Abstract
Purpose:
The plant Synedrella nodiflora (L) Gaertn is traditionally used by some Ghanaian communities to treat epilepsy. To determine if this use has merit, we studied the anticonvulsant and other neuropharmacological effects of a hydro-ethanolic extract of the whole plant using murine models.
Materials and Methods:
The anticonvulsant effect of the extract (10–1000 mg/kg) was tested on the pentylenetetrazole-, picrotoxin-, and pilocarpine-induced seizure models and PTZ-kindling in mice/rats. The effect of the extract was also tested on motor coordination using the rota-rod.
Results:
The results obtained revealed that the extract possesses anticonvulsant effects in all the experimental models of seizures tested as it significantly reduced the latencies to myoclonic jerks and seizures as well as seizure duration and the percentage severity. The extract was also found to cause motor incoordination at the higher dose of 1000 mg/kg.
Conclusions:
In summary, the hydro-ethanolic extract of the whole plant of S. nodiflora possesses anticonvulsant effects, possibly through an interaction with GABAergic transmission and antioxidant mechanisms and muscle relaxant effects. These findings thus provide scientific evidence in support of the traditional use of the plant in the management of epilepsy.
Keywords: Kindling, pentylenetetrazole, picrotoxin, pilocarpine, Synedrella nodiflora
Epilepsy is a neurological disorder characterized by recurrent seizures, which are sudden, unprovoked, and transitory episodes of abnormal hypersynchronous neuronal discharge. It is estimated that more than 50 million people worldwide are epileptic (1–2% of the world's population), out of whom 40 million are believed to be living in the developing countries.[1] The currently available antiepileptic drugs have several limitations as they provide only symptomatic relief and their use is often plagued by significant adverse effects such as rash, blood dyscrasias, vitamin K and folate deficiencies, loss of libido, hormonal dysfunction, and bone marrow hypoplasia and fetal abnormalities such cleft palate, cleft lip, congenital heart disease, slowed growth rate, and mental deficiency. Moreover, a significant proportion of patients (up to 40%) do not respond to these agents and this proportion is quite high in the developing countries.[2,3] Additionally, the high cost of newer and more effective antiepileptic drugs have led to a greater proportion of patients in Ghana and possibly other third world countries, resorting to the use of traditional medicine. There is therefore a universal and local need for continued research into the development of newer and cost effective agents for the management of this disorder.[4–6] Plant sources of drugs dominate therapy in the developing countries and have often served as an effective means of getting lead compounds, from which newer and effective drugs can be developed.
Synedrella nodiflora (L.) Gaertn. (family: Asteraceae) is an annual herb that grows to about 60–120 cm high. It is a native tropical American weed, but now dispersed pan-tropically and occurring throughout the West African region. In Ghana, the foliage is eaten by livestock, whereas in Indonesia, the foliage is eaten as a vegetable by some indigenous tribes. The plant has also been extensively used in Nigeria for cardiac problems, wound healing, and to stop bleeding.[7] In Malaysia and Indonesia, it has been used for the treatment of headaches, earaches, stomachaches, and as a liniment for rheumatism.[8] In traditional Ghanaian medicine, the whole plant is boiled and the aqueous extract drunk as required for the treatment of epilepsy. The leaves are used for the treatment of hiccup and threatened abortion.[9] The whole plant extract has been reported to possess potent anti-inflammatory activity[10] and central analgesic effects.[11] We have also demonstrated in our laboratory that the hydro-ethanolic extract of the whole plant possess antinociceptive effects possibly mediated via adenosinergic mechanisms.[12] To date there is little scientific evidence to support the traditional use of S. nodiflora in the treatment of epilepsy and the possible mechanisms involved. This study tested the general hypothesis that the plant S. nodiflora has anticonvulsant activity. We undertook to systematically evaluate the actions of an extract of S. nodiflora on chemically induced seizures and muscle tone in rodents. The hydro-ethanolic whole plant extract, similar to that traditionally used, was used in this study.
Materials and Methods
Plant collection
The whole plant of S. nodiflora was obtained from the Botanical Gardens, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana, in August 2007 and authenticated by the Department of Pharmacognosy, KNUST. A specimen voucher, FP/08/025, has been kept at the Faculty of Pharmacy Herbarium.
Preparation of extract
Samples of S. nodiflora were collected and air-dried for 7 days and powdered. Two kilograms of the powder was cold-macerated with 70% v/v of ethanol. The hydro-ethanolic extract was then evaporated to a syrupy mass under reduced pressure, air-dried, and kept in a dessicator. A 7% w/w yield was obtained. This is subsequently referred to as the extract or SNE.
Animals
Sprague-Dawley rats (150–200 g) and ICR mice (20–30 g) were purchased from Noguchi Memorial Institute for Medical Research, University of Ghana, Legon, and maintained in the Animal House of the Department of Pharmacology, KNUST, Kumasi. The animals were housed in groups of six in stainless steel cages (34 cm × 47 cm × 18 cm) with soft wood shavings as bedding, fed with normal commercial pellet diet (GAFCO, Tema), given water ad libitum and maintained under laboratory conditions (temperature 24–28°C, relative humidity 60–70%, and 12-h light–dark cycle). In other experiments conducted at the Health Science Center (HSC), Kuwait University, Balb/c and MF-1 mice of either sex (20–30 g) were obtained from the Animal Resource Center (ARC), HSC, Kuwait University, Kuwait.
All procedures and techniques used in these studies were in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals (NIH, Department of Health Services publication No. 83-23, revised 1985). The protocols for the study were approved by the Departmental and Institutional Ethics Committees.
