Abstract
Objective:
The objective of the present investigation was to prepare colon targeted curcumin microspheres using Eudragit S100 and evaluate the same for in vitro/in vivo properties.
Materials and Methods:
A “O/O solvent evaporation” technique was used in the preparation of microspheres. The influence of various process variables including stirring speed, drug:polymer ratio and percentage of emulsifier on the fabrication were investigated and the formulation was optimized. Prepared microspheres were evaluated for in vitro and in vivo properties. Surface morphology, particle size, percentage drug entrapment, percentage yield, drug polymer interaction, in vitro drug release in simulated gastrointestinal transit conditions and stability were the in vitro parameters investigated. Using an optimized formulation, drug release into the systemic circulation and organ distribution were investigated as in vivo parameters. In vivo parameters were estimated in male albino rats.
Results:
Curcumin microspheres of Eudragit S100 were successfully prepared using o/o solvent evaporation method. Microspheres prepared using 1:2 drug:polymer ratio, with a stirring speed of 1000 rpm, and using 1.0% w/v concentration of emulsifying agent was selected as an optimized formulation. The release studies with optimized formulation demonstrated that aqueous solubility of curcumin was enhanced by 8 times with the formulation. FTIR studies demonstrated no change in drug characteristics upon microsphere fabrication. The enhancement in solubility is thus due to the increase in the surface area of the drug substance and not due to a change of drug to a different physical state. This was further confirmed by scanning electron microsphere pictures. Drug release followed Korsmeyer and Peppas release model. Accelerated stability studies indicated that the drug is stable in the formulation for a period of atleast 14 weeks at room temperature. In vivo studies demonstrated a sustained drug release into the systemic circulation after oral administration of the formulation. Further, colon target was affectively achieved using the optimized formulation. Eudragit microspheres delivered most of their drug load (79.0%) to the colon, whereas with plain drug suspension only 28.0% of the total dose reached the target site.
Conclusion:
This study successfully developed curcumin microspheres that can be used effectively in the treatment of the colon cancer.
Keywords: Colon targeting, curcumin, eudragit S100, microspheres, pH sensitive
Colon is afflicted with various diseases, such as inflammatory bowel diseases, infectious diseases, as well as colon cancer. In all these conditions, colon targeting of the active drug is the need of the hour.[1] This is because after conventional oral administration, drugs generally reach systemic circulation or gets degraded in the gastrointestinal tract before even reaching colon. Colon targeted drug delivery would ensure direct treatment at the disease site, lower dose and offer less systemic side effects.[2] Additionally, colon targeting of biotechnology-based drugs proved to be particularly useful for their systemic delivery. This is particularly true with proteins and peptides.[3] The aim of this study was to develop a well-designed and improved colon-targeted delivery system for curcumin, a drug of interest in colon cancer. A microsphere formulation was selected because it can increase the intrinsic solubility of poorly soluble curcumin, and thereby its target tissue retention can be increased.[4] The objectives of present investigation were to prepare colon-targeted microspheres of curcumin using pH sensitive polymer (Eudragit S100) by solvent evaporation method, to optimize the formulation and to perform in vivo studies in rats using the optimized formulation.
Colon cancer is the third leading cause of cancer death in the USA.[5] More than 66,000 cases of colon cancer are reported to occur every year in India. Current chemotherapeutic agents used for the treatment of cancers are associated with toxicities. Thus, the use of naturally occurring dietary substances, which are nontoxic are preferred over synthetic agents. Curcumin is one such naturally occurring dietary compound, which demonstrated promise to treat colon cancer.[6–9] However, some problems have to be solved before it can come to the practical clinical utility. Several groups all around the world are working on this problem. The works aimed at increasing its aqueous solubility, reducing its stomach and intestinal degradation and achieving-site specific delivery after oral administration.[10–12] A colon-targeted delivery system of curcumin with aforementioned specific advantages is beneficial. The various strategies for colon targeting include coating with pH-dependent polymers, formulation of timed release systems, use of prodrugs, exploitation of carriers that degrade specifically by colonic bacteria, microspheres, nanospheres, and bioadhesive systems.[1,13–16] In the present investigation, the approach selected to deliver curcumin to colon was pH-sensitive microspheres. Here instead of single unit dosage forms multiunit dosage forms, that is, pH-sensitive microspheres was selected. Such a delivery system reduces the intersubject variability in absorption, lower the probability of dose dumping, and offers more reliable release of drugs.[17] Eudragit S100, a pH sensitive enteric polymer, was used to selectively deliver the drug to colon. The polymer does not dissolve in stomach and intestinal pH. However, it dissolves in the colon at pH 7.0 because of the ionization of its carboxyl functional groups and releases the drug in the colon. It thus protects curcumin from degradation in the stomach and intestines. The dosage form can increase the solubility of curcumin in colon environment, sustain the drug release, and protect the drug from abrupt degradation in the colon environment. All of these alterations can enhance the colon tissue levels, especially in the colon cancer cells in the patients and thereby can enhance the utility of the therapy. Previously a nanoparticle formulation for curcumin using the same polymer was developed and was reported to have better effect than free curcumin in colon targeting.[18] Better colon targeting was achieved with this formulation by increased cellular uptake. However, the pilot scale or manufacturing scale preparation of nanoparticles is a difficult task. Also, the data on the toxicity of formulations based on nanotechnology is still being generated. Thus, we chose to develop a microsphere formulation. The manufacturing of microspheres is practical and also this approach can result in enhanced colon targeting of curcumin by increasing its dissolution rate in the colon.
