sunitinib) 2 cycles orally 37.5 mg once daily (4 weeks on /2 weeks off). Finally the patient received the 3rd line of chemotherapy which consisted of Doxorubicin 20 mgs/m2 + Ifosfamide 2000 mgs/m2 for three days every 3 weeks. The patient had partial response but the disease progressed after chemotherapy with liver, lungs and bone metastases in 12th thoracic vertebra.
Pathologic findings
The surgically excised mammary specimens were grossly examined, fixed in 10% buffered formalin, dehydrated in ethanol, cleared in xylene and embedded in paraffin. Five-μm sections were stained with Hematoxylin and eosin. In addition, 5-μm sections were stained with CD31 (DAKO, Glostrup, DenmarkClone JC70A, 1:100), FLI-1 (Cell Marque, Rocklin, USA, Clone MRQ-1, 1:100), HIF-1α (Abcam, San Francisco, USA, ab65979, 1:100), WT-1 (Lab vision, Fremont, USA, Clone 6F-H2, 1:50), VEGF (Lab vision, Fremont, USA, Clone JH121, 1:50), and VEGF-R (R&D Systems, USA, Clone 54703, 1:100), estrogen receptors (DAKO, Glostrup, Denmark, Clone 1D5, 1:50), progesterone receptors( DAKO, Glostrup, DenmarkClone PgR 636, 1:50), and D2-40 (Invitrogen, Carlsbad, USA, Clone D2-40, 1:50) using streptavidin-biotin immunoperoxidase method. The stained sections were examined by three pathologists participated in this study.
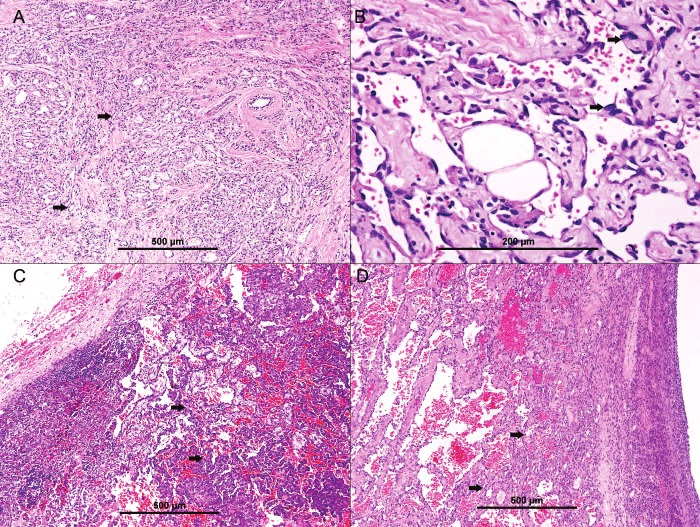
The histopathologic examination of sections from both breast tissues revealed ramifying irregular vascular structures in the breast tissue parenchyma (Figure 1A) formed of atypical endothelial cells, with hyperchromatic, pleomorphic nuclei and mitoses, lining the vascular structures (Figure 1B). Occasional multilayering was present. Focal solid areas of tumor cells were evident. Foci of tumor cell necrosis were seen. One left axillary lymph node was involved by similar neoplastic tissue (Figure 1C). The diagnosis of bilateral primary angiosarcoma of breast with lymph node metastasis was made.
Figure 1.
Primary angiosarcoma of breast. A, showing ramifying irregular vascular structures infiltrating breast parenchyma (thick arrows). B. Neoplastic vascular spaces formed of atypical endothelial cells, with hyperchromatic, pleomorphic nuclei (thick arrows). C. Metastatic angiosarcoma to the lymph node. D. metastatic angiosarcoma to the ovary.
The microscopic examination of both ovarian specimens revealed metastatic angiosarcoma (Figure 1D).