Drugs and chemicals
Pentylenetetrazole (PTZ), picrotoxin (PIC), pilocarpine (PILO), n-butyl-bromide hyoscine, phenobarbitone sodium, and baclofen were purchased from Sigma-Aldrich Inc., St. Louis, MO, USA, while diazepam was obtained from Pharm-Inter, Brussels, Belgium.
Phytochemical analysis
The extract of S. nodiflora was screened for the presence of alkaloids, reducing sugars, glycosides, saponins, and tannins as described by Trease and colleagues.[13]
PTZ-induced seizures
The anticonvulsant testing method as described by Vellucci and colleagues was used with modifications.[14] In brief, clonic seizures were induced in drug/vehicle pretreated male Sprague-Dawley rats (150–200 g) by a subcutaneous injection of 75 mg/kg PTZ into the loose skin fold on the back of the neck of the rats. The animals were pretreated with SNE (100–1000 mg/kg, p.o.) or phenobarbitone sodium (3–30 mg/kg i.p.) 30 minutes before the injection of PTZ. The control animals received 0.9% saline solution orally (0.01 ml/kg). After the PTZ injection, the animals were placed in a testing chamber (made of perspex of dimensions 15 cm×15 cm× 15 cm). A mirror angled at 45° below the floor of the chamber allowed a complete view of the convulsive event of PTZ. The behavior of the animals was captured with a camcorder (Everio™ model GZ-MG 130U, JVC, Tokyo, Japan) placed directly opposite to the mirror. The latencies to myoclonic jerks and clonic seizures and the duration of seizures were scored from the video recordings with the aid of the public domain software JWatcher™ Version 1.0 (University of California, Los Angeles, USA, and Macquarie University, Sydney, Australia; available at http://www.jwatcher.ulca.edu/). The ED50 (a measure of anticonvulsant potency) of the extract and the reference anticonvulsants were calculated from the dose–response curves of the percent seizure inhibition by the drug/extract to the vehicle-treated group. The ability of a drug/extract to prevent the seizures or delay/prolong the latency or onset of the tonic hind-limb extensions was considered as indications of anticonvulsant activity.
PIC-induced seizures
In brief, clonic-tonic seizures were induced in drug or vehicle pretreated male ICR mice (20–30 g) by an i.p. injection of 10 mg/kg PIC. The animals were pretreated with SNE (100–1000 mg/kg, p.o.) or phenobarbitone sodium (3–30 mg/kg, i.p.) 30 minutes before the injection of PIC. The control animals received 0.9% saline solution orally (0.01 ml/kg). After the PIC injection, animals were placed in the testing chamber and a video recording of the event was made as described for the PTZ experiment. The latencies to myoclonic jerks and clonic-tonic seizures and the duration and frequency of the seizures were scored from the video recordings as described above. The ED50 of the extract and diazepam was calculated as described in the PTZ test.
PILO-induced seizures
In this experiment, seizures were induced by an i.p. injection of PILO (350 mg/kg) into drug or vehicle-treated male rats. Rats were pretreated with SNE (100–1000 mg/kg p.o.) or diazepam (1–10 mg/kg i.p.) for 30 or 15 minutes, respectively, before PILO injection. To reduce peripheral autonomic effects produced by PILO, the animals were pretreated with n-butyl-bromide hyoscine (1 mg/kg i.p. 30 minutes before PILO administration). After the injection of the PILO, the animals were placed separately into the transparent plexiglass testing chamber and video recordings made as described in the PTZ experiments. The latency to and duration of seizures were scored from the video recordings as previously described. The ED50 of the extract was calculated as described in the PTZ test.
PTZ -induced kindling
To induce kindling, a 35 mg/kg dose of PTZ was injected i.p. into SNE-, diazepam-, or vehicle-treated male Sprague-Dawley rats (200–300 g) every 48 h for 32 days. After each PTZ injection, the rats were placed in the testing chamber and recorded as in the PTZ experiments described above. Seizure intensities were classified according to the Racine score[15] as follows:
Stage 0: no response
Stage 1: ear and facial twitching
Stage 2: convulsive waves throughout the body
Stage 3: myoclonic jerks, rearing
Stage 4: turning over onto one side
Stage 5: turning over onto the back, generalized tonic-clonic seizures
Each rat was considered fully kindled after showing stage 4 or 5 on two consecutive PTZ administrations. Seven days after kindling had been achieved, the rats were challenged with 35 mg/kg of PTZ, and the entire event was also recorded. The ED50 of the extract was calculated as described in the PTZ test.
Rota-rod test
The effect of SNE on motor coordination was measured using Rotamex-4/8 (Columbus Instrument, USA). During the test, Balb/c mice were selected and screened primarily at 12 rpm for four consecutive times (an hour interval) for a day. On day 2, the speed was increased to 24 rpm and mice that could stay on the rotating rod for 600 s were selected and grouped into six: three dose levels of SNE (10–1000 mg/kg, p.o.), two for baclofen (1 and 10 mg/kg, i.p.), and one as vehicle-treated. The mice, in their various groups, were trained for four consecutive days with four training sessions (at 1 h interval) per day. On the day of the experiment, each mouse was given a drug-free rotation and 30 minutes later treated with SNE, baclofen, or vehicle and tested at every 30 minutes for 2.5 h. The latency to fall was recorded as the time spent on the rotating rod. The ED50 of the extract was calculated from the concentration–response curves.
