Abstract
The underconnectivity theory of autism attributes the disorder to lower anatomical and functional systems connectivity between frontal and more posterior cortical processing. Here we review evidence for the theory and present a computational model of an executive functioning task (Tower of London) implementing the assumptions of underconnectivity. We make two modifications to a previous computational account of performance and brain activity in typical individuals in the Tower of London task (Newman et al., 2003): (1) the communication bandwidth between frontal and parietal areas was decreased and (2) the posterior centers were endowed with more executive capability (i.e., more autonomy, an adaptation is proposed to arise in response to the lowered frontal-posterior bandwidth). The autism model succeeds in matching the lower frontal-posterior functional connectivity (lower synchronization of activation) seen in fMRI data, as well as providing insight into behavioral response time results. The theory provides a unified account of how a neural dysfunction can produce a neural systems disorder and a psychological disorder with the widespread and diverse symptoms of autism.
Keywords: autism, connectivity, underconnectivity, 4CAPS, computational model, fMRI
1. Introduction
Although autism has surely been with mankind for millennia, it was systematically identified only recently by Kanner (1943), and Asperger (1944). Both of these papers were psychiatric case studies of children, and their characterizations of the behaviors in autism remain accurate and insightful to this day. Although neither of these seminal papers provided a scientific account of autism at either the behavioral or neuroscientific level, they both suggested a possible biological origin for the disorder. Despite this identification of the disorder in the 1940’s, scientific research into autism (and its funding) remained small in scale in the U.S. until the 1990’s, when new methods of cognitive and social neuroscience were developed and began to be applied to autism. Methods including genomics, eye-movement tracking, and electrophysiology held the promise of providing an understanding of the psychological and biological mechanisms that underpin the disorder. Our focus here is on the findings from another new method, neuroimaging of brain structure and of brain activity. In this paper, we propose a formal model of autism that integrates some of the recent neuroimaging findings, instantiating a cortical systems underconnectivity theory of autism.
Autism has long been an enigma in at least three ways: one way is that the symptoms (disorder of social and communicative behaviors, and a restricted range of interests) are diverse and seemingly unrelated; another way is that the syndrome does not bear an obvious correspondence to a particular biological function (such as some forms of blindness being related to damage to the visual cortex); and a third way is that occasionally autism is manifested as a perceptual advantage. However, with the rapid development of new scientific understanding of brain function that has occurred in the past two decades, it is now possible to make sense of these three aspects of the enigma: the diversity of the symptoms of autism can now be understood as a manifestation of a neural systems disorder whose impacts are widespread; the link to a biological substrate is being illuminated by functional and anatomical brain imaging as well as by genomic research; and the understanding of the brain and cognition as a complex system illuminates how a perturbation of the system can have both negative and positive impacts on system functioning. The theory we propose here attempts to provide a detailed scientific account of some aspects of the enigma.
Autism has recently been characterized as a disorder of neurological origin with abnormalities found in the coordinated functioning of brain regions. This theoretical view, the cortical underconnectivity theory, first emerged from fMRI (functional magnetic resonance imaging) measurements of cortical activation in several types of thinking tasks. These studies showed that the degree of synchronization of the activation (or functional connectivity) between frontal and posterior brain regions was lower in autism. The observation was first made in a language comprehension task (Just et al., 2004), and undersynchronization of activation during task performance has since been found between the frontal lobe and more posterior regions in a wide variety of other tasks (Darmala et al., 2010; Just et al., 2004, 2007; Kana et al., 2006, 2007, 2009; Koshino et al., 2005, 2008; Mason et al., 2008; Mizuno et al., 2011; Schipul et al., 2011a; see Schipul et al. 2011b for a recent review). We propose that the lower synchronization arises because the communication bandwidth between frontal and posterior cortical areas is lower in autism than in the typical population. We use the term bandwidth to refer to the maximal rate of data transfer supported by a communication channel, taking into account the impact of noise, consistent with Shannon’s (1949) usage.
Decreased bandwidth would clearly impact system performance when the interregional communication needs were high enough. Some of the central questions that emerge from examining underconnectivity in autism are: 1) How would a bandwidth constraint in autism affect the communication transfer between frontal and posterior regions? 2) How might a brain with autism adapt to or compensate for such an impairment? 3) Does the physical or anatomical distance between cortical areas play a key role in information transfer in autism? 4) Are there underlying structural and developmental bases for the underconnectivity? and 5) Can computational modeling of fMRI data account for the variation in synchronization (functional connectivity) in terms of variation in several structural and functional attributes of the brain? In this paper, we attempt to address these questions using a formal theory accompanied by a computational model.