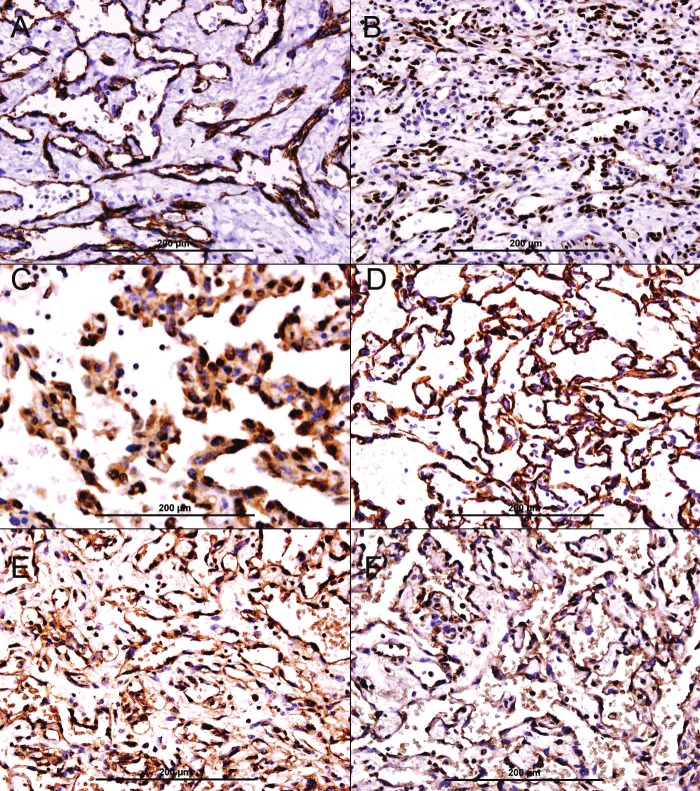
The neoplastic cells were diffusely immunoreactive to CD31 (Figure 2A), FLI-1 (Figure 2B), HIF-1α (Figure 2C), WT-1 (Figure 2D), VEGF (Figure 2E), and VEGF-R (Figure 2F). The neoplastic cells showed no immunoreactivity to estrogen receptor, progesterone receptor, and D2-40 (Data not shown).
Figure 2.
Immunohistochemical stains of angiosarcoma. A. Diffuse membranous and cytoplasmic immunoreactivity of neoplastic endothelial cells for CD31, streptavidin-biotin immunoperoxidase method. B. Diffuse nuclear immunoreactivity of neoplastic endothelial cells for FLI 1, streptavidin-biotin immunoperoxidase method. C. Diffuse nuclear and cytoplasmic immunoreactivity of neoplastic endothelial cells for HIF-1α, streptavidin-biotin immunoperoxidase method. D. Diffuse nuclear and cytoplasmic immunoreactivity of neoplastic endothelial cells for WT-1, streptavidinbiotin immunoperoxidase method. E. Diffuse cytoplasmic immunoreactivity of neoplastic endothelial cells for VEGF, streptavidin-biotin immunoperoxidase method. F. Diffuse cytoplasmic immunoreactivity of neoplastic endothelial cells for VEGF-R, streptavidin-biotin immunoperoxidase method.
Discussion
BAS is a rare cancer with unknown etiology in most cases. BAS has been described in children following radiotherapy for vascular disorders at a very young age [10]. Other documented risk factors include: xeroderma pigmentosa [11], neurofibromatosis I [12], and exposure to vinyl chloride [13]. The diagnosis of BAS may be suspected on clinical examination when red-blue discoloration of the skin is present before later stages nodular tumor growth appears. The diagnosis may be supported further by fine needle aspiration, in particular when combined with immunocytochemistry [14]. A tissue biopsy provides confirmation of the diagnosis of BAS.
We show diffuse expression of HIF1α, VEGF, VEGFR and WT-1 protein by neoplastic cells. HIF -1α is a potent transcription factor. Over expression of HIF-1α can lead to the production of various hypoxia inducible mRNAs including the mRNAs encoding VEGF, platelet-derived growth factor B, erythropoietin, Galectin-3, WT-1 protein, and transforming growth factor alpha [8]. In addition, WT-1 protein is capable of activating vascular gene transcription [15]. Hence the synergistic effect of diffuse expression of HIF1α and WT-1 protein by neoplastic cells can lead to transcription of VEGF, which is a paracrine growth factor that stimulates endothelial cells proliferation and tumor formation. We believe that HIF-1α plays a central role in development of BAS. We also show no immunoreactivity of the neoplastic cells to D2-40, which is a marker for lymphatic endothelial cells. This finding indicates that primary BAS originates from blood vessel endothelial cells rather than lymphatic endothelial cells. These are novel findings and have not been previously reported.
Moreover, we show metastasis of BAS to axillary lymph node, both ovaries, both lungs and D12, a pattern mimics breast carcinoma metastasis prototype.