Lipid peroxidative assay in PTZ-kindled rat brain homogenates
This test was done to assess the role of lipid peroxidation in PTZ-induced kindling and a possible beneficial role of SNE which has demonstrated in vitro antioxidant properties. On the day after the 35 mg/kg PTZ challenge, the kindled rats were sacrificed by decapitation and the forebrain dissected out and homogenized (100 mg/ml) in ice-cold 0.1 M phosphate buffer (pH 7.4) with Ultra-Turrax T 25 homogenizer (IKA Labortehnic, Staufen, Germany). Brain homogenate (2.5 ml) was mixed with 1 ml phosphate buffer and the mixture was then incubated in an orbital shaker incubator (BoroLabs, Aldermaston Berkshire, EC) at 37°C for 1 h.
To assay for thiobarbituric acid reactive substances, 0.1 ml of the incubated reaction mixture was placed in a test tube containing 1.5 ml of 10% trichloroacetic acid and allowed to stand for 10 minutes. Then the tubes were centrifuged at 2200 g for 10 minutes. The supernatant was separated and mixed in a tube containing 1.5 ml of 0.67% thiobarbituric acid in 20% acetic acid. The mixture was heated in a hot water bath at 85°C for 1 h to complete the reaction and allowed to cool. The intensity of the pink-colored complex formed was measured at 535 nm in a spectrophotometer (Cecil CE 7200 spectrophotometer, Cecil Instrument Limited, Milton Technical Centre, UK). In this test, absorbance decreases with increasing ability to inhibit lipid peroxidation. Phosphate buffer was used as a blank throughout the experiment.
The percentage inhibition of lipid peroxidation was calculated and presented as % inhibition of lipid peroxidation against concentration and the EC50 for each drug determined.
Data analysis
The ED50 (dose responsible for 50% of the maximal effect) and inhibitory effects of drugs were analyzed by using an iterative computer least squares method, GraphPad Prism for Windows version 5.0 (GraphPad Software, San Diego, CA, USA) with the following nonlinear regression (four-parameter logistic equation).
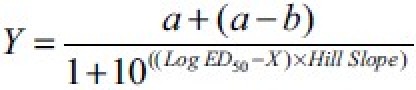
where X is the logarithm of concentration and Y is the response and starts at a and goes to b with a sigmoid shape.
The fitted midpoints (ED50s) of the curves were compared statistically using F test.[16,17] GraphPad Prism Version 5.0 for Windows (GraphPad Software) was used for all statistical analyses and ED50 determination. P < 0.05 was considered statistically significant in all analysis. The graphs were plotted using Sigma-Plot for Windows Version 11.0 (Systat Software Inc., Germany).
Results
Phytochemical analysis
Glycosides, saponins, anthracene glycosides, sterols, alkaloids, tannins, and pseudotannins were found to be present in the extract, whereas cyanogenetic and cardiac glycosides were absent.[12]
PTZ-induced seizures
PTZ induced a sequence of events starting with myoclonic jerks which was then followed by an intense clonic convulsive phase. The extract, SNE, showed significant anticonvulsant effect against seizures induced by PTZ. SNE, dose dependently, increased both the onset time of the myoclonic jerks (F3,20 = 1.481, P = 0.0250; Figure 1a) and latency to myoclonic convulsions (F3,20 = 3.999, P = 0.0230; Figure 1b) and these effects were significant at the 1000 mg/kg (P < 0.01). However, SNE produced no significant effect on the duration of the seizures (F3,20 = 1.012, P = 0.4081; Figure 2). Phenobarbitone, the reference anticonvulsant used, delayed both the onset of myoclonic jerks (F3,20 = 3.818, P = 0.0259; Figure 1a) and myoclonic seizures (F3,20 = 25.85, P < 0.001; Figure 1b) and also reduced the duration of the myoclonic seizures (F3,20 = 27.04, P < 0.001; Figure 2). The anticonvulsant effect of SNE was, however, less when compared with phenobarbitone as shown by the EC50 values [Table 1].
Figure 1a.

Effect of SNE (100–1000 mg/kg) and phenobarbitone (3–30 mg/kg) on the latencies to myoclonic jerks induced by PTZ. Each column represents the mean ± SEM (n = 5). *P < 0.05, ***P < 0.001 compared with vehicle-treated group (one-way analysis of variance followed by Newman–Keuls post hoc test)
Figure 1b.

Effect of SNE (100–1000 mg/kg) and phenobarbitone (3–30 mg/kg) on the latencies to seizures induced by PTZ. Each column represents the mean ± SEM (n = 5). *P < 0.05, ***P < 0.001 compared with vehicle-treated group (one-way analysis of variance followed by Newman–Keuls post hoc test)
Figure 2.

Effect of SNE (100–1000 mg/kg) and phenobarbitone (3–30 mg/kg) on the total duration of the seizures induced by PTZ. Each column represents the mean ± SEM (n = 5). **P < 0.01 compared with vehicle-treated group (one-way analysis of variance followed by Newman–Keul's post hoc test)
Table 1.