This article is organized into several sections below: a brief summary of previous findings, a description of underconnectivity theory and of its implementation as a computational model, and a discussion of the theory and its relation to other theories.
2. Previous biological findings
Several types of previous background findings concerning brain biology lend plausibility to underconnectivity theory, although they are not a part of the theory proper.
2.1. Abnormal maturation of the brain in autism
Consistent with autism being a developmental disorder, there is an abnormal developmental trajectory of the brain. There are several important brain maturational events that continue into early adulthood, such as synaptic pruning, elaboration of dendritic arborization (Changeux and Danchin, 1976; Huttenlocher, 1990), and increased myelination (Giedd et al., 1999; Paus et al., 1999; Yakovlev and Lecours, 1967) that could impact cortical connectivity. Abnormalities in any of several brain development mechanisms in autism (Bailey et al., 1998; Bauman and Kemper, 1985; Courchesne et al., 2001, 2003; Hazlet et al., 2005) could result in abnormal cortico-cortical connectivity (Castelli et al., 2002; Just et al., 2004, 2007; Kana et al., 2006). It has been postulated that the pruning of synapses that normally occurs during later stages of neuronal development is compromised in autism (C. Frith, 2003; Schultz and Klin, 2002). In the typical brain, initial growth and subsequent regression (due to mechanisms like neuronal loss and synaptic pruning) are timed in ways that are presumed to allow activity and experience to support the organization of functional networks (Kandel et al., 2000). The growth profiles in autism may not support an appropriate balance between maturation and experience. Whereas normal pruning could help eliminate faulty connections and optimize coordinated neural functioning, compromised pruning might fail to do so, possibly resulting in some degree of anatomical “overconnectivity” that could either increase or decrease the efficiency of communication among cortical regions. Decreased elimination of neural structures, including apoptosis, axonal pruning, and dendritic degeneration, as well as increased neurogenesis, have been suggested to occur in autism (Piven et al., 1996).
C. Frith (2003) hypothesized that the brain enlargement in autism was linked to (i.e. a marker of) abnormal connectivity, brought about by a lack of pruning. He further hypothesized that several behavioral and neural characteristics of autism might be attributable to the frontal cortex failing to adequately modulate sensory processing due to its reduced connectivity with posterior areas. Consistent with this hypothesis, C. Frith and his colleagues (Bird et al., 2006) later showed that the modulation of the synchronization between early visual areas and the fusiform face area by higher-level attentional processes was altered in autism.
Abnormalities in these maturational processes are consistent with the findings of enlarged brain size in autism in early stages of development (Aylward et al., 2002; Piven et al., 1995), with the enlargement being greatest in frontal cortex (Carper and Courchesne, 2005). It has been suggested that increased brain size during development may critically impact the relative cost and efficiency of short-distance and long-distance cortico-cortical connectivity (Lewis and Elman, 2008), with overgrowth resulting not only in conduction delays, but also in greater cell maintenance costs associated with long-distance connections. A compensatory process that would reduce the functional impact of increased brain size would be a reduction in the proportion of more costly long-distance connections (Jacobs and Jordan, 1992; Kaas, 1997, 2000; Mitchison, 1991; Ringo, 1991; Ringo et al., 1994), and there is evidence from typically-developing adult males that an inverse relationship exists between the length of interhemispheric connections and the degree of interhemispheric connectivity (Lewis et al., 2009). Thus, increased brain size at a critical stage of development in children with autism, particularly in frontal regions, could plausibly result in lasting differences in long-distance anatomical connectivity consistent with the reduced task-related frontal-posterior functional connectivity consistently seen in adults.
