Abstract
Background
Knowledge of trends in the incidence of and survival after myocardial infarction (MI) in a community setting is important to understanding trends in coronary heart disease (CHD) mortality rates.
Methods and Results
We estimated race and gender specific trends in the incidence of hospitalized MI, case-fatality and CHD mortality from community-wide surveillance and validation of hospital discharges and of in- and out-of-hospital deaths among 35 to 74 year old residents of four communities in the Atherosclerosis Risk in Communities (ARIC) Study. Biomarker adjustment accounted for change from reliance on cardiac enzymes to widespread use of troponin measurements overtime. Between 1987 and 2008, a total of 30,985 fatal or non-fatal hospitalized acute MI events occurred. Rates of CHD death among persons without a history of MI fell an average 4.7 percent per year among men and 4.3 percent per year among women. Rates of both in- and out-of-hospital CHD death declined significantly throughout the period. Age- and biomarker adjusted average annual rate of incident MI decreased 4.3 percent among white men, 3.8 percent among white women, 2.9 percent among black women, and 1.5 percent among black men. Declines in CHD mortality and MI incidence were greater in the second decade (1997–2008). Failure to account for biomarker shift would have masked declines in incidence, particularly among blacks. Age-adjusted 28-day case-fatality after hospitalized MI declined 4.2 percent per year among white men and 3.6 percent per year among black men, 2.6 percent per year among white women, and 2.4 percent per year among black women.
Conclusions
Although these findings from 4 communities may not directly generalize to blacks and whites in the entire US, we observed significant declines in MI incidence, primarily due to downward trends in rates between 1997 and 2008.
Keywords: epidemiology, mortality rates, myocardial infarction, surveillance, survival
Introduction
Recent studies suggest that trends in the incidence rate of myocardial infarction (MI) in the United States may have changed substantially in the last two decades from relatively stable rates in the 1980’s and 1990’s 1–4 to significant declines in the new millennium. 5–9 Some recent studies furthered our understanding of contemporary patterns of MI rates by examining trends by type of MI (presence or absence of ST-segment elevation) and by estimating the impact on rates brought about by the introduction of troponin measurements and new definitions of clinical events. 8, 10–13 Whether or not these recently reported trends apply similarly across race and gender groups and to what extent changes in biomarkers account for these trends is less well characterized. 14–15 Although available studies offer valuable insights into recent trends in the occurrence of MI and mortality due to coronary heart disease (CHD), additional data on trends in annual incidence of MI and CHD mortality from other large, geographically and ethnically diverse environments using a common methodology is needed and can provide valuable insights into disease trends in the population. The importance of these types of data was emphasized in a recent Institute of Medicine (IOM) report on cardiovascular disease surveillance needs in the United States. 16 We studied trends in mortality due to CHD and in the incidence of MI with and without a unique adjustment for changes in biomarkers over time from 1987 through 2008, as well as trends in short-term (28 day) case-fatality after MI from community surveillance in the Atherosclerosis Risk in Communities (ARIC) Study.
Methods
Study Population
Since 1987, The ARIC Study 17 has conducted continuous retrospective surveillance of hospital discharges for MI and deaths due to CHD occurring in or out of the hospital among residents 35 through 74 years of age in Forsyth County, North Carolina; the city of Jackson, Mississippi; eight northern suburbs of Minneapolis, Minnesota; and Washington County, Maryland with a combined study population of approximately 396,000 persons in 2008 (Table 1). Twenty-four percent of the study population was black. The trends reported here in ARIC blacks and whites in these four communities were of interest, even though the ARIC design cannot totally separate race effects from regional effects, given that sufficient numbers of blacks to yield stable estimates were only present in only 2 communities (Jackson, Mississippi and Forsyth County, North Carolina).
Table 1.
Hospitalized myocardial infarctions, coronary heart disease deaths and population estimates; age 35–74 years in the four ARIC communities. The ARIC Study 1987 through 2008 *
| Hospitalizations for myocardial infarction | Death due to Coronary Heart Disease | Population † | ||||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Total | Men | Women | Total | Men | Women | |
| Number (percent with first events) | ||||||||
| Race | ||||||||
| Black | 3937 (72) | 3140 (76) | 7077 (74) | 1206 (59) | 891 (64) | 2097 (61) | 41,138 | 52,807 |
| White | 16,423 (65) | 7485 (73) | 23,908 (67) | 4280 (47) | 1780 (54) | 6061 (49) | 147,072 | 155,497 |
| Total | 20,360 (66) | 10,625 (74) | 30,985 (69) | 5486 (49) | 2671 (57) | 8158 (52) | 188,210 | 208,304 |
| Community | ||||||||
| Forsyth County, NC | 8014 (70) | 4147 (75) | 12,161 (71) | 2163 (50) | 1000 (56) | 3163 (52) | 72,894 | 81,573 |
| Jackson, MS | 4384 (68) | 2697 (77) | 7081 (71) | 1595 (55) | 878 (68) | 2473 (60) | 31,102 | 38,494 |
| Minneapolis suburbs, MN | 4275 (64) | 2045 (73) | 6320 (67) | 974 (44) | 382 (48) | 1357 (45) | 52,454 | 56,212 |
| Washington County, MD | 3686 (59) | 1736 (67) | 5422 (62) | 754 (41) | 410 (48) | 1165 (43) | 31,760 | 32,025 |
The numbers shown in the table were estimated from sampled events that were validated: 12,947 hospitalized for myocardial infarction in men and 7128 in women; 4657 deaths due to CHD in men and 2406 in women. The percentages show how many of those with an event had no history of myocardial infarction.
Population numbers shown are of blacks and whites from 35 to 74 years of age in 2008.
Identification of Hospitalized MI Events
Hospitalized MIs were identified from electronic discharge lists obtained from all hospitals serving the four communities (n=31). Trained ARIC staff members abstracted medical records for possible events, selected on age, residence in the community and discharge code (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 402, 410–414, 427, 428, and 518.4) through random sampling within discharge code strata. See supplemental materials for a description of ICD-9-CM codes used to identify events for investigation and validation. Sampling probabilities varied by race, sex, field center, and discharge code group and were adjusted periodically. 18–19 Hospitalizations of community residents that occurred outside the study area were not included, unless transferred to and discharged from a surveillance hospital. Diagnostic information from the transferring hospital was included in the validation of events. Information obtained from medical records included: presence of chest pain, history of MI or other cardiovascular related conditions, and measures of cardiac biomarkers (total creatinine phosphokinase (CK), creatinine phosphokinase-myocardial band (CK-MB), lactate dehydrogenase, and troponin). Copies of up to three electrocardiograms were obtained and sent to the University of Minnesota Electrocardiographic Reading Center for classification according to the Minnesota code 20. A standardized computerized algorithm was applied to data on chest pain, cardiac biomarkers, and electrocardiographic evidence to determine each patient’s computer MI diagnosis 17. Criteria for each of these three diagnostic elements in the algorithm remained constant over the study period and are described in detail in the ARIC Study Surveillance Manual. 19 Cases with disagreements between the computer diagnosis and discharge diagnosis codes were reviewed by physicians on the ARIC Mortality and Morbidity Classification Committee (MMCC) for final classification. All eligible hospitalized events were classified as either definite, probable, suspect, or no MI. 17 Definite or probable MI was combined to define a MI for analysis unless otherwise specified. MI events with equivocal or abnormal biomarkers were further classified as non-ST segment elevation MI (NSTEMI) or ST-segment elevation MI (STEMI) based on the coded electrocardiograms.
A first (incident) MI was defined as one in a patient for whom the medical record either stated that there was no history of MI or did not contain any reference to a history of MI. Recurrent MI was defined as any definite or probable MI for which the medical record stated a history of MI.
Eligible hospitalizations for which the chart could not be located were deemed unclassifiable. Because missing hospital records are likely not random and may have included events that would have been validated as MI had the medical record been available, we adjusted the trends in hospitalized MI rates in sensitivity analyses to account for this possible source of bias.
Identification of CHD Deaths
For the period 1987–1998 deaths with underlying cause of death ICD-9-CM codes 250, 401, 402, 410–414, 427–429, 440, 518.4, 798, and 799 were sampled. Beginning in 1999, ICD-10-CM codes E10–14, I10–I11, I21–25, I46–51, I70, I97, J81, J96, R96, and R98–99 were sampled. Sampling fractions varied by sex, field center, and code group and were adjusted over time. Deaths among community residents occurring outside the state of residence were omitted. The number of such deaths was few (10 eligible out of state deaths in 2008) and stable over time. Deaths in nursing homes, emergency departments, and hospital admissions of persons dead on arrival were classified as out-of-hospital deaths. Trained ARIC staff reviewed death certificates that met sampling eligibility criteria. For in-hospital deaths, medical records were also reviewed. For out-of-hospital deaths, additional information was sought from the next of kin and other informants, certifying and family physicians, and coroners or medical examiners. Using standardized criteria, 17 the MMCC reviewed these data for deaths and assigned a final diagnosis, with disagreements adjudicated. Trends in CHD mortality included deaths classified as due to either definite fatal MI or definite fatal CHD. Due to state regulations that prohibited full investigation of out-of-hospital deaths in Washington County until 1995, this community was not included in computation of trends involving CHD death prior to that year.
