Abstract
The mitochondrial respiratory chain has been reported to play a role in the stabilization of HIF-1α when mammalian cells experience hypoxia, most likely through the generation of free radicals. Although previous studies have suggested the involvement of superoxide catalyzed by complex III more recent studies raise the possibility that nitric oxide (NO) catalyzed by cytochrome c oxidase (Cco/NO), which functions in hypoxic signaling in yeast, may also be involved. Herein, we have found that HEK293 cells, which do not express a NOS isoform, possess Cco/NO activity and that this activity is responsible for an increase in intracellular NO levels when these cells are exposed to hypoxia. By using PTIO, a NO scavenger, we have also found that the increased NO levels in hypoxic HEK293 cells help stabilize HIF-1α. These findings suggest a new mechanism for mitochondrial involvement in hypoxic signaling in mammalian cells.
Keywords: Cytochrome c oxidase, mitochondria, hypoxia, Hypoxic Inducible Factor, superoxide, nitric oxide
Introduction
Several studies have implicated the mitochondrial respiratory chain in the induction of hypoxic genes (hypoxic signaling) when eucaryotic cells experience hypoxia and have proposed that increased mitochondrial free radicals produced by hypoxic mitochondria are involved [5;9;20]. As yet, the mitochondrial free radical(s) involved in hypoxic signaling has (have) not been conclusively identified. Early evidence suggested the involvement of superoxide produced by complex III [8;11]. However, it has been demonstrated more recently that hypoxic mitochondria also produce nitric oxide (NO), catalyzed by cytochrome c oxidase (Cco/NO) [6;7], and there is growing evidence that cell and tissue NO levels increase under hypoxic conditions [34]. Moreover, several lines of evidence indicate a role for Cco and NO in hypoxic signaling in yeast cells [6;7;32].
Hypoxic signaling in mammalian cells has been addressed largely by examining HIF-1, a dimeric hypoxic transcription factor, whose alpha subunit, HIF-1α, is susceptible to degradation after prolyl hydroxylation in normoxic cells and which becomes stabilized under hypoxia [14]. It is now well established that mitochondria play a role in the hypoxic stabilization of HIF-1α and it has been demonstrated that cytochrome c, which is required for Cco/NO activity, helps stabilize HIF-1α in murine cell lines exposed to hypoxia [26]. Current understanding of the role of NO in HIF-1α stability is still evolving. While several early studies reported that NO destabilizes HIF-1α in hypoxic cells [15;25;33] recent studies have revealed a more complex picture in which NO levels are important. Indeed, it now appears that short term exposure to NO leads to the stabilization of HIF-1α either in normoxic or hypoxic cells while longer term exposure destabilizes HIF-1α [3;4]. Similarly, high doses of NO have been reported to stabilize HIF-1α independently of O2 concentration whereas low levels of NO destabilize HIF-1α under hypoxic conditions [27]. These latter studies have used human osteocarcinoma cells treated with exogenous NO donors (e.g., GSNO) [4] or HEK293 cells with an overexpressed transfected exogenous iNOS isoform or in the presence of exogenous NO donors [27]. Consequently, it is not clear if the intracellular NO levels produced in these studies were in the physiological range or if either cell type experienced an increase in NO from endogenous sources when exposed to hypoxia. Also unclear is whether NO produced from Cco/NO plays a role in the stabilization of HIF-1α in hypoxic cells These questions are addressed in this study with untransfected HEK293 cells.
Materials and Methods
Cell Culture
Human embryonic kidney cells, HEK293 (Invitrogen 11625-019: 293F), were grown to >80% confluence in Dulbecco’s modified eagle medium (DMEM; Gibco 10569) supplemented with 10% FBS and 1% Penicillin-Streptomycin at 37°C in a humidified atmosphere of air supplemented with 5% CO2. To harvest, confluent HEK293 adherent cells were washed once with PBS pH 7.4 and removed from the culture plate using 0.05% trypsin-EDTA solution. A 10% FBS solution was added and the harvested cells were washed twice with PBS and then suspended in the appropriate buffer.