2.2. White matter abnormalities in autism
What surely affects inter-regional cortical communication is the integrity of the white matter tracts that carry the information between different brain regions. Connectivity is usually thought of at the level of connections between individual neurons, but the phenomenon of communication among cortical centers in different parts of the brain is enormously affected by the degree of myelination of axons. The myelin sheath around axons can increase the transmission speed (and hence bandwidth) by a factor of 10 or more (Hartline and Colman, 2007), so myelination (formation of insulating white matter) and its distribution has a clear relation to cortical communication capacities, including synchronization capabilities. Several studies have reported volumetric abnormalities in white matter in autism, with enlargements in some areas and decreased volumes in other areas, and the increased brain size in young children with autism discussed above is largely driven by white matter, particularly in the frontal lobes (Carper et al., 2002, Herbert et al., 2004). For example, Herbert et al. (2003) reported overall greater white matter volume in 7 to 11-year-old children with autism and in a later study found that this deviation from normal was greatest in the radiate white matter in the frontal lobe (Herbert et al., 2004). This overgrowth of white matter is followed by reduced white matter volume in adolescence and adulthood, relative to controls (Courchesne et al., 2001, 2004; Waiter et al., 2005). In contrast, typically developing individuals show a linear increase in white matter volume between the ages of 4 and 22 years (Giedd et al., 1999). The most prominent white matter tract in the cortex, the corpus callosum, is usually smaller in autism (although the size of the effect is modest) (Chung et al., 2004; Hardan et al., 2000; Manes et al., 1999; Piven et al., 1997; Vidal et al., 2006). The corpus callosum enables communication among specialized but collaborating functional systems in the two hemispheres. Hence decreased corpus callosum size could be an index of white matter deficits that could contribute to impaired cortical connectivity. As noted above, total brain size is abnormal in autism, and this abnormality of larger brain volume has been found to be correlated with smaller corpus callosum size (Jäncke et al., 1997, 1999), suggesting multiple loci of disruption in connectivity (Ringo, 1991). The abnormalities in white matter in autism suggest a plausible neural basis for disrupted systems-level connectivity in autism.
2.3. Biological mechanisms affecting connectivity
Several microstructural processes could underpin the impairments in functional and anatomical connectivity observed in autism. A number of early neurodevelopmental processes (such as neuronal migration and axonal pathfinding) could individually or in combination result in abnormalities in the brain’s development of connectivity. Neuronal migration abnormalities have been reported in postmortem cases of autism (Bailey et al., 1998). Developmental alterations in axon number, axon pathfinding, synaptogenesis, and subsequent pruning of axons could result in abnormalities in the connectivity provided by white matter tracts (Geschwind and Levitt, 2007).
Abnormalities associated with glial cells may also play a critical role in maldevelopment of the brain structure and connections. Specialized glial cells (oligodendrocytes) enhance neural transmission by constructing insulating myelin. Other glial cells provide organizational structure to the neuronal network, managing waste and cleaning up neurotransmitters. Evidence of astroglial and microglial activation and neuroinflammation in gray and white matter (in samples taken from the middle frontal gyrus, anterior cingulate gyrus, and posterior cerebellar hemispheres) have been found in studies of autistic postmortem cases (Vargas et al., 2005). Glial cells are also involved in neural migration, structural formation of the minicolumn, minicolumn function, and apoptosis (Marin-Teva et al., 2004).
Neuronal migration abnormalities could alter the fundamental vertical organization of cortical minicolumns in autism, which would lead to fractionated and incompletely or aberrantly formed minicolumn vertical circuitry, as well as an imbalance between excitation and inhibition within and between minicolumns (Courchesne et al., 2005). Minicolumn abnormalities (more numerous and abnormally narrow minicolumns in frontal and temporal cortex) have been reported in autism (Casanova et al., 2006). Minicolumn abnormalities could create an abundance of short connective fibers relative to long ones, which may lead to a deficiency in inter-regional connectivity.
Yet another possibility is that abnormalities in neurochemistry could affect brain development and its connectivity. Regionally specific reductions in N-acetylaspartate (NAA) (assessed with magnetic resonance spectroscopy) have been reported in autism in several brain regions including cingulate gyrus, temporal gray matter, frontal and parietal white matter, hippocampal-amygdaloid complex, and cerebellum (Friedman et al., 2003; Hisaoka et al., 2001; Levitt et al., 2003; Otsuka et al., 1999). NAA is an amino acid whose presumed role in connectivity is to provide acetate for lipid and myelin synthesis in oligodendrocytes, the glial cells that myelinate axons (Baslow, 2003). Another amino acid, glutamate, also plays an important role in neurodevelopmental processes, including neuronal migration, differentiation, axon genesis, and plasticity (Coyle et al., 2002). Several authors have proposed that glutamatergic dysfunction could be a factor of relevance to autism (Carlsson 1998; Polleux and Lauder, 2004; Rubenstein and Merzenich, 2003). Irregularities in brain neurochemistry could be a potential factor in the abnormal development of connections and hence atypical brain functioning in people with autism. In summary, the disruption of cortical connectivity observed in autism could plausibly stem from one or more of the biological mechanisms described above.
3. Convergence of the brain imaging evidence implicating disrupted connectivity in autism
Aside from the lower-level biological mechanisms cited above that could underpin cortical connectivity disruption, four recent brain imaging findings more directly implicate aberrant cortical connectivity in autism, and they do so in a tightly convergent way.
