Abstract
Purpose
The goal was to understand the functions of retinopetal axons containing histamine. In prior work, type 3 histamine receptors (HR3) have been localized to the tips of ON bipolar cell dendrites in macaque retinas. Voltage-gated potassium channels have also been localized to bipolar cell dendrites, and the hypothesis tested in the present study was that these are modulated by histamine.
Methods
Whole-cell recordings of potassium currents were made from bipolar cells in slice preparations of macaque retina. In voltage-clamp mode, the cells were held at −60 mV and stepped to values from −60 to 80 mV. Recordings of the membrane potential were also made in current-clamp mode. Histamine, the HR3 agonist (R) α-methylhistamine (RAMH), tetraethyl ammonium (TEA), and 4-aminopyridine (4-AP) were applied in the superfusate.
Results
Histamine produced a dose-dependent increase in potassium currents in a subset of bipolar cells. At 5 μM, histamine increased the currents by 15% or more in the ON bipolar cells but not in the OFF bipolar cells. RAMH at 5 μM increased the amplitude of the potassium currents in the ON bipolar cells. In 10 mM TEA, potassium currents were reduced in all the bipolar cells, and there was no effect of histamine. Histamine hyperpolarized the resting membrane potential of the ON bipolar cells by 5 mV.
Conclusions
By enhancing potassium currents in the ON bipolar cells, histamine is expected to reduce the amplitude of the light responses and limit their duration. The hyperpolarization of the resting membrane potential would also reduce neurotransmitter release at their output synapses.
In primates, the retina receives input from neurons tuberomammillary nucleus of the hypothalamus.1 These are in the a subset of the neurons that are most active in the waking state and project diffusely throughout the central nervous system.2 They are tonically active, releasing histamine throughout the day in macaques.3 The axons of these neurons enter the retina through the optic disc, run in the optic fiber layer, and terminate in the inner plexiform layer (IPL).4 Only one type of histamine receptor (HR), however, has been localized to date in the macaque retina, and it is in the outer plexiform layer (OPL). Using light and electron microscopic immunohistochemistry, HR3 has been localized to the tips of the dendrites of ON bipolar cells.5 These are local circuit neurons that depolarize in response to increments of light intensity in their receptive field centers and convey this signal to the IPL. There are six types, including rod bipolar cells, ON midget bipolar cells, blue cone bipolar cells, and three types of diffuse bipolar cells.6 Based on double-labeling experiments with antibodies to a marker for ON bipolar cells, metabotropic glutamate receptor 6, they all express HR3.5
Because they are also localized to ON bipolar cell dendrites, voltage-gated potassium channels are among the potential targets for modulation by histamine. Like HR3, the Kv1.2 subunit has been localized to the tips of ON bipolar cell dendrites in goldfish retina.7 Other subunits, including Kv1.1, Kv1.3, and Kv2.1, have also been localized to ON bipolar cell dendrites in mouse retina.8 These subunits, like others in the Kv class, form potassium channels that rapidly activate after depolarization and bring the membrane potential closer to the equilibrium potential for potassium.9 Although the voltage-gated potassium channels of primate bipolar cells have not been localized anatomically, they have been described using the whole-cell patch clamp technique. Using acutely isolated bipolar cells from macaque retina, two major types of potassium currents activated by depolarization were identified. A transient current, IK(A), peaked in less than 5 ms and reached a steady state by 40 ms; this current predominated in midget bipolar cells. The delayed rectifier IK(V) activated more slowly and did not inactivate rapidly. IK(V) predominated in diffuse bipolar cells and was the only potassium current detectable in rod bipolar cells.10 Subtypes of bipolar cells vary in their potassium currents in other mammalian retinas.11
Here we report that histamine produced a dose-dependent increase in IK(V) in ON bipolar cells, including many rod bipolar cells. The selective HR3 agonist (R)-α-methylhistamine (RAMH)12 had the same effect. Histamine also hyperpolarized ON bipolar cells.
Methods
Retinal Preparations
Twenty pairs of macaque eyes (Macaca fascicularis) were purchased from Charles River BRF, Inc. (Houston, TX), or were donated by investigators at the University of Texas Health Science Center at Houston. Seven other pairs were obtained from macaques (Macaca mulatta) donated by investigators at Brooks City-Base, Texas (San Antonio, TX). Because no differences in results were apparent, the two species were considered together. The animal protocol was approved by The University of Texas Health Science Center at Houston, and all animals were treated in accordance with institutional and National Institutes of Health guidelines and with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The animals were sedated with ketamine (15 mg/kg, intramuscularly) and were euthanatized by overdose of sodium pentobarbital (100 mg/kg intravenously). Within 20 minutes postmortem, the eyes were enucleated and dissected as described previously.10 In brief, the eyes were hemisected, and the vitreous was carefully removed with fine forceps from the posterior half of the eyecup. The retinas were transported to the laboratory in dark containers filled with Ames medium (Sigma-Aldrich, St. Louis, MO) and were continuously supplied with 95% O2/5% CO2. The retinas were then removed from the eyecups, and pieces of peripheral retina were flattened, photoreceptor side up, onto a filter (pore size, 0.45 μm; Millipore, Billerica, MA). The retina and the filter paper were sectioned into 200-μm-thick slices in a chamber and fixed onto the chamber with high-vacuum grease (Dow Corning, Midland, MI). The chamber was then mounted on the stage of an upright, fixed-stage fluorescence microscope (BX51 WI; Olympus Optical, Tokyo, Japan). Retinal slices were continuously superfused with Ames medium equilibrated with 95% O2/5% CO2 at 22°C. All chemicals were dissolved in Ames medium.