Materials and Methods
Curcumin was procured from Yarrow Enterprises, Mumbai, India. Eudragit S100 was procured as a gift sample from MSN Laboratories Ltd, Hyderabad, India. Span 80, light liquid paraffin, acetone, and potassium dihydrogen phosphate were procured from Qualigen Fine Chemicals, Mumbai, India. Hydrochloric acid and ethyl acetate were procured from Rankhem Chemicals, New Delhi, India. Methanol HPLC grade, disodium hydrogen phosphate, sodium hydroxide pellets were procured from Finar Reagents, Ahmedabad, India. Male albino rats (200–250 g) were purchased from Mahaveera Enterprises, Hyderabad, India. The in vivo study was performed in accordance with the protocol approved by the Institutional Animal Ethics Committee (IAEC).
Preparation of curcumin microspheres
The Eudragit S100 microspheres were prepared by O/O emulsion solvent evaporation method.[19,20] Briefly, drug (100 mg) and the polymer (200 mg) were taken and dissolved in a mixture of 10 mL of acetone and 5 mL of ethanol; 1% Span 80 was taken in 100 mL of light liquid paraffin and kept under propeller stirrer. Solution of drug and polymer mixture was added drop by drop to liquid paraffin containing Span 80 while stirring. Stirring was continued for 3–4 h for complete removal of solvent. After that microspheres were collected by filtration with Whatman filter paper. Collected microspheres were washed with 50 mL of petroleum ether 4–5 times to remove liquid paraffin, dried, and then packed into the final container for further evaluation.
Optimization of some process parameters
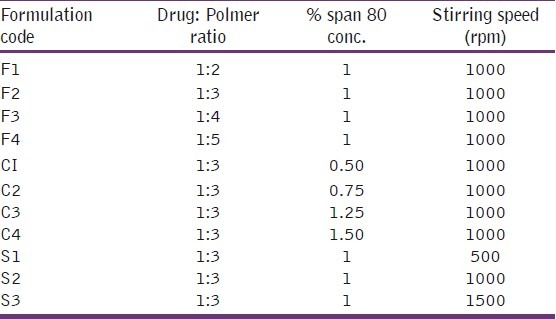
The effects of process variables, such as drug:polymer ratio, stirring rate, and emulsifier concentration on the particle size of the microspheres, drug entrapment efficiency, and drug release were studied. To determine the influence of drug–polymer ratio, Eudragit microspheres were prepared using various drug polymer ratios, that is, 1:2, 1:3, 1:4, and 1:5, while keeping the other two variables constant, that is, stirring speed at 1000 rpm and emulsifier concentration 1.0% w/v. At the end of this process, the final microspheres formed were evaluated. As the surfactant concentration influences the size of microspheres, microspheres were prepared using various emulsifier concentrations, that is, 0.50, 0.75, 1.0, 1.25, 1.50, while keeping the drug:polymer ratio at 1:3 and the stirring speed at 1000 rpm. Microspheres were prepared at various stirring rates, that is, 500, 1000, 1500 rpm, while keeping the drug polymer ratios at 1:3 and the emulsifier concentration at 1.0% w/v, and the final microspheres formed were evaluated.
In vitro characterization of curcumin microspheres
The prepared microspheres were characterized in terms of drug purity, morphology, percentage yield, drug–polymer interaction, particle size measurement (microscopy), entrapment efficiency, in vitro drug release studies, and accelerate stability. Drug purity in the prepared microspheres was assessed using a high-performance liquid chromatographic (HPLC) method described previously for curcumin and curcumin analogs in the formulations.[21] Briefly, a specific amount of the formulation was extracted for the drug, filtered, and this filtrate was injected onto HPLC. The mobile phase consisted of 70:30 methanol:water and the detection wavelength was 425 nm. The percentage yield was calculated using the following formula:
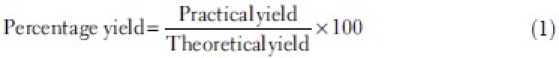
Practical yield = actual weight of prepared microspheres. Theoretical yield = total weight of all non volatile components used for preparation.
Drug–polymer interaction was investigated using fourier transform infrared spectroscopy (FTIR). FTIR of drug, polymer, placebo microspheres, and drug-loaded particles were taken. After fabrication and drying, the morphology of the particles was immediately noted using a microscope. Microspheres were characterized for particle size and particle characteristics (shape factor, Feret diameter). Under the microscope, a cross section of 50 particles was taken and subsequently different diameters of the particles were determined. The eye piece was calibrated prior to the analysis. Particle information was thus obtained. Feret diameter (F) and shape factor (Q) were determined. Feret diameter is defined as the perpendicular distance between parallel lines, tangent to the perimeter at opposite sides of a 2D object in a certain direction. The shape factor is defined as the mean shape of Na measured objects according to the following equation:

Where A is the area and U is the perimeter length of the objects. This shape factor varies between 0 and 1. For a sphere the factor is 1 and for a cube the mean shape factor is 0.668.
