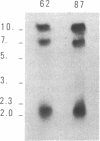
Abstract
The dynamics of telomere turnover were studied in Plasmodium, whose telomeric structures consist of linear, recognisable sequences of two distinct repeats (TTTAGGG and TTCAGGG). Independent recombinant clones containing a well-defined chromosomal extremity of Plasmodium berghei, both before and after a rare insertion event took place, were obtained from clonal parasite populations and analysed. The insertion, which splits the original telomere and causes a significant reduction in the size of the telomeric structure, is shown to consist of an integer number of subtelomeric repeats typical of P.berghei, flanked on both sides by telomere-derived motifs. Analysis of the telomeric repeat sequence heterogeneity in the otherwise homogeneous populations examined, is compatible with a model in which diversification of a given telomere is driven by the occurrence of breakpoints whose frequency rapidly increases along the telomeric tract when moving in the outward direction. The breakpoints might be due either to terminal deletions followed by random serial addition of the two repeat versions, or to recombination events. The shortening/elongation mechanism is favoured against the recombination hypothesis because of the absence of higher-order patterns in the sequence of telomeric repeats.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnot D. E., Barnwell J. W., Stewart M. J. Does biased gene conversion influence polymorphism in the circumsporozoite protein-encoding gene of Plasmodium vivax? Proc Natl Acad Sci U S A. 1988 Nov;85(21):8102–8106. doi: 10.1073/pnas.85.21.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards A., Michels P. A., Lincke C. R., Borst P. Growth of chromosome ends in multiplying trypanosomes. Nature. 1983 Jun 16;303(5918):592–597. doi: 10.1038/303592a0. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Telomeres: structure and synthesis. J Biol Chem. 1990 Apr 15;265(11):5919–5921. [PubMed] [Google Scholar]
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Dore E., Pace T., Ponzi M., Picci L., Frontali C. Organization of subtelomeric repeats in Plasmodium berghei. Mol Cell Biol. 1990 May;10(5):2423–2427. doi: 10.1128/mcb.10.5.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore E., Pace T., Ponzi M., Scotti R., Frontali C. Homologous telomeric sequences are present in different species of the genus Plasmodium. Mol Biochem Parasitol. 1986 Nov;21(2):121–127. doi: 10.1016/0166-6851(86)90015-0. [DOI] [PubMed] [Google Scholar]
- Dover G. A. DNA turnover and the molecular clock. J Mol Evol. 1987;26(1-2):47–58. doi: 10.1007/BF02111281. [DOI] [PubMed] [Google Scholar]
- Dover G. Molecular drive: a cohesive mode of species evolution. Nature. 1982 Sep 9;299(5879):111–117. doi: 10.1038/299111a0. [DOI] [PubMed] [Google Scholar]
- Dunn B., Szauter P., Pardue M. L., Szostak J. W. Transfer of yeast telomeres to linear plasmids by recombination. Cell. 1984 Nov;39(1):191–201. doi: 10.1016/0092-8674(84)90205-8. [DOI] [PubMed] [Google Scholar]
- Enea V., Galinski M., Schmidt E., Gwadz R., Nussenzweig R. S. Evolutionary profile of the circumsporozoite gene of the Plasmodium cynomolgi complex. J Mol Biol. 1986 Apr 20;188(4):721–726. doi: 10.1016/s0022-2836(86)80017-1. [DOI] [PubMed] [Google Scholar]
- Frontali C., Pizzi E. Conservation and divergence of repeated structures in Plasmodium genomes: the molecular drift. Acta Leiden. 1991;60(1):69–81. [PubMed] [Google Scholar]