Electrophysiological Recordings
The bipolar cells were visualized in the slices by using an infrared CCD camera (Rolera-XR; Q-image, Burnaby, BC, Canada) mounted on the microscope and then were monitored on a laptop computer screen with image-review software (Q-Capture Suite Software; Q-image). Whole-cell recordings were obtained with a double patch-clamp amplifier (EPC10; HEKA Elektronik, Lambrecht, Germany) in voltage- or current-clamp mode. The electrodes were pulled from borosilicate glass (BF 150-86-10; Sutter Instruments, Novato, CA) with a micropipette puller (Flaming/Brown P-97; Sutter Instruments). They had a resistance of 8 to 12 MΩ when filled with an intracellular solution containing the following: 126 mM K+-gluconate, 1 mM CaCl2, 1 mM MgCl2, 1.1 mM EGTA, 4 mM KCl, 10 mM HEPES, 1 mM Mg2+ ATP(H2O)2, 1 mM Na+3GTP(H2O)2, 0.025% Lucifer yellow, and 0.08% intracellular tracer (Neurobiotin; Vector Laboratories, Burlingame, CA) at pH 7.2. Current signals were filtered at 3 kHz and digitized at 5 kHz with a computer equipped with a data acquisition interface (LIH 1600 A/D board; HEKA Elektronik). Data acquisition software (Patchmaster; HEKA Elektronik) was used to generate voltage and current commands. Cell membrane capacitance and series resistance current were automatically compensated by the amplifier using the software. The calculated liquid junction potential was also subtracted by this software, as well.
Visualization of Cell Morphology
After the electrophysiological data were recorded, the bipolar cells were visualized with the CCD camera on the fluorescence microscope and monitored on a computer screen with commercial software (Q-Capture Suite Software; Q-image). Then the electrode was carefully pulled out from the soma, and the retinal slice was immediately fixed in fresh 4% paraformaldehyde/phosphate-buffered saline (PBS; pH 7.8), for 2 hours at room temperature. Three-dimensional cell morphology was visualized with a confocal microscope (model 510; Carl Zeiss Meditec, Thornwood, NY). Images were acquired with a 40× water-immersion objective (numerical aperture, 0.75), using the 458-nm excitation line of an argon laser and a high-pass 505-nm emission filter. Consecutive optical sections were superimposed to form a single image using laser scanning microscope computer software (Carl Zeiss Meditec), and these compressed image stacks were further processed with an image-analysis program (Photoshop 6.0; Adobe Systems, San Jose, CA) to improve the signal-to-noise ratio. Because signal intensity values were typically enhanced during processing to improve the visibility of smaller processes, the cell bodies and larger processes of some cells appeared saturated because of their higher concentration of Lucifer yellow. The background images of the retinal slices were acquired simultaneously and imaged with transmitted light. Because cells with somas situated under the surface of the slice were selected, they typically had relatively intact processes, as assessed by rotation of the stacked images.
Data Analysis
Analysis of voltage-clamp data was carried out with commercial statistical software (Igor 5.0; WaveMetrics, Lake Oswego, OR, or Sigmaplot 9.0; Systat Software, San Jose, CA). All results are given as the mean ± SEM. Statistical analyses were performed with t-tests (Excel; Microsoft, Redmond, WA). The differences were all statistically significant according to a two-tailed t-test (P < 0.05).