The surface morphology of the microspheres was observed under scanning electron microscope. To determine the encapsulation efficiency, 50 mg microspheres were accurately weighed and triturated with 10 mL of methanol and kept for 12 h. After filtration appropriate dilutions were made with methanol and the absorbance was measured with UV–Vis spectrophotometer (Shimadzu UV 1800) at 425 nm. The entrapment efficiency was calculated using the following formula:
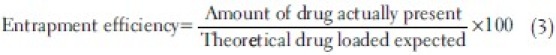
The release of curcumin from the microspheres was investigated using the USP rotating paddle dissolution apparatus (Electrolab, Mumbai, India) at 100 rpm and 37°C±0.5°C. To achieve simulated gastrointestinal transit conditions the pH of the dissolution medium was changed at various time intervals. To prevent photostability and simulate in vivo conditions, the release studies were conducted in dark. An accurately weighed amount (equivalent to 15 mg of drug content) of microspheres was added to 500 mL of dissolution medium. First 2 h the pH of the dissolution medium was maintained at 1.2 with 0.1 N HCl. After 2 h the pH of the medium was adjusted to 4.5 by adding 1.7 g of KH2PO4 and 2.225 g of Na2HPO4.2H2O and sufficient quantity of 1.0 M NaOH. The release study was carried out at this pH for 2 h. Then the pH of the dissolution medium was adjusted to 7.0 by adding 1.0 M NaOH and study was continued for 24 h.[20,22] Five milliliters of samples were withdrawn from the dissolution medium at various time intervals, that is, at 1, 2, 3, 4, 5, 6, 8, 10, 12, and 24 h and replaced with fresh dissolution medium. The samples were filtered with a microfilter. The samples were then analyzed by UV spectrophotometer. All dissolution studies were performed in triplicate. The effects of drug:polymer ratio on in vitro drug release of Eudragit microspheres were evaluated. Similarly this study was carried out with 15 mg of plain curcumin drug under similar conditions. As curcumin is known to be notorious for its lack of stability, a comparison of the release of the drug from plain curcumin and the release from microspheres is justified. Also all the release media contained antioxidants that help prevent further degradation of curcumin during the release studies. To analyze the mechanism of the drug release rate kinetics of the dosage form, the obtained data were fitted into zero-order, first-order, Higuchi, and Korsmeyer–Peppas release models. Stability studies were performed according to previously reported method.[20] The formulations were stored in amber colored glass bottles at 4°C±1°C, room temperature at 25°C±1°C, and in hot air oven at 40°C±1°C for a period of 1 month. The samples were analyzed for drug content every 10 days by spectrophotometer.
In vivo characterization
From the data of in vitro release study suitable microsphere formulation was selected to carry out in vivo studies. After procurement of albino rats, these were acclimatized for 10 days before the conducting the experiment.
Pharmacokinetic study
Animals were divided into 3 groups, each having 3 male rats weighing approximately 200–250 g. Rats were fasted for 24 h before the studies, and during the course of the studies, water was available. Group I received normal saline at a dose of 1 mL/kg by oral route, group II received curcumin suspension in water at a dose of 100 mg/kg. Group III received optimized microsphere formulation suspension in water by oral route at a dose equivalent to 100 mg/kg of curcumin drug.
Blood samples (0.5 mL) from the experimental rats were collected by retro-orbital plexus technique into a series of Eppendroff microcentrifuge tubes containing 0.2 mL of sodium citrate solution. The blood samples were collected at specific intervals 0.5, 1, 2, 3, 4, 6, 8, and 24 h. The collected blood samples were centrifuged at a speed of 3000 rpm for 10 min and plasma was separated into other microcentrifuge tube by using micropipette and stored in deep freeze. The drug was extracted from the plasma by adding 500 μL of extraction reagent (95% ethyl acetate and 5% methanol), and vortexed at high speed on a cyclo mixer for 30 s, then all tubes were centrifuged at high speed for 5 min. The organic phase was separated and collected into another microcentrifuge tube and allowed to dryness.[23] These dried samples were reconstituted with 200 μL of mobile phase methanol:phosphate-citrate buffer pH 3.0 (72:28) and analyzed at 262 nm wavelength using HPLC (Cyberlabs, Millbury, USA, and column size 4.6 × 250 mm, 5 μm). The retention time and peak areas were noted and the concentration of curcumin in samples was calculated based on the calibration curve generated in plasma. Data obtained through HPLC analysis is further used to generate pharmacokinetic parameters. The parameters, such as Ka, ke, Vd, elimination t1/2, absorption t1/2, Tmax, Cmax, clearance, and AUC were calculated.
