Abstract
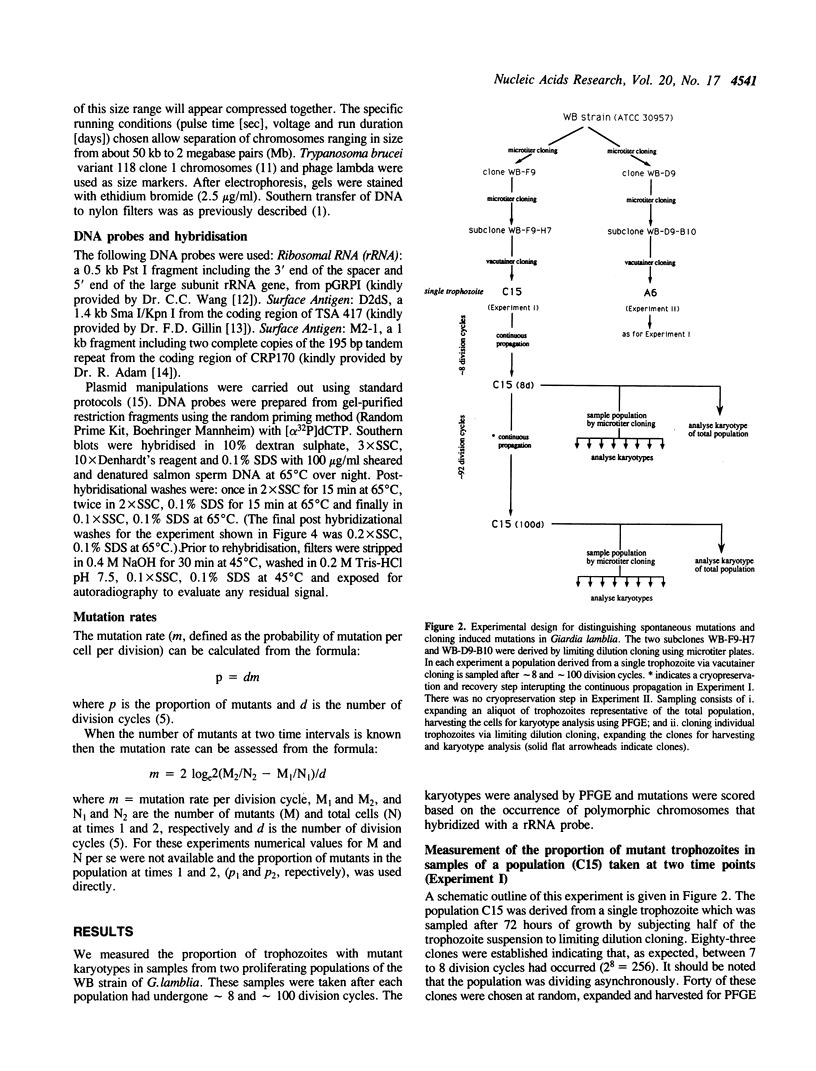
Subcloned lines of the WB strain of Giardia lamblia contain polymorphic ribosomal RNA (rRNA) encoding chromosomes (Le Blancq et al., Nucl. Acids Res. 1991, 19, 4405-4412). We show that in a continuously propagated culture of G.lamblia trophozoites the proportion of trophozoites with rearranged rRNA encoding chromosomes gradually increases, consistent with the high mutation rate of about 1% per cell per division cycle. This conclusion is based on the finding in one experiment that after about 8 division cycles 20% of the population consisted of independent mutants, while after approximately 100 division cycles 87.5% of the population were independent mutants. In a second experiment, approximately 38% and 71.5% of the trophozoites were independent mutants after approximately 9 and approximately 100 division cycles, respectively. The data show that the genome of the WB strain of G.lamblia has a highly recombinogenic phenotype. Extensive karyotype heterogeneity has also been observed among recently isolated G.lamblia strains obtained from a defined geographic area (Korman et al., J. Clin. Invest. 1992, 89, 1725-1733) suggesting that a high mutation rate might also occur in vivo.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam R. D., Aggarwal A., Lal A. A., de La Cruz V. F., McCutchan T., Nash T. E. Antigenic variation of a cysteine-rich protein in Giardia lamblia. J Exp Med. 1988 Jan 1;167(1):109–118. doi: 10.1084/jem.167.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam R. D., Nash T. E., Wellems T. E. The Giardia lamblia trophozoite contains sets of closely related chromosomes. Nucleic Acids Res. 1988 May 25;16(10):4555–4567. doi: 10.1093/nar/16.10.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam R. D., Yang Y. M., Nash T. E. The cysteine-rich protein gene family of Giardia lamblia: loss of the CRP170 gene in an antigenic variant. Mol Cell Biol. 1992 Mar;12(3):1194–1201. doi: 10.1128/mcb.12.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum K. F., Berens R. L., Jones R. H., Marr J. J. A new method for cloning Giardia lamblia, with a discussion of the statistical considerations of limiting dilution. J Parasitol. 1988 Apr;74(2):267–269. [PubMed] [Google Scholar]
- Boothroyd J. C., Wang A., Campbell D. A., Wang C. C. An unusually compact ribosomal DNA repeat in the protozoan Giardia lamblia. Nucleic Acids Res. 1987 May 26;15(10):4065–4084. doi: 10.1093/nar/15.10.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran L. M., Thompson J. K., Walliker D., Kemp D. J. Homologous recombination within subtelomeric repeat sequences generates chromosome size polymorphisms in P. falciparum. Cell. 1988 Jun 3;53(5):807–813. doi: 10.1016/0092-8674(88)90097-9. [DOI] [PubMed] [Google Scholar]