Results
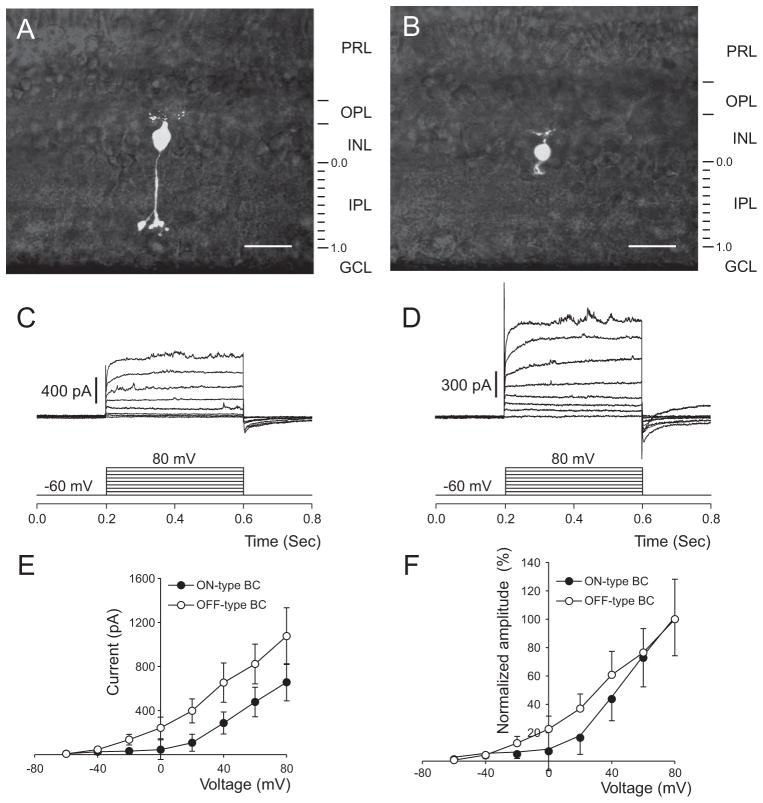
In all, 65 bipolar cells from macaques were studied. Of these, 24 were clearly identified as ON cells based on their long axons terminating in the proximal half of the IPL. Most of these had morphology characteristic of rod bipolar cells, described previously in primate retinas.13–15 That is, they had long axons terminating deep in the IPL with compact arbors and large varicosities (Fig. 1A). ON cone bipolar cells were also included in the sample. Others (n = 17) had axonal arbors restricted to the distal half of the IPL and were classified as OFF bipolar cells (Fig. 1B). The membrane potential was held at −60 mV and was stepped to more depolarized potentials up to 80 mV in increments of 10 mV. Families of outward currents generated by this procedure are illustrated in Figure 1. In general, the currents evoked by depolarization were smaller in the ON bipolar cells (Fig. 1C) than in the OFF bipolar cells (Fig. 1D), as reported.10 The two current-voltage curves are compared directly in Figure 1E. These outward currents were present in the physiological range of membrane voltages for the ON bipolar cells. On average, they were 8 ± 2 at −60 mV, 26 ± 13 at −40 mV, and 32 ± 20 at −20 mV (pA ± SEM).
Figure 1.
Voltage-dependent outward currents of macaque retinal ON and OFF bipolar cells. (A) The stacked confocal fluorescence image of a typical ON bipolar cell from our sample. This cell resembled rod bipolar cells described previously. (B) The typical OFF bipolar cell imaged by the same technique. (C) Voltage-evoked current responses on the same cell as in (A). The cell was held at −60 mV and depolarized by a series of 400-ms test pulses ranging from −60 to 80 mV. (D) Voltage-evoked current responses on the same cell as in (B). (E) The current–voltage relationships obtained from the responses in (C, D) taken 200 ms from the onset of the test pulses. (F) Normalized current amplitudes. Error bars represent the SEM. PRL, photoreceptor layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar, 20 μm.
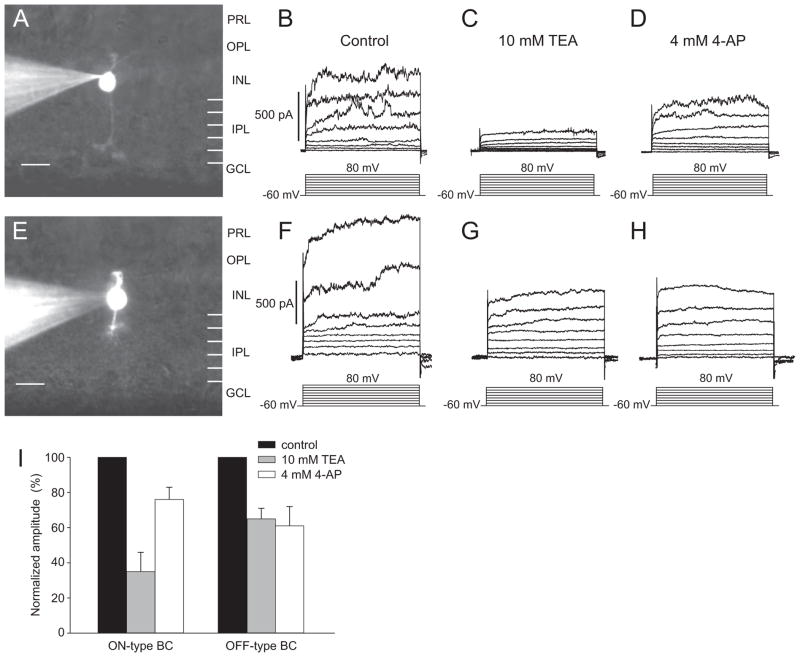
The outward currents of the two types of bipolar cells also differed in their responses to pharmacologic agents (Fig. 2). To facilitate comparisons between cells, the control currents at 80 mV were set to 1.0, and the currents after treatment with pharmacologic agents were reported as a percentage of that value. In the entire sample, the outward currents were reduced by approximately half by 10 mM tetraethyl ammonium (TEA; 54.4% ± 19.9%). The effects of 10 mM TEA were more pronounced in the ON cells (Fig. 2C) than in OFF cells (Fig. 2G). The currents in the ON cells were reduced, on average, to 37.5% ± 8.1% of their control values, but in the OFF cells they were reduced to only 70.1% ± 9.5% of their control values. The effect of 4 mM 4-AP was generally smaller. In the entire dataset, the current was reduced to 68.4% ± 9.7% of its control value. The effects of 4-AP were more pronounced in the OFF cells than in the ON cells. The currents of the OFF cells were reduced to 62.4% ± 7.3% of control values, whereas those of the ON cells were reduced to 73.0% ± 6.6%. These results are summarized in Figure 2I.