For fatal CHD events, presence or absence of a history of MI was based on information obtained from the next of kin and other informants including the certifying physician, coroner or medical examiner, or from medical records for any eligible hospitalization within 28 days before death. Vital status of hospitalized MI events after discharge was determined through linkage with National Death Index files and used for computing case fatality percentages.
Adjustment for Biomarker Change
Dramatic shifts in the use of cardiac biomarkers to diagnose MI occurred between 1987 and 2008 10–11. In the ARIC communities, the proportion of eligible hospitalizations with a troponin measurement increased from 8% in 1996 to 98% by 2001. Patterns of biomarker adoption varied by community and by hospital within community. To permit a more meaningful interpretation of trends in MI, we developed an imputation method that standardizes the event rates to a consistent usage of cardiac biomarkers. Details of this method as applied to ARIC Surveillance data are described in supplemental materials and only briefly described here. We adjusted the event rates that include hospitalized myocardial infarction for changes in biomarkers by imputing the number of events that would have occurred in a pre-troponin year (i.e. had troponin not been introduced and other biomarkers not dropped), and had the distribution of the biomarker usage combinations been the same across years. The adjustment included both an imputation and a standardization procedure. First we imputed the distribution of the pre-troponin combinations (i.e. the biomarker usage combinations that would have occurred in a pre-troponin year) and then imputed the probability of myocardial infarctions in each of the pre-troponin combinations. We then standardized the imputed probability of myocardial infarctions, and therefore the number of events, to the distribution of the pre-troponin combinations in a reference period mimicking direct adjustment. This was an extension of direct adjustment where the distribution of the pre-troponin combinations for post-1995 events was imputed, from which the probability of myocardial infarctions in each of the pre-troponin combinations could be estimated by data regarding overlaps in troponin use and other biomarkers. The last step was weighting the pre-troponin combination-specific probability of myocardial infarction by the distribution of the pre-troponin combinations in the reference period.
To validate our biomarker adjustment method procedure we used ARIC data from 1997–2008 from ARIC for those events that had (troponic AND other enzymes) whenever they had “troponin OR other enzymes”, simulating an experiment where hospitals kept collecting enzyme data as usual while adding troponins. We then completely dropped troponins for 1997–2002, an artificial “pre-troponin era”. In our artificial data we used 2002 as the standard enzyme group distribution. We estimated age and enzyme distribution-standardized “true” trends in MI attack rate from the biomarker data without troponins. Next we produced 200 datasets that simulated the dropping of other enzymes when troponin was introduced. The choice of events from which to drop some enzyme data was random, and was done independently in each of the 200 simulations. We simulated an increasing proportion over time of events that had enzymes dropped. With each of these 200 datasets we applied the biomarker adjustment algorithm to get biomarker adjusted rates and trends for the 4 race sex groups for the period 1997–2008, and then took the average over the 200 datasets and compared with “true trends”. We considered only linear trend. The true age- and enzyme distribution-adjusted trends were −4.4 and −3.7 for men and women, respectively, whereas the biomarker-adjusted values averaged over the 200 simulated ARIC-like datasets were −4.0 and −3.3, very close to the true values.
Statistical Analysis
Sampling probabilities were reviewed periodically and modified over the 22-year surveillance period for efficiency. Details of the sampling procedure are reported elsewhere. 18 Our analyses were weighted and standard errors computed by stratified random sample methodology to reflect the sampling scheme.
Annual event rates per 1000 persons specific for sex, race, and community were computed based on population denominators estimated using interpolation and extrapolation of 1980, 1990 and 2000 United States Census population estimates. Race-specific rates reported by sex were adjusted for age by the direct method using the 2000 United States total age-specific population census counts as the standard. Sex-specific rates reported were similarly adjusted for age and race. In Tables 2 and 3, we report overall 22-year age-adjusted trends by gender and race or by gender adjusted additionally for race from linear or quadratic Poisson regression models. Results by time period are from the quadratic models showing trends separately for the first (1987 through 1996) and second (1997 through 2008) decade as the average annual percent change in each time period. Figure 1 shows age- and biomarker adjusted event rates with both linear and quadratic regression model fits displayed.
Table 2.
Average annual percent change (95% confidence interval) in rates of death due to CHD or rates of hospitalized myocardial infarction, adjusted for age, The ARIC Study 1987 through 2008.* MEN
| Event type | Whites 1987–1996 |
1997–2008 | All years | Blacks 1987–1996 |
1997–2008 | All years | Total † 1987–1996 |
1997–2008 | All years |
|---|---|---|---|---|---|---|---|---|---|
| Death due to CHD | −4.0 (−5.1, −3.0) |
−9.8 (−11.1, −8.5) |
−6.5 (−7.0, −6.1) |
−0.9 (−3.3, 1.5) |
−5.3 (−7.5, −3.2) |
−3.2 (−4.1, −2.2) |
−3.4 (−4.3, −2.4) |
−8.6 (−9.7, −7.5) |
−5.7 (−6.1, −5.3) |
| Death due to CHD without history of MI |
−4.5 (−6.2, −2.8) |
−7.3 (−9.3, −5.2) |
−5.7 (−6.4, −4.9) |
−1.1 (−4.3, 2.2) |
−3.2 (−6.0, −0.4) |
−2.2 (−3.5, −0.9) |
−3.6 (−5.1, −2.1) |
−6.0 (−7.6, −4.3) |
−4.7 (−5.3, −4.1) |
| In-hospital death due to CHD |
−4.9 (−6.7, −3.2) |
−12.4 (−15.0, −9.7) |
−7.9 (−8.7, −7.1) |
−0.2 (−5.2, 4.9) |
−8.7 (−13.7, −3.7) |
−4.3 (−6.3, −2.4) |
−4.1 (−5.7, −2.4) |
−11.6 (−13.9, −9.3) |
−7.2 (−8.0, −6.5) |
| Out-of-hospital death due to CHD |
−3.4 (−4.8, −2.0) |
−8.4 (−9.9, −6.8) |
−5.6 (−6.2, −5.0) |
−1.2 (−3.9, 1.6) |
−4.1 (−6.5, −1.7) |
−2.7 (−3.8, −1.6) |
−2.8 (−4.0, −1.6) |
−7.1 (−8.4, −5.9) |
−4.9 (−5.4, −4.3) |
| First MI or death due to CHD ‡ |
−3.7 (−4.5, −3.0) |
−6.4 (−7.3, −5.4) |
−4.9 (−5.3, −4.5) |
−0.9 (−2.8, 1.1) |
−2.6 (−4.1, −1.0) |
−1.8 (−2.6, −1.0) |
−3.2 (−3.9, −2.5) |
−5.5 (−6.3, −4.6) |
−4.3 (−4.6, −3.9) |
| First MI or death due to CHD |
−1.7 (−2.6, −0.9) |
−4.0 (−5.2, −2.8) |
−2.9 (−3.4, −2.4) |
2.3 (−0.1, 4.6) |
−0.8 (−2.6, 0.9) |
0.4 (−0.5, 1.4) |
−0.9 (−1.8, −0.1) |
−3.1 (−4.1, −2.1) |
−2.1 (−2.5, −1.7) |
| First MI ‡ | −3.6 (−4.5, −2.7) |
−5.1 (−6.4, −3.8) |
−4.3 (−4.7, −3.8) |
−0.4 (−3.3, 2.6) |
−2.5 (−4.7, −0.4) |
−1.5 (−2.7, −0.4) |
−3.2 (−4.1, −2.3) |
−4.5 (−5.6, −3.4) |
−3.8 (−4.2, −3.3) |
| First MI | −1.4 (−2.4, −0.5) |
−3.7 (−5.0, −2.4) |
−2.6 (−3.1, −2.1) |
3.2 (0.4, 6.1) |
−0.5 (−2.5, 1.5) |
1.0 (−0.1, 2.1) |
−0.8 (−1.7, 0.1) |
−2.9 (−.3.9, −1.8) |
−1.9 (−2.3, −1.4) |
| First STEMI ‡ | −3.0 (−4.5,−1.5) |
−8.4 (−11.1,−5.6) |
−5.4 (−6.2,−4.5) |
4.2 (0.0,8.3) |
−7.4 (−12.1,−2.8) |
−2.2 (−3.8,−0.5) |
−2.0 (−3.4,−0.5) |
−8.0 (−10.4,−5.7) |
−4.8 (−5.6,−4.0) |
| First STEMI | −1.2 (−2.7,0.3) |
−8.8 (−11.2,−6.4) |
−4.8 (−5.5,−4.0) |
6.3 (2.2,10.5) |
−5.9 (−10.2,−1.6) |
−0.7 (−2.3,0.9) |
−0.2 (−1.6,1.2) |
−7.9 (−10.0,−5.9) |
−4.0 (−4.7,−3.3) |
| First NSTEMI ‡ | −6.3 (−7.6,−5.1) |
−3.5 (−5.2,−1.8) |
−4.8 (−5.5,−4.1) |
−4.0 (−7.8,−0.2) |
−0.4 (−3.1,2.3) |
−2.0 (−3.6,−0.4) |
−6.0 (−7.2,−4.8) |
−2.7 (−4.2,−1.3) |
−4.3 (−4.9,−3.7) |
| First NSTEMI | −2.1 (−3.4,−0.9) |
−0.8 (−2.4,0.8) |
−1.4 (−2.1,−0.7) |
1.1 (−2.6,4.8) |
2.2 (−0.2,4.6) |
1.8 (0.3,3.2) |
−1.7 (−2.9,−0.5) |
−0.1 (−1.4,1.3) |
−0.8 (−1.4,−0.2) |
| Recurrent MI ‡ | −4.1 (−5.6, −2.7) |
−9.3 (−11.4, −7.2) |
−6.3 (−7.0, −5.6) |
−2.2 (−5.6, 1.2) |
−2.8 (−6.3, 0.8) |
−2.5 (−4.1, −0.9) |
−4.0 (−5.3, −2.7) |
−8.0 (−9.8, −6.2) |
−5.7 (−6.4, −5.1) |
Average annual percent change from quadratic regression models. Negative numbers indicate a decrease, and positive numbers an increase.