Hypoxic Shifts
Confluent HEK293 cells were placed in a Biospherix Pro-ox C- Chamber inside a 37°C incubator with fresh media without FBS supplement. O2 concentration in the Biospherix C-Chamber was controlled using the Pro-ox Model C21 Biospherix regulator that balances air and N2 to achieve the desired O2 concentration while CO2 is regulated independently and held constant at 5% for all experiments. Cells were shifted from atmospheric O2 concentrations to 2%, 1%, or 0% O2 and maintained at the desired O2 concentration for varying lengths of time (typically 3 hours). The media used during the hypoxic shifts were DMEM or Phenol-free DMEM (Gibco 31053) with 100 mg/L Sodium Pyruvate added to match Gibco 10569 DMEM composition.
Measurement of NO production
NO Electrode Assay
HEK293 adherent cells were harvested and then suspended in Electrode Assay Buffer (75 mM mannitol, 25 mM sucrose, 100 mM KCl, 10 mM KH2PO4, 10 mM Tris, 50 μM EDTA, adjusted to a final pH of 7.0) with 2% n-dodecyl-R-D maltoside to solubilize the cells. The cell lysate was clarified by centrifuged at 3,000 x g, treated with 1X phosphatase inhibitor cocktail (Sigma P5726) to stabilize activity [13], aliquoted for single use, and stored in liquid nitrogen. Samples were assayed immediately after thawing. Cell lysate NO production was measured simultaneously with O2 consumption, with the Innovative Instruments inNOII system using the TMPD/ascorbate method as previously described [2]. Briefly, measurements were performed at 30°C in a closed WPI NOCHM-4 multi-port thermostatic measurement chamber using amiNO-700 and OXY-2 Clark-type electrodes. Cell lysate (3 mg/mL), superoxide dismutase (5000 units), 10 mM ADP, 10 μM mammalian cytochrome c, 0.5 mM ascorbate, and 0.5 mM TMPD were added to the assay chamber; the O2 concentration was drawn down to 0% by the respiring lysate, and then sodium nitrite (NaNO2) (1 mM) was added to the assay chamber to initiate NO production. The NO scavenger, PTIO (2-Phenyl-4,4,5-tetramethylimidazoline-3-oxide-1-oxyl) was used to validate the NO specificity of the electrode signal. The NO electrode was calibrated with a series of known concentrations of NO (10, 20, 40, 80, 100 nM) generated by mixing 1 μM NaNO2 with 0.1 M KI2 and 0.1M H2SO4.
Measurement of NO Levels using DAF-2DA Fluorescent dye
Confluent HEK293 cells were harvested and resuspended in Reaction Buffer (PBS pH 7.4, 110 mg/L Sodium Pyruvate, 4.5 g/L glucose). The fluorescent probe, DAF-2DA (4,5-Diaminofluorescein diacetate; Cayman Chemical 85165), was added to a concentration of 11 μM [18] and 150 μL (~20 x 104 cells per well) of each fraction was added in replicate to a black 96-well plate. NO related fluorescence signals (Ex/Em 485/538) were measured following 1 hour of normoxia (atmospheric O2, 5% CO2, 37°C) [30] and following up to 3 hours of hypoxia (1% O2, 5% CO2, balanced N2, 37°C). Changes in NO levels were determined by calculating the difference in fluorescence in cells prior to entering the hypoxic chamber and immediately after removal.
Measurement of HIF
Nuclear Protein isolation
Nuclear and cytoplasmic extracts were obtained using a Nuclear Extract Kit (Active Motif 40010) per kit instructions. Briefly, media was aspirated from adherent HEK293 cells and the cells were washed and scraped with ice-cold PBS containing Phosphatase Inhibitor (Active Motif 102146). They were then pelleted, resuspended, and incubated in Hypotonic Buffer (Active Motif 100505). Next, detergent (Active Motif 100512) was added, the sample was vortexed 10 seconds at the highest setting, and the sample was centrifuged for 30 seconds at 14,000 x g. The supernatant (cytoplasmic fraction) was removed and saved. The nuclear pellet was lysed (Lysis Buffer, Active Motif 100534) and the nuclear protein fraction was collected. All protein fractions were stored at −80°C and used within one week of collection.