EC50 (mg/kg) of SNE, diazepam, and phenobarbitone in PTZ-, PIC-, and PILO-induced seizures and PTZ kindling in murine models of seizure

PIC-induced seizures
As with PTZ, PIC induced tonic-clonic convulsive episodes preceded by myoclonic jerks in mice. The extract showed significant anticonvulsant effect against the seizures induced by PIC. SNE significantly and dose dependently delayed the latencies to myoclonic jerks (F4,20 = 6.959, P = 0.0011; Figure 3) and tonic-clonic seizures (F4,20 = 7.111, P = 0.0010; Figure 3) induced by PIC. SNE, however, dose dependently increased the frequency of convulsions (F4,20 = 7.562, P = 0.0007; Figure 4) but reduced the total duration of seizures significantly (F4,20 = 15.26, P < 0.0001; Figure 4). Similar to SNE, the reference drug, phenobarbitone also significantly and dose dependently delayed the latencies to myoclonic jerks (F3,16 = 4.593, P = 0.0167; Figure 3) and tonic-clonic seizures induced by PIC (F3,16 = 7.682, P = 0.0021; Figure 3). Furthermore, phenobarbitone also dose dependently increased the frequency of convulsions (F3,16 = 13.20, P = 0.001; Figure 4), but this effect was significant only at the 30 mg/kg (P < 0.001), Finally, phenobarbitone dose dependently reduced the duration of seizures (F3,16 = 6.208, P = 0.0053; Figure 4). In comparison to phenobarbitone, the anticonvulsant effect of SNE was less potent as revealed by their EC50 values [Table 1].
Figure 3.

Effect of SNE (100–1000 mg/kg) (a) and phenobarbitone (3–30 mg/kg) on the latency to myoclonic jerks and seizures induced by PIC. Each column represents the mean ± SEM (n=5). *P < 0.05, **P < 0.01, ***P < 0.001 compared with vehicle-treated group (one-way analysis of variance followed by Newman–Keul's post hoc test)
Figure 4.

Effect of SNE (100–1000 mg/kg) and phenobarbitone (3–30 mg/kg) on the frequency (a) and duration (b) of the convulsions induced by PIC. Each column represents the mean ± SEM (n=5). *P < 0.05, ***P < 0.001 compared with vehicle-treated group (one-way analysis of variance followed by Newman–Keul's post hoc test)
PILO-induced status epilepticus
PILO induced clonic convulsive episodes preceded by myoclonic jerks in rats. Both SNE and diazepam produced no significant effect (SNE, P = 0.6634; diazepam, P = 0.373; Figure 5a) on the latency to first myoclonic jerks compared with the vehicle treatment. SNE, however, dose dependently reduced the total duration of seizures (F3,20 = 7.235, P = 0.0018) in mice that were pretreated with it. Diazepam, a drug commonly used in the emergency room to abort status epilepticus (SE), was used as the reference drug and it also dose dependently and significantly reduced the total duration of seizures (F3,20 = 26.78, P < 0.0001; Figure 5) at 3 mg/kg, while 10 mg/kg completely abolished seizure occurrence (P < 0.001; Figure 5b).
Figure 5.

Effect of SNE (100–1000 mg/kg) (a) and diazepam (1–10 mg/kg) (b) on the latency to and total duration of seizures induced by PILO. Each column represents the mean ± SEM (n=6). *P < 0.05, **P < 0.01, ***P < 0.001 compared with vehicle-treated group (one-way analysis of variance followed by Newman–Keuls post hoc test)
PTZ-induced kindling
In the vehicle-treated group, repeated administration of 35 mg/kg of PTZ on alternate days caused a gradual increase in the convulsant's responses as scored by the Racine scale. By the 7th day, the score had increased from 0 to 3, reaching a peak severity on the Racine score of 5 by the 15th day which was maintained for the remaining duration (~2 weeks) of the study [Figure 6]. SNE significantly depressed the kindled seizures at all the dose levels tested (F3,16 =11.48, P = 0.0003; Figure 6) and none of the animals in these treatment groups achieved seizure score 5, even after 16 injections of PTZ (35 mg/kg, days 16–32). The percent severity of seizures (calculated from the AUC) shows that SNE attenuated PTZ kindled seizure activity by reducing the 5 score on the Racine scale by between 50% and 70% (i.e., between 100 and 1000 mg/kg). Diazepam also produced a significant dose-dependent depression of the kindled seizure activity (F3,16 = 224.10, P < 0.0003; Figure 6) and the percent severity of seizures was significantly reduced by 70% (for 0.1–0.3 mg/kg) and by 80% at 1 mg/kg [Figure 6].
Figure 6.

The dose–response effects of SNE (100–1000 mg/kg) (a and b) and DZP (0.1–1.0 mg/kg) (c and d) on the PTZ-kindled rats. The left panels show the time course of effects over the 32-day period and the right panels show the percent severity of seizures calculated from the AUCs for the test duration. Values are means ± SEM (n=5). *P < 0.05, ** P < 0.01, ***P < 0.001 compared with vehicle-treated group (two-way analysis of variance followed by Bonferroni's post hoc test). †P<0.05, †††P<0.001 compared with vehicle-treated group (one-way analysis of variance followed by Newman–Keul's post hoc test)
Rota-rod test
Figure 7 presents the effect of treatment with SNE and baclofen on the motor coordination using the rota-rod method. The administration of SNE, 30 minutes after the initial zero reading, produced no significant difference between the vehicle-treated and 10 and 100 mg/kg doses during the 2-h period of the experiment. SNE 1000 mg/kg, however, produced a significant reduction (88.75%, F3,16 = 34.54, P < 0.0001; Figure 7a) in the time spent on the rota-rod at 24 rpm over the 2-h period decreased by analysis of the AUC also revealed a similar trend [Figure 7b]; 1 mg/kg baclofen, a reference muscle relaxant, produced no significant effect on the skeletal muscle, whereas 10 mg/kg produced a significant depression of 87.5% (F2,12 = 5.908, P = 0.00164; Figure 7c). An analysis of the AUC showed the same effect [Figure 7d].
