Abstract
Apoptosis is an important physiological process. Normally, a healthy cell maintains a delicate balance between pro- and anti-apoptotic factors, allowing it to live and proliferate. It is thus not surprising that disturbance of this delicate balance may result in disease. It is a well known fact that apoptosis also contributes to several acquired forms of hearing impairment. Noise-induced hearing loss is the result of prolonged exposure to excessive noise, triggering apoptosis in terminally differentiated sensory hair cells. Moreover, hearing loss caused by the use of therapeutic drugs such as aminoglycoside antibiotics and cisplatin potentially may result in the activation of apoptosis in sensory hair cells leading to hearing loss due to the “ototoxicity” of the drugs. Finally, apoptosis is a key contributor to the development of presbycusis, age-related hearing loss. Recently, several mutations in apoptosis genes were identified as the cause of monogenic hearing impairment. These genes are TJP2, DFNA5 and MSRB3. This implies that apoptosis not only contributes to the pathology of acquired forms of hearing impairment, but also to genetic hearing impairment as well. We believe that these genes constitute a new functional class within the hearing loss field. Here, the contribution of apoptosis in the pathology of both acquired and genetic hearing impairment is reviewed.
Keywords: noise-induced hearing loss, presbycusis, monogenic hearing loss, ototoxicity, apoptosis
1. Introduction
The hearing apparatus is a complex system that relies on the integrated functioning of many tissues and cell types in the inner ear. Therefore, it is not surprising that mutations in a large variety of different genes have been described as the cause of hearing impairment (HI). It is estimated that approximately 1 in 1000 children are born deaf and that in almost half of these cases the hearing loss is attributable to genetic factors (Marazita et al., 1993). Not only is congenital deafness a common problem, but acquired deafness, the loss or impairment of auditory function during a lifetime, is as well. Presbycusis, the decline of hearing ability with increasing age, is a prominent example. Above the age of 65, approximately 40 % of the elderly show elevated hearing threshold levels (Ries, 1994), making age related hearing impaired (ARHI) the most common sensory disorder. Other important factors include noise-induced HI and ototoxicity, hearing loss associated with clinical drug treatment.
In essence, two major routes lead to cellular death: apoptosis and necrosis. Apoptosis is an active, energy requiring process that is initiated by specific pathways in the cell (Kerr et al., 1972), while necrosis is a passive one requiring no energy and results in the rupture of the cell body. During necrosis, the cellular content is spilled onto adjacent cells thereby possibly triggering inflammatory responses. The correct differentiation between these two death routes is important as different pathways are triggered during both processes. Necrosis and apoptosis are easily distinguishable through differentially activated biochemical processes. However, the gold standard is still morphological analysis. For a pictoral representation of the molecular pathways activated during apoptosis, see figure 1.
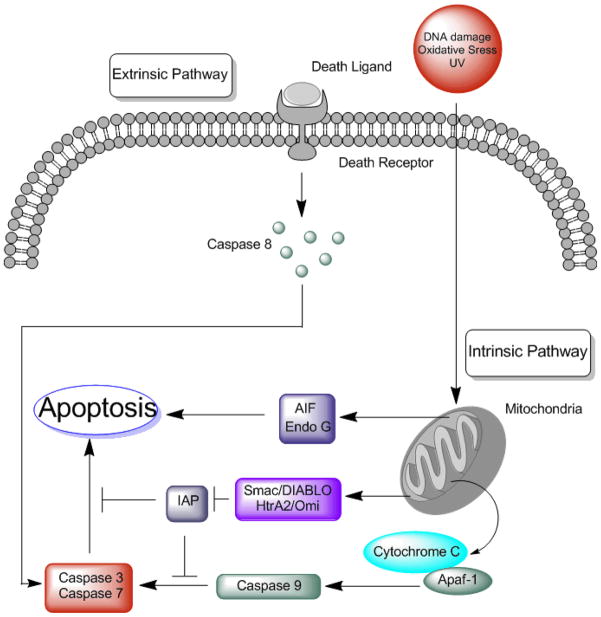
Figure 1.
Apoptosis can be induced through at least two pathways. The extrinsic pathway is activated through death receptors that reside on the plasma membrane. Binding of a death ligand to its receptor causes activation of caspase 8 that is then able to activate effector caspases such as caspase 3 and caspase 7. The intrinsic pathway on the other hand is activated from the inside of the cell. Stimuli such as DNA damage, oxidative stress and irradiation cause mitochondrial damage resulting in permeability changes of the outer mitochondrial membrane. This change causes the release of several pro apoptotic factors into the cytosol that trigger and amplify the apoptotic cascade. These proteins are then able to induce caspase-dependent and caspase-independent pathways. Release of endonuclease EndoG and apoptosis inducing factor (AIF) induce a caspase-independent apoptosis. On the other hand, release of cytochrome c proteins leads to oligomerization of apoptosis protease activating factor 1 (apaf-1) causing the formation of the so called apoptosome. This structure activates the initiator caspase 9, which in turn activates effector caspases such as caspase 3 and caspase 7. Meanwhile, Smac/DIABLO and HtrA2/Omi complexes counteract the inhibitory effects of inhibitor of apoptosis molecules (IAP) thereby enhancing activation of the apoptotic cascade.
The pathology of hearing loss has been studied extensively. Recent findings have led us to conclude that deregulation of homeostatic and apoptotic programs is an important contributing factor to the genesis of HI. Apoptosis is one of the death pathways a cell is able to follow should it encounter stress situations. In a normal functioning cell, a delicate balance of apoptosis-inducing and -inhibiting factors exists, making sure the cell is able to live and proliferate. However, in stress situations this balance is disturbed and through an internal messaging system the cell may enter the apoptotic death program. The proper functioning of the apoptotic circuitry is very critical in the development and maintenance of a multicellular organism. It is no surprise that defects in the regulation of apoptotic pathways may lead to disease.
The involvement of apoptosis in the development of acquired hearing loss has been long studied. Recently however, a new link between apoptosis and monogenic forms of hearing loss has emerged. Here, we will elaborate on the involvement of apoptosis in ARHI, noise-induced hearing loss, ototoxicity and we will discuss the recent link with monogenic forms of hearing loss.
2. Apoptosis in noise-induced hearing loss
Noise-induced hearing loss is recognized as a major cause of sensorineural hearing loss. Worldwide, approximately 16% of all hearing impairments are due to continued exposure to loud noises (Nelson et al., 2005) and it is expected that this estimate will rise in the next years. In addition to the traditional risks of factory work and the military, the popularity of clubs and discos and the increasing use of MP3 players have brought prolonged exposure to hazardous noises into the recreational area. Understanding the molecular pathologies that underlie this hearing loss is thus very important in order to design rational preventive strategies.
A key observation about the development of noise-induced hearing loss came from the study of animal models. Whenever animals were subjected to continuous loud noises of sufficient sound intensity (>100 dB), they exhibited permanent hearing loss accompanied by large lesions in their cochleae. These lesions were characterized by morphologically abnormal cells most prominently among the terminally differentiated sensory hair cells. In particular, outer hair cells seemed to be very sensitive to noise trauma.
Cochlear damage following noise exposure occurs through two major routes. The first one is direct mechanical damage, which leads to both hair cell loss through mechanical disruption of the stereocilia and direct damage to supporting and sensory cells (Slepecky, 1986). The other route involves biochemical pathways leading to cell death through either apoptosis or necrosis.
The first studies evaluating the type of cochlear cell death following intense noise exposure date back to the late 1980’s. Swollen outer hair cells were observed in cochleae of animals subjected to very loud noises (120 dB). As this is a hallmark of necrosis, it was assumed that necrosis was the major cause of cell death (Saunders et al., 1985). However, later studies revealed that next to necrosis, apoptosis is also a key mediator of noise-induced hearing loss. Nuclear condensations were observed in cochlear cells of guinea pigs, chinchillas and rats following noise exposure (Hu et al., 2002b; Hu et al., 2000; Hu et al., 2009; Niu et al., 2003; Wang et al., 2007). These nuclear changes suggested that the cells were dying from apoptosis. TUNEL labeling experiments confirmed these initial experiments and indicated that apoptosis is probably the more important cell death route in sensory hair cells in response to noise exposure (Pirvola et al., 2000). These results were further strengthened by the observation that several biochemical apoptotic markers are activated in outer hair cells of noise-insulted cochleae (Hu et al., 2002a), such as the caspase cascade (Han et al., 2006; Nicotera et al., 2003), a key mediator of apoptosis. Moreover, several members of apoptosis-inducing gene families were activated in the immediate hours following the insult. These include the tumor necrosis factor receptor family, the B-cell leukemia/lymphoma 2 family (BCL2), the tumor necrosis receptor-associated factor and the inhibitor of the apoptosis protein family (Hu et al., 2009) and the BCL-2-associated death (BAD) promoter (Vicente-Torres et al., 2006).