Adjusted also for race
Trends also adjusted for changes in cardiac biomarkers over time.
Table 3.
Average annual percent change (95% confidence interval) in rates of death due to CHD or rates of hospitalized myocardial infarction, adjusted for age, The ARIC Study 1987 through 2008.* WOMEN
| Event type | Whites 1987–1996 |
1997–2008 | All years | Blacks 1987–1996 |
1997–2008 | All years | Total † 1987–1996 |
1997–2008 | All years |
|---|---|---|---|---|---|---|---|---|---|
| Death due to CHD | −1.6 (−3.3, 0.1) |
−10.9 (−12.8, −9.0) |
−5.8 (−6.5, −5.1) |
−3.2 (−6.0, −0.4) |
−5.1 (−7.5, −2.6) |
−4.0 (−5.2, −2.9) |
−2.3 (−3.7, −0.9) |
−8.6 (−10.0, −7.1) |
−5.2 (−5.8, −4.6) |
| Death due to CHD without history of MI |
0.4 (−2.0, 2.9) |
−10.2 (−12.8, −7.6) |
−4.7 (−5.6, −3.7) |
−3.1 (−6.3, 0.1) |
−4.2 (−7.2, −1.2) |
−3.6 (−5.0, −2.2) |
−1.1 (−3.0, 0.8) |
−7.7 (−9.6, −5.7) |
−4.3 (−5.1, −3.5) |
| In-hospital death due to CHD |
−2.7 (−5.0, −0.4) |
−13.5 (−16.3,−10.7) |
−7.2 (−8.2, −6.3) |
−7.1 (−11.9, −2.4) |
−5.1 (−9.2, −1.1) |
−6.0 (−7.9, −4.1) |
−4.3 (−6.5, −2.1) |
−10.6 (−12.9, −8.3) |
−6.9 (−7.8, −6.0) |
| Out-of-hospital death due to CHD |
−0.3 (−3.0, 2.4) |
−8.8 (−11.4, −6.2) |
−4.4 (−5.5, −3.4) |
0.2 (−3.2, 3.5) |
−5.2 (−8.3, −2.1) |
−2.6 (−4.0, −1.2) |
−0.3 (−2.3, 1.8) |
−7.2 (−9.2, −5.3) |
−3.7 (−4.6, −2.9) |
| First MI or death due to CHD ‡ |
−2.4 (−3.7, −1.2) |
−5.5 (−6.9, −4.2) |
−3.9 (−4.5, −3.4) |
−2.0 (−4.3, 0.3) |
−5.0 (−6.9, −3.0) |
−3.5 (−4.4, −2.6) |
−2.4 (−3.5, −1.3) |
−5.3 (−6.4, −4.2) |
−3.8 (−4.3, −3.3) |
| First MI or death due to CHD |
−0.1 (−1.7, 1.4) |
−3.3 (−4.8, −1.7) |
−1.8 (−2.5, −1.1) |
0.3 (−2.0, 2.7) |
0.2 (−1.7, 2.2) |
0.3 (−0.8, 1.3) |
−0.1 (−1.4, 1.2) |
−1.9 (−3.1, −0.7) |
−1.1 (−1.7, −0.5) |
| First MI ‡ | −3.0 (−4.5, −1.5) |
−4.8 (−6.5, −3.1) |
−3.8 (−4.5, −3.1) |
−2.4 (−5.6, 0.7) |
−3.3 (−5.8, −0.8) |
−2.9 (−4.2, −1.5) |
−2.8 (−4.2, −1.5) |
−4.2 (−5.7, −2.8) |
−3.5 (−4.1, −2.9) |
| First MI | −0.9 (−2.4, 0.6) |
−2.5 (−4.2, −0.9) |
−1.7 (−2.4, −1.0) |
0.8 (−2.0, 3.6) |
0.9 (−1.4, 3.1) |
0.8 (−0.4, 2.1) |
−0.5 (−1.8, 0.8) |
−1.2 (−2.5, 0.1) |
−0.9 (−1.5, −0.3) |
| First STEMI ‡ | −2.2 (−4.5,0.2) |
−6.9 (−10.5,−3.3) |
−4.4 (−5.6,−3.1) |
−0.8 (−5.2,3.6) |
−5.5 (−11.2,0.1) |
−3.3 (−5.2,−1.3) |
−1.9 (−3.9,0.2) |
−6.5 (−9.5,−3.5) |
−4.1 (−5.1,−3.0) |
| First STEMI | −0.5 (−3.0,1.9) |
−7.3 (−10.8,−3.8) |
−3.8 (−4.9,−2.7) |
0.4 (−4.1,5.0) |
−2.6 (−8.3,3.2) |
−1.2 (−3.3,0.9) |
−0.4 (−2.6,1.7) |
−5.8 (−8.8,−2.8) |
−3.1 (−4.1,−2.1) |
| First NSTEMI ‡ | −5.5 (−7.4,−3.5) |
−3.7 (−6.0,−1.5) |
−4.5 (−5.4,−3.6) |
−4.4 (−8.3,−0.6) |
−3.5 (−6.6,−0.3) |
−3.9 (−5.5,−2.2) |
−5.1 (−6.9,−3.3) |
−3.5 (−5.4,−1.7) |
−4.2 (−5.1,−3.4) |
| First NSTEMI | −1.4 (−3.3,0.5) |
−0.5 (−2.4,1.5) |
−0.9 (−1.8,0.0) |
−0.0 (−3.5,3.5) |
1.5 (−1.1,4.1) |
0.9 (−0.7,2.4) |
−1.0 (−2.7,0.7) |
0.3 (−1.2,1.9) |
−0.2 (−1.0,0.5) |
| Recurrent MI ‡ | −5.8 (−8.0, −3.7) |
−6.4 (−9.4, −3.4) |
−5.9 (−7.1, −4.8) |
−1.9 (−7.0, 3.2) |
−10.8 (−15.8, −5.8) |
−6.0 (−7.8, −4.1) |
−4.7 (−6.8, −2.7) |
−7.5 (−10.0, −4.9) |
−5.8 (−6.8, −4.9) |
Average annual percent change from quadratic regression models. Negative numbers indicate a decrease, and positive numbers an increase.
Adjusted also for race
Trends also adjusted for changes in cardiac biomarkers over time.