Gastrointestinal distribution study
Rats were fasted for 24 h before the studies, and during the course of the studies, water was available. Animals were divided into 3 groups, each having 8 male rats weighing approximately 200–250 g. Group I normal saline at a dose of 1 mL/kg by oral route. Group II received curcumin suspension in water at a dose of 100 mg/kg. Group III received optimized microsphere formulation by oral route at a dose equivalent to 100 mg/kg of curcumin drug. The formulations were orally administered in suspension form followed by sufficient volume of drinking water. After 2, 4, 6, and 8 h, the rats were killed by deep ether anesthesia. Immediately after death, the entire gastrointestinal (GI) tract was removed, and blood vessels and fatty tissues were separated. The GI tract was segmented into stomach, small intestine, and colon. The contents of the lumen were removed by gentle pressure, and organs were washed with normal saline to remove the remaining luminal contents.[24] The organs were cut into small pieces and homogenized along with a small amount of phosphate buffer saline. One milliliter of ethyl acetate was added to homogenate and kept for 30 min. After that contents were centrifuged and the supernatant liquid was separated, evaporated to dryness, and reconstituted with mobile phase. Then the concentrations in the tissues were analyzed by HPLC and the drug content in different parts of GI tract at different time intervals was calculated.
Results and Discussion
Colon targeting of curcumin to treat local cancers has been previously investigated by Suresh Kumar et al[25] and Dandekar et al.[18] The first study investigated curcumin pellets for colon delivery while the second investigated colon-targeted nanoparticles. The aim of both the studies was to achieve targeted delivery of this drug to colon. Thus, the utilization of these delivery systems in colon cancer has been investigated. Although both the studies demonstrated promising results, either there are lacunae in their studies or some disadvantages existed with the technologies developed. In case of the curcumin pellet, although the drug reach may be achieved, the dissolution rate of the drug may not be surpassed. Poor dissolution is one of the main disadvantages that lead to low target tissue levels with curcumin. For effective therapy, higher tissue drug levels should be achieved. In the second study, where a nanoparticle formulation has been developed, the main drawback is the technological limitation. The scale-up of nanoparticles is not well-known and also toxicity of nanoparticles is not yet thoroughly investigated. The same group has also investigated the toxicity of this particular nanoformulation to achieve the colon targeting of the drug. It has been demonstrated that the formulation is not toxic. However, the manufacturability and scale-up issues are not addressed. With the existing literature, it can clearly be mentioned that the technological hurdles faced to pragmatically produce this formulation are enormous. Thus, we choose to develop a microparticle formulation and evaluate the same. Manufacturability and scale-up of microspheres is well known to the pharmaceutical industry.
Curcumin microspheres were successfully prepared using O/O emulsion solvent evaporation method. The curcumin microspheres were fabricated by changing various parameters, such as percentage of span 80 concentration (0.50%–1.50%), stirring speed (500, 1000, 1500 rpm), and drug:polymer ratio (1:2, 1:3, 1:4, 1:5) as shown in Table 1. As assessed by HPLC method, the drug remained pure in the formulation at the end of fabrication.
Table 1.
Formulation codes
To prepare pH-dependent microspheres, O/O emulsion solvent evaporation technique was used as it yields consistently uniform-sized, smooth and spherical microspheres.[20,26] A mixture of acetone and ethanol was used as it is an effective solvent for the polymer and drug. At the end of fabrication, FTIR analysis revealed that there was no interaction between the drug and the polymer, thus these polymers can be conveniently used in further development of colon-targeted curcumin microspheres. Further FTIR studies indicated that the drug crystallinity did not change upon fabrication. There was no formation of amorphous drug upon fabrication. Bands due to carbonyl bond stretches were studied to infer the physical state of the drug. FTIR data in the region (1800-1500 cm-1) were compared for pure curcumin and curcumin encapsulated in the microspheres [Figure 1].[27] As expected, two main characteristic C=O bands [Figure 1b] were observed for crystalline curcumin at 1653 and 1590 cm–1, assigned to the aliphatic and aromatic carbonyl stretching bands, respectively. Microsphere formulation also yielded peaks at the same wavelengths [Figure 1a]. Virgin Eudragit and the placebo microparticles did not show any peak at these wavelengths. Crystallinity was further confirmed with SEM pictures. Drug crystals appeared on the surface of the microspheres [Figure 2]. Petroleum ether was used to wash the microspheres as it removes liquid paraffin without affecting the integrity of the microspheres. The morphology of the particles was noted immediately after the fabrication. The particles were spherical to approximately spherical. The Feret diameters of the F1, F2, F3, and F4 microspheres were 76, 86, 122, and 1148 μm, respectively. The shape factor was also determined and this was 0.952, 0.975, 0.935, and 0.931 for F1, F2, F3, and F4 microspheres, respectively. The shape factor suggests that all the microparticles are spherical. The prepared microspheres were spherical as observed in SEM photographs [Figure 2].
Figure 1.