Two important factors seem to determine which cell death pathway is activated following intense noise exposure. The first is sound intensity level. Noises of 105 dB seem to favor necrosis, while much louder noises (120 dB) seem to favor apoptosis (Hu et al., 2000). Another factor is the time lapse between noise exposure and morphological analysis. Outer hair cells immediately start dying during the acoustic insult and continue to do so until at least 30 days thereafter (Hamernik et al., 1984; Yang et al., 2004). Apoptosis is the primary contributor to the expansion of the lesion immediately after the insult (Hu et al., 2002b). However, 4 days after the insult, apoptotic events start to diminish and by day 30, necrosis and apoptosis contribute equally to the still ongoing cellular death (Yang et al., 2004).
The pathways that lead to apoptosis in sensory hair cells have been partly elucidated (Figure 2). Generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) is involved in the sequence of events following noise exposure (Ohlemiller et al., 1999); (Henderson et al., 2006). Indeed, ROS can be demonstrated in the cochlea long after noise exposure has been terminated (Yamashita et al., 2004a), possibly accounting for the delayed and continued damage that can be observed morphologically. Such oxidative stress can damage mitochondria which, in turn, release pro-apoptotic factors (Yamashita et al., 2004a). Indeed, apoptosis inducing factor (AIF) and endoG are released by mitochondria into the cytosol of cochlear cells following noise exposure (Yamashita et al., 2004b). These molecules are then translocated into the nucleus and trigger an apoptotic response. Several studies specifically identified the JNK signaling pathway as a mediator of apoptosis in outer hair cells (Wang et al., 2007). JNK is part of the MAP kinase pathway and is able to induce apoptosis. This part of the MAP kinase pathway is activated in response to several cellular stresses such as osmotic shock, heat shock, inflammatory cytokines, UV radiation and oxidative stress. Interestingly, ROS formation seems to be an important activator of the JNK pathway (Lo et al., 1996).
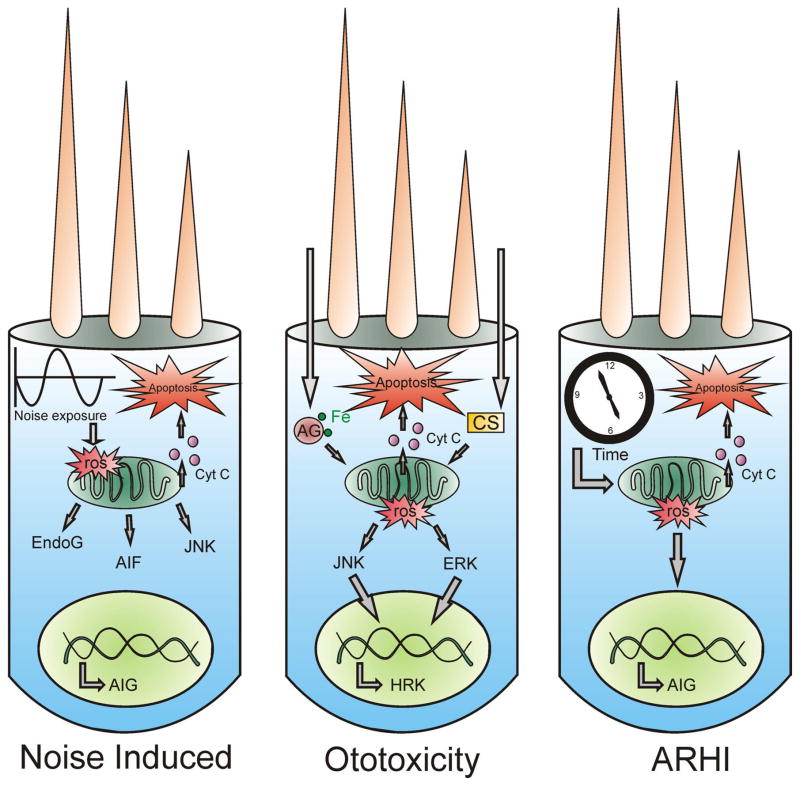
Figure 2.
Outer hair cells are vulnerable for different apoptosis-inducing stimuli. Depicted here are three outer hair cells that encounter various insults and as a result activate the apoptotic pathway. The left cell represents a noise-insulted cell. Noise exposure leads to an elevated generation of ROS, which causes mitochondria to release the pro-apoptotic factors EndoG and AIF. Moreover, ROS production is able to activate the JNK kinase system, leading to the transcription of several apoptosis inducing genes (AIG) in the nucleus. This causes the mitochondria to release cytochrome c and ultimately leads to apoptosis. In the case of ototoxicity, either aminoglycosides (AG) or cisplatin (CS) cause a rise in ROS formation. These ROS activate the JNK or ERK MAP kinase cascade, leading to transcription of AIG such as HRK (harakiri), which ultimately leads to activation of the apoptotic cell death program. The right cell depicts an ageing outer hair cell. The ageing theory predicts that in the course of time ROS concentration rises either due to depletion of antioxidant defenders or due to an elevated ROS formation. This causes mitochondrial damage and subsequent release of pro-apoptotic factors that finally induce apoptosis.
On the basis of these mechanistic studies, several small molecules and drugs with protective roles against noise-induced traumata have been identified. Indeed, if necrosis and especially apoptosis are key mediators of these cochlear lesions, then inhibitors of apoptotic and necrotic cell death should be able to attenuate the damage following acoustic overstimulation. Wang et al. showed that intracochlear perfusion of riluzole, an inhibitor of apoptosis and necrosis (Lang-Lazdunski et al., 2000; Ettaiche et al., 1999), protects the cochlea from damage by acoustic trauma (Wang et al., 2002). Other groups reported protective effects through blockage of the JNK pathway. For example, a noise exposed group of mice fed with all trans retinoic acid (ATRA), a compound with anti-apoptosis potential through inhibition of the JNK pathway, showed greater cochlear preservation with less apoptotic cells compared to a control group (Ahn et al., 2005; Shim et al., 2009). Another study reported that blocking the JNK pathway using locally delivered d-JNK-1 through the round window membrane prevented hair cell death and permanent hearing loss following noise trauma (Wang et al., 2007). In agreement with the hypothesis of ROS formation and mitochondrial damage, protection with antioxidant molecules, such as Q-ter (CoEnzyme Q10 Terclatrate, a water soluble form of the lipophilic antioxidant coQ10) (Fetoni et al., 2009), idebenone (synthetic analogue of coenzyme Q10) (Sergi et al., 2006), trolox (water soluble analogue of alpha tocopherol), salicylates (Yamashita et al., 2005), NAC (N-Acetyl-cysteine) (Ohinata et al., 2003), edaravone (Tanaka et al., 2005), D-methionine (Campbell et al., 2007), glutathione (Hight et al., 2003) and ferulic acid (Fetoni et al., 2010) will decrease auditory thresholds shifts after noise exposure and decrease the amount of apoptosis in hair cells.
The conclusion from these data is that outer hair cells are the primary target for cell death following excessive noise exposure. Apoptosis seems to be a major cause of cell death that occurs immediately during the noise exposure and continues to do so several days thereafter. Apoptotic events then decrease over time and contribute together with necrosis equally to the still ongoing cell death.
3. Hearing loss due to ototoxicity is the result of apoptosis
Certain clinically useful and essential pharmacological therapies may result in temporary or permanent hearing loss due to interactions of the drug and cells of the inner ear. The most commonly used drugs with ototoxic effects are aminoglycosides antibiotics, platinum-based chemotherapeutica (cisplatin and carboplatin), loop diuretcs, salicylates, macrolide antibiotics and antimalarials (Arslan et al., 1999; Humes, 1999). For the scope of this review, we will only discuss aminoglycosides and cisplatin because the effects of these drugs on the hearing apparatus are the most severe and, in contrast to other ototoxins, cause permanent hearing loss.
3.1 Aminoglycoside antibiotics
3.1.1. Background
Aminoglycoside antibiotics (such as amikacin, gentamicin, kanamycin, neomycin) were discovered in the 1940s and have been widely used ever since. Back then, these ‘new’ antibiotics were considered a blessing as they could be used in the treatment of tuberculosis and otherwise incurable infectious diseases. Since then, clinical trials reported reversible nephrotoxic and irreversible ototoxic effects in humans, which were confirmed in animals. These antibiotics have always been a popular choice due to the low cost and high effectiveness of the treatment. Depending on dose and duration of treatment, the incidence of cochleotoxicity may range between a few percent to 33% (Chen et al., 2007). Nowadays, at least in developed countries, the use of these antibiotics has declined but remains essential for specific indications in emergencies, cystic fibrosis and tuberculosis. In developing countries however, the use of aminoglycosides is still common practice primarily due to economical reasons.