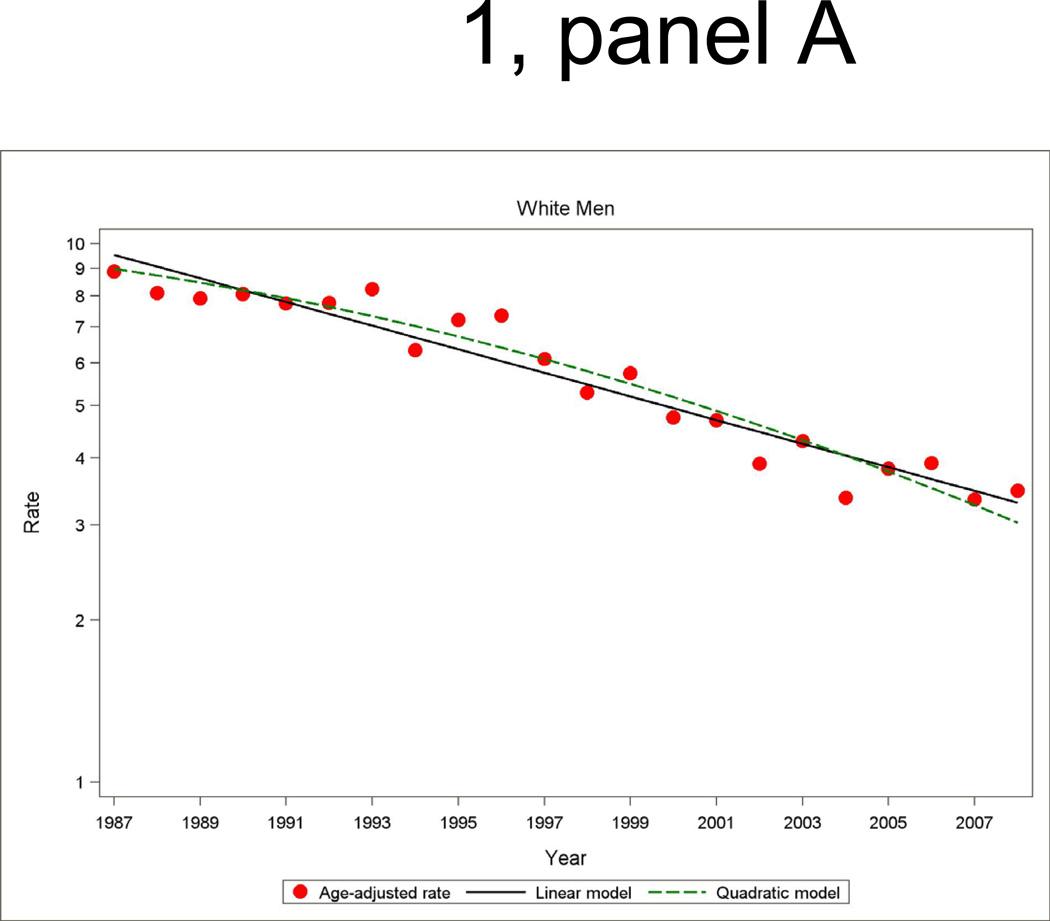
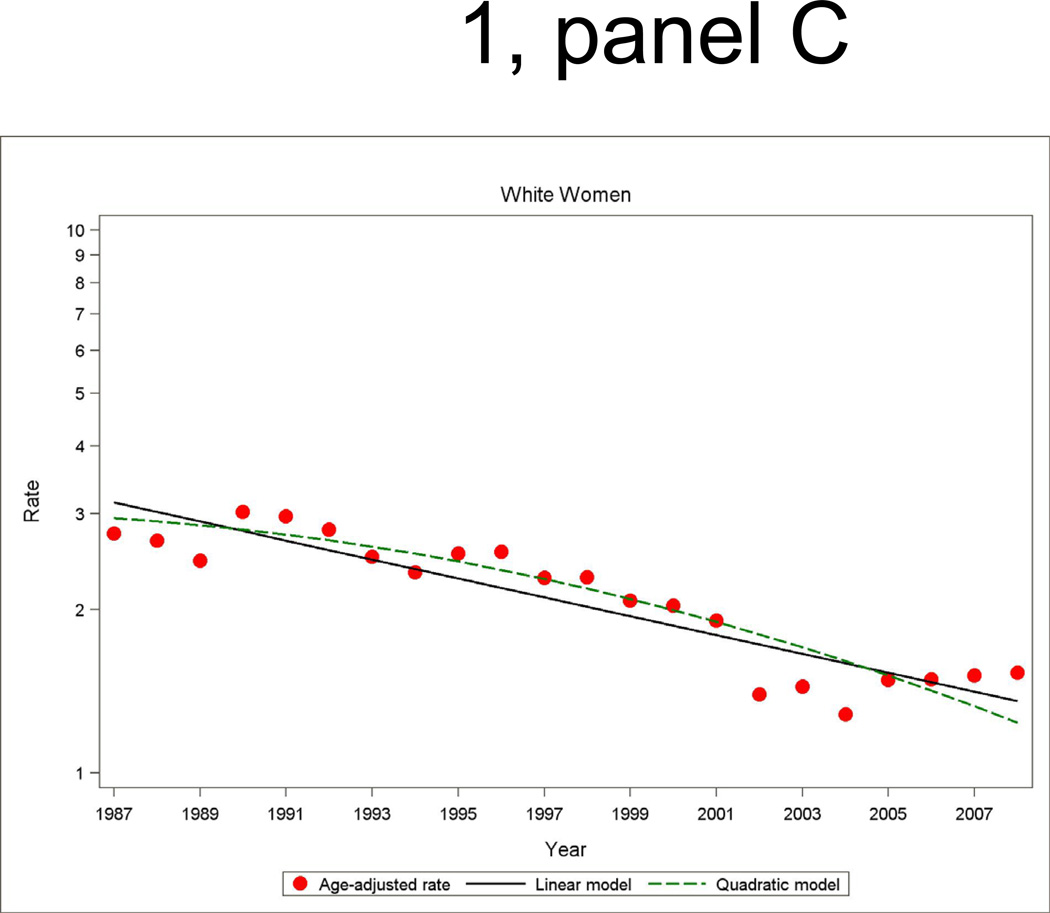
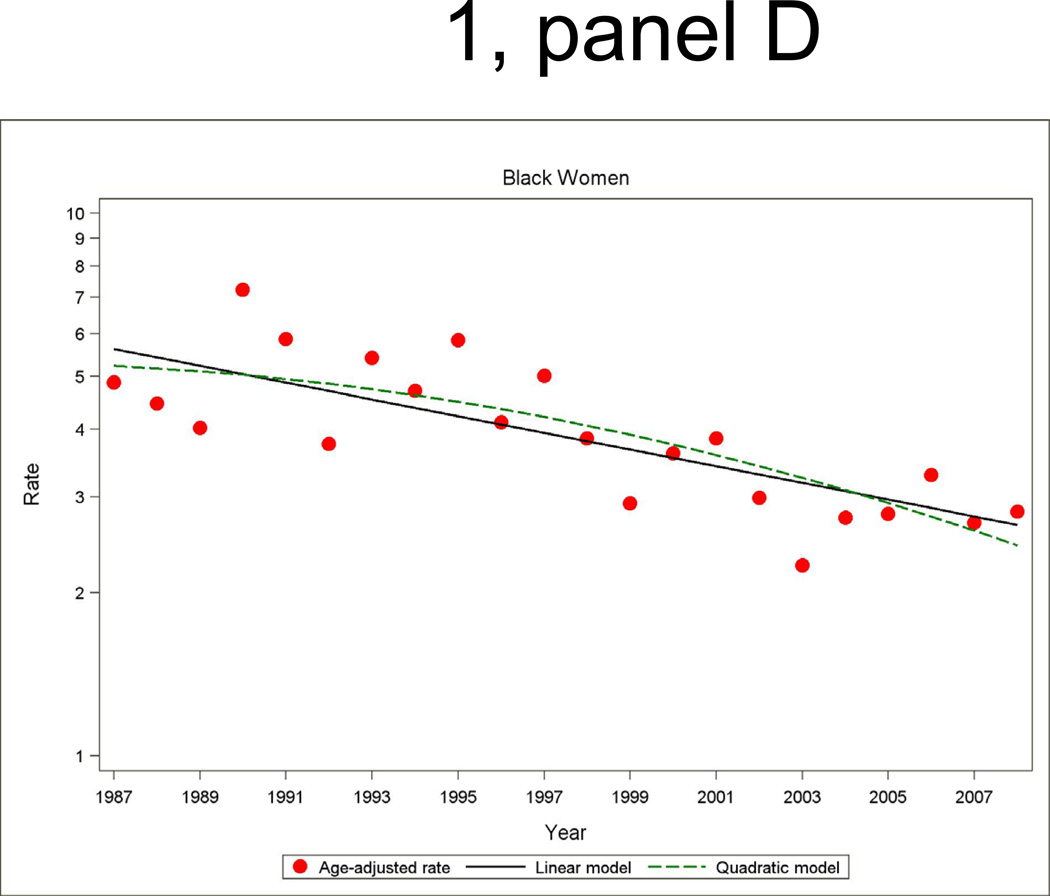
Figure 1.