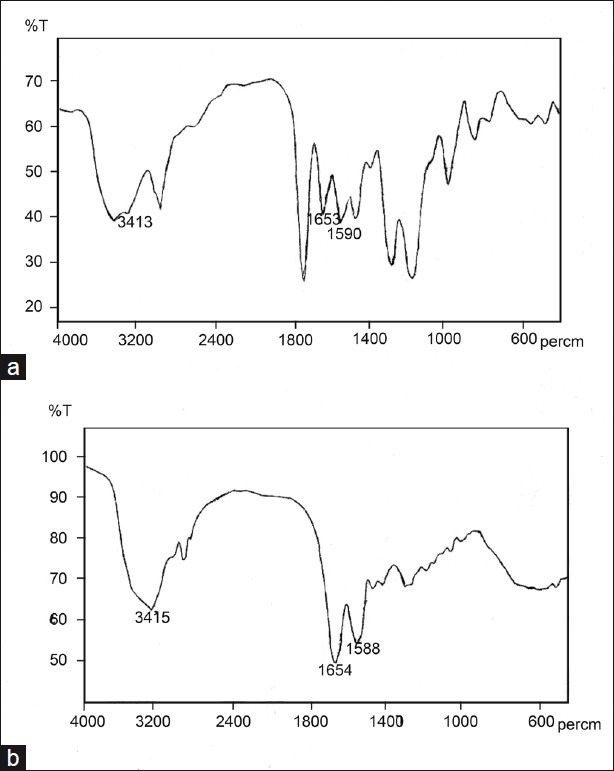
FTIR spectrum of (a) Curcumin microspheres (b) Curcumin
Figure 2.

Scanning electron microscopic (SEM) pictures of Curcumin microspheres
Microspheres were prepared using various drug:polymer ratios, that is, 1:2, 1:3, 1:4, and 1:5, while keeping the other two variables, that is, stirring speed of 1000 rpm and span 80 concentration 1.0% w/v constant [Table 2]. The mean diameter of the Eudragit S100 microspheres at increasing polymer concentrations from 1:2 to 1:5 increased from 76.12 to 147.9 μm. This increase in particle size of the microspheres may be due to the fact that the viscosity increases with increasing polymer concentrations, which resulted in larger emulsion droplets and finally it results in greater microsphere size. Similarly microspheres were prepared at various stirring rates, that is, 500, 1000, and 1500 rpm, while keeping the drug:polymer ratio at 1:3 and the emulsifier concentration at 1.0% w/v. The mean diameters of microspheres prepared using various agitation speeds, that is, 500, 1000, and 1500 rpm, were 93.83, 86.15, and 41.79 μm, respectively. High stirring rate provides the shearing force needed to reduce the size of emulsion droplet size, resulting in decrease in size of microspheres. The stirring speed of 1000 rpm was found to be optimum as the drug loading efficiency was 70.85% at this speed and also the higher stirring speed resulted in irregularly shaped microspheres. Microspheres also were prepared using various emulsifier concentrations, while keeping the drug:polymer ratio at 1:3 and the stirring speed at 1000 rpm. At Span 80 concentration 0.50%, the particles were irregular in shape as this concentration is not enough to stabilize the emulsion droplets. But as the concentration of Span 80 increases from 0.75% to 1.50%, the average particle size was found to be decreased from 91.62 to 42.79 μm. As emulsifier is a surface active agent, an increase in its concentration will allow to stabilize a greater surface area, thus leading to smaller particle size. The results indicate that the best drug percentage entrapment efficiency was observed at a drug:polymer ratio of 1:3, a stirring rate of 1000 rpm, and 1.0% w/v emulsifier concentration [Tables 3 and 4].
Table 2.
Effect of drug:polymer ratio on particle size, % yield, and entrapment efficiency of curcumin microspheres

Table 3.
Effect of stirring speed on particle size, % yield, and entrapment efficiency of curcumin microspheres

Table 4.
Effect of Span 80 concentration on particle size, % yield, and entrapment efficiency of curcumin microspheres

Release studies demonstrated interesting results. Based on the different transit times present from stomach to colon, the release profile was carried out with different pH conditions similar to in vivo conditions. The cumulative percent drug release curve [Figure 3] of the Eudragit S100 microspheres shows that the drug release was less than 10% up to 4 h (ie, at pH 1.2 for first 2 h and at pH 4.5 for next 2 h) and the drug release rate was increased when the pH of the medium was adjusted to 7.0. The release rate of curcumin from microspheres decreased as the polymer concentration increased. This may be due to the fact that the higher polymer content resulted in larger particle size that results in reduced surface area available for drug release. The release profile of curcumin microspheres was compared with plain curcumin. It was observed that the cumulative percentage release with plain drug was 12% within 24 h, but with microsphere formulation it was 96%. This suggests that the solubility of poorly soluble curcumin was enhanced with the present microsphere formulation. Furthermore, it has been confirmed by the FTIR studies that the drug did not change its crystallinity after being encapsulated in the microsphere and thus the enhanced dissolution is not due to alterations in the solid-state of the curcumin. Based on this in vitro release study F1 formulation (1:2 drug:polymer ratio) was selected to carry out in vivo studies as the drug release was 92.52% within 12 h (which was highest than other formulations), as it was reported that the normal transit time along the entire gut of fasted rat was below 8 h.[28] Drug release mechanisms were determined by fitting in vitro drug release data to various kinetic models [Table 5]. By comparing regression values (R2) for zero-order, first-order, Higuchi model, and Korsmeyer–Peppas model, it is concluded that all formulations gave good fit to the Korsmeyer–Peppas model. The diffusion exponent (n) values were greater than 1, so the drug release follows super case II transport. This type of modeling drug release will help analyze the release when the release mechanism is not well known or when the release involves more than one type of phenomenon.[29] The stability studies were performed for 30 days and the formulations were found to be stable in varying temperatures (data not shown). The results were further verified with one-way ANOVA method, the accelerated stability test data were found significant for F (3.323) at 5% level of significance (P < 0.05).