Figure 2.
Voltage-dependent inhibition of the potassium currents of bipolar cells by 10 mM TEA and 4 mM 4-AP. (A–D) Pharmacologic characterization of currents in an ON bipolar cell. (A) Digital camera image of an ON bipolar cell. (B) Voltage-evoked currents of the same cell. (C) Currents after 10 mM TEA. (D) Currents after 4 mM 4-AP. (E–H) Similar experiments were conducted on an OFF bipolar cell. (E) Digital camera image of an OFF bipolar cell. (F) Voltage-evoked current of the same cell. (G) Currents after 10 mM TEA. (H) Currents after 4 mM 4-AP. (I) Effects of 10 mM TEA and 4 mM 4-AP are compared with controls. Outward currents of ON (n = 14) and OFF (n = 7) bipolar cells were evoked by depolarizing steps from −60 mV to 80 mV. Values were normalized by setting the control values for steps to 80 mV to 1.0. PRL, photoreceptor layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar, 5 μm.
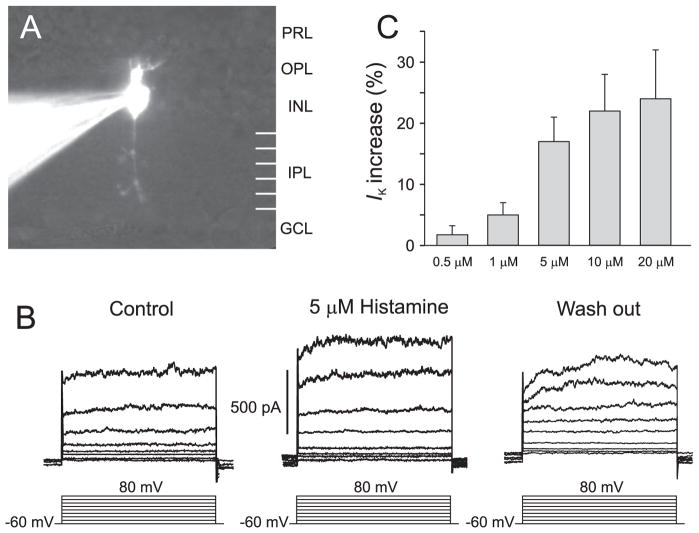
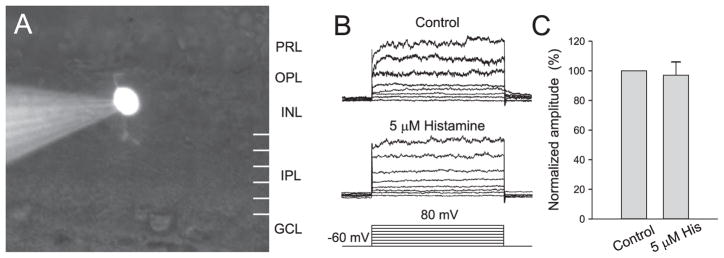
The responses to histamine applied in the superfusate are illustrated in Figure 3. In a subset of bipolar cells, the outward current was increased by histamine. All identified ON cells studied with voltage clamp and 5 μM histamine (n = 15) showed increases in the outward currents of more than 15%; on average, the increase was 19.0% ± 4.2%. Because the currents were small and variable in the physiological range, statistically significant effects of histamine were detectable only after large depolarizations. Figures 3A and 3B show a representative example. Figure 3C shows that the effect was dose dependent. Using the criterion of a 15% increase in outward current to identify ON cells, cells whose morphology was unknown were also included in the dose-response calculations. In ON cells, there was a statistically significant increase in response to doses of histamine as low as 1 μM. The effect of histamine on OFF cells was not statistically significant (Fig. 4).