Active HIF-1 Analysis
Active HIF results were obtained using a HIF-1 Activation Assay Kit (Active Motif 47096) per kit instructions. Briefly, the kit contains a 96-well plate with immobilized HRE oligonucleotide (5’-TACGTGCT-3’) from the EPO gene. Nuclear extract (20 μg/well) was incubated with Binding Buffer and bound HIF was detected by absorbance at 450 nm with HIF-1α antibody followed by an HRP conjugate antibody.
Total HIF-1α Analysis
Nuclear and/or cytoplasmic protein fractions were run on a 4–20% polyacrylamide gel and transferred to a PVDF membrane. The membrane was blocked with 5% Blotto-PBS Solution and washed in PBS with 0.1% Tween-20. The following antibodies were used: anti-HIF-1α (BD-Bioscience 610958 at 1:1000); anti-tubulin (Santa Cruz sc-58666 at 1:2,000 or NeoMarkers at 1:8000); anti-Histone H2B (Cell Signaling 2934S at 1:30,000); and anti-mouse HRP (ThermoFisher PI31430 at 1:20,000). Western Lightning (PerkinElmer NEL105001) was used to visualize bands on Kodak X-OMAT Blue XB Imaging Film. Bands were quantified using a previously described Photoshop Method [29].
Results and Discussion
HEK293 cells possess Cco/NO activity
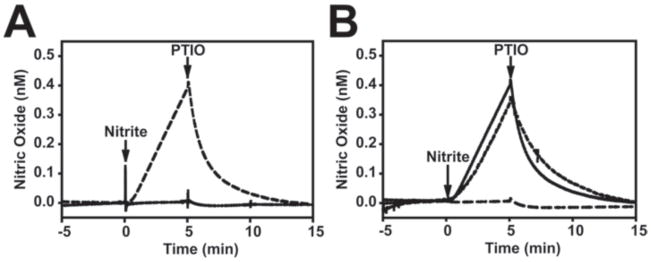
Although Cco/NO activity is enhanced under hypoxic conditions it is operable over a wide range of O2 levels [2;7] and functions at physiological NO2− concentrations (~10 μM) [2]. The NO2−-reductase activity of Cco and other proteins (e.g., xanthine oxidase, and hemoglobin) [6;16;23] are generally assayed at NO2− concentrations that exceed 1 mM in order to achieve high signal to noise ratios. So far, Cco/NO activity has been demonstrated in yeast, rat, mouse brain, plant, and human endothelial cell mitochondria as well as with purified Cco [2;6;17;21;32]. In order to determine if HEK293 cells also possess Cco/NO activity we used the previously described TMPD/ascorbate assay [2]. To improve signal level assays are generally done at O2 concentrations below 15 μM O2. As shown in Figure 1A, solubilized HEK293 cell lysates begin producing NO in this assay immediately upon the addition of NO2−. This signal returns to baseline with the addition of the NO scavenger, PTIO. It is notable that no activity is observed when the phosphatase inhibitor cocktail is omitted from the lysate during cell lysate preparation. As expected, NO synthesis is inhibited by azide but not L-NAME (Figure 1B).
Figure 1. HEK293 cells have Cco/NO activity.
(A) HEK293 cell lysates were prepared either with (dashed line) or without (solid line) phosphatase inhibitor. NO production was measured under anoxic conditions with the Innovative Instruments inNOII system as described in Materials and Methods using 3 mg/mL cell lysate. 1 mM NaNO2 was added as indicated. The NO scavenger, PTIO, was added at the time indicated to confirm the specificity of the observed signal. (B) The effects of sodium azide (5 mM; long dashed line) or L-NAME (1 mM; short dashed line) on Cco/NO activity in phosphatase inhibitor- treated HEK293 cell lysates were measured as described in Panel (A).
To our knowledge this is the first report which demonstrates that HEK293 cells possess Cco/NO activity. It is likely that the failure of an earlier study to find this activity in HEK293 cells transfected with iNOS [31] was the result the dephosphorylation of Cco during detergent lysis of the cells. Indeed, as shown in Figure 1B, no Cco/NO activity could be measured in the absence of a phosphatase inhibitor. This finding suggests that Cco/NO activity, like Cco/H2O activity, is regulated by phosphorylation [1;13].