First, the synchronization of activation (or functional connectivity) between frontal and posterior regions of the cortex is lower in autism than in control groups during task performance across a number of different domains of thought, including language (Just et al., 2004; Kana et al., 2006; Mason et al. 2008; Mizuno et al., 2011), executive function (Just et al., 2007), social processing (Kana et al., 2009; Koshino et al., 2008; Schipul et al., in press), working memory (Koshino et al., 2005; Koshino et al., 2008), high-level inhibition (Kana et al., 2007; Solomon et al. 2009), and visuospatial processing (Darmala et al., 2010). The lower functional connectivity in autism measured during the time that a task is being performed reflects the lower degree of coordination between the psychologically-driven activation modulation in two regions.
In contrast to this task-related functional connectivity, a number of studies have focused on spontaneous low-frequency fluctuations in BOLD signal intensity by low-pass filtering and partialling of task-driven effects from the time series data prior to calculating functional connectivity. Such studies have shown evidence of both reduced (Jones et al. 2010; Villalobos et al., 2005) and increased (Mizuno et al., 2006; Noonan et al., 2009; Turner et al., 2006; Shih et al. 2010) task-free (or “intrinsic”) functional connectivity in autism. Although the significance of these low frequency interregional correlations in BOLD signal remains unclear, both increases and decreases in functional connectivity measured in this way could plausibly reduce the bandwidth of communication for task-relevant processing (see Schipul et al., 2011, for a discussion).
A related type of functional connectivity can be measured in a resting state, when the “task” consists of relaxed, internally-generated thought. The functional connectivity between frontal and posterior areas (of the default network, or regions more active during rest than an externally imposed task) is lower in autism (Cherkassky et al., 2006). Even when high frequency fluctuations in activation are filtered from the time course of activation during rest (in an effort to limit the measurement to spontaneous physiological changes rather than cognitively-driven modulations of activation), frontal-posterior connectivity is found to be reduced in autism (Assaf et al., 2010; Kennedy and Courchesne, 2008; Monk et al. 2009; Weng et al., 2010). Synchronization of EEG activity across cortical areas within the alpha range (8–10 Hz) measured during rest have also provided converging findings of lower synchronization in autism between frontal and posterior regions (Coben et al., 2008; Murias et al., 2007). Coben et al. (2008) interpreted their EEG results as indicating “…dysfunctional integration of frontal and posterior brain regions in autistics along with a pattern of neural underconnectivity.”
In sum, studies of task-relevant functional connectivity associated with psychological processing measured with fMRI consistently find reduced frontal-posterior synchronization of activation across many different types of thinking, appearing to be domain general, although they tend to occur in more complex tasks, particularly those that involve frontal participation. fMRI and EEG studies of resting-state synchronization group differences are consistent with this finding, but are not the central focus here.
Second, as described above, there are white matter volumetric abnormalities in autism, implicating cortico-cortical connection abnormality as a key characteristic of autism (e.g. Carper et al., 2002; Courchesne et al., 2001; Herbert et al., 2004). These studies indicate excess white matter volume in autism in some regions (such as the frontal radiate white matter) and diminished volume in other regions (such as the corpus callosum).
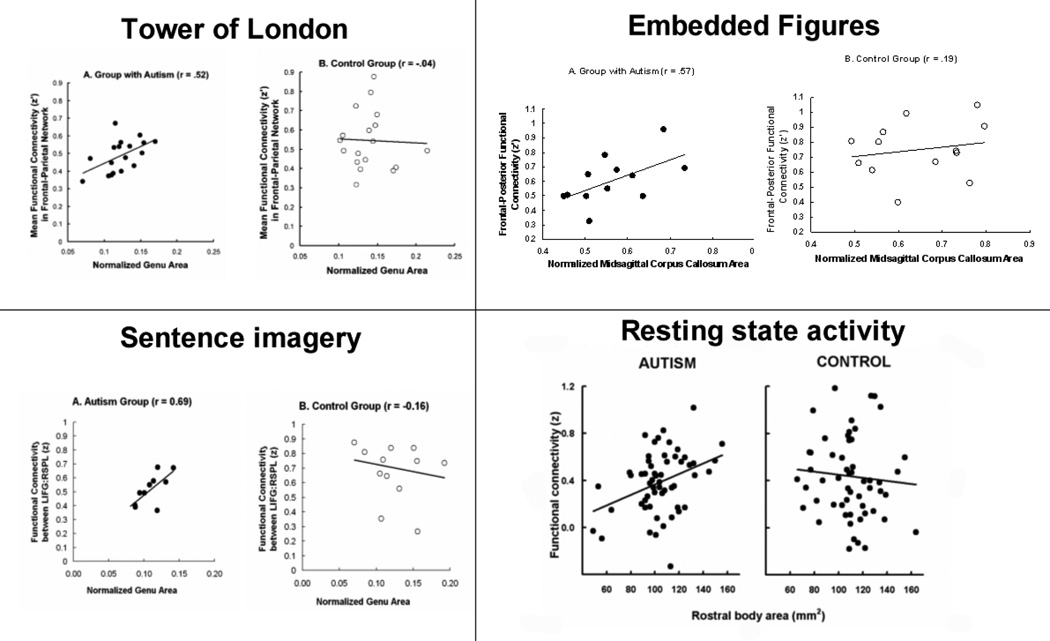