Figure 3.
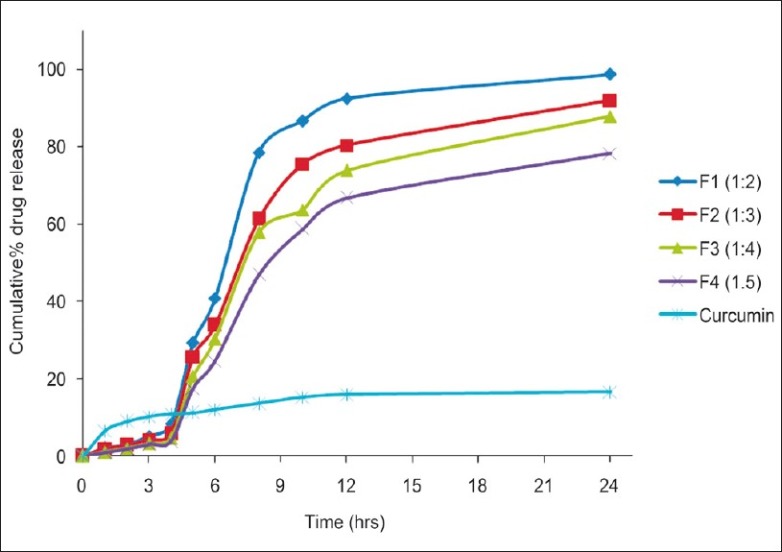
Percent cumulative in vitro drug release profile of curcumin from Eudragit microspheres containing different drug-polymer ratios and plain curcumin drug in simulated gastrointestinal transit conditions i.e., at pH 1.2 for first 2 h, pH 4.5 for 2-4 h and at pH 7.0 for 4- 24 h
Table 5.
Model applied and the R2 values for different microsphere formulations
The HPLC method for curcumin was successfully developed. The retention time of curcumin was 11 min [Figure 4]. From the plasma profile it was shown that the maximum concentration (Cmax) values for plain curcumin and curcumin microspheres were 1.016 and 1.820 μg/mL, respectively, and time to reach maximum concentration (Tmax) values were 3 and 8 h, respectively [Figure 5]. The Cmax value with curcumin microsphere formulation was more compared to plain curcumin and this may be due to its increased solubility. The Tmax was 8 h for curcumin microspheres while it was 3 h for plain curcumin. It may be due to the fact that the drug release from microspheres occurs after reaching the colon. On the other hand, drug release and absorption from plain curcumin occurs from stomach, intestines, and probably colon also. The pharmacokinetic parameters were given in Table 6. Gastrointestinal distribution study of optimized formulations F1 was carried out in albino rats to establish its colon-targeting potential. The results showed that maximum concentration of curcumin, that is, 65.41% was observed after 2 h in stomach after oral administration of plain curcumin and little amount reached the small intestine, and no drug was found in colon [Figure 6]. Only 28.93% of total administered drug reached the colon after 8 h. The curcumin-loaded microspheres were found to be intact in the upper part of the GI tract. After 8 h of administration of curcumin microspheres, the maximum percentage of drug (78.97%) was observed in the colon, and negligible amount of drug was found in the stomach and a little amount in small intestine. These in vivo studies revealed that the prepared curcumin-loaded Eudragit S100 microspheres were effective to deliver curcumin directly to colon in high concentrations, which would improve the prospects for a successful treatment outcome. Thus, in this study a curcumin microsphere formulation useful in the treatment of colon cancer has been developed.
Figure 4.
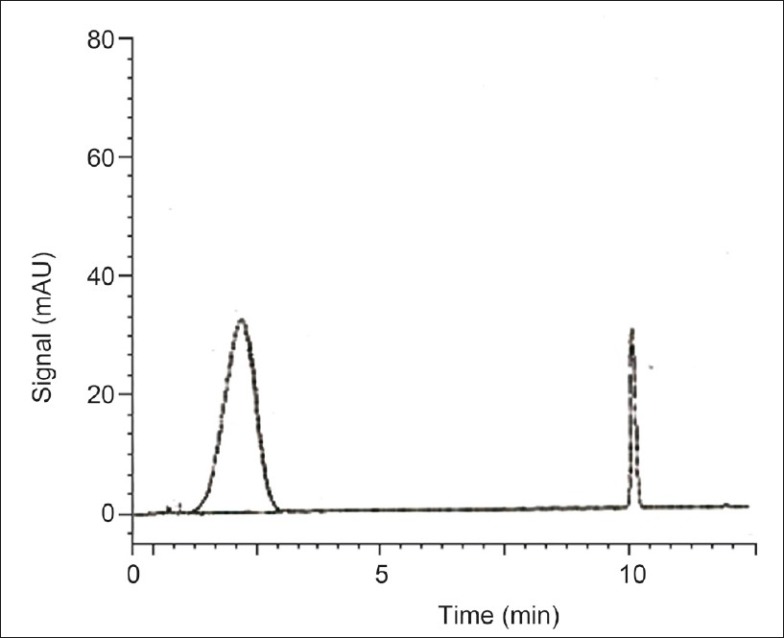
HPLC chromatogram of curcumin in Plasma
Figure 5.