Figure 3.
Effect of histamine on outward currents of ON bipolar cells. (A) Digital camera image of an ON bipolar cell. PRL, photoreceptor layer; INL, inner nuclear layer; GCL, ganglion cell layer. (B) Effect of 5 μM histamine on outward currents of the cell shown in (A). (C) Effects of 0.5, 1, 5, 10, and 20 μM histamine on outward currents of ON bipolar cells.
Figure 4.
Lack of effect of histamine on OFF bipolar cells. (A) Digital camera image of an OFF bipolar cell. PRL, photoreceptor layer; INL, inner nuclear layer; GCL, ganglion cell layer. (B) Histamine at 5 μM had no effect on the outward current of the cell shown in (A). (C) Histamine had no statistically significant effects on the outward currents of morphologically identified OFF bipolar cells (n = 7).
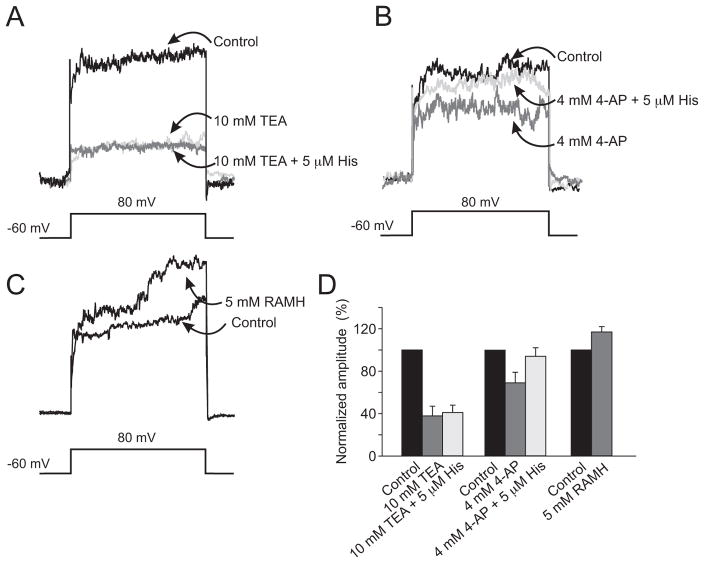
Figure 5 shows the results of a series of experiments designed to identify the component of the outward current sensitive to histamine and to identify the histamine receptor mediating the effect. There was no effect of histamine in the presence of 10 mM TEA (Fig. 5A) and no statistically significant difference between the responses before and after histamine in any of the 34 cells tested with TEA. The effect of histamine was not blocked by 4 mM 4-AP (Fig. 4B). In the presence of 4-AP, histamine enhanced the outward currents in the identified ON cells (n = 7) and in the dataset generally (n = 37), but not in the OFF cells. Figure 5D shows that the effect of histamine was mimicked by the sensitive, highly specific HR3 agonist RAMH. This experiment was repeated on 12 additional cells. On average, the increase in the outward current was 9.6%.
Figure 5.
Effects of pharmacologic agents on the responses to histamine. (A) Superimposed current traces obtained during 400-ms depolarizing pulses from −60 to 80 mV; black: control responses. In the presence of 10 mM TEA (dark gray), 5 μM histamine (light gray) had no additional effect on the outward currents recorded from the ON bipolar cells. (B) The experiment was repeated with 4 mM 4-AP. Compared with the control trace (black), there was a smaller reduction in the outward currents after 4-AP (dark gray). Outward currents were enhanced by 5 μM histamine (light gray), despite the presence of 4 mM 4-AP. (C) Group data showing the effects of 5 μM histamine in controls (black), with 10 mM TEA (dark gray) and 4 mM 4-AP (light gray) on outward currents. Error bars represent SD. (D) Effect of the selective, specific HR3 agonist RAMH (5 μM) on the outward current recorded from an ON bipolar cell.
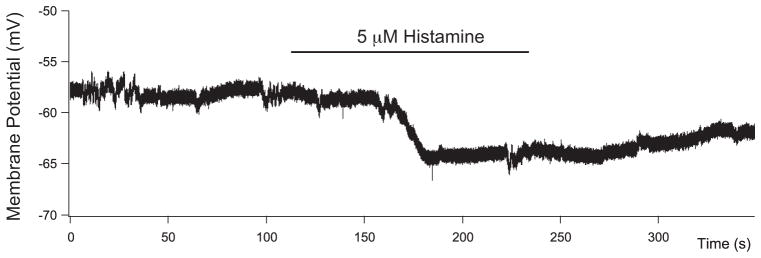
Figure 6 shows the effect of 5 μM histamine on the resting membrane potential measured in the current clamp mode. This cell was hyperpolarized by 5 mV, and the effect was slow, requiring 2 minutes to develop and outlasting the application of histamine. The experiment was repeated 22 times. In the ON cells, the mean resting potential was −46.4 ± 4.2 mV; with 5 μM histamine, it was −51.4 ± 3.8 mV (P < 0.00001). Thus, histamine hyperpolarized the membrane by 5 mV. It is possible to account for this change by an effect on the voltage-gated potassium conductance, assuming that the increases seen with large depolarizations also occurred in the physiological range of membrane potentials. With an input impedance of 1 GΩ for a primate bipolar cell in a slice preparation and the measured voltage-gated potassium conductance in control conditions of 26 pA at −40 mV, a 20% increase would produce a 5.2 mV hyperpolarization in the membrane potential. In OFF bipolar cells (n = 10), there was no significant difference between the resting potential before and after histamine application.