Third, a consistent relationship has been found in autism between an anatomical property of the brain’s white matter pertaining to communication (corpus callosum size) and the synchronization of the activation between frontal and posterior cortical areas. Several studies have found that the reduced size of the corpus callosum in individuals with autism was correlated with their lower degree of functional connectivity, in different types of thinking tasks (Just et al., 2007; Kana et al., 2006, 2009; Schipul et al., in press) as well as in a resting state (Cherkassky et al., 2006). More specifically, the functional connectivity between frontal and posterior regions in such studies has been shown to be correlated with the size of the corpus callosum segment that connects these regions, as shown in Figure 1. This figure illustrates that the phenomenon occurs in diverse types of studies which differ in detail but nevertheless produce a similar outcome. The corpus callosum size disruption in these studies is interpreted here not just as a measure of an anatomical deficit in a particular pathway, but as a more general index of white matter disruption in the cortex. The white matter disruption in autism can be seen as imposing a constraint on the communication bandwidth between frontal and posterior brain regions and hence limiting their degree of synchronization. (The white matter in control participants is assumed not to impose such a constraint, and accordingly, no correlation emerges among control participants between the size of the relevant portion of the corpus callosum and the frontal-posterior functional connectivity.)
Figure 1.
Correlation in 4 studies between the relevant portion of the corpus callosum and the mean functional connectivity between frontal and posterior areas for the Autism group (A) and a lack of correlation for the Control group (B).
Fourth, several recent diffusion tensor imaging studies of the properties of white matter have found deficiencies in the connective tracts in autism. One study of children and adolescents with autism found reduced fractional anisotropy in white matter (indicating a lower degree of coherence of directionality) adjacent to the ventromedial prefrontal cortices, anterior cingulate gyri, temporoparietal junctions, and in the corpus callosum (Barnea-Goraly et al., 2004). DTI studies examining a broader age range have found that reduced fractional anisotropy in autism persists into adulthood in areas within and near the cortico-cortical white matter tracts, particularly in the corpus callosum and in the frontal and temporal lobes (Alexander et al., 2007; Keller et al., 2007; Lee et al., 2007). At very young ages (2 to 3 years) there is some evidence of increased fractional anisotropy in autism, particularly in the frontal lobe and corpus callosum (Ben Bashat et al., 2007), consistent with evidence presented above for early overgrowth of white matter. By five years of age, however, fractional anisotropy is found to be reduced in children with autism for tracts that interconnect cortical regions within the frontal lobes (Sundaram et al., 2008) although it is equivalent to that found in typically developing children for tracts connecting the frontal lobes to more posterior cortical regions. In older children and adolescents (10- to 18-year-olds) analyses of specific frontal-posterior tracts indicate reduced fractional anisotropy in autism (Sahyoun et al., 2010) and autism-related differences in hemispheric lateralization of fractional anisotropy in the arcuate fasciculus connecting frontal, parietal, and temporal cortices (Fletcher et al., 2010; Knaus et al., 2010). Cumulatively, these studies provide clear evidence of disruption in autism of the white matter that provides the anatomical connectivity among brain regions, and suggest that deficits in frontal-posterior connectivity may increase with development. The DTI finding that seems most relevant to the theory proposed below is the very substantially reduced fractional anisotropy in an area of the left anterior corona radiata, consistent with either the left uncinate fasciculus (connecting the frontal and temporal lobes), or with the left inferior frontal-occipital fasciculus, observed in a large sample of 52 adults and adolescents with autism (compared to age and IQ-matched controls) (Keller and Just, 2009a). The critical relevance of white matter disruption in autism is that the white matter properties are key determinants of the conduction velocity and hence the bandwidth of the communication channels (Waxman, 1980).