Age- and biomarker-adjusted rate (per 1000 persons) in first hospitalized myocardial infarction or death due to CHD without prior myocardial infarction, and age-adjusted trends by linear or quadratic Poisson regression, men and women 35 to 74 years of age, the ARIC Study 1987 through 2008.
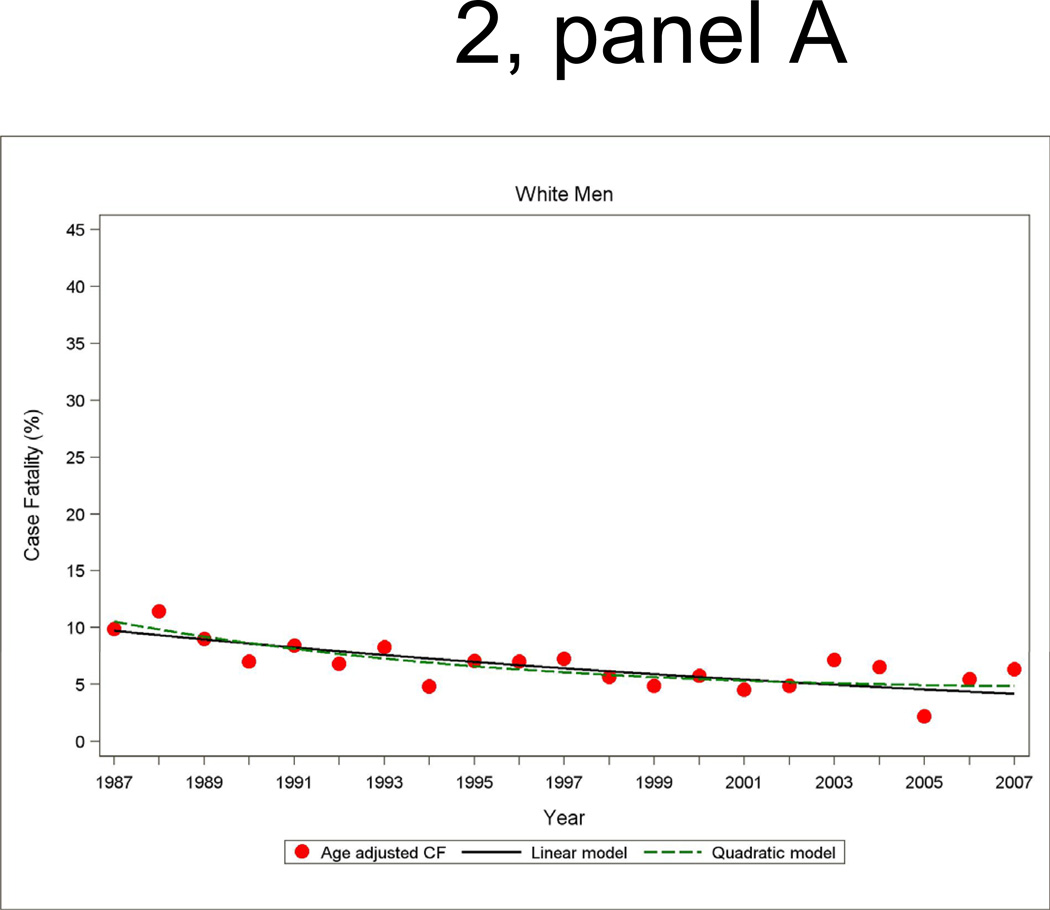
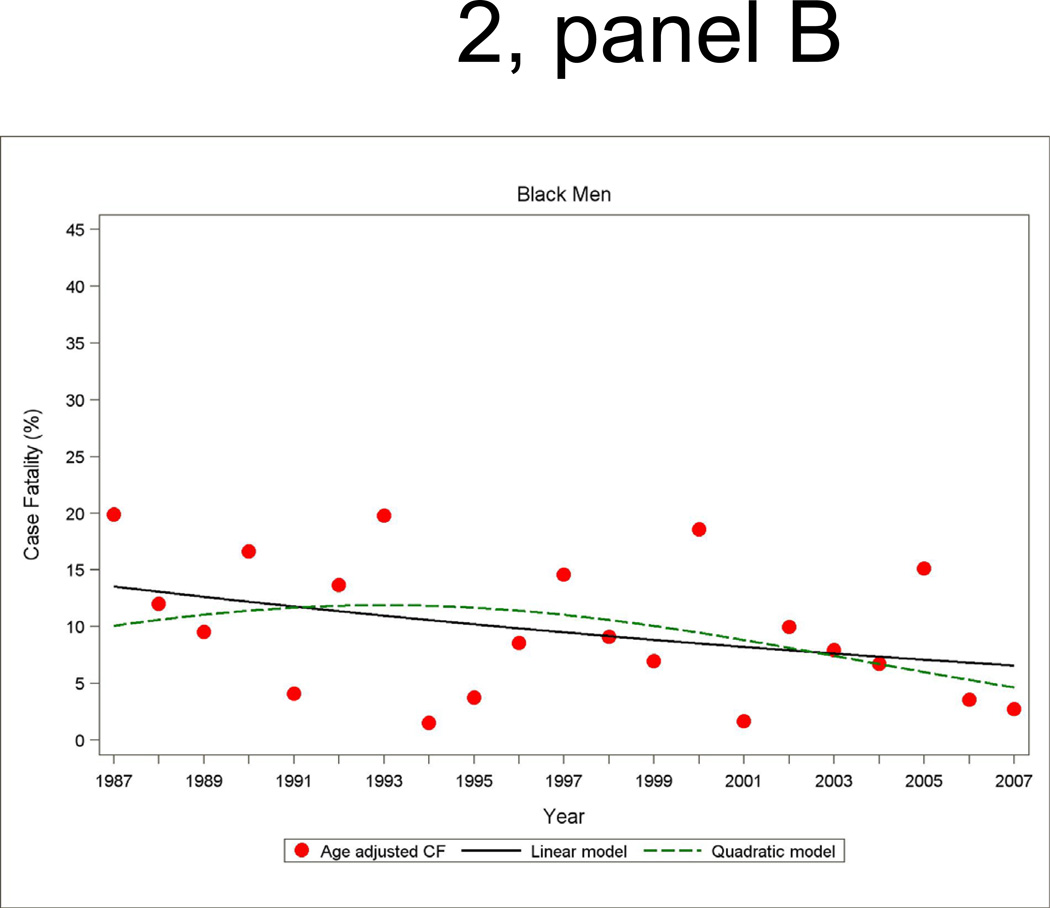
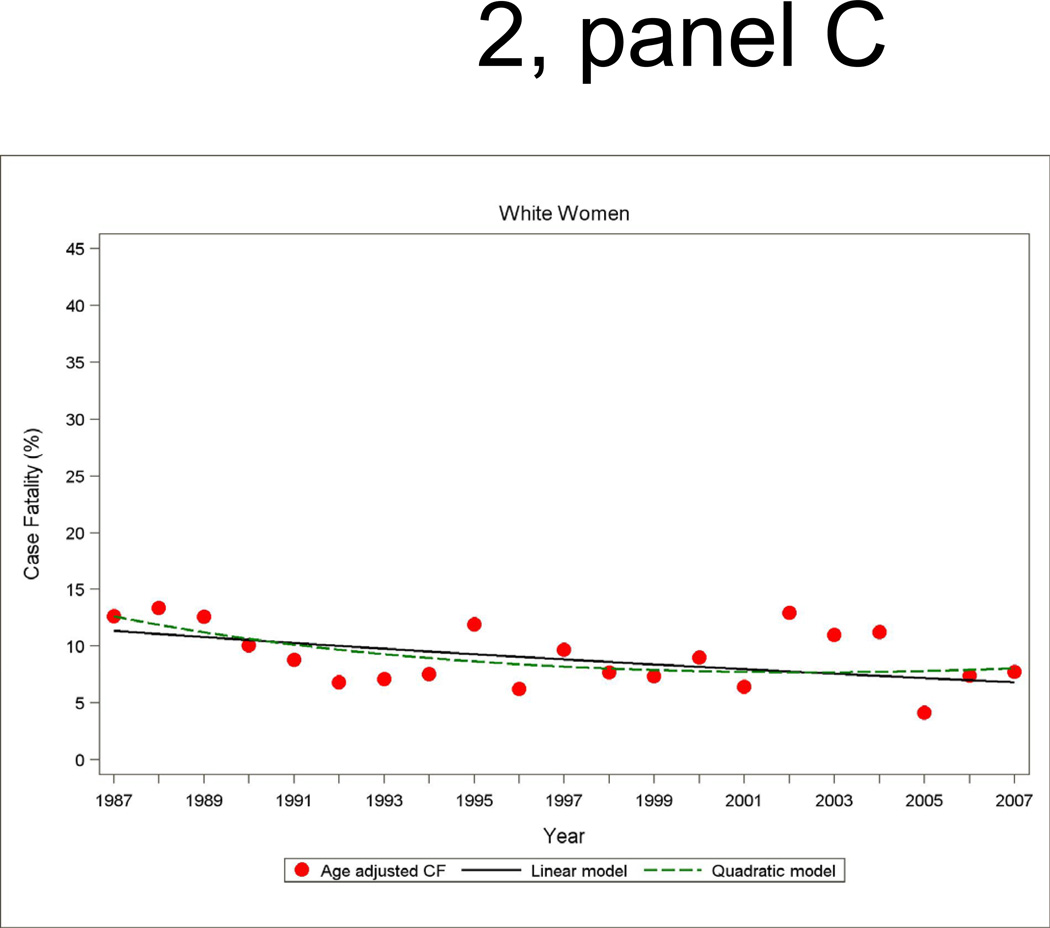
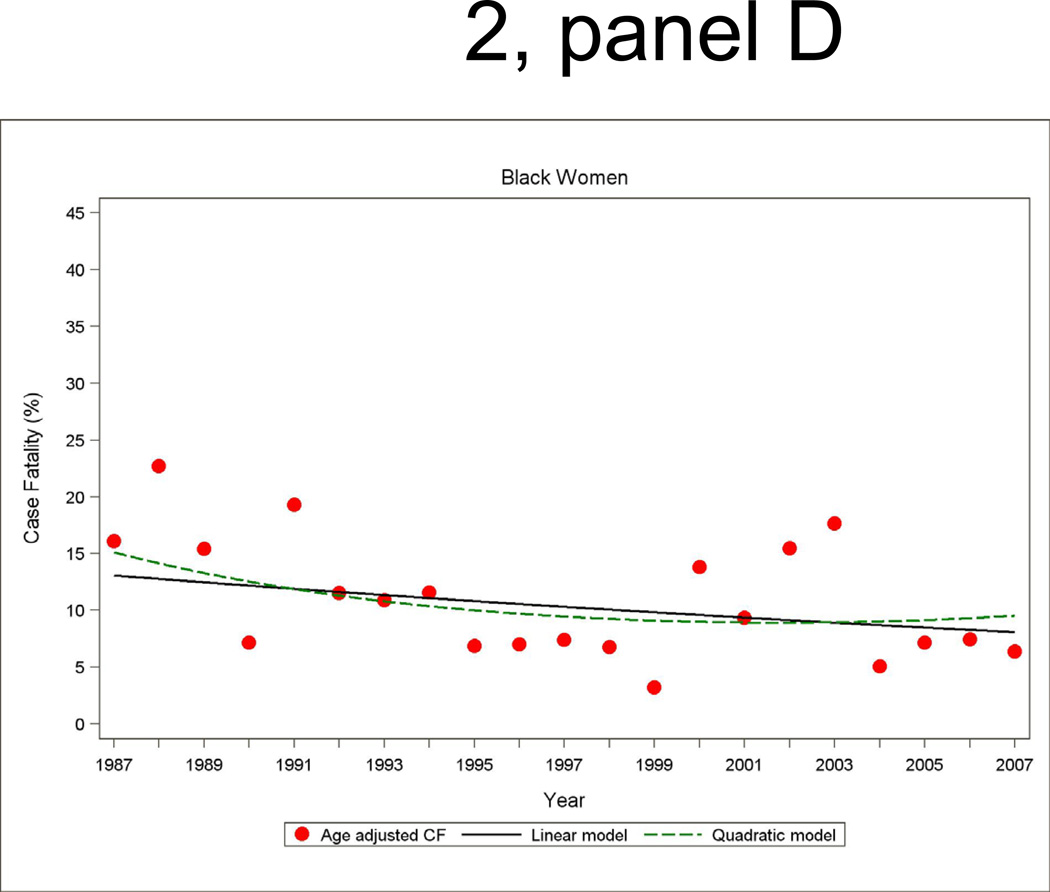
Annual 28-day and one-year case fatality percentages specific for sex and race were computed based on denominators of those who were hospitalized with a MI or a combined hospitalized plus fatal CHD event. Race-specific percentages reported by sex were adjusted for age by the direct method using the ARIC combined hospitalized MI plus fatal CHD events as the standard. Figure 2 shows age-adjusted 28-day case fatality for hospitalized MI with both linear and quadratic logistic regression model fits displayed. The statistical packages SUDAAN Logistic was used for case fatality trends analysis and SUDAAN Loglink for event rate trends analysis
Figure 2.
Age-adjusted 28-day case fatality percentage for hospitalized MI events, and age-adjusted trends by linear or quadratic Poisson regression, men and women 35 to 74 years of age, the ARIC Study 1987 through 2008.
Results
Between 1987 and 2008, a total of 30,985 fatal or non-fatal hospitalized acute MI events (based on stratified random sample of 20,075 hospitalizations investigated) occurred among residents age 35 through 74 years in the four study communities in ARIC (Table 1). Of these, 69% were in persons with no recorded history of MI. There were an estimated 8158 deaths due to CHD (on the basis of 7063 deaths sampled), including both in-hospital and out-of-hospital deaths.
The average annual percent age-and race-adjusted decline (95 percent confidence interval [CI]) in rates of death due to CHD was 5.7 percent (95% CI: −6.1, −5.3) in men and 5.2 percent (95% CI: −5.8, −4.6) in women (Tables 2 and 3). Among men, the decline was non-linear, with the decline steeper in the latter half of the study period (1997 through 2008) than in the first 10 years (1987 through 1996), −8.6 percent (95% CI: −9.7, −7.5) per year and −3.4 percent (95% CI: −4.3, −2.4) per year, respectively (p<0.01). The overall downward age-adjusted trend in total CHD mortality among men was statistically significant in both ARIC black men (−3.2 percent (95% CI: −4.1, −2.2) per year) and ARIC white men (−6.5 percent (95% CI: −7.0, −6.1) per year), with the