3.1.2. Apoptosis through use of aminoglycoside antibiotics
Usage of aminoglycosides induces cellular death in both cochlear and vestibular systems, which translates into hearing loss and vertigo. The damage is mainly irreversible and affects predominantly hair cells of the cochlea and type I sensory cells in the vestibulum. Specifically, outer hair cells in the basal turn of the cochlea seem to be the most vulnerable and the first to die, resulting in a high-frequency loss in patients treated with these antibiotics. The more resilient inner hair cells generally start dying much later, often after destruction of most outer hair cells (Suzuki et al., 2008). Much effort has been put in the identification in the type of cell death induced after systemic treatment with one of these antibiotics. Now, the consensus is that the observed cell death predominantly occurs through apoptosis (Forge, 1985; Forge et al., 2000; Li et al., 1995; Nakagawa et al., 1998), reinforced by observations such as an increased hair cell loss after the administration of XIAP (X-linked inhibitor of apoptosis proteins) inhibitors (Tabuchi et al., 2007).
3.1.3. Mechanism of aminoglycoside-induced apoptosis and possible therapies
The mechanism of gentamicin-induced cochlear apoptosis has in part been attributed to generation of ROS (Clerici et al., 1996) and RNS (Hong et al., 2006). Indeed, gentamicin seems to increase ROS after the formation of an iron-aminoglycoside complex both in-vitro (Lesniak et al., 2005) and after systemic treatment of mice with kanamycin in-vivo (Jiang et al., 2005; Sha et al., 1999). Consistent with this idea and suggesting a causal relationship between oxidative stress and cochlear cell death is the fact that iron chelators and antioxidant therapies attenuate gentamicin ototoxicity (Kawamoto et al., 2004; Sha et al., 2000; Song et al., 1998). These data suggest that mitochondria also play a key role in the aminoglycoside-induced apoptotic cell death and may be a primary target of these drugs. Indeed, Dehne et al. reported an increased mitochondrial membrane permeability potential in cell culture which resulted in the release of pro-apoptotic factors in the cytosol after aminoglycosides were added to the medium (Dehne et al., 2002). Also, supplementation of L-carnitine (LCAR), a neuroprotective agent that plays a role in mitochondrial functioning, is able to prevent outer hair cell damage after gentamicin administration (Kalinec et al., 2005). Interestingly, the JNK cascade couples oxidative stress to apoptosis (Mielke et al., 2000). In-vivo experiments show activation of the JNK pathway after administraton of aminoglycosides, which in turn triggers the apoptosis program of vestibular and cochlear cells. Concordantly, inhibitors of the JNK cascade such as CEP-1347 (Ylikoski et al., 2002) and estradiol attenuate hair cells loss following gentamicin administration (Nakamagoe et al., 2010). However, Kalinec et al. found that gentamicin in fact inhibits JNK phosphorylation but activates ERK1/2, another MAP kinase which in turn leads to upregulation of HRK and finally results in cell death (Kalinec et al., 2005). It is clear that no consensus exists as to which MAP kinase cascade is activated through gentamicin ototoxicity. Furthermore, evidence for both caspase-dependent and caspase-independent hair cell death after kanamycin administration in-vivo has been presented (Jiang et al., 2006). Many factors are probably in play in determining modes of cell death such as the model system used (in-vivo versus in-vitro) and the effective concentration or duration of drug treatment. However, it is safe to say that gentamicin ototoxicity causes loss of both vestibular cells and cochlear hair cells predominantly through an apoptotic process. The apoptotic program is triggered through mitochondrial damage due to generation of ROS which consequently activates various pathways resulting in apoptotic cell death.
3.2. Cisplatin
3.2.1. Background
Cisplatin is a platinum-based chemotherapeuticum, commonly used in clinical practice in the treatment of different types of cancers such as osteosarcoma, testicular, ovarian, bladder, head and neck and lung cancers (Hill et al., 2008). Cisplatin is taken up by the tumor cell, after which DNA crosslinks are formed through covalent bindings of the platinum atom to purine bases. This process triggers transcription inhibition, cell cycle arrest and apoptosis (Rybak et al., 2007a). Several side effects arise from the use of cisplatin due to its non-specific cytotoxic actions. The most severe and often dose limiting side effects are neurotoxicity, nephrotoxicty and ototoxicity. While neurotoxicity and nephrotoxicity can be mitigated by appropriate clinical regimens, ototoxicity seems inevitable and permanent. Ototoxic effects occur in 26–68% of patients treated with cisplatin (Musial-Bright et al., 2011). An increased risk of permanent hearing loss is reported for younger children, larger doses, pre-existing hearing loss and renal disease (Rybak et al., 2007b).
3.2.2. Effect of cisplatin on cochlear tissue
The ototoxic effects of cisplatin are classified in two major categories. A first acute reaction induces a reversible effect on the hearing apparatus through inhibition of transduction currents and voltage-dependant Ca2+ currents. Effects on the stria vascularis may contribute to the overall pattern of early cisplatin damage but such effects are initially also reversible. On the other hand, cisplatin induces long term, irreversible changes in the cochlear morphology resulting in an irreversible bilateral, sensorineural high frequency loss (Musial-Bright et al., 2011). These cochleotoxic effects of cisplatin have been well described (Estrem et al., 1981; Laurell et al., 1989) and result from a predominant destruction of the outer hair cells of the organ of Corti starting from the basal turn of the cochlea (Alam et al., 2000). Spiral ganglion cells may degenerate (Lee et al., 2003) but inner hair cells are less consistently affected. Permanent damage to the lateral wall structures (stria vascularis and spiral ganglion) of the inner ear occurs to a lesser extent. In contrast to aminoglycoside-induced ototoxicity, the vestibular system does not seem to suffer. All affected cells die predominantly through an apoptotic death program.
3.2.3. Mechanisms of cisplatin induced apoptosis
Cisplatin augments free radical formation in the inner ear (Dehne et al., 2001; Kopke et al., 1997; Tsutsumishita et al., 1998), a process that partly depends on the availability of iron (Dehne et al., 2001). Just as in aminoglycoside toxicity, it is assumed that the formation of these free radicals is the basis for cisplatin induced ototoxicity as antioxidant therapy protects (Campbell et al., 1996; Church et al., 1995; Dickey et al., 2004; Wu et al., 2005). An important source of these ROS in the inner ear appears to be NADPH oxidase 3 (NOX3), which is a superoxide-generating NADPH oxidase that is upregulated after cisplatin treatment (Banfi et al., 2004; Mukherjea et al., 2006). Excessive ROS generation could then deplete the antioxidant defense mechanisms of cochlear cells, damaging mitochondria and causing them to release cytochrome c thus triggering the apoptotic death program through activation of caspases (Rybak et al., 2007a).
In conclusion, both aminoglycosides and cisplatin cause a sensorineural, initially high-frequency hearing loss. The severity of the toxicity is related to both the duration of the treatment and the dose of the drug. The outer hair cells of the basal turn of the cochlea are highly sensitive to both drugs and seem to die from apoptosis which, in both cases, appears to be triggered through an elevated ROS formation (Figure 2).
4. Apoptosis in ARHI
ARHI is the result of genetic predispositions combined with various insults to the inner ear accumulated during a lifetime. The environmental causes contributing to ARHI are very heterogeneous and may include, for example, smoking, exposure to loud noise and ototoxic drugs. Histopathologically and pathophysiologically, ARHI does not follow a single pattern. It may variably be accompanied by an age-dependant loss of sensory hair cells, spiral ganglion cells, degeneration of stria vascularis cells and stiffening of the basilar membrane (Gates et al., 2005). The relative contributions of these individual pathologies shape the type presbycusis: for example, sensorineural-based on loss of hair cells and spiral ganglion cells or metabolic-based on functional deficits in stria vascularis.