Together these converging empirical findings suggest that alterations in cortical connectivity and the communication among cortical regions may be part of the pervasive core processing deficits in autism (Belmonte et al., 2004; Courchesne and Pierce, 2005a; 2005b; Herbert et al., 2004; Just et al., 2004; Keller et al., 2007; Rippon et al., 2007). Below we describe a theory of autism based on disruption of cortical connectivity.
4. Underconnectivity theory
The cortical underconnectivity theory that we have previously proposed in the context of specific tasks (Just et al., 2004, 2007) posits that inter-regional (systems level) connective circuitry in the brain is disrupted in autism, and that patterns of thought that are particularly dependent on integration of frontal and more posterior contributions are disrupted. Furthermore, the theory attributes disruptions in psychological functions such as Theory of Mind and executive processing to such underconnectivity. The theory proposes a causal link between the anatomical, physiological (brain activity), and psychological phenomena. Specifically, the theory posits that the communication bandwidth among cortical areas, particularly between frontal and posterior areas, is lower in autism than in typical participants. Thus, any facet of psychological or neurological function that is dependent on the coordination or integration of frontal brain regions with more posterior regions is susceptible to disruption, particularly when the computational demand is large (i.e. the task is complex and requires integration of different types of cortical computations).
Underconnectivity is proposed as a unifying theory for explaining a range of deficits at the levels of psychological function, cortical function, and cortical anatomy. The considerable heterogeneity of autism is attributed to the heterogeneity of connectivity disturbances. This is an initial attempt at an exhaustive theory, in the sense that no other independent factors that do not stem from or underlie connectivity aberrations are presumed to underlie autism.
4.1. Bandwidth limits in autism
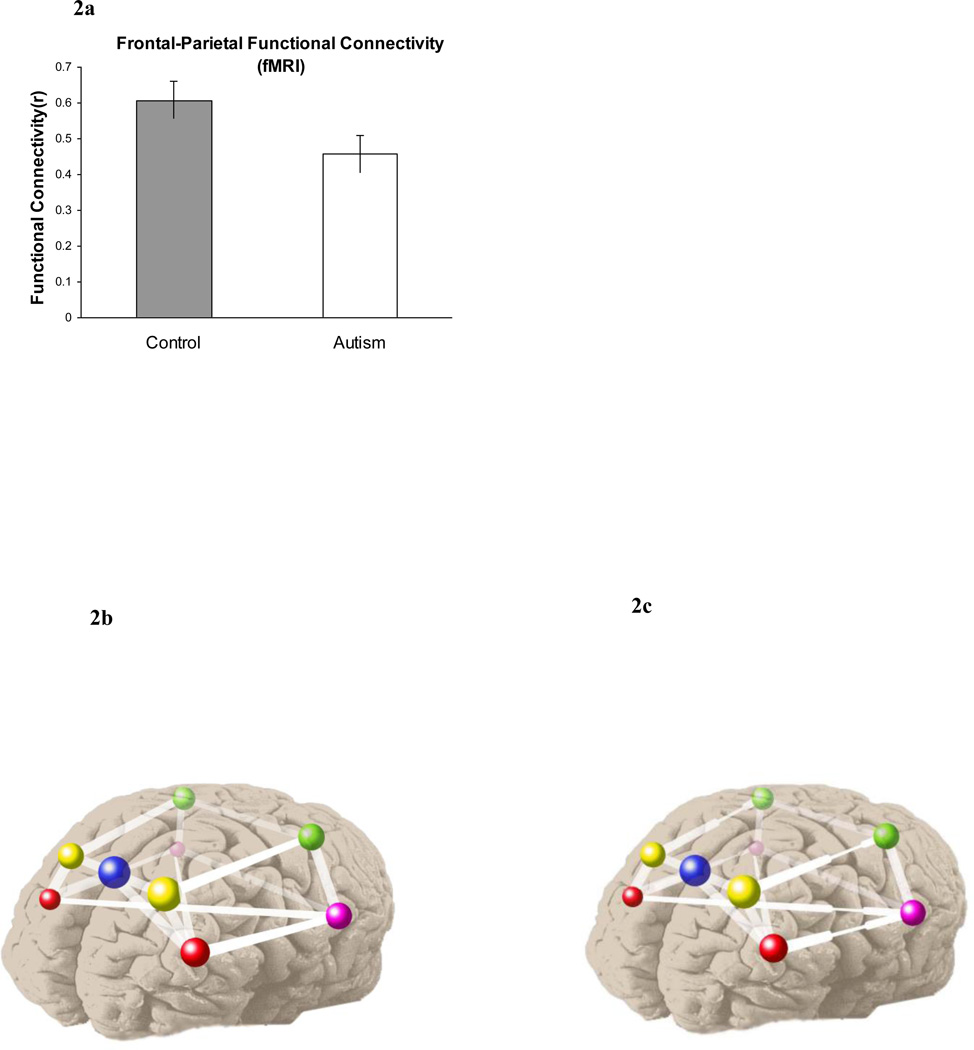
A critical factor in the performance of a communicating network is bandwidth: the amount of information that can be transmitted between nodes per unit time. Brain imaging research has definitively shown that human thought involves co-activation of a network of cortical areas whose activity is coordinated (synchronized), and the coordination is based on inter-regional communication, using the white matter tracts that provide the anatomical connectivity. The fMRI findings emerging in the last few years indicate that the synchronization between frontal and posterior areas is lower in autism (Just et al., 2004, 2007; Kana et al., 2006, 2007; Koshino et al., 2005; Villalobos et al., 2005). For example, in a Tower of London (TOL) problem-solving task, which entails activation of both frontal and parietal areas, the synchronization between the frontal and parietal areas is lower in autism