percent decline per year among ARIC white men generally about twice that of ARIC black men regardless of time period. Of note, is the statistically significant age-adjusted decline in total CHD mortality rates of 5.3 percent (95% CI: −7.5, −3.2) per year among ARIC black men from 1997 through 2008 compared to a non-statistically significant decline of just 0.9 percent (95% CI: −3.3, 1.5) per year in the preceding 10 years from 1987 through 1996. Among women, the age- and race-adjusted trends in total CHD mortality rates were generally similar to those in men (i.e. downward trends in rates greater in the more recent period (p<0.01)). The age-adjusted trends in CHD deaths not preceded by a MI history mirrored those of total CHD mortality.
Rates of out-of-hospital and in-hospital mortality due to CHD declined significantly among both men and women (Tables 2 and 3). Age- and race-adjusted declines in rates of out-of-hospital mortality due to CHD were smaller in percentage compared to in-hospital CHD deaths. Adjusted percent declines in both in-hospital and out-of-hospital CHD death rates were substantially greater in the more recent time period (1997 through 2008) compared to the previous decade (1987 through 1996).
Among men and women, the age- and biomarker-adjusted rate of combined first hospitalization for acute MI or fatal CHD among patients with no history of MI had a significant age-adjusted decline from 1987 to 2008 (Figure 1 and Tables 2 and 3). The overall age-adjusted trend predicted by the fitted quadratic models in annual incidence rates showed a decline of 4.9 percent (95% CI: −5.3, −4.5) per year among ARIC white men, 3.9 percent (95% CI: −4.5, −3.4) per year among ARIC white women, 3.5 percent (95% CI: −4.4, −2.6) per year among ARIC black women, and 1.8 percent (95% CI: −2.6, −1.0) per year among ARIC black men. The average trend for the two decades separately from quadratic regressions models show that the decline in incidence of MI and fatal CHD was generally twice as large in the latter decade compared to the first decade. The difference in the average annual percent change between the two decades was statistically significant for ARIC white men and women (p<0.01). However, the trends comparing the first and second decade among ARIC black men and women did not reach statistical significance (p>0.10). Nevertheless, age-adjusted declines in biomarker adjusted MI and fatal CHD incidence were statistically significant in all four race-gender groups in the most recent time period (1996 through 2008).