Despite the low number of primary BAS, Gennaro et al. [16] found in their series that VEGF-R expression is highly related to low- and intermediate-grade tumors and may play a role in predicting a particular patient's clinical course. In our finding, VEGF-R was expressed by almost all neoplastic cells and associated with an intermediate grade.
Furthermore, hormonal influence has also been postulated to affect the incidence of BAS. Brentani et al. [17] reported the presence of estrogen, progesterone, and glucocorticoid receptors in certain cases. In support of these observations, although it is a rare tumor, BAS is often reported in young, fertile women and is occasionally seen during pregnancy [18]. This is compatible with our findings where BAS develops in a young female, but estrogen and progesterone receptors are not expressed in neoplastic cells. The role of pregnancy in the development and progression of this neoplasm is suggested since our patient felt tumor mass during her pregnancy. The compromised immunity and placental growth factors might play a role in its pathogenesis. In addition, the patient noticed a rapid increase in tumor size during lactation which is another indication of hormonal effect on tumor growth.
Bilateral involvement of the disease can develop either as another primary tumor or due to metastatic spread. However, it has been recommended that if contralateral breast disease is detected in patients without further indication of tumor dissemination, the lesion should be treated as another primary angiosarcoma [19]. In our case, the patient showed bilateral disease when both breasts are examined by ultrasound and MRI and before the appearance of distant metastasis, suggesting that the lesion found in the right breast was possibly another primary lesion. Until now, there have been only two other such cases reported previously [20].
On imaging examinations, patients with angiosarcoma usually present with large, noncalcified, ill-defined masses that are often nonspecific by mammography and ultrasound. This results in a misleadingly high incidence of negative diagnoses [21].
Moreover, due to the extensive vascularity of the lesion, needle or open biopsy of the tumor can result in massive hemorrhage, frequently making histological diagnoses difficult. Previous reports have demonstrated that MRI shows increased enhancement on T1-weighted images, as well as high-intensity T2-weighted signals [22], that are suggestive of multiple draining vessels and blood lakes as histological hallmarks of the tumor. In the present case, MRI examination revealed a clear high-intensity tumor, enabling us to pay close attention to the contralateral breast lesions, which showed only equivocal findings upon physical examination and mammography.
The age and clinical appearance of our patient is in concordance Donnell et al. [23], series of 40 cases of primary BAS, the age at presentation ranged from 16 to 69 years, with a mean of 34 years. The manner of presentation was usually a mobile, painless, well-circumscribed mass which clinically felt like either a carcinoma or benign breast disease. Occasionally, cases presented as a bulky hemorrhagic mass, with even purple discoloration of the skin, suggesting a vascular neoplasm. It should be mentioned that another type of angiosarcoma of the breast - secondary angiosarcoma arising from radiotherapy - tends to involve the skin, and its typical simultaneous symptom is edema of the upper extremity (Stewart-Treves syndrome) [24]. By definition, primary BAS does not have any such antecedent. Microscopically, primary BAS cells root in parenchymatous tissue which is deeper, however, angiosarcoma cells caused by radiotherapy tend to concentrate in the dermis.
Metastatic angiosarcoma to the lung, skin, maxillary gingiva, liver, bone, spleen, and ovary have been reported in the literatures [25]. The reported 3-year overall survival and disease free survival is 38%, and 14% respectively [26]. Tumor size and grade are thought to be the most important prognostic factors, but Nascimento et al. [27] find no correlation between histological grade and patient's outcome, which is in line with angiosarcoma at other sites. Surgical biopsy and immunohistochemistry are the gold standard to establish the diagnosis of primary BAS.
Surgery is still considered the most applicable approach. Axillary lymph node dissection is not essential in the management of this tumor as it characteristically metastasizes via blood vessels, and hardly via lymphatics, but in our case we have left axillary lymph node metastasis hence left axillary clearance and right axillary lymph node sampling were done.
We support wide local excision as the best treatment, followed by adjuvant chemotherapy and radiation therapy if there are microscopically involved margins. Silverman et al. [28] noted the statistical superiority of adjuvant chemotherapy to the more poorly differentiated tumors.