than in a control group, as shown in Figure 2a. (We describe this study in considerable detail below, as it is the one that is later modeled). We attribute the lower synchronization to a lower communication bandwidth in autism between frontal and posterior areas, depicted schematically in Figure 2b and 2c. The model presented below demonstrates that a bandwidth constraint, along with increased parietal autonomy, results in a reduction of frontal-parietal synchronization. (In addition to the group difference in the functional connectivity between regions, there was also a behavioral group effect, namely an interaction arising because the autism group took longer to respond for the more difficult problems. This second result can also be seen as an outcome of a frontal-parietal bandwidth constraint in autism, as described below.)
Figure 2.
2a. Lower frontal-parietal functional connectivity in autism in the Tower of London task (from Just et al., 2007). 2b. Schematic depiction of typical systems connectivity. 2c. Schematic depiction of lower bandwidth between frontal and posterior cortical centers in autism.
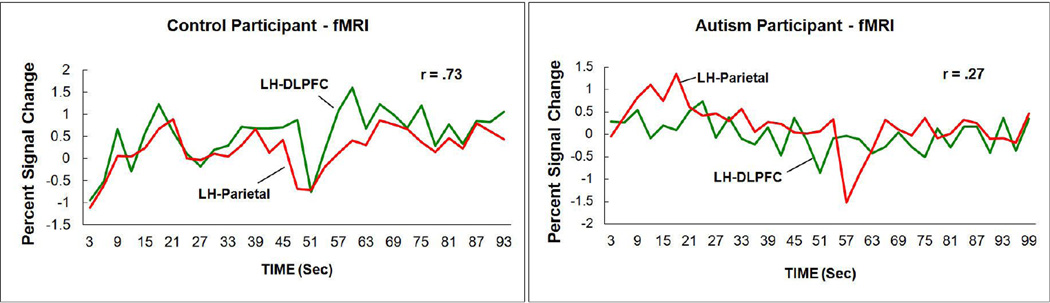
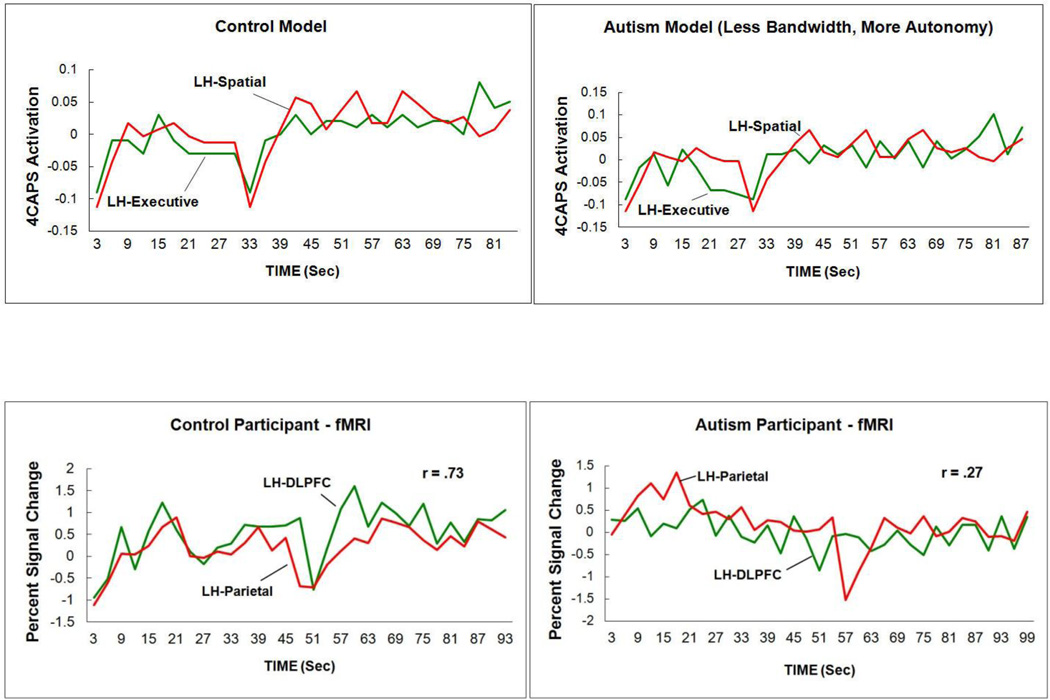
The lower synchronization (functional connectivity) in autism plotted in Figure 2a is an average taken over the 18 participants in each group and over multiple pairs of activated frontal-parietal pairs of regions of interest. The basic data on which the average is based are the correlations of the activation time series between two activated regions in the time intervals during which the task is being performed. The correlations measure the degree to which the activation levels in the two regions rise and fall together. To illustrate the coordination of two regions, Figure 3 plots the time courses of a frontal region (left DLPFC) and a parietal region (left Parietal) of one control participant (left panel) and one participant with autism (right panel), showing the rise and fall of the activation level across time (measured once every 3 sec in this case, and once every sec in more recent studies). Visual inspection and the correlation coefficients indicate that the two curves are less correlated for the participant with autism (and Figure 2a shows the mean frontal-parietal correlation for each group in the study). This reduced synchronization in autism between frontal and posterior areas in TOL problem solving exemplifies functional underconnectivity in a task involving executive processing in a visuospatial domain.
Figure 3.
Higher frontal-parietal functional connectivity between the two activation time series in a control participant (correlation r = .73) (left panel) in the Tower of London task than in an autism participant (r = .27) (right panel) (from Just et al., 2007).