Figure 7.

The dose–response effects of SNE (10–1000 mg/kg) (a and b) and baclofen (1–10 mg/kg) (c and d) on the rota-rod. The left panels show the time course of the time spent on the rotating rod at 24 rpm over a 120-min period and the right panels show the motor coordination effect calculated from the AUCs of the time course curves. Values are means ± SEM (n=5). **P < 0.01, ***P < 0.001 compared with vehicle-treated group (two-way analysis of variance followed by Bonferroni's post hoc test). ††P < 0.01 compared with vehicle-treated group (one-way analysis of variance followed by Newman–Keul's post hoc test)
Lipid peroxidative assay in PTZ-kindled rat brain homogenates
SNE (100–1000 mg/kg-1) dose dependently inhibited lipid peroxidation in the PTZ-kindled rats, whereas diazepam (0.1–1.0 mg/kg) gave an opposite effect [Figure 8].
Figure 8.

Percentage inhibition of PTZ induced lipid peroxidation in PTZ-kindled rats by SNE (100–1000 mg/kg) and diazepam (0.1–1.0 mg/kg). Each point represents the mean ± SEM (n=5)
Discussion
The outcome of this study provides evidence that the hydro-ethanolic extract of the whole plant of S. nodiflora possesses anticonvulsant and possibly sedative effects and impairs motor coordination in murine experimental models. The effectiveness of the plant's extract in the convulsion paradigms used suggests that the herb can be used in both petit and grand mal types of epilepsy.[18]
The study revealed that SNE inhibited PTZ-induced seizures. The PTZ test represents a valid model for human generalized and absence seizures.[19] PTZ has been used experimentally to study seizure phenomenon and to identify pharmaceuticals that may control seizure susceptibility. The exact mechanism of the epileptogenic action of PTZ at the neuronal level is still unclear, but it has been generally reported to produce seizures by inhibiting gamma-aminobutyric acid (GABA) neurotransmission.[20] Enhancement of GABAergic neurotransmission has been shown to inhibit or attenuate seizures, while inhibition of GABAergic neurotransmission or activity is known to promote and facilitate seizure. Anticonvulsant agents such as diazepam and phenobarbitone inhibit PTZ-induced seizure by enhancing the action of GABAA receptors, thus facilitating the GABA-mediated opening of chloride channels.[21–23] Postsynaptic GABAA receptors are multiunit complexes with binding sites for the endogenous ligand GABA, benzodiazepines, barbiturates, and other ligands with a central chloride ion channel.[24] Thus, the inhibition of PTZ-induced seizures by SNE suggests that SNE may produce this effect by enhancing GABAergic neurotransmission although it is also possible that it could have done so by depressing glutamate-mediated excitation.
PIC, a GABAA-receptor antagonist, produces seizures by blocking the chloride-ion channels linked to GABAA receptors, thus preventing the entry of chloride ions into neurons. This leads to decreased GABA transmission and activity in the brain. Thus, convulsions arising from PIC are due to the decreased GABAA-receptor-mediated inhibition which tips the balance in favor of the glutamate-mediated excitatory transmission.[21,25] The ability of SNE to attenuate seizures induced by PIC may possibly be due to an interaction with GABAA receptors and/or GABA neurotransmission. Phenobarbitone, a reference anticonvulsant, produced similar effects on PIC-induced seizures. Phenobarbitone is known to enhance GABAergic neurotransmission by increasing chloride ion flux through the chloride channels of GABAA receptors.[23,26] Since SNE mimicked, to a large extent, the anticonvulsant actions of phenobarbitone, it is possible that SNE antagonizes PIC-induced seizures by opening the chloride channels associated with GABAA receptors. It is also possible to achieve these effects by suppressing glutamate-mediated excitation but this has to be verified by studying the effects of SNE on pure glutamate-mediated excitatory postsynaptic responses in a relevant brain region. Thus, further in vitro studies are needed to unequivocally determine the exact mechanisms by which SNE attenuate these seizures.
SNE also showed anticonvulsant effects against PILO-induced seizures. PILO, a cholinergic agonist, is widely used in studies of epilepsy as a model of experimentally induced limbic seizures.[27] PILO-induced seizures and SE produces many alterations in the central nervous system neurotransmission involving significant decrease in M1, M2, and GABAergic receptor densities.[28] Decreased acetylcholinesterase and increased superoxide dismutase and catalase enzymatic activities in the rat striatum, frontal cortex, and hippocampus have also been reported.[28] There is ample evidence to show that lipid peroxidation levels are increased during the acute period of PILO-induced seizures and SE in adult rats,[28,29] suggesting the involvement of free radical in the PILO-induced brain damage. This is further supported by the fact that certain antioxidants, such as ascorbic acid, have shown anticonvulsant activity against PILO-induced SE.[29,30] Thus, the ability of SNE to attenuate seizures induced by PILO in rats could be attributed to cholinergic antagonism at the M1 or M2 receptors or increase in GABA and/or its receptor densities or through antioxidant mechanisms. Data from our laboratory (unpublished) indicate that SNE has a marked antioxidant and free radical scavenging effect in vitro. It is thus possible that it used antioxidant mechanisms to attenuate the PILO-induced seizures in rats.