Different animals have served as models for the different types of presbycusis, among them several mouse strains. Most of these strains show clear signs of ARHI but differences exist. Commonly used strains for studying ARHI are BALB/c and C57BL/6J. As in most human ARHI forms, these mice display a sensorineural type of ARHI that is accompanied with degeneration of the organ of Corti, stria vascularis and the spiral ligament. However, an often raised criticism against these models is that they already show first signs of hearing loss in early adulthood (Ohlemiller, 2006). Part of this early onset is explained through the presence of genetic variants. An example of a predisposing allele is cadherin 23ahl (Cdh23ahl). This allele encodes a protein present in the tip links of outer hair cells and is associated with a rapid progression of ARHI (Johnson et al., 2000; Ohlemiller, 2006). Another often used strain is CBA/J (Sha et al., 2008). These mice do not have the Cdh23ahl allele and seem to develop high frequency loss more slowly. The high frequency losses seem to coincide well with loss of hair cells at the base of the cochlea (Sha et al., 2008). However, an oddity observed in this strain is an early low frequency 4 kHz loss at 3 months of age, which is not seen in the other mice models. It is clear that a single mouse model for human ARHI does not exist and that trade-offs are always necessary when making a choice about which model to use. Morphological analyses of CBA/J mice show that the outer hair cell loss is attributable to a combination of both necrotic and apoptotic cell death (Sha et al., 2009). Other studies showed TUNEL-positive hair cells and spiral ganglion cells in aged gerbil and mice cochlea (Someya et al., 2007; Usami et al., 1997), suggesting that apoptosis is also involved in the development of ARHI.
Gene expression experiments in ageing cochlea of CBA/CaJ mice revealed altered gene expressions of several apoptosis-related genes. These altered expressions probably tip the scale in favor of apoptosis, causing cellular death. Although genes involved in the activation of both the extrinsic and intrinsic apoptotic pathways (Figure 1) were differentially regulated (Sha et al., 2009), the changes in the extrinsic pathways were definitely more clear-cut than those of the intrinsic pathways (Tadros et al., 2008). Together, these experiments show that both intrinsic and extrinsic apoptotic cell death programs are activated in ARHI.
It is widely accepted that oxidative stress plays a role in the pathology of ageing and that mitochondria are important sources of ROS production. As ARHI is considered an ageing disease, this suggests that mitochondria might also be implicated in the pathology of ARHI. In line with this theory is that antioxidant defenders such as mitochondrial superoxide dismutase 2 (SOD2) decrease significantly with age in all cell types of the organ of Corti, suggesting that oxidative imbalances indeed contribute to ARHI (Jiang et al., 2007). Moreover, studies in C57BL/6J mice fed with an antioxidant supplemented diet (α lipoic acid, coenzyme Q10, N-acetyl-L-cysteine) show significantly lower ABR hearing thresholds when compared to thresholds from control fed mice (Someya et al., 2009). Furthermore, hearing tests in C57BL/6J mice overexpressing mitochondrial targeted catalase (MCAT) showed reduced ABR thresholds compared to WT mice (Someya et al., 2009). These experiments in C57BL/6J mice provide evidence that mitochondrial derived ROS may play a causal role in ARHI. It was hypothesized that these ROS induce DNA damage, which results in the upregulation of P53 causing chronic activation of the mitochondrial BAK pathway, ultimately resulting in the triggering of apoptotic cell death (Someya et al., 2010). However, it should be noted that studies performed in C57BL/6J are not necessarily representative for the mechanism in age-related hearing loss, as this strain displays an accelerated form of hearing loss. Other studies point to a possible role of the non-mitochondrial SAP/JNK and p38 MAP kinase in the induction of apoptosis during the generation of ARHI (Sha et al., 2009). Despite limitations in the various models of ARHI it appears that ROS formation and apoptosis are key events in the pathology of ARHI. The exact pathways leading to the activation of apoptosis are not clearly defined and it is plausible that in reality multiple pathways are co-activated as ARHI is the product of a multifactorial process (Figure 2).
5. Apoptosis in monogenic forms of hearing loss
The search for the genetic factors contributing to hereditary HI has been very successful with the identification of many loci and causative genes during the past two decades. This is demonstrated by the fact that until now, 24 dominant, 41 recessive and 2 X-linked genes have already been described (http://hereditaryhearingloss.org/). The functions of these genes are very diverse and a single common pathway for deafness does not seem to exist. Instead, these genes cluster in different functional classes. For example, the Usher complex proteins located in the stereocilia are involved in processes such as motor activity and cell adhesion. Other proteins are implicated in K+ homeostasis, tight junction formation, or synaptic transmission. Recently, mutations in a new class of genes involved in apoptotic pathways have been identified as the cause of deafness.
5.1. DFNA5
DFNA5 was identified in 1998 as a gene causing an autosomal dominant, sensorineural form of hearing loss that starts in the high frequencies (Van Laer et al., 1998). At the genomic level, the gene spans 60kB and translates an mRNA transcript of 1491bp. To date, four different genomic mutations have been identified that on the RNA level always lead to skipping of exon 8 (Bischoff et al., 2004; Cheng et al., 2007; Van Laer et al., 1998; Yu et al., 2003). DFNA5 is expressed in all tissues investigated so far, albeit at a low level. Higher expression levels were reported in placental tissue (Van Laer et al., 1998). Functional studies showed that transfection of mutant DFNA5 causes cell death whereas transfection of WT DFNA5 does not (Van Laer et al., 2004). The mutations leading to DFNA5-induced hearing loss are thus gain of function mutations. Analysis of the DFNA5 protein revealed two regions separated by a hinge region. Morphological and flow cytometrical analysis revealed that the first region induces apoptosis after transfection in mammalian cells (Figure 3) while the second region probably has a regulatory function on the apoptosis-inducing region (Op de Beeck et al., 2011). As the apoptosis-inducing region is present in both WT and mutant DFNA5, apoptosis induction probably is an intrinsic feature of the protein. This is in line with the observation that DFNA5 is frequently epigenetically silenced in primary tumors of gastric, colorectal and breast cancer (Akino et al., 2007; Fujikane et al., 2009; Kim et al., 2008a; Kim et al., 2008b). Indeed, shutting down a possible cell death pathway results in a proliferative advantage for a tumor. Several other lines of evidence point to an important role of DFNA5 in tumor biology. DFNA5 mRNA was significantly downregulated in an etoposide-resistant melanoma cell line (MeWo ETO 1) compared to a non resistant MeWo cell line. Transfecting these cells with a DFNA5 expression vector led to an increase in the sensitivity for etoposide. This increased sensitivity was accompanied with augmented caspase 3 activation, suggesting that DFNA5 might be implicated in the apoptotic pathway (Lage et al., 2001). Moreover, DFNA5 was identified as a transcriptional target of P53 (Masuda et al., 2006). These data raised the hypothesis that DFNA5 might be a tumor suppressor gene. Indeed, transfection of DFNA5 in a gastric cancer cell line decreased the number of colonies when compared to cells transfected with empty vector (Akino et al., 2007). Alternatively, knockdown of DFNA5 in a breast cancer cell line resulted in an increased cell growth and colony size (Kim et al., 2008b). All tumor suppressor genes share the ability to reduce the possibility of an uncontrolled cellular proliferation. This is in line with the apoptosis-inducing feature of DFNA5: reducing the expression of DFNA5 through methylation may lead to the inactivation of a possible cell death route resulting in a proliferative advantage.
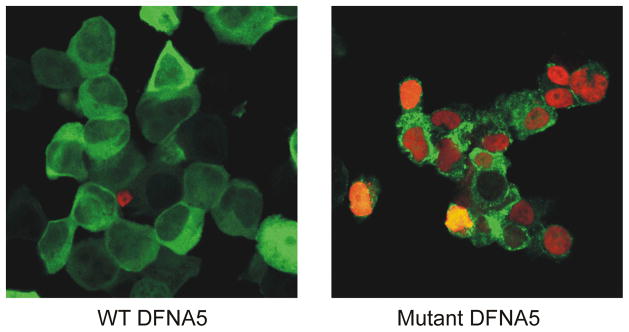
Figure 3.
Confocal images of TUNEL stained HEK293T cells transfected with either WT or mutant DFNA5-EGFP. Red stained nuclei are TUNEL positive. Cells transfected with WT DFNA5 display a healthy appearance with little to no TUNEL positive nuclei. In contrast, cells transfected with mutant DFNA5 display an unhealthy appearance with clear TUNEL positive nuclei, indicative of apoptotic cell death.
It is hypothesized that the truncating DFNA5 mutations constitutively activate the apoptosis-inducing feature causing cell death of the terminally differentiated auditory sensory epithelium which, over time, results in a sensorineural HI. The phenotype of DFNA5 patients is compatible with this theory. Audiograms of DFNA5 patients show an early loss of high frequencies that progresses to the mid frequencies and at the endpoint also affects the lower frequencies. The high frequency loss may be explained through the typical tonotopy of the cochlea. Indeed, it appears that basal hair cells, which are essential for high frequencies, are more susceptible to apoptotic cell death and probably die first as they do in drug-induced and noise-induced hearing loss. The apoptosis-inducing feature of mutant DFNA5 might then cause continued stress on cells of the sensory epithelium, causing the more resilient cells, located at the cochlear apex, to die. The fact that DFNA5 patients show relatively good speech recognition clearly suggests that DFNA5-induced hearing loss is not an auditory neuropathy, but that the lesion is located at the sensory epithelium of the organ of Corti.
