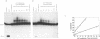Abstract
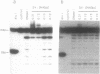
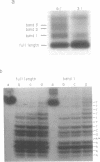
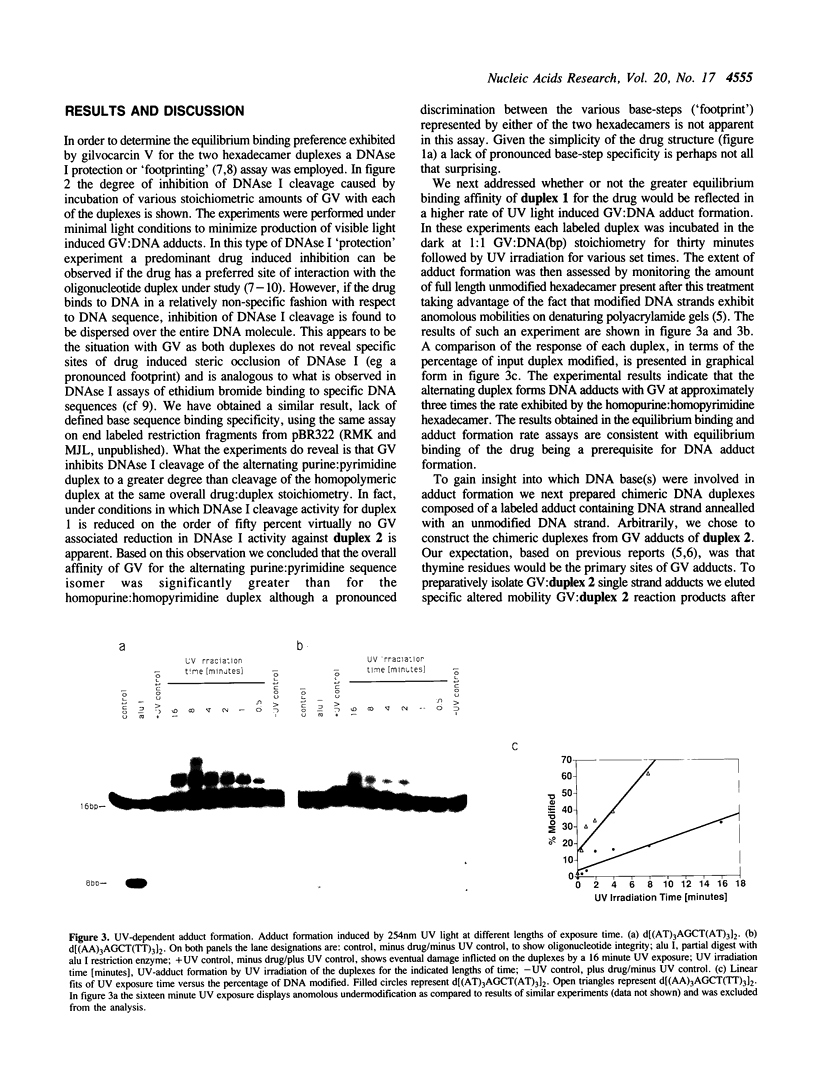
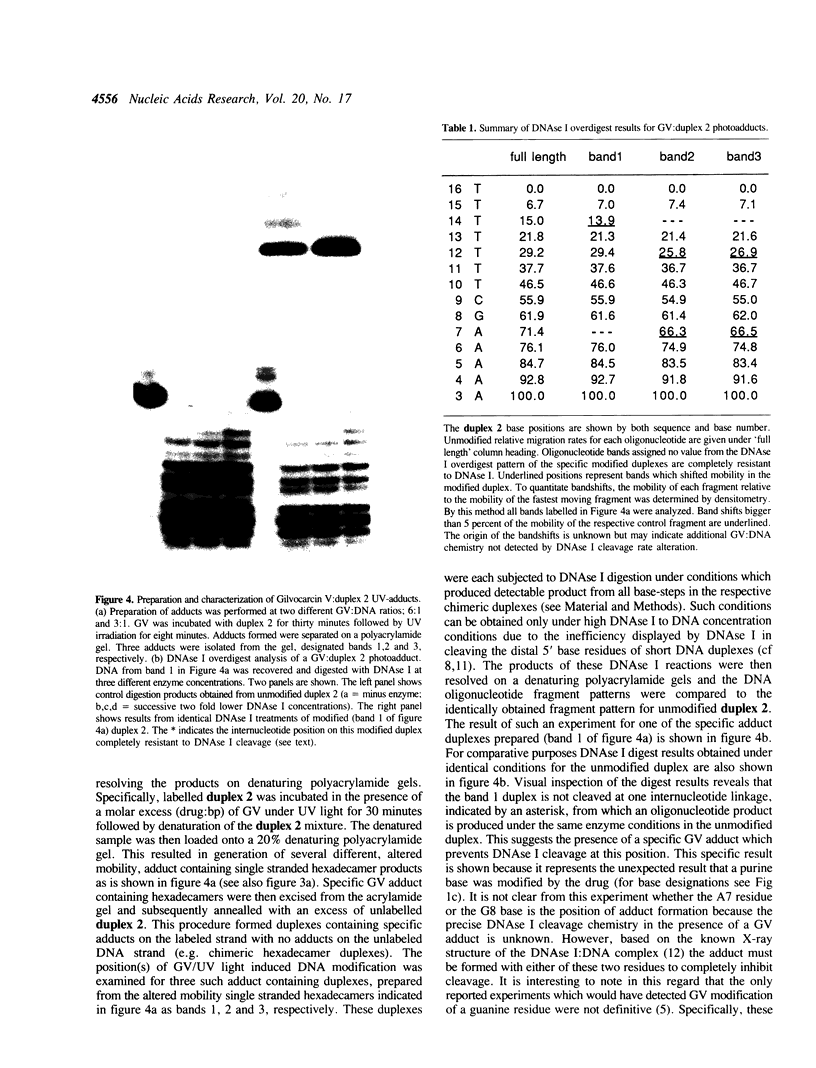
The relative degree of both equilibrium binding and of ultraviolet light induced adduct formation for the antitumor antibiotic gilvocarin V with two hexaecamer DNA sequence isomers, d[ATATATAGCTATATAT]2 and d[AAAAAAAGCTTTTTTT]2, was assessed. The experiments reveal that gilvocarin V binds, under equilibrium conditions, and reacts, in the presence of exogenously applied UV light, more efficiently with the alternating purine:pyrimidine sequence hexadecamer than the homopurine:homopyrimidine duplex at identical gilvocarcin V to DNA duplex ratios. DNAse I digests of adduct containing duplexes derived from the d[AAAAAAAGCTTTTTTT]2 duplex, identified and isolated using gel shift assays employing denaturing polyacrylamide gels, confirm that gilvocarcin V adducts can be formed with thymine residues but suggest that adduct formation with either adenine or guanine residues is also possible.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alegria A. E., Krishna C. M., Elespuru R. K., Riesz P. An ESR study of the visible light photochemistry of gilvocarcin V. Photochem Photobiol. 1989 Mar;49(3):257–265. doi: 10.1111/j.1751-1097.1989.tb04104.x. [DOI] [PubMed] [Google Scholar]
- Balitz D. M., O'Herron F. A., Bush J., Vyas D. M., Nettleton D. E., Grulich R. E., Bradner W. T., Doyle T. W., Arnold E., Clardy J. Antitumor agents from Streptomyces anandii: gilvocarcins V, M and E. J Antibiot (Tokyo) 1981 Dec;34(12):1544–1555. doi: 10.7164/antibiotics.34.1544. [DOI] [PubMed] [Google Scholar]
- Drew H. R. Structural specificities of five commonly used DNA nucleases. J Mol Biol. 1984 Jul 15;176(4):535–557. doi: 10.1016/0022-2836(84)90176-1. [DOI] [PubMed] [Google Scholar]