Endogenous NO levels increase in hypoxic HEK293 cells
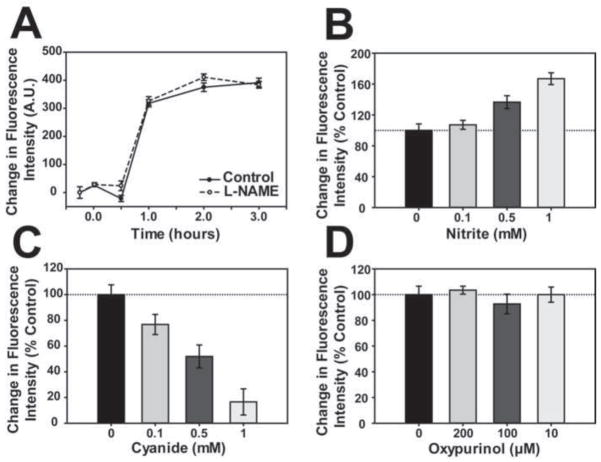
HEK293 cells have been used extensively to examine mitochondrial involvement in hypoxic signaling and mammalian gene induction and it has been reported that HEK293 cells transfected with i-NOS release NO into the surrounding medium when exposed to hypoxia [31]. While interesting, it is unlikely that these findings with HEK293 cells transfected with an exogenous i-NOS are relevant to normal HEK293 cells because these cells by themselves do not express any NOS isoforms [10]. Similarly, these previous findings do not address whether intracellular NO levels change in hypoxic untransformed HEK293 cells or whether NO generated from an endogenous source affects HIF-1 stability in these cells when they are exposed to hypoxia. To examine these questions we first determined whether exposure to hypoxia increases intracellular NO levels in HEK293 cells by using an NO sensitive dye, DAF-2DA. Previously, we and others have reported that DAF-2DA is specific for NO and NO oxidation products and does not react to any measureable extent with other reactive oxygen species (e.g., superoxide and hydrogen peroxide) in vivo under our assay conditions [22;35]. As shown in Figure 2A, HEK293 cells experience an increase in NO-related signals upon exposure to hypoxia. This increase begins after about 30 minutes of exposure to hypoxia, plateaus after about 2 hours, and is unaffected by added L-NAME, a general NOS inhibitor. Moreover, the level of NO produced is elevated in a dose-dependent manner by added exogenous NO2− (Figure 2B) and is reduced by cyanide (Figure 2C), which inhibits Cco/NO activity [2]. Oxypurinol, a xanthine oxidase inhibitor, has no effect on the increase in hypoxic NO production (Figure 2D). Together, these findings indicate that hypoxia increases endogenous NO levels in HEK293 cells, which do not express a NOS isoform, and suggest that new NO synthesis, catalyzed by Cco/NO, is responsible for the increase. These results also do not support the view that the increase in intracellular NO observed merely reflects the release on NO from Cco, rather than new NO synthesis by Cco/NO, because cyanide inhibits the increase in intracellular NO levels. Indeed, if Cco bound NO is merely released from Cco under hypoxia then NO levels should increase in the presence of cyanide, as the enzyme becomes reduced [31].
Figure 2. Effect of hypoxia on NO levels in HEK293 cells.
(A) NO levels were measured in HEK293 cells at different times after exposure to hypoxia (1% O2) using the NO-scavenging fluorescent probe, DAF-2DA, in the presence (open circles; solid line) or absence (closed circles; dashed line) of 1 mM L-NAME. (B-D) NO levels were measured in HEK293 cells using DAF-2DA as described in above, following 3 hours of hypoxic incubation (1% O2) in the presence of the indicated concentrations of (B) NaNO2, (C) potassium cyanide, or (D) oxypurinol. For all assays, the change in fluorescence intensity is represented as the percent of signal compared to the untreated hypoxic control.