Figure 6.
Histamine hyperpolarizes ON bipolar cells. Histamine at 5 μM produced a slowly developing hyper-polarization of approximately 5 mV in an ON bipolar cell under current-clamp recording conditions (0 pA).
Discussion
Histamine increased the delayed rectifier component of the voltage-gated potassium current in the ON bipolar cells from macaque retina; the OFF bipolar cells were unaffected. Another indication that the histamine effect was selective is that it was completely blocked by 10 mM TEA, but not by 4 mM 4-AP, a blocker of the transient outward current. This effect was also dose dependent and highly sensitive; significant enhancement was observed with histamine concentrations as low as 1 μM. The finding that the selective HR3 agonist mimics the effect suggests that the sites of action are the HR3 immunoreactive puncta on the dendrites of macaque ON bipolar cells.5 Taken together, these findings suggest that ON bipolar cells are among the targets of histamine released from retinopetal axons in macaques.4
Voltage-gated potassium currents of ON bipolar cells are targets of neuromodulators in other species. A relatively low concentration of dopamine increases the voltage-dependent potassium currents of ON bipolar cells in goldfish retina,16 and agonists of cannabinoid receptor 1 block this effect.17 In zebrafish ON bipolar cells, however, a higher concentration of dopamine decreases the potassium currents.18 In rat retina, somatostatin inhibits the voltage- and calcium-dependent potassium channels of rod bipolar cells.19
Many other effects of histamine on voltage-dependent potassium currents of neurons have been reported. Typically, these are decreases in leak conductance, decreases in the currents mediating the afterhyperpolarization, or increases in the hyperpolarization-activated current.20 This is the first description of an effect of histamine on the delayed rectifier of a neuron, but histamine has a similar effect on the delayed rectifier of guinea pig atrial cells.21 There, histamine enhances a slow component of the delayed rectifier current that is observed during strong depolarizations. The effect of histamine on the guinea pig atrial cells is mediated by HR1, but in macaque ON bipolar cells the receptor is HR3.5
Histamine also hyperpolarized the resting membrane potential of the bipolar cells by 5 mV on average. This hyperpolarization may be mediated, at least in part, by the increases in voltage-gated potassium conductance because these channels are likely to be open at the resting membrane potential. Another possible mechanism underlying the effect of histamine on the resting membrane potential is an increase in chloride conductance, as described previously in the hypothalamus22 and thalamus23 of mammals. In arthropod retinas, this effect of histamine is mediated by the histamine-gated chloride channel hclA.24 The homologue in primates is the beta subunit of the glycine receptor, which has been localized to puncta in both the OPL and IPL of macaque retina.25 Hyperpolarization-activated and cyclic nucleotide–gated (HCN) channels are found in rod bipolar cells of rats,26 and these may also mediate the change in membrane potential produced by histamine. However, HCN channels have not been detected in human bipolar cells,27 and it is uncertain whether they are present in monkey bipolar cells because the stimulation protocol used in the present study would not have activated these channels.
The 5-mV hyperpolarization produced by histamine in the bipolar cell membrane potential is relatively small but was expected to have important consequences for neurotransmitter release based on results from other mammalian retinas. Two studies of rat retinal slices have been published in which the voltage dependence of synaptic transmission from rod bipolar cells to third-order cells was analyzed. Hartveit28 measured the frequency of inhibitory postsynaptic currents originating from A17 amacrine cells and found that glutamate release was negligible at −60 mV but substantial at −50 mV. Using a pipette solution with 100 times greater calcium-buffering capacity, Singer and Diamond29 found that −40 mV was the threshold for glutamate release onto AII amacrine cells. The level of calcium buffer used in the present study was intermediate between the two. Taken together, the results from these studies suggest that there is a pool of synaptic vesicles that are relatively sensitive to calcium buffering and that these are released at membrane potentials more depolarized than −60 mV. The hyperpolarization in the resting membrane potential produced by histamine in ON bipolar cells would tend to decrease the rate of glutamate release from this pool.