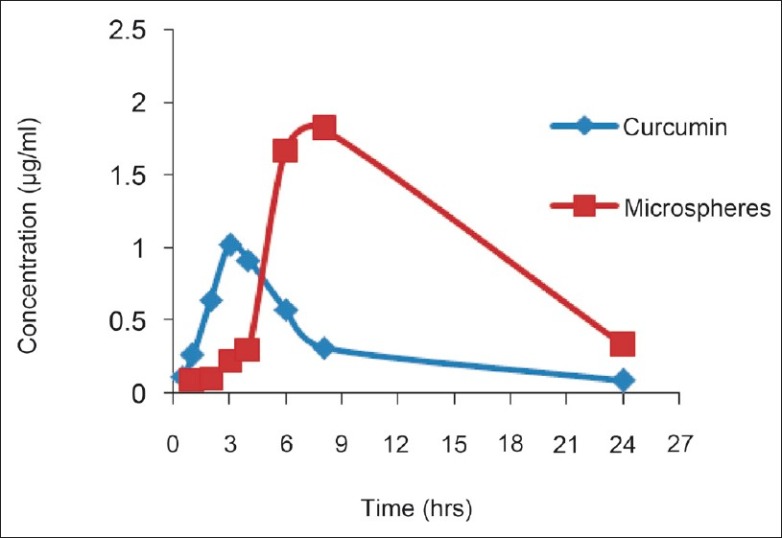
Plasma drug concentration profile after oral administration of curcumin microspheres and plain curcumin
Table 6.
Pharmacokinetic parameters
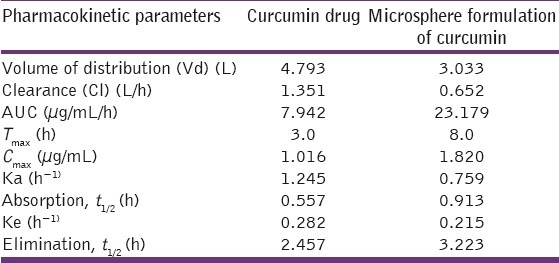
Figure 6.
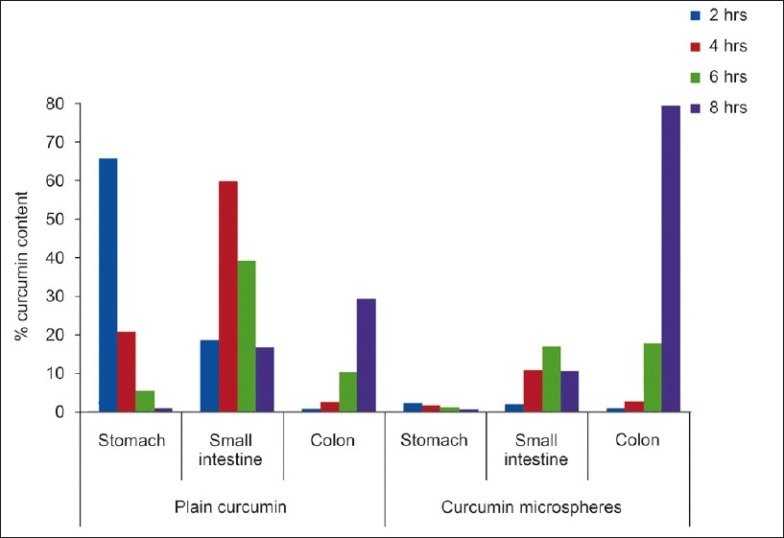
Curcumin content in isolated organs of albino rats after oral administration of curcumin loaded Eudragit microspheres and plain curcumin
Acknowledgments
The authors of the manuscript would acknowledge the management of Mother Teresa College of Pharmacy for providing partial support for the work. One of the Authors, Dr. A. V. Jithan would like to acknowledge the Department of Science and Technology (DST), Government of India. This project is partly funded under a SERC-DST Fast Track Proposal for Young Scientists awarded to Dr. A. V. Jithan.
Footnotes
Source of Support: This project is partly funded under a SERC-DST Fast Track Proposal for Young Scientists awarded to Dr. A. V. Jithan
Conflict of Interest: None declared.
