Abstract
Aim:
To evaluate and compare the pH and antibacterial property of Ca(OH)2 combined with iodine potassium iodide (IKI) or chlorhexidine (CHX) on E. faecalis and to assess and compare their effect on fracture resistance of root dentin.
Materials and Methods:
CHX (0.5%) The following test materials were used: Group I Calcium hydroxide + saline, Group II Calcium hydroxide + CHX (0.5%) and Group III Calcium hydroxide + IKI (2%). For antibacterial activity, 60 root dentin blocks (20 in each group) were infected by E. faecalis followed by placement of medicaments. At the end of 24 h and 7 days, 10 samples from each group were randomly chosen and assessed for antibacterial activity. For evaluation of root strength, 30 teeth were used and stored in sterile saline after placement of medicament. At the end of 30 days, samples were subjected to fracture resistance testing on the Universal Strength Testing Machine. Hounsfield strength testing machine, UK pH of the various calcium hydroxide combinations was determined with a digital pH meter.
Statistical analysis:
Kruskal Wallis test, Mann Whitney U test, and one-way ANOVA test for intergroup comparison and Wilcoxon's signed rank test and student's paired t test for intragroup comparison.
Results:
Group III showed significantly greater antibacterial activity against E. faecalis, followed by group II and control group. There was no statistically significant change in the pH and root strength values among all the groups.
Conclusion:
The present study revealed that IKI or CHX in combination with Ca(OH)2 is an effective medicament against E. faecalis.
Keywords: Calcium hydroxide, fracture resistance, intracanal medicaments, pH
Introduction
The main objectives of endodontic therapy are to eliminate bacteria from the root canal and to prevent the regrowth of residual microorganisms. Antimicrobial agents are recommended for intracanal antisepsis and to prevent the growth of microorganisms between appointments.[1]
E. faecalis plays a major role in the etiology of persistent periradicular lesions after root canal treatment. Calcium hydroxide [Ca(OH)2] has been widely used in endodontics. However, E. faecalis has been reported to be resistant to the antimicrobial effect of calcium hydroxide as a result of their ability to penetrate the dentinal tubules and adapt to the changing environment. Therefore, the search for a better alternative has led to various formulations of calcium hydroxide, using different vehicles, and newer antimicrobial agents such as chlorhexidine (CHX) and iodine potassium iodide (IKI).[2] The action of calcium hydroxide is dependent on its pH, and also the high alkalinity may affect the strength of root dentin when placed for longer periods of time.
Therefore, the aim of the present in vitro study was to evaluate and compare the pH and antibacterial property of Ca(OH)2 combined with IKI or CHX on E. faecalis, when used as an intracanal medicament, and to assess and compare their effect on fracture resistance of root dentin.
Materials and Methods
Test materials
Calcium hydroxide + saline (0.9%)
Calcium hydroxide + CHX (0.5%)
Calcium hydroxide + IKI (iodine 2%, potassium iodide 4%)
Seventy-five human permanent, non-carious, single-rooted teeth, extracted for therapeutic reasons, were collected and stored in distilled water until further use.
All the specimens were disinfected with 5% sodium hypochlorite for 15 min, and then cleaned with a rubber cup and prophy brush and pumice. Among those, 45 teeth were used for assessing antibacterial activity and 30 teeth were used for assessing root strength.
Assessment of antibacterial activity
The crowns were amputated at their cervical limit, and 4-mm height root dentin blocks were obtained and enlarged to a standard diameter of 1.2 mm, using an ISO O12 bur.[3] [Figure 1]
Figure 1.
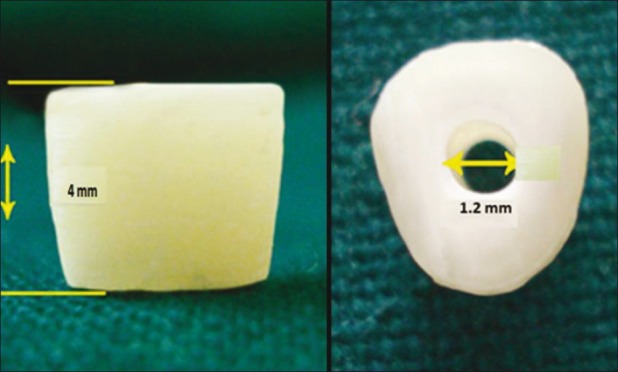
Standardization of dentin block
Dentin blocks assay
Organic and inorganic debris, including smear layer, were removed from the dentin blocks (dbs) by treatment with 17% EDTA for 10 min. The dbs were immersed in an ultrasonic bath of distilled water for 5 min to remove all traces of chemicals used and sterilized by autoclaving (121°C for 15 min) in Tryptone Soya Broth (TSB), (HiMedia Lab, Mumbai, India) to allow optimal penetration of the nutrient broth into the dentinal tubules.