Oral administration of SNE to rats decreased the progression of epileptogenesis induced by PTZ kindling. PTZ-induced kindling is an acknowledged experimental model of human epilepsy and useful for the study of seizure mechanisms.[31] It is associated with cognitive deficit,[32] changes in emotional behavior,[33] and neuronal cell loss in hippocampal CA1, CA3, CA4 structures, dentate gyrus, and the hilus.[34–36] It has been reported that free radical generation due to increased activity of the glutamate plays a fundamental role in neuronal cell death associated with PTZ kindling in rats.[37–39] Free radicals have been implicated in a number of seizure models including PTZ kindling[40,41] and some antioxidants have been shown to be effective in these seizure models.[42,43] When the production of free radicals increases or the defense mechanism of the body decreases, lipid peroxidation at polyunsaturated sites on biological membranes occurs leading to cell dysfunction.[44] SNE's ability to prevent full kindling by PTZ in rats may be as a result of its ability to inhibit lipid peroxidation and/or scavenge free radicals. This possibility is supported by the in vitro experiments whereby SNE was observed to dose dependently inhibit lipid peroxidation in brains from PTZ kindled rats. Additional in vitro experiments also show that SNE possesses antilipid peroxidation activity and free radical scavenging effects (unpublished data). Thus, it is possible that SNE slowed the progression of epileptogenesis through antioxidant mechanisms. By contrast, diazepam, while slowing the progression of epileptogenesis, did not show any antioxidant properties.
The ability of SNE to significantly reduce the time spent on the rotating rod at 1000 mg/kg suggests that at higher doses the extract impairs motor coordination. The accelerating rota-rod is a valid animal model used to assess the effects of drugs on motor coordination in rodents.[45] Time spent on the rotating rod is presumably affected by both sedation and loss of muscle tone.[46] It is however known that some medications such as benzodiazepines cause a reduction in muscle tone through central mechanisms independent of sedation.[46,47] Therefore, the effect observed for SNE at 1000 mg/kg could be due to either sedation or centrally mediated muscle relaxation or both. Similar to SNE, baclofen, a GABAB receptor agonist and the reference muscle relaxant used in this study, also significantly reduced the time spent on the rotating bar.[48,49]
Additional experiments in our labs (unpublished data) also show that similar to diazepam, SNE increased the duration of sleep induced by pentobarbitone. Sedation can be induced by activation of GABAA receptors, mainly those containing α2-subunits, and most benzodiazepines use this mechanism to produce sedation.[47] This sedative effect thus further strengthens the possibility that SNE may produce its CNS effect by modulating the GABAergic system.
Conclusion
In conclusion, the hydro-ethanolic extract of the whole plant of S. nodiflora possesses anticonvulsant and motor impairment effects in rodents, possibly by an interaction with GABAergic neurotransmission. An antioxidant action may also contribute to its anticonvulsant effects. This study thus lends support to the folkloric use of this plant in Ghana for the treatment of epilepsy. Future experiments will separate, purify, and attempt to identify the chemical entity or entities in the extract that are responsible for the CNS effects of S. nodiflora.
Acknowledgments
The authors wish to express their gratitude to Mr. Thomas Ansah of the Department of Pharmacology, KNUST, Kumasi for his technical assistance. Thanks are also due to staff of the ARC, HSC, Kuwait University.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.McNamara JO, Bonhaus DW, Shin CY. Cambridge, UK: Cambridge University Press; 1993. The kindling model of epilepsy. Epilepsy: Models, Mechanisms and Concepts; pp. 27–47. [Google Scholar]
- 2.Regesta G, Tanganelli P. Clinical aspects and biological bases of drug-resistant epilepsies. Epilepsy Res. 1999;34:109–22. doi: 10.1016/s0920-1211(98)00106-5. [DOI] [PubMed] [Google Scholar]
- 3.Kwan P, Brodie MJ. Epilepsy after the first drug fails: substitution or add-on? Seizure. 2000;9:464–8. doi: 10.1053/seiz.2000.0442. [DOI] [PubMed] [Google Scholar]
- 4.Loscher W. Current status and future directions in the pharmacotherapy of epilepsy. Trends Pharmacol Sci. 2002;23:113–8. doi: 10.1016/S0165-6147(00)01974-X. [DOI] [PubMed] [Google Scholar]
- 5.McNamara JO. Emerging insights into the genesis of epilepsy. Nature. 1999;399(6738 Suppl):A15–22. doi: 10.1038/399a015. [DOI] [PubMed] [Google Scholar]
- 6.Brodie MJ, Kwan P. The star systems: overview and use in determining antiepileptic drug choice. CNS Drugs. 2001;15:1–12. doi: 10.2165/00023210-200115010-00001. [DOI] [PubMed] [Google Scholar]