- Gasparro F. P., Knobler R. M., Edelson R. L. The effects of gilvocarcin V and ultraviolet radiation on pBR322 DNA and lymphocytes. Chem Biol Interact. 1988;67(3-4):255–265. doi: 10.1016/0009-2797(88)90062-2. [DOI] [PubMed] [Google Scholar]
- Knobler R. M., Edelson R. L. Cutaneous T cell lymphoma. Med Clin North Am. 1986 Jan;70(1):109–138. doi: 10.1016/s0025-7125(16)30972-5. [DOI] [PubMed] [Google Scholar]
- Knobler R. M., Graninger W., Graninger W., Lindmaier A., Trautinger F., Smolen J. S. Extracorporeal photochemotherapy for the treatment of systemic lupus erythematosus. A pilot study. Arthritis Rheum. 1992 Mar;35(3):319–324. doi: 10.1002/art.1780350311. [DOI] [PubMed] [Google Scholar]
- Lane M. J., Dabrowiak J. C., Vournakis J. N. Sequence specificity of actinomycin D and Netropsin binding to pBR322 DNA analyzed by protection from DNase I. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3260–3264. doi: 10.1073/pnas.80.11.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook A. H., Freundlich B., Jegasothy B. V., Perez M. I., Barr W. G., Jimenez S. A., Rietschel R. L., Wintroub B., Kahaleh M. B., Varga J. Treatment of systemic sclerosis with extracorporeal photochemotherapy. Results of a multicenter trial. Arch Dermatol. 1992 Mar;128(3):337–346. [PubMed] [Google Scholar]
- Tse-Dinh Y. C., McGee L. R. Light-induced modifications of DNA by gilvocarcin V and its aglycone. Biochem Biophys Res Commun. 1987 Mar 30;143(3):808–812. doi: 10.1016/0006-291x(87)90320-2. [DOI] [PubMed] [Google Scholar]
- Vowels B. R., Cassin M., Boufal M. H., Walsh L. J., Rook A. H. Extracorporeal photochemotherapy induces the production of tumor necrosis factor-alpha by monocytes: implications for the treatment of cutaneous T-cell lymphoma and systemic sclerosis. J Invest Dermatol. 1992 May;98(5):686–692. doi: 10.1111/1523-1747.ep12499907. [DOI] [PubMed] [Google Scholar]