References
- 1.Krishnaiah YSR, Satyanarayana S. Advances in controlled and novel drug delivery: Colon specific drug delivery systems. New Delhi: CBS Publishers and Distributors; 2001. pp. 89–119. [Google Scholar]
- 2.Kinget R, Kalala W, Vervoort L, van den Mooter G. Colonic drug targeting. J Drug Target. 1998;6:129–49. doi: 10.3109/10611869808997888. [DOI] [PubMed] [Google Scholar]
- 3.Jain D, Panda AK, Majumdar DK. Eudragit S100 entrapped insulin microspheres for oral delivery. AAPS PharmSciTech. 2005;6:E100–7. doi: 10.1208/pt060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woo JS, Piao MG, Li DX, Ryu D, Choi JY, Kim J, et al. Development of cyclosporin A-loaded hyaluronic microsphere with enhanced oral bioavailability. Int J Pharm. 2007;345:134–41. doi: 10.1016/j.ijpharm.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 5.Pisani P, Bray F, Parkin DM. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int J Cancer. 2002;97:72–81. doi: 10.1002/ijc.1571. [DOI] [PubMed] [Google Scholar]
- 6.Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170–81. doi: 10.1016/j.canlet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Chauhan DP. Chemotherapeutic potential of curcumin for colorectal cancer. Curr Pharm Des. 2002;8:1695–706. doi: 10.2174/1381612023394016. [DOI] [PubMed] [Google Scholar]
- 8.Chen A, Xu J, Johnson AC. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene. 2006;25:278–87. doi: 10.1038/sj.onc.1209019. [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–98. [PubMed] [Google Scholar]
- 10.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 11.Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, Williams ML, et al. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev. 2002;11:105–11. [PubMed] [Google Scholar]
- 12.Sharma RA, Steward WP, Gescher AJ. Pharmacokinetics and pharmacodynamics of curcumin. Adv Exp Med Biol. 2007;595:453–70. doi: 10.1007/978-0-387-46401-5_20. [DOI] [PubMed] [Google Scholar]
- 13.Chourasia MK, Jain SK. Pharmaceutical approaches to colon targeted drug delivery systems. J Pharm Pharm Sci. 2003;6:33–66. [PubMed] [Google Scholar]
- 14.Sarasija S, Hota A. Colon-specific drug delivery systems. Indian J Pharm Sci. 2000;62:1–8. [Google Scholar]
- 15.Vijaya R, Prabhakaran L, Purushothaman M. Colon targeted drug delivery system- an overview. Pharma infonet. 2010;8 [Google Scholar]
- 16.Vyas SP, Khar RK. Controlled drug delivery: concepts and advances. New Delhi: Vallabh Prakashan; 2005. Systems for colon specific delivery; pp. 218–53. [Google Scholar]
- 17.Asghar LF, Chandran S. Multiparticulate formulation approach to colon specific drug delivery: Current perspectives. J Pharm Pharm Sci. 2006;9:327–38. [PubMed] [Google Scholar]
- 18.Prajakta D, Ratnesh J, Chandan K, Suresh S, Grace S, Meera V, et al. Curcumin loaded pH-sensitive nanoparticles for the treatment of colon cancer. J Biomed Nanotechnol. 2009;5:445–55. doi: 10.1166/jbn.2009.1038. [DOI] [PubMed] [Google Scholar]
- 19.Basu SK, Adhiyaman R. Preparation and characterization of nitrendipine loaded Eudragit RL 100 microspheres prepared by an emulsion–solvent evaporation method. Trop J Pharm Res. 2008;7:1033–41. [Google Scholar]
- 20.Sunil KJ, Gopal R, Saraf DK, Agrawal GP. The preparation and evaluation of albendazole microspheres for colonic delivery. Pharm Tech. 2004;28:66–71. [Google Scholar]
- 21.Gogu PK, Jithan AV. Preparation and in vitro/in vivo characterization of spray dried microsphere formulation encapsulating 4-chlorocurcumin. Indian J Pharm Sci. 2010;72:346–52. doi: 10.4103/0250-474X.70481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paharia A, Yadav AK, Rai G, Jain SK, Pancholi SS, Agrawal GP. Eudragit-coated pectin microspheres of 5-fluorouracil for colon targeting. AAPS PharmSciTech. 2007;8:12. doi: 10.1208/pt0801012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heath DD, Pruitt MA, Brenner DE, Rock CL. Curcumin in plasma and urine: quantitation by high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;783:287–95. doi: 10.1016/s1570-0232(02)00714-6. [DOI] [PubMed] [Google Scholar]
- 24.Rahman Z, Kohli K, Zhang SQ, Khar RK, Ali M, Charoo NA, et al. In vivo evaluation in rats of colon-specific microspheres containing 5-fluorouracil. J Pharm Pharmacol. 2008;60:615–23. doi: 10.1211/jpp.60.5.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suresh Kumar R, Munikumar , Ganesh GN, Jawahar J, Nagasamyvenkatesh D, Senthil V, et al. Formulation and evaluation of pectin-hydroxypropylmethylcellulose coated curcumin pellets for colon delivery. Asian J Pharm. 2009;3:138–42. [Google Scholar]
- 26.Dandagi PM, Mastiholimath VS, Gadad AP, Kulkarni AR, Konnur BK. pH-sensitive mebeverine microspheres for colon delivery. Indian J Pharm Sci. 2009;71:464–8. doi: 10.4103/0250-474X.57303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanphui P, Rajesh Goud N, Khandavalli UB, Bhanoth S, Nangia A. United Kingdom: The Royal Society of Chemistry; 2011. New polymorphs of curcumin.Electronic Supplementary Material (ESI) for Chemical Communications; pp. S1–12. [DOI] [PubMed] [Google Scholar]
- 28.Cifty K, Groves MJ. Delivery of antitumor compounds, to the rat colon: in vitro and in vivo evaluation. Int J Pharm. 1996;145:157–64. [Google Scholar]
- 29.Jose S, Dhanya K, Cinu TA, Aleykutty NA. Multiparticulate system for colon targeted delivery of ondansetron. Indian J Pharm Sci. 2010;72:58–64. doi: 10.4103/0250-474X.62237. [DOI] [PMC free article] [PubMed] [Google Scholar]