Some authors propose that if negative surgical margins can be achieved, breast sarcoma should be managed by conservative surgery with postoperative irradiation to a microscopic tumoricidal dose (50 Gy) to the whole breast, and at least 60 Gy to the tumor bed. Tumor necrosis factor -α and α-interferon have been adopted in adjuvant therapy and showed an efficacy of about 84%; however, this would need to be verified. Despite of what is mentioned above; Gennaro et al. [16] suggest the use of a targeted therapy in the adjuvant setting when VEGF-R is expressed. Our patient developed distant metastasis to ovaries, lungs, liver and bone hence she was given adjuvant chemotherapy and radiation therapy.
In conclusion, HIF- 1α, WT-1 and VEGF are possible protagonists in the development of bilateral primary angiosarcoma of breast. The neoplastic process involves endothelial cell of blood vessels lineage rather than lymphatic lineage. Painless breast tumors in young women that are highly vascular at the time of biopsy should be considered as malignant until proven otherwise. Tissue biopsy is the gold standard in the diagnosis of primary BAS.
References
- 1.McGowan TS, Cummings BJ, O'Sullivan B, Catton CN, Miller N, Panzarella T. An analysis of 78 breast sarcoma patients without distant metastases at presentation. Int J Radiat Oncol Biol Phys. 2000;46:383–390. doi: 10.1016/s0360-3016(99)00444-7. [DOI] [PubMed] [Google Scholar]
- 2.Chen KT, Kirkegaard DD, Bocian JJ. Angiosarcoma of the breast. Cancer. 1980;46:368–371. doi: 10.1002/1097-0142(19800715)46:2<368::aid-cncr2820460226>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.van Geel AN, den Bakker MA. Bilateral angiosarcoma of the breast in a fourteen-year-old child. Rare Tumors. 2009;28:e38. doi: 10.4081/rt.2009.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou SA, Wei H, Ding K. A Rare Case of Metachronous Bilateral Angiosarcoma of the Breast. Breast Care (Basel) 2009;4:405–407. doi: 10.1159/000261506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchant LK, Orel SG, Perez-Jaffe LA, Reynolds C, Schnall MD. Bilateral angiosarcoma of the breast on MR imaging. AJR Am J Roentgenol. 1997;169:1009–1010. doi: 10.2214/ajr.169.4.9308452. [DOI] [PubMed] [Google Scholar]
- 6.Khoshim M, Sadiq S, Ajarim D, Jamjoom ZA. Bilateral angiosarcoma of the breast--a case report. Jpn J Surg. 1991;21:693–695. doi: 10.1007/BF02471057. [DOI] [PubMed] [Google Scholar]
- 7.Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007;19:2003–2012. doi: 10.1016/j.cellsig.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Greijer AE, van der Groep P, Kemming D, Shvarts A, Semenza GL, Meijer GA, van de Wiel MA, Belien JA, van Diest PJ, van der Wall E. Upregulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1) J Pathol. 2005;206:291–304. doi: 10.1002/path.1778. [DOI] [PubMed] [Google Scholar]
- 9.Dohi S, Ohno S, Ohno Y, Kyo S, Soma G, Sugiyama H, Inoue M. WT1 expression correlates with angiogenesis in endometrial cancer tissue. Anticancer Res. 2010;30:3187–3192. [PubMed] [Google Scholar]
- 10.Cancellieri A, Eusebi V, Mambelli V, Ricotti G, Gardini G, Pasquinelli G. Well-differentiated angiosarcoma of the skin following radiotherapy. Report of two cases. Pathol Res Pract. 1991;187:301–306. doi: 10.1016/S0344-0338(11)80788-0. [DOI] [PubMed] [Google Scholar]
- 11.Marcon I, Collini P, Casanova M, Meazza C, Ferrari A. Cutaneous angiosarcoma in a patient with xeroderma pigmentosum. Pediatr Hematol Oncol. 2004;21:23–26. [PubMed] [Google Scholar]
- 12.Elli M, Can B, Ceyhan M, Pinarli FG, Dagdemir A, Ayyildiz HS, Gürsel B, Dagçinar A. Intrathoracic malignant peripheral nerve sheath tumor with angiosarcoma in a child with NF1. Tumori. 2007;93:641–644. doi: 10.1177/030089160709300625. [DOI] [PubMed] [Google Scholar]