Infecting the dentin blocks
E. faecalis was used as the test organism in this study. Isolated overnight pure culture of E. faecalis (ATCC 29212) suspension was prepared on TSB. This culture suspension was adjusted to match the turbidity equivalent to 0.5 McFarland standards. Subsequently, 30 TSB-containing test tubes having two dbs each were infected with 100 μL of the bacterial suspension and incubated at 37°C for 2 weeks during which time the broth was regularly changed at 2 day intervals. After 2 weeks, the infected dbs were taken and washed in sterile saline to remove any remnants of the incubation broth.[4]
Sixty dbs were then divided into two experimental groups of 20 blocks each and a control group having 20 dbs. Each of them was treated with above-mentioned medicaments, and the canal lumen on the top and bottom surfaces of the individual blocks were sealed with paraffin wax and incubated at 37°C in a sterile airtight container.
At the end of 24 h, 10 samples each from the three test groups were randomly chosen. Paraffin wax was removed and subsequently the medication was carefully rinsed from the dbs using sterile saline solution. Dentin samples from each block were harvested by drilling inside the canal lumen of each db with a round bur (ISO 016), [Figure 2] and dentin shavings were collected in 1 mL of TSB. Then, 100 μL of this broth was taken and was serially diluted with 500 μL of sterile saline and plated on TS agar and incubated. The culture plates were incubated up to 24 h at 37°C to detect any bacterial growth, and the number of bacterial colonies formed [Figures 3 and 4] was counted with the help of digital colony counter. The same procedure was repeated for all three test groups at the end of 7 days. The results were tabulated and statistically analyzed.
Figure 2.

Harvesting of dentin shavings
Figure 3.
Colony-forming units in all groups at the end of 24 h
Figure 4.
Colony-forming units in all groups at the end of 7 days
Evaluation of root strength
Thirty teeth were used for evaluation of root strength, 10 teeth in each group. The teeth were sectioned at that level perpendicular to the long axis of the tooth with a diamond disc under constant water cooling to obtain a standard root length of 13 mm. The roots were then biomechanically prepared up to an apical size 40 with the help of H files (Mani, Inc., Tochigi, Japan), and copious irrigation with sterile saline was completed between file systems. Medicaments were incorporated into the canals with the help of lentulospirals. Following incorporation of medicament, the roots were sealed apically with bonded composite and cervically with a cotton pellet and bonded composite. The dbs were stored in sterile 0.9% saline solution at room temperature.[5] At the end of 30 days, the medicament from the dentin blocks was removed and mounted in acrylic resin such that 9 mm of the root was embedded in acrylic resin and 4 mm remained above the surface.[6] The samples were then subjected to fracture resistance testing on the Universal Strength Testing Machine at a cross-head speed of 2 mm/min.[7] The mean compressive force required to fracture the samples was noted and tabulated.
Evaluation of pH
Calcium hydroxide pastes were prepared by mixing calcium hydroxide powder with saline (0.9%), CHX (0.5%), and IKI. Using these pastes, aqueous solutions of calcium hydroxide to a final concentration of 0.1 M were prepared. The pH was determined with digital pH meter calibrated to pH 7 with standard buffer solutions before use, immediately after preparation of the solution, and after 7 days. The solution was maintained at 37°C for measurement after 7 days. Three readings per sample were taken and the mean was calculated.[8]
Results
The results were tabulated and statistically analyzed using Kruskal Wallis test, Mann Whitney U test and one-way ANOVA test for intergroup comparison, and Wilcoxon's signed rank test and student's paired t test for intragroup comparison. A ‘P’ value of 0.05 or less was considered as statistically significant.