The age- and biomarker-adjusted incidence of hospitalizations for MI had significant adjusted declines over the 22-year period. The overall downward trend showed a similar pattern to the trend in combined incident hospitalized MI and fatal CHD, although a test for differences in the average change in trends between the decades did not reach statistical significance. Of note is that among black men and women in ARIC, the lack of a statistically significant downward trend in first hospitalized MI in the earlier time period transitioned to significant declines in MI incidence during the more recent period (1997 through 2008), of −2.5 percent (95% CI: −4.7, −0.4) per year and −3.3 percent (95% CI: −5.8, −0.8) per year, respectively. An examination of trends in recurrent MI revealed significant declines overall, with the declines in men in the period 1997 through 2008 greater than in the earlier decade (p<0.01).
The impact of biomarker change adjustment was particularly notable in investigating trends within and across race-gender groups (Tables 2 and 3). The statistically significant declines in incidence of hospitalized MI events among ARIC blacks in the most recent time period found in the biomarker adjusted rates were masked when shifts in biomarkers were not considered. For example, the age-adjusted average annual percent change in first hospitalized MI over the 22-year surveillance period among black men in ARIC showed an increase of 1.0 percent (95% CI: −0.1, 2.1) per year before accounting for the use of more sensitive biomarkers. After adjustment for biomarker change over time a significant downward trend of 1.5 percent (95% CI: −2.7, −0.4) per year was revealed.
The annual incidence rate of STEMI had age- and biomarker-adjusted declines among men and women (Tables 2 and 3). For men, the decline was greater in the period from 1997 through 2008 (−8.0 percent (95% CI: −10.4, −5.7) per year) than in the prior decade (−2.0 percent (95% CI: −3.4, −0.5) per year) (p<0.01). The age- and biomarker-adjusted incidence of NSTEMI also declined over the 22-year period. Without biomarker adjustment, the rate of decline in NSTEMI was about half that observed for STEMI.
The trends in 28-day case fatality percentages among hospitalized MI cases are shown in Figure 2 and Table 4. The overall decline in 28-day case fatality among men for hospitalized cases was similar in the both decades, although a greater improvement in 28-day case fatality in the more recent decade occurred among black men in ARIC. For men the age- and race adjusted annual percent change in 28-day case fatality for hospitalized MI was −4.4 percent (95% CI: −7.5, −1.2) per year from 1987 to 1996 and −3.3 percent (95% CI: −6.5, −0.2) per year from 1997 to 2007 (Table 4). Among women, the significant decline in 28-day case fatality seen from 1987 through 1996 was no longer significant in the more recent decade.
Table 4.
Average annual percent change (95% confidence interval) in proportion of events not surviving 28 days (28 day case fatality), adjusted for age. The ARIC Study 1987 through 2008.
| Whites | Blacks | Total * | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1987–1996 | 1997–2007 | All years | 1987–1996 | 1997–2007 | All years | 1987–1996 | 1997–2007 | All years | |
| MEN | |||||||||
| Hospitalized MI patients only | |||||||||
|
28-day case fatality |
−5.9 (−12.0, 0.2) |
−1.8 (−7.6, 4.0) |
−4.2 (−5.9, −2.4) |
1.4 (−2.3, 5.1) |
−8.7 (−12.4, −5.0) |
−3.6 (−6.4, −0.8) |
−4.4 (−7.5, −1.2) |
−3.3 (−6.5, −0.2) |
−3.9 (−5.4, −2.5) |
| Hospitalized MI patients plus out of hospital fatal CHD events | |||||||||
|
28-day case fatality |
−1.9 (−5.0, 1.1) |
−4.8 (−7.4, −2.1) |
−3.1 (−3.8, −2.4) |
−1.2 (−3.3, 1.0) |
−4.6 (−7.1, −2.0) |
−2.8 (−3.8, −1.7) |
−1.8 (−3.5, 0.0) |
−4.7 (−6.5, −2.8) |
−3.0 (−3.6, −2.4) |
| WOMEN | |||||||||
| Hospitalized MI patients only | |||||||||
|
28-day case fatality |
−4.6 (−11.8, 2.6) |
0.0 (−5.6, 5.6) |
−2.5 (−4.4, −0.6) |
−5.0 (−7.7, 2.4) |
0.1 (−4.4, 4.6) |
−2.4 (−5.7, 0.9) |
−4.8 (−7.3, −2.3) |
−0.1 (−3.7, 3.5) |
−2.6 (−4.3, −0.9) |
| Hospitalized MI patients plus out of hospital fatal CHD events | |||||||||
|
28-day case fatality |
−1.6 (−4.0, 0.9) |
−4.7 (−7.1, −2.3) |
−2.9 (−4.0, −1.8) |
−3.2 (−4.3, −2.0) |
−0.1 (−2.1, 1.8) |
−1.6 (−3.0, −0.2) |
−2.3 (−3.3, −1.3) |
−2.6 (−4.1, −1.0) |
−2.4 (−3.3, −1.6) |
Adjusted also for race
Trends in a modified definition of MI not including biomarkers (presence of evolving diagnostic Q wave patterns on serial electrocardiograms, or as any evidence of any diagnostic Q wave or ST-segment elevation on any electrocardiograms and a history of chest pain of cardiac origin) yielded similar patterns to those seen in Tables 2 and 3. Accounting for lack of data on out-of-hospital deaths among community residents for whom neither an informant interview or physician questionnaire was available had little effect on the overall patterns of CHD mortality trends. Similarly, adjustment of hospitalized MI events for missing records did not appreciably change the original trend estimates.
Discussion
We found that the age-adjusted CHD mortality rates declined among 35 to 74 year olds in four geographically and ethnically diverse communities in the ARIC Study from 1987 through 2008. Although the event trends observed in the ARIC communities may not be representative of the entire US, the decline in CHD mortality rate was statistically significant for both blacks and whites in ARIC. This decline in CHD mortality in the ARIC communities was similar to that reported from national vital statistics. 21–23 However, the three-fold acceleration of the decline in CHD mortality rates in the most recent decade (1997 to 2008) in the ARIC communities was greater than the 2-fold acceleration of the decline in early 2000s reported using United States statistics. 23 The Framingham Study cohort and a community surveillance study in Worcester, Massachusetts also reported that the decline CHD mortality and sudden cardiac death has accelerated in recent decades. 24–25
A major determinant of the accelerated decline in CHD mortality observed is the concomitant decline in MI incidence. After accounting for shifts in biomarkers over time, the incidence of hospitalized MI declined an average 3.8 percent per year in men and 3.5 percent per year in women. The percent declines during the most recent time period (1997 through 2008) were approximately twice those of the previous decade and were most dramatic among blacks. Our findings corroborate those reported in the Kaiser Permanente Northern California health care system 7 where the incidence of MI increased from 1999 to 2000, and then decreased each year thereafter through 2008. A greater decline in STEMI compared to non-STEMI found in the Kaiser Permanente study is in agreement with the trends in STEMI and non-STEMI we observed in the four ARIC communities. However, the Kaiser data were not reported by ethnicity, were not adjusted for change in use of cardiac biomarkers, and represented trends in non-validated events based solely on discharge diagnosis codes or through billing claims.
Recent reports from Olmsted County, Minnesota 8 and Worcester, Massachusetts 9 indicate that trends in incidence of MI varied by the presence or absence of ST elevation. In Olmsted County, incidence rates of STEMI declined by 41% from 1987 through 2006, whereas the incidence rates of non-STEMI increased by a similar percentage. In Olmsted County, when all MIs were included irrespective of the biomarker used for diagnosis, the incidence rates of MI did not change between 1987 and 2006. In analysis restricted to those cases meeting only CK and CK-MB criteria, they found a significant temporal decline in the incidence of MI of about 1 percent per year. However, this method of adjustment may not adequately account for the addition of new biomarkers and the elimination of older biomarkers in some hospitals.
Our findings agree with a recent study of hospitalization rates among the Medicare Fee-for-Service beneficiaries.5 From 2002 to 2007, white men experienced a 24 percent decrease in hospitalized MI rates, whereas black men experienced a decline of 18 percent. Direct comparisons between Medicare data and ARIC are limited because Medicare events were non-validated, lacked a differentiation between incident and recurrent events, and were restricted to individuals over age 65 years. However, the Medicare findings agree well with those from the National Hospital Discharge survey showing a decline in hospitalization rates for acute MI from 1996 to 2005 after a period of stability in rates between 1987 and 1995. 6
Our results on case fatality trends agree with previous studies reporting steady improvements in age-and sex-adjusted mortality after MI in recent decades 7–8, although we found the decline among women to be less consistent than that observed in men. Strengths of our study include its population-based design, inclusion of multiple ethnically diverse communities, standardized event validation procedures and innovative application of standard statistical adjustment methods for accounting for shifts in biomarkers use over time. However, conducting on-going community surveillance and validation of hospitalizations among all residents in multiple communities over 22-years presents challenges to maintaining comparability across time. Hospitalizations of community residents occurring in hospitals occurring out of state are only included if the patient was transferred to a surveillance hospital. Although this may be a source of bias in our study, data from death certificate surveillance suggest that the relatively few out of state events have little impact on our trend estimates. Also due to small numbers of blacks in two of the four communities, event rates for blacks only represent those occurring in two communities. This may limit the generalizability of our findings.