- 7.Idu M, Onyibe HI. Medicinal Plants of Edo State, Nigeria. Res J Med Plants. 2007;2:32–41. [Google Scholar]
- 8.Burkill HM. 2nd ed. Royal Botanical Gardens Kew; 1985. The Useful Plants of West Tropical Africa. [Google Scholar]
- 9.Mshana NR, Abbiw DK, Addae-Mensah I. Traditional Medicine and Pharmacopeia: Scientific, Technical and Research Commission of the Organization of African Union. 2000 [Google Scholar]
- 10.Abad MJ, Bermejo P, Carretero E, Martínez-Acitores C, Noguera B, Villar A. Antiinflammatory activity of some medicinal plant extracts form Venezuela. J Ethnopharmacol. 1996;55:63–8. doi: 10.1016/s0378-8741(96)01478-x. [DOI] [PubMed] [Google Scholar]
- 11.Forestieri AM, Monforte MT, Ragusa S, Trovato A, Iauk L. Antiiflammatory, Analgesic and Antipyretic Activity in Rodents of Plant Extracts used in African Medicine. Phytother Res. 1996;10:100–6. [Google Scholar]
- 12.Woode E, Amoateng P, Ansah C, Duweijua M. Anti-nociceptive effects of an ethanolic extract of the whole plant of Synedrella nodiflora (L.) Gaertn in mice: Involvement of adenosinergic mechanisms. J Pharmacol Toxicol. 2009;4:17–29. [Google Scholar]
- 13.Trease GE, Evans WC. London: Baulliere Tinall Ltd; 1989. A Textbook of Pharmacognosy. [Google Scholar]
- 14.Vellucci SV, Webster RA. Antagonism of caffeine-induced seizures in mice by Ro 15-1788. Eur J Pharmacol. 1984;97:289–93. doi: 10.1016/0014-2999(84)90462-x. [DOI] [PubMed] [Google Scholar]
- 15.Racine RJ. Modification of seizure activity by electrical stimulation. I. After-discharge threshold. Electroencephalogr Clin Neurophysiol. 1972;32:269–79. doi: 10.1016/0013-4694(72)90176-9. [DOI] [PubMed] [Google Scholar]
- 16.Motulsky HJ, Christopoulos A. A practical guide to curve fitting. San Diego, CA: GraphPad Software Inc; 2003. Fitting model to biological data using linear and nonlinear regression. [Google Scholar]
- 17.Miller JR. San Diego, CA: GraphPad Software Inc; 2003. GraphPad Version 4.0. Step-by-Step Examples. [Google Scholar]
- 18.Mahomed IM, Ojewole JA. Anticonvulsant activity of Harpagophytum procumbens DC [Pedaliaceae] secondary root aqueous extract in mice. Brain Res Bull. 2006;69:57–62. doi: 10.1016/j.brainresbull.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Loscher W, Schmidt D. Which animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Epilepsy Res. 1988;2:145–81. doi: 10.1016/0920-1211(88)90054-x. [DOI] [PubMed] [Google Scholar]
- 20.De Sarro G, Ferreri G, Gareri P, Russo E, De Sarro A, Gitto R, et al. Comparative anticonvulsant activity of some 2,3-benzodiazepine derivatives in rodents. Pharmacol Biochem Behav. 2003;74:595–602. doi: 10.1016/s0091-3057(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 21.Gale K. Role of GABA in the genesis of chemoconvulsant seizures. Toxicol Lett. 1992;64-65:417–28. doi: 10.1016/0378-4274(92)90215-6. [DOI] [PubMed] [Google Scholar]
- 22.Olsen RW. The GABA postsynaptic membrane receptor-ionophore complex.Site of action of convulsant and anticonvulsant drugs. Mol Cell Biochem. 1981;39:261–79. doi: 10.1007/BF00232579. [DOI] [PubMed] [Google Scholar]
- 23.Meldrum BS. Epilepsy and gamma-aminobutyric acid-mediated inhibition. Int Rev Neurobiol. 1975;17:1–36. doi: 10.1016/s0074-7742(08)60205-6. [DOI] [PubMed] [Google Scholar]
- 24.Olsen RW, Leeb-Lundberg F. Convulsant and anticonvulsant drug binding sites related to GABA-regulated chloride ion channels. Adv Biochem Psychopharmacol. 1981;26:93–102. [PubMed] [Google Scholar]
- 25.Leidenheimer NJ, Browning MD, Harris RA. GABAA receptor phosphorylation: Multiple sites, actions and artifacts. Trends Pharmacol Sci. 1991;12:84–7. doi: 10.1016/0165-6147(91)90509-q. [DOI] [PubMed] [Google Scholar]
- 26.Davies PA, Hanna MC, Hales TG, Kirkness EF. Insensitivity to anaesthetic agents conferred by a class of GABA(A) receptor subunit. Nature. 1997;385:820–3. doi: 10.1038/385820a0. [DOI] [PubMed] [Google Scholar]
- 27.Turski L, Cavalheiro EA, Czuczwar SJ, Turski WA, Kleinrok Z. The seizures induced by pilocarpine: behavioral, electroencephalographic and neuropathological studies in rodents. Pol J Pharmacol Pharm. 1987;39:545–55. [PubMed] [Google Scholar]
- 28.Freitas RM, Sousa FC, Vasconcelos SM, Viana GS, Fonteles MM. Pilocarpine-induced status epilepticus in rats: Lipid peroxidation level, nitrite formation, GABAergic and glutamatergic receptor alterations in the hippocampus, striatum and frontal cortex. Pharmacol Biochem Behav. 2004;78:327–32. doi: 10.1016/j.pbb.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Xavier SM, Barbosa CO, Barros DO, Silva RF, Oliveira AA, Freitas RM. Vitamin C antioxidant effects in hippocampus of adult Wistar rats after seizures and status epilepticus induced by pilocarpine. Neurosci Lett. 2007;420:76–9. doi: 10.1016/j.neulet.2007.04.056. [DOI] [PubMed] [Google Scholar]