- 13.Kirchner SG, Heller RM, Kasselberg AG, Greene HL. Infantile hepatic hemangioendothelioma with subsequent malignant degeneration. Pediatr Radiol. 1981;11:42–45. doi: 10.1007/BF00972043. [DOI] [PubMed] [Google Scholar]
- 14.Boucher LD, Swanson PE, Stanley MW, Silverman JF, Raab SS, Geisinger KR. Cytology of angiosarcoma. Findings in fourteen fine-needle aspiration biopsy specimens and one pleural fluid specimen. Am J Clin Pathol. 2000;114:210–219. doi: 10.1309/PXMU-LF05-3894-W29F. [DOI] [PubMed] [Google Scholar]
- 15.Kirschner KM, Sciesielski LK, Scholz H. Wilms’ tumour protein Wt1 stimulates transcription of the gene encoding vascular endothelial cadherin. Pflugers Arch. 2010;460:1051–1061. doi: 10.1007/s00424-010-0873-6. [DOI] [PubMed] [Google Scholar]
- 16.Gennaro M, Valeri B, Casalini P, Carcangiu ML, Gronchi A, Conti AR, Agresti R, Greco M. Angiosarcoma of the breast and vascular endothelial growth factor receptor. Tumori. 2010;96:930–935. [PubMed] [Google Scholar]
- 17.Brentani MM, Pacheco MM, Oshima CT. Steroid receptor in breast angiosarcoma. Cancer. 1983;51:2105–2111. doi: 10.1002/1097-0142(19830601)51:11<2105::aid-cncr2820511125>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 18.Staingaszner LC, Enzinger FM, Taylor HB. Hemangiosarcoma of the breast. Cancer. 1965;18:352–361. doi: 10.1002/1097-0142(196503)18:3<352::aid-cncr2820180311>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 19.Chen KTK, Kirkengaard DD, Bocian JJ. Angiosarcoma of the breast. Cancer. 1981;46:368–371. doi: 10.1002/1097-0142(19800715)46:2<368::aid-cncr2820460226>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Gupta S, Chopra P, Sharma LK. Bilateral angiosarcoma of the breast: an overview. Aust N Z J Surg. 1990;60:341–345. doi: 10.1111/j.1445-2197.1990.tb07381.x. [DOI] [PubMed] [Google Scholar]
- 21.Liberman L, Dershaw DD, Kaufman RJ, Rosen PP. Angiosarcoma of the breast Radiology. 1992;183:649–654. doi: 10.1148/radiology.183.3.1584913. [DOI] [PubMed] [Google Scholar]
- 22.Bernathova M, Jaschke W, Pechlahner C, Zelger B, Bodner G. Primary angiosarcoma of the breast associated with Kasabach-Merritt syndrome during pregnancy. Breast. 2006;15:255–258. doi: 10.1016/j.breast.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Donnell RM, Rosen PP, Lieberman PH, Kaufman RJ, Kay S, Braun DW Jr, Kinne DW. Angiosarcoma and other vascular tumors of the breast. Am J Surg Path. 1981;5:629–642. doi: 10.1097/00000478-198110000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Donnell RM, Rosen PP, Lieberman PH, Kaufman RJ, Kay S, Braun DW Jr, Kinne DW. Angiosarcoma of the breast: the experience of the European Institute of Oncology and a review of the literature. Breast Cancer Res Treat. 2007;105:81–85. doi: 10.1007/s10549-006-9429-z. [DOI] [PubMed] [Google Scholar]
- 25.Tiwary SK, Singh MK, Prasad R, Sharma D, Kumar M, Shukla VK. Primary angiosarcoma of the breast. Surgery. 2007;141:821–822. doi: 10.1016/j.surg.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez Ortega A, Gil Gil JM, Urruticoetxea A, Serra Payr óJM. Angiosarcoma of the breast. Two cases following breast conserving treatment for invasive carcinoma. Clin Transl Oncol. 2006;8:536–539. doi: 10.1007/s12094-006-0055-3. [DOI] [PubMed] [Google Scholar]
- 27.Nascimento AF, Raut CP, Fletcher CD. Primary angiosarcoma of the breast: clinicopathologic analysis of 49 cases, suggesting that grade is not prognostic. Am J Surg Pathol. 2008;32:1896–1904. doi: 10.1097/PAS.0b013e318176dbc7. [DOI] [PubMed] [Google Scholar]
- 28.Silverman LR, Deligdisch L, Mandeli J, Greenspan EM. Chemotherapy for angiosarcoma of the breast: case report of 30 year survival and analysis of the literature. Cancer Invest. 1994;12:145–155. doi: 10.3109/07357909409024870. [DOI] [PubMed] [Google Scholar]