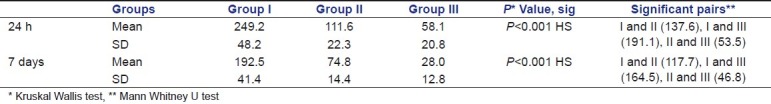
Table 1 shows the mean and standard deviation and intergroup comparison of the bacterial colonies (CFU's) in various experimental groups at the end of 24 h and 7 days. Group III showed the highest antibacterial activity followed by group II and group I, both at the end of 24 h and 7 days [Graph 1]. The values were statistically highly significant (P<0.001). On pair-wise comparison by Mann Whitney U test, Groups I and II, Groups I and III, and Groups II and III were found to be statistically significant (P<0.05).
Table 1.
Descriptive statistics of inter group comparison of bacterial colonies at the end of 24 h and 7 days
Graph 1.

Intergroup comparison of bacterial colonies at the end of 24 h and 7 days
Table 2 shows the mean and standard deviation of the bacterial colonies (CFU's) within the groups I, II and III at the end of 24 h and 7 days. Group I, group II, and group III showed an increase in the antibacterial activity at the end of 7 days as compared with that at the end of 24 h, which were statistically significant with P value of 0.02, 0.007, and 0.005, respectively.
Table 2.
Descriptive statistics of intra group comparison of bacterial colonies among different groups at the end of 24 h and 7 days

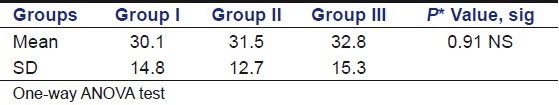
Table 3 shows the mean, standard deviation, and group wise significance of the compressive force values in control and experimental groups at the end of 30 days. The highest mean compressive force value required to fracture the root specimens was observed for Group III and the lowest value was observed with Group I [Graph 2]. This was, however, found to be statistically insignificant (P = 0.9).
Table 3.
Descriptive statistics of inter group comparison of compressive force values among different groups at the end of 30 days
Graph 2.

Intergroup comparison of mean compressive force values at the end of 30 days
Table 4 shows intragroup and intergroup comparison and significance of pH values immediately after mixing and at the end of 7 days. There was no statistically significant difference in the mean pH values among all the experimental groups with P value of 0.83 immediately after mixing and 0.95 at the end of 7 days.
Table 4.
Descriptive statistics of intra and inter group comparison of pH values immediately after mixing and at the end of 7 days
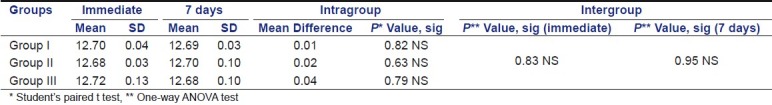
On intragroup comparison, group I, group II and group III showed no significant change in pH values after 7 days when compared to values immediately after mixing, with P value of 0.82, 0.63, and 0.79, respectively [Graph 3].
Graph 3.

Intergroup comparison of mean pH values immediately after mixing and at the end of 7 days
Discussion
Bacteria are the main causative factors in the development of pulpal and periapical inflammation. Therefore, one of the most important objectives of endodontic treatment is to eliminate bacteria from the infected root canal system.[1]
Calcium hydroxide [Ca(OH)2], discovered by Herman in 1920, has been advocated and used as an intracanal medicament since ages. Its antimicrobial properties have been attributed to its high pH (11–12.5); its dissociation into the highly interactive and lethal hydroxyl ions, which kill bacterial cells by damaging the cytoplasmic membrane, protein denaturation, and damaging the DNA; its ability to absorb carbon dioxide, which deprives capnophilic bacteria; and its physical presence, which prevents the ingress of bacteria, either coronally or apically. In spite of all these antimicrobial actions, calcium hydroxide has been shown to be incapable in eliminating E. faecalis and certain other organisms, which are present deep within the dentinal tubules as it needs direct contact with the bacteria for action, tends to get neutralized due to the dentin buffering system, inherent ability of certain bacteria to resist its high pH, and its low diffusibility and solubility.[9]
A variety of antimicrobial agents have been tested for their ability to eradicate calcium hydroxide-resistant microorganisms (especially E. faecalis) from root canals and dentinal tubules, including irrigants, such as IKI,[10] CHX,[11] and sodium hypochlorite. These in vitro studies have shown that phenol compounds such as IKI and CHX kill E. faecalis in the dentinal tubules more effectively. Therefore, supplementing the antibacterial activity of Ca(OH)2 with IKI or CHX preparations may be one way to improve the efficacy of intracanal medicament.