5.2. TJP2
The causative gene for DFNA51-associated hearing loss was discovered through arrayCGH, which allows for the identification of complex mutations such as microdeletions or microduplications. The disease-causing mutation was identified as an inverted genomic duplication of TJP2. This leads to overexpression of TJP2, resulting in a non-syndromic, autosomal dominant, progressive hearing loss, starting at the high frequencies, but eventually altering the complete auditory spectrum. TJP2 encodes tight junction protein 2. In the inner ear, the gene is mainly expressed at the membrane between hair cells and supporting cells. Patients harboring the duplication have an approximate 1.7 fold overexpression of the mRNA compared to normal controls. In-vitro overexpression of TJP2 leads to a reduced phosphorylation of glycogen synthase kinase 3β (GSK-3β), resulting in its activation. It is hypothesized that the overexpression in patients also leads to an increased amount of the activated, unphosphorylated form of GSK-3β, as in-vitro studies suggest. Real time PCR experiments showed that overexpression of TJP2 results in the differential expression of several apoptosis-related genes such as BCL2L11, IL6, Rel and TSPO in patients with a mutation, probably due to altered activity of GSK-3β. This altered expression might shift the overall homeostatic balance to apoptosis, causing hearing loss (Walsh et al., 2010).
5.3. MSRB3
MSRB3, the gene responsible for DFNB74-associated hearing loss, was recently identified. Affected family members have a c.265T>G transversion in exon 4 of the gene. The transversion segregates with the hearing loss in all six known DFNB74 families but was absent in 262 DNA samples from normal hearing controls. The gene has four isoforms. Isoform A contains an endoplasmic reticulum localization signal at the N-terminal, the other three isoforms contain a mitochondrial localization signal. MSRB3 is ubiquitously expressed in almost all tissues tested thus far. In the organ of Corti of mice MSRB3 showed strong signals in inner as well as outer hair cells and to a lesser extent also in supporting cells. The gene encodes a methionine sulfoxide reductase which is involved in repairing oxidatively damaged proteins. Malfunction of the protein may thus result in an accumulation of damaged proteins, which could trigger endoplasmic reticulum stress and an apoptotic response in inner hair cells resulting in HI (Ahmed et al., 2011).
6. Summary
The hearing apparatus is a complex system where failure of one component potentially results in hearing loss. In the early 1990s the first genes for monogenic hearing loss were discovered through the use of genetic linkage techniques. Now dozens of genes responsible for HI have been described and functionally analyzed. As expected, the functions of these genes are very diverse ranging from gap junction and motor proteins to ion channels reflecting the complex hearing machinery. Although the pathology of HI is very complicated, extensive genetic and molecular biological studies have provided considerable insight into underlying mechanisms of cell death.
Apoptosis of sensory hair cells is an important contributor to several acquired hearing pathologies. The outer hair cells in particular appear to be very susceptible for various apoptotic stimuli. For example, in the case of noise-induced hearing loss, excessive noise exposure triggers the activation of apoptotic cell death programs in these outer hair cells. Likewise, use of certain therapeutic drugs such as aminoglycoside antibiotics and cisplatin triggers apoptosis in these sensory cells, resulting in permanent hearing loss. Finally, apoptosis is a key event in the pathology of ARHI, probably due to the accumulation of ROS, which are also implicated in noise- and drug-induced hearing loss.
As apoptosis clearly contributes to these forms of acquired hearing impairment, it is not surprising that several apoptosis genes were recently identified as the cause of monogenic deafness. These genes are TJP2, MSRB3 and DFNA5. The fact that these genes are ubiquitously expressed throughout many tissues and yet only cause HI might be explained by a high sensitivity of the sensory epithelium to stress and to deregulations of their apoptotic programs. As the hair cells are terminally differentiated it is not surprising that these mutations result in a phenotype of permanent hearing loss.
We feel that the recent discovery of apoptosis-related deafness genes is only the tip of the iceberg and expect that many more mutations in such genes will be revealed in monogenic deafness families.
Acknowledgments
This work was supported by the ‘Fonds voor Wetenschappelijk Onderzoek Vlaanderen’ (FWO grant G.0245.10N). K.O.D.B. holds a predoctoral research position with the ‘Instituut voor de Aanmoediging van Innovatie door Wetenschap en Technologie in Vlaanderen (IWT)’. Dr. Schacht’s research on drug-induced hearing loss is supported by grant R01 DC003685 from the National Institute for Deafness and Other Communication Disorders, National Institutes of Health.
Abbreviations
- ABR
Auditory brainstem response
- AIF
Apoptosis inducing factor
- Apaf-1
Apoptotic protease activating factor 1
- ARHI
Age related hearing impairment
- ATRA
All trans retinoic acid
- BAD
Bcl-2-associated death
- BCL2
B-cell lymphoma 2
- Cdh23
Cadherin 23
- DFNA5
Deafness, autosomal dominant5
- DNA
Deoxyribonuleic acid
- EndoG
Endonuclease G
- ERK
Extracellular signal regulated kinase
- GSK-3β
Glycogen synthase kinase 3β
- HI
Hearing impairment
- Hz
Hertz
- IL6
Interleukin 6
- JNK
Jun N-terminal Kinase
- LCAR
L-carnitine
- MAP kinase
Mitogen activated protein kinase
- NOX3
NADPH oxidase 3
- MSRB3
methionine sulfoxide reductase B3
- Q-ter
Co-Enzyme Q10 terclatrate
- RNA
Ribonucleic acid
- ROS
Reactive oxygen species
- RNS
Reactive nitrogen species
- SOD2
Superoxide dismutase 2
- TJP2
Tight junction protein 2
- TSPO
Translocator protein
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- XIAP
X-linked inhibitor of apoptosis proteins
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Ahmed ZM, Yousaf R, Lee BC, Khan SN, Lee S, Lee K, Husnain T, Rehman AU, Bonneux S, Ansar M, Ahmad W, Leal SM, Gladyshev VN, Belyantseva IA, Van Camp G, Riazuddin S, Friedman TB, Riazuddin S. Functional null mutations of MSRB3 encoding methionine sulfoxide reductase are associated with human deafness DFNB74. Am J Hum Genet. 2011;88:19–29. doi: 10.1016/j.ajhg.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JH, Kang HH, Kim YJ, Chung JW. Anti-apoptotic role of retinoic acid in the inner ear of noise-exposed mice. Biochem Biophys Res Commun. 2005;335:485–90. doi: 10.1016/j.bbrc.2005.07.114. [DOI] [PubMed] [Google Scholar]
- Akino K, Toyota M, Suzuki H, Imai T, Maruyama R, Kusano M, Nishikawa N, Watanabe Y, Sasaki Y, Abe T, Yamamoto E, Tarasawa I, Sonoda T, Mori M, Imai K, Shinomura Y, Tokino T. Identification of DFNA5 as a target of epigenetic inactivation in gastric cancer. Cancer Sci. 2007;98:88–95. doi: 10.1111/j.1349-7006.2006.00351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam SA, Ikeda K, Oshima T, Suzuki M, Kawase T, Kikuchi T, Takasaka T. Cisplatin-induced apoptotic cell death in Mongolian gerbil cochlea. Hear Res. 2000;141:28–38. doi: 10.1016/s0378-5955(99)00211-7. [DOI] [PubMed] [Google Scholar]