HIF-1 expression levels in hypoxic cells are affected by Cco/NO inhibitors and endogenously produced NO
Under hypoxia NO has been reported previously to both increase and decrease HIF-1α stability. Early studies suggested that this dual effect of NO was concentration dependent where high concentrations of NO stabilize HIF-1α irrespective of O2 concentration and low levels destabilize HIF-1α in hypoxic cells [27]. More recent studies have reported that acute exposure to NO stabilizes HIF-1α while longer term exposure to NO destabilizes HIF-1α [3;4]. Because these latter studies made use of an exogenous NO donor it appears that the two phases are a reflection of the amount of NO released to the cells and that the latter phase is a reflection of the reduced release kinetics from the NO donor. Consequently, it is not clear how these results translate to the changes in endogenous NO synthesis during hypoxia that are reported in this study.
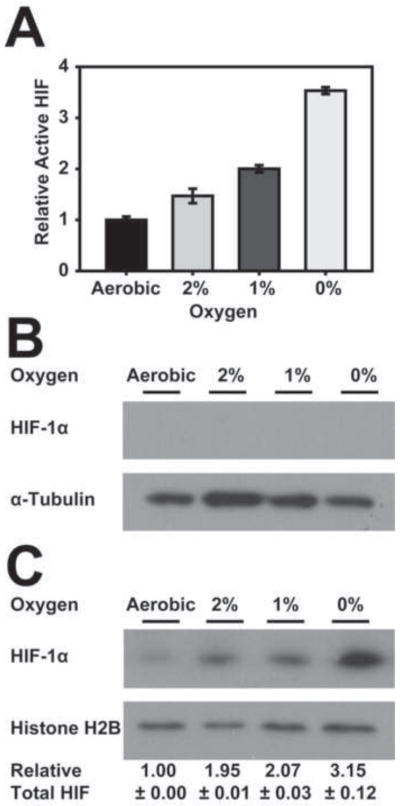
In order to ask if the increase in endogenously produced NO in hypoxic cells affects HIF-1 levels we first looked at the expression of HIF in the HEK293 cell line used above. Cells grown in an air- 5% CO2 mixture were exposed to reduced O2 levels of 2% O2, 1% O2, or 0% O2 with 5% CO2 and balanced N2 for 3 hours. Then, both active HIF-1 levels and HIF-1α subunit levels were determined. From Figure 3A it is clear that this cell line expressed HIF activity at nominal levels under aerobic conditions and that this activity increased under hypoxic (1 or 2% O2) or anoxic conditions. As expected, immunoblot analysis of both cytosolic and nuclear fractions revealed the complete absence of HIF-1α in the cytosol at all O2 concentrations, indicating that the increase in HIF-1 activity coincides with an increase in nuclear but not cytosolic HIF-1α levels (Figure 3B,C).
Figure 3. HIF-1 expression levels increase as O2 concentrations decrease.
HEK293 cells were shifted to 0%, 1%, or 2% O2 for 3 hours, as described in Materials and Methods or were left at atmospheric O2 levels. An Active Motif® nuclear extraction kit was used to produce cytosolic and nuclear extracts. (A) The nuclear extract was examined using an Active Motif® HIF-1 ELISA assay for active HIF-1 levels. (B) The cytosolic fraction was analyzed by immunoblotting using anti-HIF-1α and anti-α-Tubulin antibodies. (C) The nuclear fraction was analyzed by immunoblotting using anti-HIF-1α and anti-Histone H2B antibodies. Quantitation was as described in Materials and Methods.
Having demonstrated that HEK293 cells have Cco/NO activity, which is responsible for the increase in endogenous NO levels in hypoxic cells, we next asked if this Cco/NO affects HIF-1 and HIF-1α levels under hypoxia. This was done first by examining whether azide, a Cco/NO inhibitor, affects HIF-1 levels in hypoxic cells. From Figure 4A it is clear that active HIF-1 levels do not increase in cells treated with azide, either in the presence or absence of L-NAME. The finding that azide affects the hypoxic stabilization of HIF-1 supports and extends a previous observation that azide inhibits the accumulation of HIF-1α in hypoxic HEK293 cells [12]. We next asked if the increased NO levels generated by Cco/NO in hypoxic cells affect HIF-1 or HIF-1α levels. This was done by using the cell permeable NO scavenger, PTIO, to deplete intracellular NO levels in HEK293 cells exposed to hypoxia (1% O2). From Figure 4B it is clear that added PTIO diminishes active HIF levels in a concentration dependent manner. This decrease in active HIF levels parallels a decrease in HIF-1α levels in these cells (Figure 4C).