CHX has a wide spectrum antimicrobial action against a variety of organisms including E. faecalis. Previous studies have shown that 0.2% CHX in combination with calcium hydroxide exhibits good antibacterial activity,[12,13] and its efficacy is dependent on the concentration. In our study, concentration of CHX was increased from 0.2% to 0.5% in combination with Ca(OH)2 to improve the antibacterial property.
In endodontics, a 2% preparation of IKI has been used, which has shown to be less toxic or tissue irritating. Its antibacterial property may be attributed to the presence of iodine, which is rapidly bactericidal, fungicidal, and sporicidal. The antimicrobial action of iodine is rapid, even at low concentrations. It is thought that iodine attacks key groups such as proteins, nucleotides, and fatty acids, resulting in cell death.[14]
Hence, the aim of this in vitro study was to evaluate the antibacterial effect of combinations of Ca(OH)2 with IKI (2%) and CHX (0.5%) against E. faecalis. As the antibacterial effect of Ca(OH)2 is pH dependent, it becomes important to measure any change in pH after adding IKI and CHX to Ca(OH)2. It has been established that long-term exposure to Ca(OH)2 alters the strength of root dentin. Thus, another objective of our study was to assess if addition of IKI and CHX to Ca(OH)2 has any effect on strength of root dentin.
Antibacterial activity
In the present study, the db assay proposed by Haapasalo and Orstavik,[3] which is the most commonly used in vitro model for infection of dentinal tubules to test endodontic antiseptic irrigants and medicaments, was used, which was modified by using human, single rooted permanent teeth instead of bovine teeth, to simulate the clinical scenario.
The procedure of fabricating the dbs was done in a meticulous way such that the standard height of each dentin block was 4 mm. Canals were enlarged to a standard diameter of 1.2 mm using an ISO 012 bur. In a study conducted by Haapasalo M and Orstavik D, removal of cementum from the dbs before infection resulted in blocks which were better standardized and more easily infected.[3] Hence, in our study, cementum from outer surface of dentin blocks was removed prior to infecting them with E. faecalis.
E. faecalis was chosen as the test organism because it is the bacterium most often associated with persistent root canal infections and it is resistant to the commonly used Ca(OH)2 medication.[15] It has been widely used in previous in vitro studies.[11,16] Good dentin penetration by E. faecalis after an incubation period of 2 weeks has been shown in several studies.[3] Hence, dentin blocks were incubated in a broth containing E. faecalis for a period of 2 weeks for infection.
Antibacterial efficacy of the medicaments was evaluated at the end of 24 h and 7 days, as these time periods commonly correspond to the time intervals scheduled between the appointments during endodontic procedures.[4]
In our study, Table 1 shows intergroup comparison of bacterial colonies (CFU's) at the end of 24 h and 7 days. Group III showed the highest antibacterial activity, followed by group II, and then group I, with higher activity at the end of 7 days as compared to the activity at the end of 24 h. The results of this study confirm the previous observations that combinations of Ca(OH)2 with CHX (0.5%) and IKI (2%) are more effective against E. faecalis than Ca(OH)2 and saline mixture in vitro.[4]
Table 2 shows intragroup comparison of bacterial colonies (CFU's) at the end of 24 h and 7 days. The 24-h experiments showed a somewhat slower disinfection by medicament, which was in accordance to previous study.[4] Slower disinfection by combinations may be a result of some initial inhibitory effect of Ca(OH)2 on IKI and CHX. However, after 1 week, there was an effective disinfection of the tubules. The final disinfecting effect of the various combinations of medicaments suggests that Ca(OH)2 does not negatively affect the disinfecting effect of IKI and CHX.
Evaluation of pH
The antimicrobial effectiveness of Ca(OH)2 is based on its ability to release OH- ions.[17] The higher pH inhibits enzyme activities that are essential to bacterial life, i.e. metabolism, growth, and cellular division.