The change in resting membrane potential produced by histamine might also influence synaptic transmission from horizontal cells to ON bipolar cells. GABAA receptors have been localized to ON bipolar cell dendrites in macaque retinas,30,31 as have the cation-coupled chloride cotransporters,32 a finding suggesting chloride equilibrium potential there is relatively depolarized. By hyperpolarizing ON bipolar cell dendrites, histamine would shift the membrane potential of this compartment away from the chloride equilibrium potential, and, as a result, GABA would be expected to produce larger depolarizations of ON bipolar cell dendrites and to generate stronger inhibitory surrounds.
In summary, a number of changes in the physiology of ON bipolar cells would be expected during the daytime, when endogenous histamine is released from retinopetal axons of primates. In darkness or in steady light, the membrane potential would be slightly hyperpolarized, resulting in a lower rate of glutamate release. A larger IK(V) might be expected to decrease the amplitude and shorten the duration of the depolarizing responses to increments in light intensity, as it does in other species.33 It is possible that surround responses mediated by feed-forward inhibition from horizontal cells would be enhanced. Because histamine is released in the IPL but the receptors are in the OPL, these effects must be mediated by volume transmission.
Acknowledgments
Supported by National Eye Institute Grants EY06472, EY10608, EY02520, and EY04446; the Retina Research Foundation; and Research to Prevent Blindness.
The authors thank Roy Jacoby, Vanessa Jensen, and Tami Andrus for their assistance in obtaining the macaque eye tissue used for these experiments; Alice Chuang for assistance with the statistical analysis; and Douglas Baxter, Ruth Heidelberger, Brian Reed, and Yin Liu for valuable discussions.
Footnotes
Disclosure: Y.-C. Yu, None; H. Satoh, None; S.M. Wu, None; D.W. Marshak, None
References
- 1.Labandeira-Garcia JL, Guerra-Seijas MJ, Gonzalez F, Perez R, Acuna C. Location of neurons projecting to the retina in mammals. Neurosci Res. 1990;8:291–302. doi: 10.1016/0168-0102(90)90035-d. [DOI] [PubMed] [Google Scholar]
- 2.Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Prell GD, Khandelwal JK, Burns RS, Green JP. Diurnal fluctuation in levels of histamine metabolites in cerebrospinal fluid of rhesus monkey. Agents Actions. 1989;26:279–286. doi: 10.1007/BF01967291. [DOI] [PubMed] [Google Scholar]
- 4.Gastinger MJ, O’Brien JJ, Larsen NB, Marshak DW. Histamine immunoreactive axons in the macaque retina. Invest Ophthalmol Vis Sci. 1999;40:487–495. [PMC free article] [PubMed] [Google Scholar]
- 5.Gastinger MJ, Barber AJ, Vardi N, Marshak DW. Histamine receptors in mammalian retinas. J Comp Neurol. 2006;495:658–667. doi: 10.1002/cne.20902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wässle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- 7.Yazulla S, Studholme KM. Differential distribution of Shaker-like and Shab-like K+-channel subunits in goldfish retina and retinal bipolar cells. J Comp Neurol. 1998;396:131–140. doi: 10.1002/(sici)1096-9861(19980622)396:1<131::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 8.Klumpp DJ, Song EJ, Pinto LH. Identification and localization of K+ channels in the mouse retina. Vis Neurosci. 1995;12:1177–1190. doi: 10.1017/s0952523800006805. [DOI] [PubMed] [Google Scholar]
- 9.Hille B. Ion Channels of Excitable Membranes. 3. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 10.Han Y, Jacoby RA, Wu SM. Morphological and electrophysiological properties of dissociated primate retinal cells. Brain Res. 2000;875:175–186. doi: 10.1016/s0006-8993(00)02614-7. [DOI] [PubMed] [Google Scholar]
- 11.Hu HJ, Pan ZH. Differential expression of K+ currents in mammalian retinal bipolar cells. Vis Neurosci. 2002;19:163–173. doi: 10.1017/s0952523802191140. [DOI] [PubMed] [Google Scholar]
- 12.Arrang JM, Garbarg M, Lancelot JC, et al. Highly potent and selective ligands for histamine H3-receptors. Nature. 1987;327:117–123. doi: 10.1038/327117a0. [DOI] [PubMed] [Google Scholar]
- 13.Chan TL, Martin PR, Clunas N, Grünert U. Bipolar cell diversity in the primate retina: morphologic and immunocytochemical analysis of a new world monkey, the marmoset Callithrix jacchus. J Comp Neurol. 2001;437:219–239. doi: 10.1002/cne.1280. [DOI] [PubMed] [Google Scholar]