Figure 4. Azide and PTIO prevent the accumulation of HIF-1 in hypoxic cells.
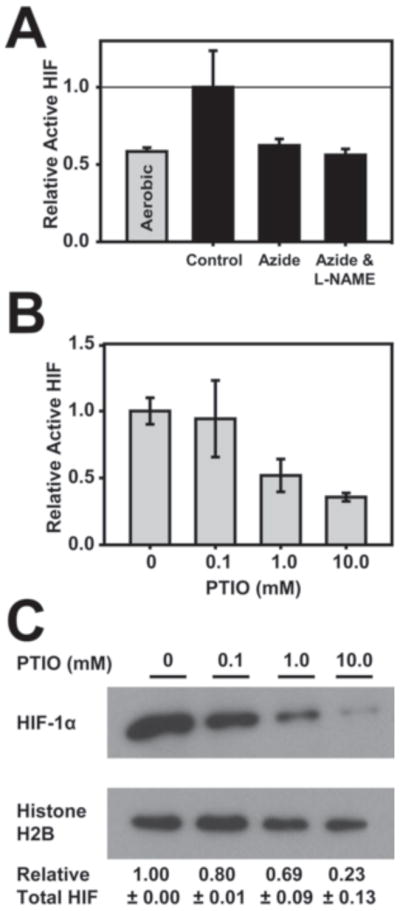
HEK293 cells were shifted to 1% O2 for 3 hours as described in Materials and Methods or were left at atmospheric O2 levels (A) in the presence or absence sodium azide (5 mM) and/or L-NAME (1 mM), as indicated or (B) in the presence of PTIO at the indicated concentrations. Active HIF-1 levels were measured as described in the legend to Figure 3. (C) Nuclear HIF-1α levels in the PTIO samples were evaluated by immunoblot analysis using anti-HIF-1α and anti-Histone H2B antibodies. Quantitation was as described in Materials and Methods.
Together, these findings support the conclusion that NO produced by Cco/NO in hypoxic HEK293 cells plays a role in the stabilization of HIF-1α. Previous efforts to understand the effects of NO on HIF-1α stability under hypoxia have focused largely on the effects of NO on the prolyl hydroxylases (PHDs) that hydroxylate HIF-1α. They have revealed that exposing cells to exogenous NO-donors reduces HIF-1α hydroxylation [28] and that NO has an inhibitory effects on PHDs 1–3 in vitro. It is not yet clear how NO inhibits PHDs but it has been proposed to compete with O2 for binding to the ferrous iron at the active site on these enzymes [28]. It is not yet known if NO also functions in the nitrosylation or tyrosine nitration of HIF-1 protein side chains in hypoxic cells or if NO exerts its effect on HIF-1α indirectly by affecting proteins with which it interacts or which function in other HIF-1α posttranslational modifications. In this latter regard it is interesting that the hypoxic deacetylation of HIF-1α is catalyzed by SIRT1 [24] and that NO modulates the deacetylase activity of SIRT1 [19].
Mitochondrial involvement in hypoxic signaling
It is clear that the identification of mitochondria as the intracellular source of NO generated in hypoxic cells is crucial to understanding the role of NO in HIF-1α stabilization and hypoxic signaling. Indeed, the findings reported here support a new model for mitochondrial involvement in hypoxic signaling [32]. This model takes into account the finding that hypoxic mitochondria produce both superoxide and NO and proposes that the predominant mitochondrially-generated signals in hypoxic cells are either peroxynitrite (ONOO-) or NO. Because these free radicals are highly reactive and unstable it is unlikely that they diffuse over the great distances that separate the mitochondrial reticulum and the nucleus. Indeed, it is more likely that these free radicals initiate signaling via S-nitrosylation, or tyrosine nitration and that these modifications activate the signaling pathway between the mitochondrion and HIF-1 in the nucleus. Attempts to identify these proteins are currently underway.