- Arslan E, Orzan E, Santarelli R. Global problem of drug-induced hearing loss. Ann N Y Acad Sci. 1999;884:1–14. doi: 10.1111/j.1749-6632.1999.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–72. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- Bischoff AMLC, Luijendijk MWJ, Huygen PLM, van Duijnhoven G, De Leenheer EMR, Oudesluijs G, Van Laer L, Cremers FPM, Cremers CWRJ, Kremer H. A second mutation identified in the DFNA5 gene in a Dutch family. A clinical and genetic evaluation. Audiol Neuro-otol. 2004;9:34–36. doi: 10.1159/000074185. [DOI] [PubMed] [Google Scholar]
- Campbell KC, Rybak LP, Meech RP, Hughes L. D-methionine provides excellent protection from cisplatin ototoxicity in the rat. Hear Res. 1996;102:90–8. doi: 10.1016/s0378-5955(96)00152-9. [DOI] [PubMed] [Google Scholar]
- Campbell KC, Meech RP, Klemens JJ, Gerberi MT, Dyrstad SS, Larsen DL, Mitchell DL, El-Azizi M, Verhulst SJ, Hughes LF. Prevention of noise-and drug-induced hearing loss with D-methionine. Hear Res. 2007;226:92–103. doi: 10.1016/j.heares.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Chen Y, Huang WG, Zha DJ, Qiu JH, Wang JL, Sha SH, Schacht J. Aspirin attenuates gentamicin ototoxicity: from the laboratory to the clinic. Hear Res. 2007;226:178–82. doi: 10.1016/j.heares.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Cheng J, Han DY, Dai P, Sun HJ, Tao R, Sun Q, Yan D, Qin W, Wang HY, Ouyang XM, Yang SZ, Cao JY, Feng GY, Du LL, Zhang YZ, Zhai SQ, Yang WY, Liu XZ, He L, Yuan HJ. A novel DFNA5 mutation, IVS8+4 A>G, in the splice donor site of intron 8 causes late-onset non-syndromic hearing loss in a Chinese family. Clin Genet. 2007;72:471–7. doi: 10.1111/j.1399-0004.2007.00889.x. [DOI] [PubMed] [Google Scholar]
- Church MW, Kaltenbach JA, Blakley BW, Burgio DL. The comparative effects of sodium thiosulfate, diethyldithiocarbamate, fosfomycin and WR-2721 on ameliorating cisplatin-induced ototoxicity. Hear Res. 1995;86:195–203. doi: 10.1016/0378-5955(95)00066-d. [DOI] [PubMed] [Google Scholar]
- Clerici WJ, Hensley K, DiMartino DL, Butterfield DA. Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear Res. 1996;98:116–24. doi: 10.1016/0378-5955(96)00075-5. [DOI] [PubMed] [Google Scholar]
- Dehne N, Lautermann J, Petrat F, Rauen U, de Groot H. Cisplatin ototoxicity: involvement of iron and enhanced formation of superoxide anion radicals. Toxicol Appl Pharmacol. 2001;174:27–34. doi: 10.1006/taap.2001.9171. [DOI] [PubMed] [Google Scholar]
- Dehne N, Rauen U, de Groot H, Lautermann J. Involvement of the mitochondrial permeability transition in gentamicin ototoxicity. Hear Res. 2002;169:47–55. doi: 10.1016/s0378-5955(02)00338-6. [DOI] [PubMed] [Google Scholar]
- Dickey CA, De Mesquita DD, Morgan D, Pennypacker KR. Induction of memory-associated immediate early genes by nerve growth factor in rat primary cortical neurons and differentiated mouse Neuro2A cells. Neurosci Lett. 2004;366:10–4. doi: 10.1016/j.neulet.2004.04.089. [DOI] [PubMed] [Google Scholar]
- Estrem SA, Babin RW, Ryu JH, Moore KC. Cis-diamminedichloroplatinum (II) ototoxicity in the guinea pig. Otolaryngol Head Neck Surg. 1981;89:638–45. doi: 10.1177/019459988108900424. [DOI] [PubMed] [Google Scholar]
- Ettaiche M, Fillacier K, Widmann C, Heurteaux C, Lazdunski M. Riluzole improves functional recovery after ischemia in the rat retina. Invest Ophthalmol Vis Sci. 1999;40:729–36. [PubMed] [Google Scholar]
- Fetoni AR, Piacentini R, Fiorita A, Paludetti G, Troiani D. Water-soluble Coenzyme Q10 formulation (Q-ter) promotes outer hair cell survival in a guinea pig model of noise induced hearing loss (NIHL) Brain Res. 2009;1257:108–16. doi: 10.1016/j.brainres.2008.12.027. [DOI] [PubMed] [Google Scholar]
- Fetoni AR, Mancuso C, Eramo SL, Ralli M, Piacentini R, Barone E, Paludetti G, Troiani D. In vivo protective effect of ferulic acid against noise-induced hearing loss in the guinea-pig. Neuroscience. 2010;169:1575–88. doi: 10.1016/j.neuroscience.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Forge A. Outer hair cell loss and supporting cell expansion following chronic gentamicin treatment. Hear Res. 1985;19:171–82. doi: 10.1016/0378-5955(85)90121-2. [DOI] [PubMed] [Google Scholar]
- Forge A, Li L. Apoptotic death of hair cells in mammalian vestibular sensory epithelia. Hear Res. 2000;139:97–115. doi: 10.1016/s0378-5955(99)00177-x. [DOI] [PubMed] [Google Scholar]
- Fujikane T, Nishikawa N, Toyota M, Suzuki H, Nojima M, Maruyama R, Ashida M, Ohe-Toyota M, Kai M, Nishidate T, Sasaki Y, Ohmura T, Hirata K, Tokino T. Genomic screening for genes upregulated by demethylation revealed novel targets of epigenetic silencing in breast cancer. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0600-1. [DOI] [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbycusis. Lancet. 2005;366:1111–20. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- Hamernik RP, Turrentine G, Roberto M, Salvi R, Henderson D. Anatomical correlates of impulse noise-induced mechanical damage in the cochlea. Hear Res. 1984;13:229–47. doi: 10.1016/0378-5955(84)90077-7. [DOI] [PubMed] [Google Scholar]
- Han W, Shi X, Nuttall AL. AIF and endoG translocation in noise exposure induced hair cell death. Hear Res. 2006;211:85–95. doi: 10.1016/j.heares.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- Hight NG, McFadden SL, Henderson D, Burkard RF, Nicotera T. Noise-induced hearing loss in chinchillas pre-treated with glutathione monoethylester and R-PIA. Hear Res. 2003;179:21–32. doi: 10.1016/s0378-5955(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Hill GW, Morest DK, Parham K. Cisplatin-induced ototoxicity: effect of intratympanic dexamethasone injections. Otol Neurotol. 2008;29:1005–11. doi: 10.1097/MAO.0b013e31818599d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SH, Park SK, Cho YS, Lee HS, Kim KR, Kim MG, Chung WH. Gentamicin induced nitric oxide-related oxidative damages on vestibular afferents in the guinea pig. Hear Res. 2006;211:46–53. doi: 10.1016/j.heares.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Hu BH, Guo W, Wang PY, Henderson D, Jiang SC. Intense noise-induced apoptosis in hair cells of guinea pig cochleae. Acta Otolaryngol. 2000;120:19–24. [PubMed] [Google Scholar]
- Hu BH, Henderson D, Nicotera TM. F-actin cleavage in apoptotic outer hair cells in chinchilla cochleas exposed to intense noise. Hear Res. 2002a;172:1–9. doi: 10.1016/s0378-5955(01)00361-6. [DOI] [PubMed] [Google Scholar]
- Hu BH, Henderson D, Nicotera TM. Involvement of apoptosis in progression of cochlear lesion following exposure to intense noise. Hear Res. 2002b;166:62–71. doi: 10.1016/s0378-5955(02)00286-1. [DOI] [PubMed] [Google Scholar]
- Hu BH, Cai Q, Manohar S, Jiang H, Ding D, Coling DE, Zheng G, Salvi R. Differential expression of apoptosis-related genes in the cochlea of noise-exposed rats. Neuroscience. 2009;161:915–25. doi: 10.1016/j.neuroscience.2009.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes HD. Insights into ototoxicity. Analogies to nephrotoxicity. Ann N Y Acad Sci. 1999;884:15–8. doi: 10.1111/j.1749-6632.1999.tb00278.x. [DOI] [PubMed] [Google Scholar]
- Jiang H, Sha SH, Schacht J. NF-kappaB pathway protects cochlear hair cells from aminoglycoside-induced ototoxicity. J Neurosci Res. 2005;79:644–51. doi: 10.1002/jnr.20392. [DOI] [PubMed] [Google Scholar]
- Jiang H, Sha SH, Forge A, Schacht J. Caspase-independent pathways of hair cell death induced by kanamycin in vivo. Cell Death Differ. 2006;13:20–30. doi: 10.1038/sj.cdd.4401706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Talaska AE, Schacht J, Sha SH. Oxidative imbalance in the aging inner ear. Neurobiol Aging. 2007;28:1605–12. doi: 10.1016/j.neurobiolaging.2006.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Erway LC. A major gene affecting age-related hearing loss is common to at least ten inbred strains of mice. Genomics. 2000;70:171–80. doi: 10.1006/geno.2000.6377. [DOI] [PubMed] [Google Scholar]