- Galinski M. R., Arnot D. E., Cochrane A. H., Barnwell J. W., Nussenzweig R. S., Enea V. The circumsporozoite gene of the Plasmodium cynomolgi complex. Cell. 1987 Jan 30;48(2):311–319. doi: 10.1016/0092-8674(87)90434-x. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989 Jan 26;337(6205):331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985 Dec;43(2 Pt 1):405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987 Dec 24;51(6):887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990 May 31;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Dempster M., Dunlop M. G., Thompson A. M., Green D. K., Allshire R. C. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990 Aug 30;346(6287):866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Horowitz H., Haber J. E. Subtelomeric regions of yeast chromosomes contain a 36 base-pair tandemly repeated sequence. Nucleic Acids Res. 1984 Sep 25;12(18):7105–7121. doi: 10.1093/nar/12.18.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz H., Thorburn P., Haber J. E. Rearrangements of highly polymorphic regions near telomeres of Saccharomyces cerevisiae. Mol Cell Biol. 1984 Nov;4(11):2509–2517. doi: 10.1128/mcb.4.11.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz H., Thorburn P., Haber J. E. Rearrangements of highly polymorphic regions near telomeres of Saccharomyces cerevisiae. Mol Cell Biol. 1984 Nov;4(11):2509–2517. doi: 10.1128/mcb.4.11.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger D., Philippsen P. Many yeast chromosomes lack the telomere-specific Y' sequence. Mol Cell Biol. 1989 Dec;9(12):5754–5757. doi: 10.1128/mcb.9.12.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson D. D., Spangler E. A., Blackburn E. H. Dynamics of telomere length variation in Tetrahymena thermophila. Cell. 1987 Jul 31;50(3):477–483. doi: 10.1016/0092-8674(87)90501-0. [DOI] [PubMed] [Google Scholar]
- Morin G. B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989 Nov 3;59(3):521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Pace T., Ponzi M., Dore E., Frontali C. Telomeric motifs are present in a highly repetitive element in the Plasmodium berghei genome. Mol Biochem Parasitol. 1987 Jun;24(2):193–202. doi: 10.1016/0166-6851(87)90106-x. [DOI] [PubMed] [Google Scholar]
- Pace T., Ponzi M., Dore E., Janse C., Mons B., Frontali C. Long insertions within telomeres contribute to chromosome size polymorphism in Plasmodium berghei. Mol Cell Biol. 1990 Dec;10(12):6759–6764. doi: 10.1128/mcb.10.12.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pays E., Laurent M., Delinte K., Van Meirvenne N., Steinert M. Differential size variations between transcriptionally active and inactive telomeres of Trypanosoma brucei. Nucleic Acids Res. 1983 Dec 10;11(23):8137–8147. doi: 10.1093/nar/11.23.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pays E., Steinert M. Control of antigen gene expression in African trypanosomes. Annu Rev Genet. 1988;22:107–126. doi: 10.1146/annurev.ge.22.120188.000543. [DOI] [PubMed] [Google Scholar]
- Pizzi E., Liuni S., Frontali C. Detection of latent sequence periodicities. Nucleic Acids Res. 1990 Jul 11;18(13):3745–3752. doi: 10.1093/nar/18.13.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta A. F., Zakian V. A. Recombination occurs during telomere formation in yeast. Nature. 1989 Feb 2;337(6206):429–433. doi: 10.1038/337429a0. [DOI] [PubMed] [Google Scholar]
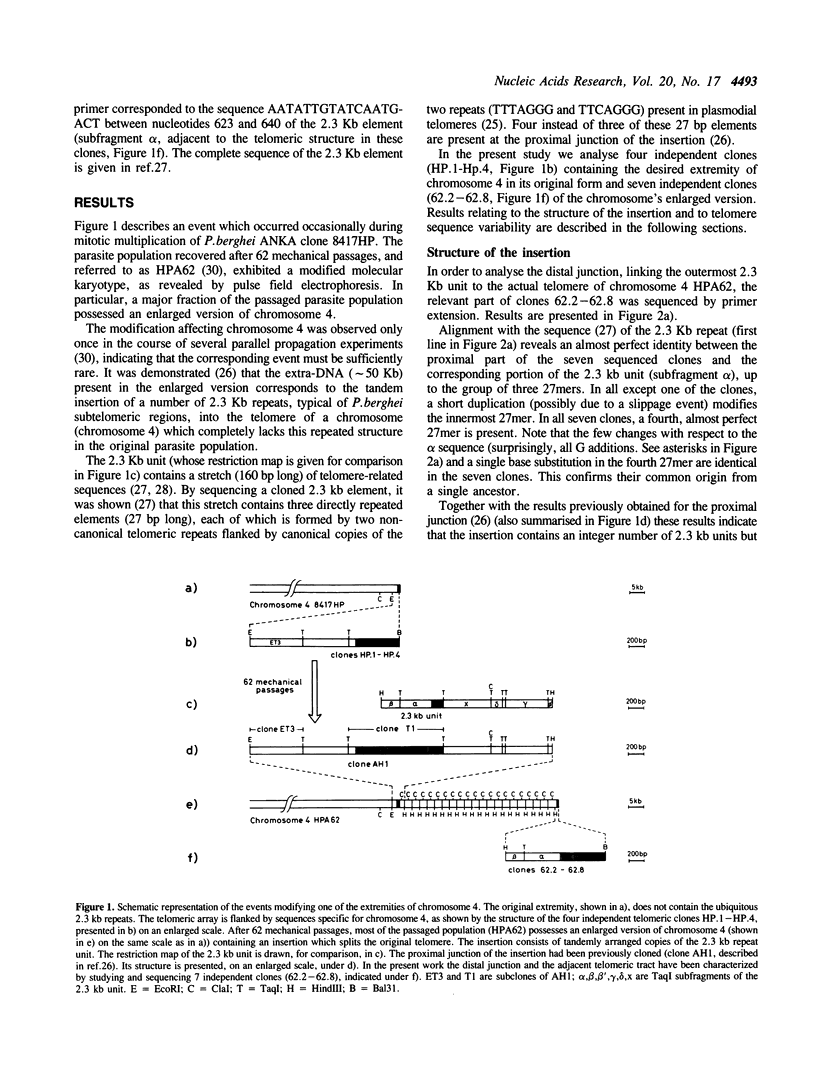
- Ponzi M., Janse C. J., Dore E., Scotti R., Pace T., Reterink T. J., van der Berg F. M., Mons B. Generation of chromosome size polymorphism during in vivo mitotic multiplication of Plasmodium berghei involves both loss and addition of subtelomeric repeat sequences. Mol Biochem Parasitol. 1990 Jun;41(1):73–82. doi: 10.1016/0166-6851(90)90098-7. [DOI] [PubMed] [Google Scholar]
- Ponzi M., Pace T., Dore E., Frontali C. Identification of a telomeric DNA sequence in Plasmodium berghei. EMBO J. 1985 Nov;4(11):2991–2995. doi: 10.1002/j.1460-2075.1985.tb04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V. Chromosomal polymorphisms and gene expression in Plasmodium falciparum. Exp Parasitol. 1989 Jan;68(1):121–125. doi: 10.1016/0014-4894(89)90018-0. [DOI] [PubMed] [Google Scholar]