Highlights.
HEK293 mammalian cells, which lack a NOS isoform, make nitric oxide under hypoxia
Cytochrome c oxidase produces nitric oxide in hypoxic HEK293 cells
Nitric oxide from cytochrome c oxidase stabilizes HIF-1α in hypoxic cells
A new mode of mitochondrial involvement in hypoxic signaling in mammals is proposed
Acknowledgments
This work was supported by National Institutes of Health grant GM30228 to R.O. P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Acin-Perez R, Gatti DL, Bai Y, Manfredi G. Protein phosphorylation and prevention of cytochrome oxidase inhibition by ATP: coupled mechanisms of energy metabolism regulation. Cell Metab. 2011;13:712–719. doi: 10.1016/j.cmet.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ball KA, Castello PR, Poyton RO. Low intensity light stimulates nitrite-dependent nitric oxide synthesis but not oxygen consumption by cytochrome c oxidase: Implications for phototherapy. J Photochem Photobiol B. 2011;102:182–191. doi: 10.1016/j.jphotobiol.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Berchner-Pfannschmidt U, Tug S, Kirsch M, Fandrey J. Oxygen-sensing under the influence of nitric oxide. Cell Signal. 2010;22:349–356. doi: 10.1016/j.cellsig.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Berchner-Pfannschmidt U, Yamac H, Trinidad B, Fandrey J. Nitric oxide modulates oxygen sensing by hypoxia-inducible factor 1-dependent induction of prolyl hydroxylase 2. J Biol Chem. 2007;282:1788–1796. doi: 10.1074/jbc.M607065200. [DOI] [PubMed] [Google Scholar]
- 5.Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Castello PR, David PS, McClure T, Crook Z, Poyton RO. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 2006;3:277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Castello PR, Woo DK, Ball K, Wojcik J, Liu L, Poyton RO. Oxygen-regulated isoforms of cytochrome c oxidase have differential effects on its nitric oxide production and on hypoxic signaling. Proc Natl Acad Sci USA. 2008;105:8203–8208. doi: 10.1073/pnas.0709461105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 9.Dirmeier R, O'Brien KM, Engle M, Dodd A, Spears E, Poyton RO. Exposure of yeast cells to anoxia induces transient oxidative stress. Implications for the induction of hypoxic genes. J Biol Chem. 2002;277:34773–34784. doi: 10.1074/jbc.M203902200. [DOI] [PubMed] [Google Scholar]
- 10.Eissa NT, Yuan JW, Haggerty CM, Choo EK, Palmer CD, Moss J. Cloning and characterization of human inducible nitric oxide synthase splice variants: a domain, encoded by exons 8 and 9, is critical for dimerization. Proc Natl Acad Sci USA. 1998;95:7625–7630. doi: 10.1073/pnas.95.13.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- 13.Helling S, Vogt S, Rhiel A, Ramzan R, Wen L, Marcus K, Kadenbach B. Phosphorylation and kinetics of mammalian cytochrome c oxidase. Mol Cell Proteomics. 2008;7:1714–1724. doi: 10.1074/mcp.M800137-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. 1996;271:32253–32259. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- 15.Huang LE, Willmore WG, Gu J, Goldberg MA, Bunn HF. Inhibition of hypoxia-inducible factor 1 activation by carbon monoxide and nitric oxide. Implications for oxygen sensing and signaling. J Biol Chem. 1999;274:9038–9044. doi: 10.1074/jbc.274.13.9038. [DOI] [PubMed] [Google Scholar]
- 16.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igamberdiev AU, Hill RD. Plant Mitochondrial Function During Anaerobiosis. Ann Bot (Lond) 2008;103:259–268. doi: 10.1093/aob/mcn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- 19.Kornberg MD, Sen N, Hara MR, Juluri KR, Nguyen JV, Snowman AM, Law L, Hester LD, Snyder SH. GAPDH mediates nitrosylation of nuclear proteins. Nat Cell Biol. 2010;12:1094–1100. doi: 10.1038/ncb2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwast KE, Burke PV, Staahl BT, Poyton RO. Oxygen sensing in yeast: evidence for the involvement of the respiratory chain in regulating the transcription of a subset of hypoxic genes. Proc Natl Acad Sci USA. 1999;96:5446–5451. doi: 10.1073/pnas.96.10.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacza Z, Kozlov AV, Pankotai E, Csordas A, Wolf G, Redl H, Kollai M, Szabo C, Busija DW, Horn TF. Mitochondria produce reactive nitrogen species via an arginine-independent pathway. Free Radic Res. 2006;40:369–378. doi: 10.1080/10715760500539139. [DOI] [PubMed] [Google Scholar]