- Drake J. W. A constant rate of spontaneous mutation in DNA-based microbes. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7160–7164. doi: 10.1073/pnas.88.16.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin F. D., Diamond L. S. Entamoeba histolytica and Giardia lamblia: effects of cysteine and oxygen tension on trophozoite attachment to glass and survival in culture media. Exp Parasitol. 1981 Aug;52(1):9–17. doi: 10.1016/0014-4894(81)90055-2. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Hagblom P., Harwood J., Aley S. B., Reiner D. S., McCaffery M., So M., Guiney D. G. Isolation and expression of the gene for a major surface protein of Giardia lamblia. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4463–4467. doi: 10.1073/pnas.87.12.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin F. D., Reiner D. S. Attachment of the flagellate Giardia lamblia: role of reducing agents, serum, temperature, and ionic composition. Mol Cell Biol. 1982 Apr;2(4):369–377. doi: 10.1128/mcb.2.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesdiener K., Garciá-Anoveros J., Lee M. G., Van der Ploeg L. H. Chromosome organization of the protozoan Trypanosoma brucei. Mol Cell Biol. 1990 Nov;10(11):6079–6083. doi: 10.1128/mcb.10.11.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Royle N. J., Wilson V., Wong Z. Spontaneous mutation rates to new length alleles at tandem-repetitive hypervariable loci in human DNA. Nature. 1988 Mar 17;332(6161):278–281. doi: 10.1038/332278a0. [DOI] [PubMed] [Google Scholar]
- Keister D. B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77(4):487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Korman S. H., Le Blancq S. M., Deckelbaum R. J., Van der Ploeg L. H. Investigation of human giardiasis by karyotype analysis. J Clin Invest. 1992 Jun;89(6):1725–1733. doi: 10.1172/JCI115774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blancq S. M., Kase R. S., Van der Ploeg L. H. Analysis of a Giardia lamblia rRNA encoding telomere with [TAGGG]n as the telomere repeat. Nucleic Acids Res. 1991 Oct 25;19(20):5790–5790. doi: 10.1093/nar/19.20.5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blancq S. M., Korman S. H., Van der Ploeg L. H. Frequent rearrangements of rRNA-encoding chromosomes in Giardia lamblia. Nucleic Acids Res. 1991 Aug 25;19(16):4405–4412. doi: 10.1093/nar/19.16.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzi M., Janse C. J., Dore E., Scotti R., Pace T., Reterink T. J., van der Berg F. M., Mons B. Generation of chromosome size polymorphism during in vivo mitotic multiplication of Plasmodium berghei involves both loss and addition of subtelomeric repeat sequences. Mol Biochem Parasitol. 1990 Jun;41(1):73–82. doi: 10.1016/0166-6851(90)90098-7. [DOI] [PubMed] [Google Scholar]
- Szostak J. W., Wu R. Unequal crossing over in the ribosomal DNA of Saccharomyces cerevisiae. Nature. 1980 Apr 3;284(5755):426–430. doi: 10.1038/284426a0. [DOI] [PubMed] [Google Scholar]