- 14.Grünert U, Martin PR, Wässle H. Immunocytochemical analysis of bipolar cells in the macaque monkey retina. J Comp Neurol. 1994;348:607–627. doi: 10.1002/cne.903480410. [DOI] [PubMed] [Google Scholar]
- 15.Kolb H, Linberg KA, Fisher SK. Neurons of the human retina: a Golgi study. J Comp Neurol. 1992;318:147–187. doi: 10.1002/cne.903180204. [DOI] [PubMed] [Google Scholar]
- 16.Fan SF, Yazulla S. Modulation of voltage-dependent K+ currents (IK(V)) in retinal bipolar cells by ascorbate is mediated by dopamine D1 receptors. Vis Neurosci. 1999;16:923–931. doi: 10.1017/s095252389916512x. [DOI] [PubMed] [Google Scholar]
- 17.Fan SF, Yazulla S. Reciprocal inhibition of voltage-gated potassium currents (I K(V)) by activation of cannabinoid CB1 and dopamine D1 receptors in ON bipolar cells of goldfish retina. Vis Neurosci. 2005;22:55–63. doi: 10.1017/S0952523805221089. [DOI] [PubMed] [Google Scholar]
- 18.Yu CJ, Li L. Dopamine modulates voltage-activated potassium currents in zebrafish retinal on bipolar cells. J Neurosci Res. 2005;82:368–376. doi: 10.1002/jnr.20637. [DOI] [PubMed] [Google Scholar]
- 19.Petrucci C, Resta V, Fieni F, Bigiani A, Bagnoli P. Modulation of potassium current and calcium influx by somatostatin in rod bipolar cells isolated from the rabbit retina via sst2 receptors. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:680–694. doi: 10.1007/s002100100422. [DOI] [PubMed] [Google Scholar]
- 20.Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol. 2001;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto Y, Ogura T, Uemura H, Saito T, Masuda Y, Nakaya H. Histamine H1-receptor-mediated modulation of the delayed rectifier K+ current in guinea-pig atrial cells: opposite effects on IKs and IKr. Br J Pharmacol. 1999;128:1545–1553. doi: 10.1038/sj.bjp.0702918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatton GI, Yang QZ. Ionotropic histamine receptors and H2 receptors modulate supraoptic oxytocin neuronal excitability and dye coupling. J Neurosci. 2001;21:2974–2982. doi: 10.1523/JNEUROSCI.21-09-02974.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KH, Broberger C, Kim U, McCormick DA. Histamine modulates thalamocortical activity by activating a chloride conductance in ferret perigeniculate neurons. Proc Natl Acad Sci U S A. 2004;101:6716–6721. doi: 10.1073/pnas.0400817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stuart AE, Borycz J, Meinertzhagen IA. The dynamics of signaling at the histaminergic photoreceptor synapse of arthropods. Prog Neurobiol. 2007;82:202–227. doi: 10.1016/j.pneurobio.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Grünert U, Wässle H. Immunocytochemical localization of glycine receptors in the mammalian retina. J Comp Neurol. 1993;335:523–537. doi: 10.1002/cne.903350405. [DOI] [PubMed] [Google Scholar]
- 26.Ma YP, Cui J, Hu HJ, Pan ZH. Mammalian retinal bipolar cells express inwardly rectifying K+ currents (IKir) with a different distribution than that of Ih. J Neurophysiol. 2003;90:3479–3489. doi: 10.1152/jn.00426.2003. [DOI] [PubMed] [Google Scholar]
- 27.Ohkuma M, Kawai F, Horiguchi M, Miyachi E. Patch-clamp recording of human retinal photoreceptors and bipolar cells. Photochem Photobiol. 2007;83:317–322. doi: 10.1562/2006-06-15-RA-923. [DOI] [PubMed] [Google Scholar]
- 28.Hartveit E. Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. J Neurophysiol. 1999;81:2923–2936. doi: 10.1152/jn.1999.81.6.2923. [DOI] [PubMed] [Google Scholar]
- 29.Singer JH, Diamond JS. Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. J Neurosci. 2003;23:10923–10933. doi: 10.1523/JNEUROSCI.23-34-10923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haverkamp S, Grünert U, Wässle H. The cone pedicle, a complex synapse in the retina. Neuron. 2000;27:85–95. doi: 10.1016/s0896-6273(00)00011-8. [DOI] [PubMed] [Google Scholar]
- 31.Vardi N, Sterling P. Subcellular localization of GABAA receptor on bipolar cells in macaque and human retina. Vision Res. 1994;34:1235–1246. doi: 10.1016/0042-6989(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 32.Vardi N, Zhang LL, Payne JA, Sterling P. Evidence that different cation chloride cotransporters in retinal neurons allow opposite responses to GABA. J Neurosci. 2000;20:7657–7663. doi: 10.1523/JNEUROSCI.20-20-07657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao BQ, MacLeish PR, Victor JD. The intrinsic dynamics of retinal bipolar cells isolated from tiger salamander. Vis Neurosci. 1998;15:425–438. doi: 10.1017/s0952523898153051. [DOI] [PubMed] [Google Scholar]