The ultimate measure of successful public health and clinical efforts to reduce the major cause of mortality in the United States comes from community-based studies of disease incidence rates. 26–27 ARIC findings on declining incidence trends of hospitalized MI and out of hospital CHD death and steady improvements in 28-day case fatality, viewed together with other reports from community based studies, large national databases, and large health maintenance organizations, strongly support the conclusion that the past decade has seen a new era of impact from primary prevention efforts in the United States. 5, 7–8, 28 This conclusion could not be made 10 years ago when, although coronary heart disease mortality rates were falling, incidence of hospitalized MI remained static 1, 29. Our novel approach to accounting for changing diagnostic biomarkers over time adds new evidence in support of this conclusion. Maintaining the decline in incidence of MI that has gained momentum in the new millennium and continuing the decline in death due coronary heart disease will require continued efforts to promote cardiovascular health at the community level.
Supplementary Material
Acknowledgements
The authors thank the staff and participants of the ARIC Study for their important contributions.
Funding Sources: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None
References
- 1.Rosamond WD, Chambless LE, Folsom AR, Cooper LS, Conwill DE, Glegg L, Wang CH, Heiss G. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987 to 1994. N Engl J Med. 1998;339:861–867. doi: 10.1056/NEJM199809243391301. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg RJ, Yarzebski J, Lessard D, Gore J. A two-decade (1975 to 1995) long experience in the incidence, in-hospital and long-term case-fatality rates of acute myocardial infarction: a community-wide perspective. J Am Coll Cardiol. 1999;33:1533–1539. doi: 10.1016/s0735-1097(99)00040-6. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, Jacobsen SJ, Weston SA, Goraya T, Killian BB, Reeder GS, Kottke TE, Yawn BP, Frye RL. Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med. 2002;136:341–348. doi: 10.7326/0003-4819-136-5-200203050-00005. [DOI] [PubMed] [Google Scholar]
- 4.McGovern PG, Pankow JS, Shahar E, Doliszny KM, Folsom A, Blackburn H, Luepker R. Recent trends in acute coronary heart disease — mortality, morbidity, medical care, and risk factors. N Engl J Med. 1996;334:884–890. doi: 10.1056/NEJM199604043341403. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Normand ST, Wang Y, Drye EE, Schreiner BS, Krumholz HM. Recent declines in hospitalizations for acute myocardial infarction for Medicare fee-for-service beneficiaries: Progress and continuing challenges. Circulation. 2010;121:1322–1328. doi: 10.1161/CIRCULATIONAHA.109.862094. [DOI] [PubMed] [Google Scholar]
- 6.Fang J, Alderman MH, Keenan NL, Ayala C. Acute myocardial infarction hospitalization in the United States, 1979 to 2005. Amer J of Med. 2010;123:259–266. doi: 10.1016/j.amjmed.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 8.Roger VL, Weston SA, Gerber Y, Killian BB, Dunlay SM, Jaffe AS, Bell MR, Kors J, Yawn BP, Jacobsen SJ. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121:863–869. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McManus DD, Gore JM, Yarzebski J, Spencer F, Lessard D, Goldberg RJ. Recent trends in the incidence, treatment, and Outcomes of patients with STEMI and NSTEMI. Amer J Med. 2011;124:40–47. doi: 10.1016/j.amjmed.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alpert JS, Thygesen K, Antman E, Bassand J. Myocardial infarction redefined-a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–969. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 11.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 12.Parikh NI, Gona P, Larson MG, Fox CS, Benjamin EJ, Murabito JM, O'Donnell CJ, Vasan RS, Levy D. Long-term trends in myocardial infarction incidence and case fatality in the National Heart, Lung, and Blood Institute's Framingham Heart study. Circulation. 2009;119:1203–1210. doi: 10.1161/CIRCULATIONAHA.108.825364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salomaa V, Koukkunen H, Ketonen M, Immonen-Raiha P, Karja-Koskenkari P, Mustonen J, Lehto S, Torppa J, Lehtonen A, Tuomilehto J, Kesaniemi YA, Pyorala K. A new definition for myocardial infarction: what difference does it make? Eur Heart J. 2005;26:1719–1725. doi: 10.1093/eurheartj/ehi185. [DOI] [PubMed] [Google Scholar]
- 14.Yeh RW, Go AS. Rethinking the epidemiology of acute myocardial infarction. Arch Intern Med. 2010;170:759–764. doi: 10.1001/archinternmed.2010.88. [DOI] [PubMed] [Google Scholar]
- 15.Luepker RV, Berger AK. Is acute myocardial infarction disappearing? Circulation. 2010;121:1280–1282. doi: 10.1161/CIR.0b013e3181d98478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.IOM (Institute of Medicine) A Nationwide Framework for Surveillance of Cardiovascular and Chronic Lung Disease. Washington, DC: The National Academy of Science Press; 2011. [PubMed] [Google Scholar]
- 17.White AD, Folsom AR, Chambless LE, Sharret AR, Yany K, Conwill DE, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 18.Rosamond W, Chambless L, Sorlie P, Bell E, Weitzman S, Smith J, Folsom A. Trends in the sensitivity, positive predictive value, false-positive rate, and comparability ratio of hospital discharge diagnosis codes for acute myocardial infarction in four United States communities, 1987 to 2000. Am J Epidimiol. 2004;160:1137–1146. doi: 10.1093/aje/kwh341. [DOI] [PubMed] [Google Scholar]
- 19.ARIC Investigators. Manual 3: Surveillance Component Procedures Manual of Operations. 2009 http://www.cscc.unc.edu/aric/displaydata.php?pg_id=19.
- 20.Edlavitch SA, Crow R, Burke GL, Huber J, Prineas R, Blackburn H. The effect of the number of electrocardiograms analyzed on cardiovascular disease surveillance: the Minnesota Heart Study (MHS) J Clin Epidemiol. 1990;43:93–99. doi: 10.1016/0895-4356(90)90061-s. [DOI] [PubMed] [Google Scholar]
- 21.National Heart Lung, and Blood Institute. Morbidity and Morality: 2009 Chart Book on Cardiovascular, Lung, and Blood Disorders. Bethesda, MD: National Institutes of Health; 2009. [Google Scholar]
- 22.Lloyd-Jones D AR, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart Disease and Stroke Statistics-2010 Update: A report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 23.Ford ES, Capewell S. Coronary heart disease morality among young adults in the U.S. from 1980 through 2002. J Am Coll Cardiol. 2007;50:2128–2132. doi: 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 24.Fox KA, Evans JC, Larson GG, Kannel WB, Levy D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: The Framingham Heart Study. Circulation. 2004;110:522–527. doi: 10.1161/01.CIR.0000136993.34344.41. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg RJ, Glatfelter K, Burbank-Schmidt E, Lessard D, Gore JM. Trends in community mortality due to coronary heart disease. Am Heart J. 2006;151:501–507. doi: 10.1016/j.ahj.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 26.National Heart Lung, and Blood Institute. Final Report from the Strategic Planning Workshop on Cardiovascular Program: Translation, Implementation, and Community Research. Bethesda, MD: National Health Lung and Blood Institute; 2006. [Google Scholar]
- 27.Goff D, Brass L, Braun L, Croft J, Flesch J, Fowkes G, Hong Y, Howard V, Huston S, Jencks S, Luepker R, Manolio T, O'Donnell C, Robertson R, Rosamond W, Rumsfeld J, Sidney S, Zheng Z. Essential features of a surveillance system to support the prevention and management of heart disease and stroke: A scientific statement from the American Heart Association. Circulation. 2007;115:127–155. doi: 10.1161/CIRCULATIONAHA.106.179904. [DOI] [PubMed] [Google Scholar]
- 28.Myerson M, Coady S, Taylor H, Rosamond W, Goff D. Declining Severity of Myocardial Infarction 1987–2002: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2009;119:503–514. doi: 10.1161/CIRCULATIONAHA.107.693879. [DOI] [PubMed] [Google Scholar]
- 29.Levy D, Thom T. Death rates from coronary disease-Progress and puzzling paradox. N Engl J Med. 1998;339:915–917. doi: 10.1056/NEJM199809243391309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.