- Kalinec GM, Fernandez-Zapico ME, Urrutia R, Esteban-Cruciani N, Chen S, Kalinec F. Pivotal role of Harakiri in the induction and prevention of gentamicin-induced hearing loss. Proc Natl Acad Sci U S A. 2005;102:16019–24. doi: 10.1073/pnas.0508053102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Sha SH, Minoda R, Izumikawa M, Kuriyama H, Schacht J, Raphael Y. Antioxidant gene therapy can protect hearing and hair cells from ototoxicity. Mol Ther. 2004;9:173–81. doi: 10.1016/j.ymthe.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Chang X, Yamashita K, Nagpal JK, Baek JH, Wu G, Trink B, Ratovitski EA, Mori M, Sidransky D. Aberrant promoter methylation and tumor suppressive activity of the DFNA5 gene in colorectal carcinoma. Oncogene. 2008a;27:3624–34. doi: 10.1038/sj.onc.1211021. [DOI] [PubMed] [Google Scholar]
- Kim MS, Lebron C, Nagpal JK, Chae YK, Chang X, Huang Y, Chuang T, Yamashita K, Trink B, Ratovitski EA, Califano JA, Sidransky D. Methylation of the DFNA5 increases risk of lymph node metastasis in human breast cancer. Biochem Biophys Res Commun. 2008b;370:38–43. doi: 10.1016/j.bbrc.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopke RD, Liu W, Gabaizadeh R, Jacono A, Feghali J, Spray D, Garcia P, Steinman H, Malgrange B, Ruben RJ, Rybak L, Van de Water TR. Use of organotypic cultures of Corti’s organ to study the protective effects of antioxidant molecules on cisplatin-induced damage of auditory hair cells. Am J Otol. 1997;18:559–71. [PubMed] [Google Scholar]
- Lage H, Helmbach H, Grottke C, Dietel M, Schadendorf D. DFNA5 (ICERE-1) contributes to acquired etoposide resistance in melanoma cells. FEBS Lett. 2001;494:54–59. doi: 10.1016/s0014-5793(01)02304-3. [DOI] [PubMed] [Google Scholar]
- Lang-Lazdunski L, Heurteaux C, Mignon A, Mantz J, Widmann C, Desmonts J, Lazdunski M. Ischemic spinal cord injury induced by aortic cross-clamping: prevention by riluzole. Eur J Cardiothorac Surg. 2000;18:174–81. doi: 10.1016/s1010-7940(00)00430-9. [DOI] [PubMed] [Google Scholar]
- Laurell G, Engstrom B. The ototoxic effect of cisplatin on guinea pigs in relation to dosage. Hear Res. 1989;38:27–33. doi: 10.1016/0378-5955(89)90125-1. [DOI] [PubMed] [Google Scholar]
- Lee JE, Nakagawa T, Kim TS, Iguchi F, Endo T, Dong Y, Yuki K, Naito Y, Lee SH, Ito J. A novel model for rapid induction of apoptosis in spiral ganglions of mice. Laryngoscope. 2003;113:994–9. doi: 10.1097/00005537-200306000-00015. [DOI] [PubMed] [Google Scholar]
- Lesniak W, Pecoraro VL, Schacht J. Ternary complexes of gentamicin with iron and lipid catalyze formation of reactive oxygen species. Chem Res Toxicol. 2005;18:357–64. doi: 10.1021/tx0496946. [DOI] [PubMed] [Google Scholar]
- Li L, Nevill G, Forge A. Two modes of hair cell loss from the vestibular sensory epithelia of the guinea pig inner ear. J Comp Neurol. 1995;355:405–17. doi: 10.1002/cne.903550307. [DOI] [PubMed] [Google Scholar]
- Lo YY, Wong JM, Cruz TF. Reactive oxygen species mediate cytokine activation of c-Jun NH2-terminal kinases. J Biol Chem. 1996;271:15703–7. doi: 10.1074/jbc.271.26.15703. [DOI] [PubMed] [Google Scholar]
- Marazita ML, Ploughman LM, Rawlings B, Remington E, Arnos KS, Nance WE. Genetic epidemiological studies of early-onset deafness in the U.S. school-age population. Am J Med Genet. 1993;46:486–91. doi: 10.1002/ajmg.1320460504. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Futamura M, Kamino H, Nakamura Y, Kitamura N, Ohnishi S, Miyamoto Y, Ichikawa H, Ohta T, Ohki M, Kiyono T, Egami H, Baba H, Arakawa H. The potential role of DFNA5, a hearing impairment gene, in p53-mediated cellular response to DNA damage. J Hum Genet. 2006;51:652–64. doi: 10.1007/s10038-006-0004-6. [DOI] [PubMed] [Google Scholar]
- Mielke K, Herdegen T. JNK and p38 stresskinases--degenerative effectors of signal-transduction-cascades in the nervous system. Prog Neurobiol. 2000;61:45–60. doi: 10.1016/s0301-0082(99)00042-8. [DOI] [PubMed] [Google Scholar]
- Mukherjea D, Whitworth CA, Nandish S, Dunaway GA, Rybak LP, Ramkumar V. Expression of the kidney injury molecule 1 in the rat cochlea and induction by cisplatin. Neuroscience. 2006;139:733–40. doi: 10.1016/j.neuroscience.2005.12.044. [DOI] [PubMed] [Google Scholar]
- Musial-Bright L, Fengler R, Henze G, Hernaiz Driever P. Carboplatin and ototoxicity: hearing loss rates among survivors of childhood medulloblastoma. Childs Nerv Syst. 2011;27:407–13. doi: 10.1007/s00381-010-1300-1. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Yamane H, Takayama M, Sunami K, Nakai Y. Apoptosis of guinea pig cochlear hair cells following chronic aminoglycoside treatment. Eur Arch Otorhinolaryngol. 1998;255:127–31. doi: 10.1007/s004050050027. [DOI] [PubMed] [Google Scholar]
- Nakamagoe M, Tabuchi K, Uemaetomari I, Nishimura B, Hara A. Estradiol protects the cochlea against gentamicin ototoxicity through inhibition of the JNK pathway. Hear Res. 2010;261:67–74. doi: 10.1016/j.heares.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Nelson DI, Nelson RY, Concha-Barrientos M, Fingerhut M. The global burden of occupational noise-induced hearing loss. Am J Ind Med. 2005;48:446–58. doi: 10.1002/ajim.20223. [DOI] [PubMed] [Google Scholar]
- Nicotera TM, Hu BH, Henderson D. The caspase pathway in noise-induced apoptosis of the chinchilla cochlea. J Assoc Res Otolaryngol. 2003;4:466–77. doi: 10.1007/s10162-002-3038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X, Shao R, Canlon B. Suppression of apoptosis occurs in the cochlea by sound conditioning. Neuroreport. 2003;14:1025–9. doi: 10.1097/01.wnr.0000070830.57864.32. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Miller JM, Schacht J. Protection from noise-induced lipid peroxidation and hair cell loss in the cochlea. Brain Res. 2003;966:265–73. doi: 10.1016/s0006-8993(02)04205-1. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Wright JS, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neurootol. 1999;4:229–36. doi: 10.1159/000013846. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK. Contributions of mouse models to understanding of age- and noise-related hearing loss. Brain Res. 2006;1091:89–102. doi: 10.1016/j.brainres.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Op de Beeck K, Van Camp G, Thys S, Cools N, Callebaut I, Vrijens K, Van Nassauw L, Van Tendeloo VF, Timmermans JP, Van Laer L. The DFNA5 gene, responsible for hearing loss and involved in cancer, encodes a novel apoptosis-inducing protein. Eur J Hum Genet. 2011 doi: 10.1038/ejhg.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Xing-Qun L, Virkkala J, Saarma M, Murakata C, Camoratto AM, Walton KM, Ylikoski J. Rescue of hearing, auditory hair cells, and neurons by CEP-1347/KT7515, an inhibitor of c-Jun N-terminal kinase activation. J Neurosci. 2000;20:43–50. doi: 10.1523/JNEUROSCI.20-01-00043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries PW. Prevalence and characteristics of persons with hearing trouble: United States, 1990–91. Vital Health Stat. 1994;10:1–75. [PubMed] [Google Scholar]
- Rybak LP, Ramkumar V. Ototoxicity. Kidney Int. 2007a;72:931–5. doi: 10.1038/sj.ki.5002434. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res. 2007b;226:157–67. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Saunders JC, Dear SP, Schneider ME. The anatomical consequences of acoustic injury: A review and tutorial. J Acoust Soc Am. 1985;78:833–60. doi: 10.1121/1.392915. [DOI] [PubMed] [Google Scholar]