- 30.Tejada S, Sureda A, Roca C, Gamundí A, Esteban S. Antioxidant response and oxidative damage in brain cortex after high dose of pilocarpine. Brain Res Bull. 2007;71:372–5. doi: 10.1016/j.brainresbull.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Mason CR, Cooper RM. A permanent change in convulsive threshold in normal and brain-damaged rats with repeated small doses of pentylenetetrazole. Epilepsia. 1972;13:663–74. doi: 10.1111/j.1528-1157.1972.tb04401.x. [DOI] [PubMed] [Google Scholar]
- 32.Becker A, Grecksch G, Matthies H. The influence of diazepam on learning processes impaired by pentylenetetrazol kindling. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:492–6. doi: 10.1007/BF00169138. [DOI] [PubMed] [Google Scholar]
- 33.Duncan JS. Seizure-induced neuronal injury: Human data. Neurology. 2002;59(Suppl 5):S15–20. doi: 10.1212/wnl.59.9_suppl_5.s15. [DOI] [PubMed] [Google Scholar]
- 34.Franke H, Kittner H. Morphological alterations of neurons and astrocytes and changes in emotional behavior in pentylenetetrazol-kindled rats. Pharmacol Biochem Behav. 2001;70:291–303. doi: 10.1016/s0091-3057(01)00612-8. [DOI] [PubMed] [Google Scholar]
- 35.Pavlova TV, Iakovlev AA, Stepanichev MIu, Mendzheritskiĭ AM, Guliaeva NV. Pentylenetetrazole kindling induces activation of caspase-3 activation in rat brain. Zh Vyssh Nerv Deiat Im I P Pavlova. 2003;53:110–2. [PubMed] [Google Scholar]
- 36.Pohle W, Becker A, Grecksch G, Juhre A, Willenberg A. Piracetam prevents pentylenetetrazole kindling-induced neuronal loss and learning deficits. Seizure. 1997;6:467–74. doi: 10.1016/s1059-1311(97)80022-2. [DOI] [PubMed] [Google Scholar]
- 37.Rocha L, Briones M, Ackermann RF, Anton B, Maidment NT, Evans CJ, et al. Pentylenetetrazole-induced kindling: Early involvement of excitatory and inhibitory systems. Epilepsy Res. 1996;26:105–13. doi: 10.1016/s0920-1211(96)00046-0. [DOI] [PubMed] [Google Scholar]
- 38.Rauca C, Zerbe R, Jantze H. Formation of free hydroxyl radicals after pentylenetetrazol-induced seizure and kindling. Brain Res. 1999;847:347–51. doi: 10.1016/s0006-8993(99)02084-3. [DOI] [PubMed] [Google Scholar]
- 39.Becker A, Tiedge A, Grecksch GA. Diazepam--its effects on the development of pentylenetetrazol kindling, related learning impairments, and neuronal cell loss. Pharmacol Res. 1997;35:27–32. doi: 10.1006/phrs.1996.0116. [DOI] [PubMed] [Google Scholar]
- 40.Sejima H, Ito M, Kishi K, Tsuda H, Shiraishi H. Regional excitatory and inhibitory amino acid concentrations in pentylenetetrazol kindling and kindled rat brain. Brain Dev. 1997;19:171–5. doi: 10.1016/s0387-7604(96)00492-5. [DOI] [PubMed] [Google Scholar]
- 41.Gupta YK, Veerendra Kumar MH, Srivastava AK. Effect of Centella asiatica on pentylenetetrazole-induced kindling, cognition and oxidative stress in rats. Pharmacol, Biochem Behav. 2003;74:579–85. doi: 10.1016/s0091-3057(02)01044-4. [DOI] [PubMed] [Google Scholar]
- 42.Willimore LJ, Rubin JJ. Antiperoxidant pretreatment and iron-induced epileptiform discharges in the rat: EEG and histopathologic studies. Neurology. 1981;31:63–9. doi: 10.1212/wnl.31.1.63. [DOI] [PubMed] [Google Scholar]
- 43.Kabuto H, Yokoi I, Ogawa N. Melatonin inhibits iron-induced epileptic discharges in rats by suppressing peroxidation. Epilepsia. 1998;39:237–43. doi: 10.1111/j.1528-1157.1998.tb01367.x. [DOI] [PubMed] [Google Scholar]
- 44.Gupta YK, Sharma M, editors. New Delhi: Society of Biosciences/jamai Hamdard/Asiatech Publ; 1999. Oxidative stress in neurological disorders. [Google Scholar]
- 45.Bohlen M, Cameron A, Metten P, Crabbe JC, Wahlsten D. Calibration of rotational acceleration for the rotarod test of rodent motor coordination. J Neurosci Methods. 2009;178:10–4. doi: 10.1016/j.jneumeth.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Green AR, Hainsworth AH, Misra A, Debens TA, Jackson DM, Murray TK, et al. The interaction of AR-A008055 and its enantiomers with the GABAA receptor complex and their sedative, muscle relaxant and anticonvulsant activity. Neuropharmacology. 2001;41:167–74. doi: 10.1016/s0028-3908(01)00053-3. [DOI] [PubMed] [Google Scholar]
- 47.Rang HP, Dale MM, Ritter JM, Moore PK. Muscle spasms and centrally acting muscle relaxants. In: Hunter L, editor. Pharmacology. 5th ed. London: Churchill Livingstone; 2003. p. 559. [Google Scholar]
- 48.Waldman HJ. Centrally acting skeletal muscle relaxants and associated drugs. J Pain Symptom Management. 1994;9:434–41. doi: 10.1016/0885-3924(94)90199-6. [DOI] [PubMed] [Google Scholar]
- 49.Kuroiwa M, Kitano Y, Takasuna K, Manabe S, Saito T. Muscle relaxant and neurotoxic activities of intrathecal baclofen in rats. Pharmacol Res. 2009;60:392–6. doi: 10.1016/j.phrs.2009.06.010. [DOI] [PubMed] [Google Scholar]