- Said A., Timperman G., Wéry M. Influence de la maintenance d'une souche de Plasmodium berghei Anka sur la gamétogénèse. Ann Soc Belg Med Trop. 1986;66(2):123–131. [PubMed] [Google Scholar]
- Shampay J., Blackburn E. H. Generation of telomere-length heterogeneity in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1988 Jan;85(2):534–538. doi: 10.1073/pnas.85.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shampay J., Szostak J. W., Blackburn E. H. DNA sequences of telomeres maintained in yeast. Nature. 1984 Jul 12;310(5973):154–157. doi: 10.1038/310154a0. [DOI] [PubMed] [Google Scholar]
- Shippen-Lentz D., Blackburn E. H. Telomere terminal transferase activity from Euplotes crassus adds large numbers of TTTTGGGG repeats onto telomeric primers. Mol Cell Biol. 1989 Jun;9(6):2761–2764. doi: 10.1128/mcb.9.6.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. P. Evolution of repeated DNA sequences by unequal crossover. Science. 1976 Feb 13;191(4227):528–535. doi: 10.1126/science.1251186. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Long range periodicities in mouse satellite DNA. J Mol Biol. 1975 May 5;94(1):51–69. doi: 10.1016/0022-2836(75)90404-0. [DOI] [PubMed] [Google Scholar]
- Vernick K. D., McCutchan T. F. Sequence and structure of a Plasmodium falciparum telomere. Mol Biochem Parasitol. 1988 Mar;28(2):85–94. doi: 10.1016/0166-6851(88)90055-2. [DOI] [PubMed] [Google Scholar]
- Walmsley R. W., Chan C. S., Tye B. K., Petes T. D. Unusual DNA sequences associated with the ends of yeast chromosomes. Nature. 1984 Jul 12;310(5973):157–160. doi: 10.1038/310157a0. [DOI] [PubMed] [Google Scholar]
- Wang S. S., Zakian V. A. Sequencing of Saccharomyces telomeres cloned using T4 DNA polymerase reveals two domains. Mol Cell Biol. 1990 Aug;10(8):4415–4419. doi: 10.1128/mcb.10.8.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. S., Zakian V. A. Telomere-telomere recombination provides an express pathway for telomere acquisition. Nature. 1990 May 31;345(6274):456–458. doi: 10.1038/345456a0. [DOI] [PubMed] [Google Scholar]
- Weiden M., Osheim Y. N., Beyer A. L., Van der Ploeg L. H. Chromosome structure: DNA nucleotide sequence elements of a subset of the minichromosomes of the protozoan Trypanosoma brucei. Mol Cell Biol. 1991 Aug;11(8):3823–3834. doi: 10.1128/mcb.11.8.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G. L., Blackburn E. H. Developmentally programmed healing of chromosomes by telomerase in Tetrahymena. Cell. 1991 Nov 15;67(4):823–832. doi: 10.1016/0092-8674(91)90077-c. [DOI] [PubMed] [Google Scholar]
- Yu G. L., Bradley J. D., Attardi L. D., Blackburn E. H. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature. 1990 Mar 8;344(6262):126–132. doi: 10.1038/344126a0. [DOI] [PubMed] [Google Scholar]
- Zahler A. M., Prescott D. M. Telomere terminal transferase activity in the hypotrichous ciliate Oxytricha nova and a model for replication of the ends of linear DNA molecules. Nucleic Acids Res. 1988 Jul 25;16(14B):6953–6972. doi: 10.1093/nar/16.14.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakian V. A., Blanton H. M. Distribution of telomere-associated sequences on natural chromosomes in Saccharomyces cerevisiae. Mol Cell Biol. 1988 May;8(5):2257–2260. doi: 10.1128/mcb.8.5.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakian V. A. Structure and function of telomeres. Annu Rev Genet. 1989;23:579–604. doi: 10.1146/annurev.ge.23.120189.003051. [DOI] [PubMed] [Google Scholar]
- de Lange T., Shiue L., Myers R. M., Cox D. R., Naylor S. L., Killery A. M., Varmus H. E. Structure and variability of human chromosome ends. Mol Cell Biol. 1990 Feb;10(2):518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]