- Sergi B, Fetoni AR, Paludetti G, Ferraresi A, Navarra P, Mordente A, Troiani D. Protective properties of idebenone in noise-induced hearing loss in the guinea pig. Neuroreport. 2006;17:857–61. doi: 10.1097/01.wnr.0000221834.18470.8c. [DOI] [PubMed] [Google Scholar]
- Sha SH, Schacht J. Formation of reactive oxygen species following bioactivation of gentamicin. Free Radic Biol Med. 1999;26:341–7. doi: 10.1016/s0891-5849(98)00207-x. [DOI] [PubMed] [Google Scholar]
- Sha SH, Schacht J. Antioxidants attenuate gentamicin-induced free radical formation in vitro and ototoxicity in vivo: D-methionine is a potential protectant. Hear Res. 2000;142:34–40. doi: 10.1016/s0378-5955(00)00003-4. [DOI] [PubMed] [Google Scholar]
- Sha SH, Kanicki A, Dootz G, Talaska AE, Halsey K, Dolan D, Altschuler R, Schacht J. Age-related auditory pathology in the CBA/J mouse. Hear Res. 2008;243:87–94. doi: 10.1016/j.heares.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SH, Chen FQ, Schacht J. Activation of cell death pathways in the inner ear of the aging CBA/J mouse. Hear Res. 2009;254:92–9. doi: 10.1016/j.heares.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim HJ, Kang HH, Ahn JH, Chung JW. Retinoic acid applied after noise exposure can recover the noise-induced hearing loss in mice. Acta Otolaryngol. 2009;129:233–8. doi: 10.1080/00016480802226155. [DOI] [PubMed] [Google Scholar]
- Slepecky N. Overview of mechanical damage to the inner ear: noise as a tool to probe cochlear function. Hear Res. 1986;22:307–21. doi: 10.1016/0378-5955(86)90107-3. [DOI] [PubMed] [Google Scholar]
- Someya S, Yamasoba T, Weindruch R, Prolla TA, Tanokura M. Caloric restriction suppresses apoptotic cell death in the mammalian cochlea and leads to prevention of presbycusis. Neurobiol Aging. 2007;28:1613–22. doi: 10.1016/j.neurobiolaging.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Someya S, Xu J, Kondo K, Ding D, Salvi RJ, Yamasoba T, Rabinovitch PS, Weindruch R, Leeuwenburgh C, Tanokura M, Prolla TA. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc Natl Acad Sci U S A. 2009;106:19432–7. doi: 10.1073/pnas.0908786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Prolla TA. Mitochondrial oxidative damage and apoptosis in age-related hearing loss, 2010. Mech Ageing Dev. 2010;131:480–6. doi: 10.1016/j.mad.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BB, Sha SH, Schacht J. Iron chelators protect from aminoglycoside-induced cochleo- and vestibulo-toxicity. Free Radic Biol Med. 1998;25:189–95. doi: 10.1016/s0891-5849(98)00037-9. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Ushio M, Yamasoba T. Time course of apoptotic cell death in guinea pig cochlea following intratympanic gentamicin application. Acta Otolaryngol. 2008;128:724–31. doi: 10.1080/00016480701714244. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Pak K, Chavez E, Ryan AF. Role of inhibitor of apoptosis protein in gentamicin-induced cochlear hair cell damage. Neuroscience. 2007;149:213–22. doi: 10.1016/j.neuroscience.2007.06.061. [DOI] [PubMed] [Google Scholar]
- Tadros SF, D’Souza M, Zhu X, Frisina RD. Apoptosis-related genes change their expression with age and hearing loss in the mouse cochlea. Apoptosis. 2008;13:1303–21. doi: 10.1007/s10495-008-0266-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Takemoto T, Sugahara K, Okuda T, Mikuriya T, Takeno K, Hashimoto M, Shimogori H, Yamashita H. Post-exposure administration of edaravone attenuates noise-induced hearing loss. Eur J Pharmacol. 2005;522:116–21. doi: 10.1016/j.ejphar.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Tsutsumishita Y, Onda T, Okada K, Takeda M, Endou H, Futaki S, Niwa M. Involvement of H2O2 production in cisplatin-induced nephrotoxicity. Biochem Biophys Res Commun. 1998;242:310–2. doi: 10.1006/bbrc.1997.7962. [DOI] [PubMed] [Google Scholar]
- Usami S, Takumi Y, Fujita S, Shinkawa H, Hosokawa M. Cell death in the inner ear associated with aging is apoptosis? Brain Res. 1997;747:147–50. doi: 10.1016/s0006-8993(96)01243-7. [DOI] [PubMed] [Google Scholar]
- Van Laer L, Huizing EH, Verstreken M, van Zuijlen D, Wauters JG, Bossuyt PJ, Van de Heyning P, McGuirt WT, Smith RJH, Willems PJ, Legan PK, Richardson GP, Van Camp G. Nonsyndromic hearing impairment is associated with a mutation in DFNA5. Nat Genet. 1998;20:194–197. doi: 10.1038/2503. [DOI] [PubMed] [Google Scholar]
- Van Laer L, Vrijens K, Thys S, Van Tendeloo VFI, Smith RJH, Van Bockstaele DR, Timmermans JP, Van Camp G. DFNA5: hearing impairment exon instead of hearing impairment gene? J Med Genet. 2004;41:401–6. doi: 10.1136/jmg.2003.015073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Torres MA, Schacht J. A BAD link to mitochondrial cell death in the cochlea of mice with noise-induced hearing loss. J Neurosci Res. 2006;83:1564–72. doi: 10.1002/jnr.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T, Pierce SB, Lenz DR, Brownstein Z, Dagan-Rosenfeld O, Shahin H, Roeb W, McCarthy S, Nord AS, Gordon CR, Ben-Neriah Z, Sebat J, Kanaan M, Lee MK, Frydman M, King MC, Avraham KB. Genomic duplication and overexpression of TJP2/ZO-2 leads to altered expression of apoptosis genes in progressive nonsyndromic hearing loss DFNA51. Am J Hum Genet. 2010;87:101–9. doi: 10.1016/j.ajhg.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Dib M, Lenoir M, Vago P, Eybalin M, Hameg A, Pujol R, Puel JL. Riluzole rescues cochlear sensory cells from acoustic trauma in the guinea-pig. Neuroscience. 2002;111:635–48. doi: 10.1016/s0306-4522(02)00004-0. [DOI] [PubMed] [Google Scholar]
- Wang J, Ruel J, Ladrech S, Bonny C, van de Water TR, Puel JL. Inhibition of the c-Jun N-terminal kinase-mediated mitochondrial cell death pathway restores auditory function in sound-exposed animals. Mol Pharmacol. 2007;71:654–66. doi: 10.1124/mol.106.028936. [DOI] [PubMed] [Google Scholar]
- Wu YJ, Muldoon LL, Neuwelt EA. The chemoprotective agent N-acetylcysteine blocks cisplatin-induced apoptosis through caspase signaling pathway. J Pharmacol Exp Ther. 2005;312:424–31. doi: 10.1124/jpet.104.075119. [DOI] [PubMed] [Google Scholar]
- Yamashita D, Jiang HY, Schacht J, Miller JM. Delayed production of free radicals following noise exposure. Brain Res. 2004a;1019:201–9. doi: 10.1016/j.brainres.2004.05.104. [DOI] [PubMed] [Google Scholar]
- Yamashita D, Miller JM, Jiang HY, Minami SB, Schacht J. AIF and EndoG in noise-induced hearing loss. Neuroreport. 2004b;15:2719–22. [PubMed] [Google Scholar]
- Yamashita D, Jiang HY, Le Prell CG, Schacht J, Miller JM. Post-exposure treatment attenuates noise-induced hearing loss. Neuroscience. 2005;134:633–42. doi: 10.1016/j.neuroscience.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Yang WP, Henderson D, Hu BH, Nicotera TM. Quantitative analysis of apoptotic and necrotic outer hair cells after exposure to different levels of continuous noise. Hear Res. 2004;196:69–76. doi: 10.1016/j.heares.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Xing-Qun L, Virkkala J, Pirvola U. Blockade of c-Jun N-terminal kinase pathway attenuates gentamicin-induced cochlear and vestibular hair cell death. Hear Res. 2002;163:71–81. doi: 10.1016/s0378-5955(01)00380-x. [DOI] [PubMed] [Google Scholar]
- Yu C, Meng X, Zhang S, Zhao G, Hu L, Kong X. A 3-nucleotide deletion in the polypyrimidine tract of intron 7 of the DFNA5 gene causes nonsyndromic hearing impairment in a Chinese family. Genomics. 2003;82:575–579. doi: 10.1016/s0888-7543(03)00175-7. [DOI] [PubMed] [Google Scholar]