Preservation of high alkalinity for longer periods of time is regarded as one of the major advantages of Ca(OH)2. As IKI and CHX were added to Ca(OH)2 in the present study, their possible inhibitory effect on the alkalinity, in combination with Ca(OH)2, was evaluated by using digital pH meter, calibrated to pH 7 with standard buffer solutions before use.[6]
Table 4 shows intergroup and intragroup comparison of pH values immediately after mixing and at the end of 7 days. In this experiment, it was observed that the alkalinity of the mixtures remained unchanged with both freshly prepared and 7-day old combinations. As the addition of CHX or IKI did not affect the alkalinity of the products, it can be assumed that combinations also have the potential to be used as long-term intracanal medications.
Root strength
The high pH and antimicrobial properties of Ca(OH)2, combined with the permeability of dentin, may account for its effectiveness as an intracanal inter-appointment medicament. Furthermore, it has been suggested that Ca(OH)2 medicament may be responsible for changes in the physical properties of dentin.
A common protocol for the management of infected mature teeth with apical periodontitis is to place calcium hydroxide in the root canal system for short periods of time, ranging from 1 to 4 weeks. A study by Sahebi et al. found that Ca(OH)2 mixed with saline, when placed in root canal for 30 days, can reduce the strength of the root dentin significantly.[7]
Thus, this study was undertaken to evaluate the effect of addition of CHX and IKI to Ca(OH)2 on strength of human permanent root dentin, after placing in the root canal for a period of 30 days.
Human mature permanent teeth were chosen in the study as an increase in frequency of fracture with immature teeth has been reported because of incomplete root development and subsequent thinner dentinal walls. Irrigation of root canals by sodium hypochlorite has been reported to reduce the modulus of elasticity and flexural strength of dentin, and this was attributed to the loss of organic substance from the dentin. Hence, only saline was used in this study to irrigate the root canals during the canal preparation phase of the experiment. The compressive force required to fracture the specimens was evaluated by using Universal Strength Testing Machine.[7]
Table 3 shows the intergroup comparison of compressive force values required to fracture the root dentin. The mean compressive force values required to fracture the root specimens of all three groups were comparable. This means that the strength of the root was not significantly reduced with 30 days application of Ca(OH)2 in combination with CHX and IKI. Therefore, it appears that the standard protocol of up to 30 days application of above Ca(OH)2 combinations for infected mature teeth with apical periodontitis is safe and need not be adjusted.
Conclusion
The antibacterial effect of IKI or CHX in combination with Ca(OH)2 may prove to be of benefit in the treatment of certain types of persistent infections in primary cases and particularly in re-treatment cases where E. faecalis is the most common isolate. However, complete eradication of the bacteria is still not guaranteed with the use of these agents.
Another advantage noted in our study was that these combinations did not adversely affect the strength of root dentin. Hence, its use may be beneficial during the treatment of nonvital immature permanent teeth where longstanding contact of the medicaments is inevitable.
We recommend further controlled studies on in vivo models to confirm our observations and ascertain their true clinical effects.