- 22.Li B, Skinner C, Castello PR, Kato M, Easlon E, Xie L, Li T, Lu SP, Wang C, Tsang F, Poyton RO, Lin SJ. Identification of potential calorie restriction-mimicking yeast mutants with increased mitochondrial respiratory chain and nitric oxide levels. J Aging Res. 2011;2011:673185. doi: 10.4061/2011/673185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the effects of oxygen on xanthine oxidase-mediated nitric oxide formation. J Biol Chem. 2004;279:16939–16946. doi: 10.1074/jbc.M314336200. [DOI] [PubMed] [Google Scholar]
- 24.Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell. 2010;38:864–878. doi: 10.1016/j.molcel.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Christou H, Morita T, Laughner E, Semenza GL, Kourembanas S. Carbon monoxide and nitric oxide suppress the hypoxic induction of vascular endothelial growth factor gene via the 5' enhancer. J Biol Chem. 1998;273:15257–15262. doi: 10.1074/jbc.273.24.15257. [DOI] [PubMed] [Google Scholar]
- 26.Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab. 2005;1:393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mateo J, Garcia-Lecea M, Cadenas S, Hernandez C, Moncada S. Regulation of hypoxia-inducible factor-1alpha by nitric oxide through mitochondria-dependent and -independent pathways. Biochem J. 2003;376:537–544. doi: 10.1042/BJ20031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metzen E, Zhou J, Jelkmann W, Fandrey J, Brune B. Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Mol Biol Cell. 2003;14:3470–3481. doi: 10.1091/mbc.E02-12-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller LP. Quantifying western blots without expensive commercial quantification software. Aug 1, 2007. pp. 1–8. Online. Ref Type: Online Source. [Google Scholar]
- 30.Nakatsubo N, Kojima H, Kikuchi K, Nagoshi H, Hirata Y, Maeda D, Imai Y, Irimura T, Nagano T. Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofluoresceins. FEBS Lett. 1998;427:263–266. doi: 10.1016/s0014-5793(98)00440-2. [DOI] [PubMed] [Google Scholar]
- 31.Palacios-Callender M, Hollis V, Mitchison M, Frakich N, Unitt D, Moncada S. Cytochrome c oxidase regulates endogenous nitric oxide availability in respiring cells: a possible explanation for hypoxic vasodilation. Proc Natl Acad Sci USA. 2007;104:18508–18513. doi: 10.1073/pnas.0709440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poyton RO, Ball KA, Castello PR. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol Metab. 2009;20:332–340. doi: 10.1016/j.tem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Sogawa K, Numayama-Tsuruta K, Ema M, Abe M, Abe H, Fujii-Kuriyama Y. Inhibition of hypoxia-inducible factor 1 activity by nitric oxide donors in hypoxia. Proc Natl Acad Sci USA. 1998;95:7368–7373. doi: 10.1073/pnas.95.13.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Faassen EE, Bahrami S, Feelisch M, Hogg N, Kelm M, Kim-Shapiro DB, Kozlov AV, Li H, Lundberg JO, Mason R, Nohl H, Rassaf T, Samouilov A, Slama-Schwok A, Shiva S, Vanin AF, Weitzberg E, Zweier J, Gladwin MT. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev. 2009;29:683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou X, He P. Improved measurements of intracellular nitric oxide in intact microvessels using 4,5-diaminofluorescein diacetate. Am J Physiol Heart Circ Physiol. 2011;301:H108–H114. doi: 10.1152/ajpheart.00195.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]