Acknowledgments
The authors would like to acknowledge Dr. Sadashiva Shetty, Principal of Bapuji Dental College, for his support in making this study a success. Dr.Veena,Assistant Professor,Department of Microbiology,JJMMC,Davangere And Dr.Manjappa,Professor & Head of Dept. of Analytical Chemistry, UBDT Engineering College, Davangere for their technical support extended during the conductance of this study
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Kayaoglu G, Omurlu H, Akca G, Gurel M, Gencay O, Sorkun K, et al. Antibacterial activity of propolis versus conventional endodontic disinfectants against Enterococcus faecalis in infected dentinal tubules. J Endod. 2011;37:376–81. doi: 10.1016/j.joen.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 2.Möller AJ, Fabricius L, Dahlén G, Sundqvist G, Happonen RP. Apical periodontitis development and bacterial response to endodontic treatment. Experimental root canal infections in monkeys with selected bacterial strains. Europ J Oral Sci. 2004;112:207–15. doi: 10.1111/j.1600-0722.2004.00135.x. [DOI] [PubMed] [Google Scholar]
- 3.Haapasalo M, Orstavik D. In vitro infection and disinfection of dentinal tubules. J of Dent Res. 1987;66:137–59. doi: 10.1177/00220345870660081801. [DOI] [PubMed] [Google Scholar]
- 4.Siren EK, Haapasalo MP, Waltimo TM, Orstavik D. In vitro antibacterial effect of calcium hydroxide combined with chlorhexidine or iodine potassium iodide on Enterococcus faecalis. Eur J Oral Sci. 2004;112:326–31. doi: 10.1111/j.1600-0722.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 5.Doyon GE, Dumsha T, Fraunhofer JA. Fracture resistance of human root dentin exposed to intracanal calcium hydroxide. J Endod. 2005;31:895–7. doi: 10.1097/01.don.0000194542.02521.af. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg B, Murray PE, Namerow K. The effect of calcium hydroxide root filling on dentin fracture strength. Dent Traumatol. 2007;23:26–9. doi: 10.1111/j.1600-9657.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- 7.Sahebi S, Moazami F, Abbott P. The effects of short term calcium hydroxide application on the strength of dentine. Dent Traumatol. 2010;26:43–6. doi: 10.1111/j.1600-9657.2009.00834.x. [DOI] [PubMed] [Google Scholar]
- 8.Pacios MG, Casa ML, Bulacio MA, Lopez ME. Influence of different vehicles on the pH of calcium hydroxide pastes. J Oral Sci. 2004;46:107–11. doi: 10.2334/josnusd.46.107. [DOI] [PubMed] [Google Scholar]
- 9.Rao R Nageshwar, Kidiyoor HK, Hegde C. Efficacy of calcium hydroxide-chlorhexidene paste against Enterococcus faecalis - An in vitro study. J Endod. 2004;16:61–4. [Google Scholar]
- 10.Lin S, Kfir A, Laviv A, Sela G, Fuss Z. The in vitro antibacterial effect of iodine potassium iodide and calcium hydroxide in infected dentinal tubules at different time intervals. J Contemp Dent Pract. 2009;10:59–66. [PubMed] [Google Scholar]
- 11.Vahdaty A, Ford TP, Wilson RF. Efficacy of chlorhexidine in disinfecting dentinal tubules in vitro. Endod Dent Traumatol. 1993;9:243–8. doi: 10.1111/j.1600-9657.1993.tb00280.x. [DOI] [PubMed] [Google Scholar]
- 12.Zehnder M, Grawehr M, Hasselgren G, Waltimo T. Tissue-dissolution capacity and dentin-disinfecting potential of calcium hydroxide mixed with irrigating solutions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:608–13. doi: 10.1016/s1079-2104(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 13.Jhamb S, Nikhil V, Singh V. An in vitro study of antibacterial effect of calcium hydroxide and chlorhexidine on Enterococcus faecalis. Ind J Dent Res. 2010;21:512–4. doi: 10.4103/0970-9290.74222. [DOI] [PubMed] [Google Scholar]
- 14.Athanassiadis B, Abbott PV, Walsh LJ. The use of calcium hydroxide, antibiotics and biocides as antimicrobial medicaments in endodontics. Aust Dent J Suppl. 2007;52(1 Suppl):S64–S82. doi: 10.1111/j.1834-7819.2007.tb00527.x. [DOI] [PubMed] [Google Scholar]
- 15.Safavi KE, Spånberg LS, Langeland K. Root canal dentinal tubule disinfection. J Endod. 1990;16:207–10. doi: 10.1016/s0099-2399(06)81670-5. [DOI] [PubMed] [Google Scholar]
- 16.Heling I, Steinberg D, Kenig S, Gavrilovich I, Sela MN, Friedman M. Efficacy of a sustained-release device containing chlorhexidine and Ca(OH)2 in preventing secondary infection of dentinal tubules. Int Endod J. 1992;25:20–4. doi: 10.1111/j.1365-2591.1992.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 17.Haenni S, Schmidlin PR, Mueller B, Sener B, Zehnder M. Chemical and antimicrobial properties of calcium hydroxide mixed with irrigating solutions. Int Endod J. 2003;36:100–5. doi: 10.1046/j.1365-2591.2003.00629.x. [DOI] [PubMed] [Google Scholar]