Such functional underconnectivity of a very similar form has also been observed in autism in a number of diverse domains. One of these was a social task involving face perception and working memory, exhibiting reduced synchronization between the fusiform face area and a frontal area (Koshino et al., 2008). Another was a Theory of Mind task in which the intentions of animated geometric objects were being inferred from their motions, exhibiting reduced functional connectivity between the medial frontal area and the right posterior superior temporal area (both associated with Theory of Mind processing) (Kana et al., 2009). Another study was a sentence comprehension task in which participants had to construct a visual image in order to evaluate whether the sentence was true or false, requiring coordinating between a frontal language area and a parietal spatial processing area (Kana et al., 2006). Yet another was a task requiring complex inhibition, displaying reduced frontal-parietal connectivity (Kana et al., 2007). Finally, even in a resting state when participants were not performing any assigned task, the functional underconnectivity in autism between frontal and posterior regions continued to be manifested (Cherkassky et al., 2006). The diversity of these tasks speaks to the generality of the functional underconnectivity phenomenon.
Figures 2a and 3 depict the main phenomenon for which the modeling attempts to provide a postulated mechanism. The goal of the modeling was to evaluate whether a decrease in the synchronization of the activation in two co-activating areas could arise from a lowered communication bandwidth.
A key assumption is that lowering the bandwidth of a complex system will tend to produce an adaptation in the system. For example, in adaptive computer networks, agents in a collaborative environment can switch to a more autonomous mode of processing when inter-agent communication is impaired (Stone and Veloso, 1999), and communications networks using connections of variable reliability can switch to an asynchronous mode when the bandwidth decreases (Fall, 2003). Analogously, the underconnectivity theory of autism predicts that decreased cortical bandwidth could result in concomitant adaptations in cortical functioning. In particular, the model explores increased parietal autonomy which may arise as an adaptation to decreased frontal-parietal bandwidth. Moreover, it is also plausible that decreased frontal-posterior bandwidth could give rise to increased functional connectivity among posterior regions.
5. Computational modeling of brain function and cognition
As functional imaging is increasingly providing finer detail about brain activation, computational modeling provides a theory-building workspace where the new pieces of information about underlying mechanisms can be brought together and their co-functioning can be examined (Just and Varma, 2007). In this workspace, the component mechanisms can be specified in detail, and their ability to account for the observed phenomena can be tested, as a few initial attempts have shown (Anderson et al., 2004; Arbib et al., 2000; Horwitz and Tagamets, 1999; Just et al.,, 1999; Just and Varma, 2007). Below we describe a computational model developed in the 4CAPS neuroarchitecture that accounts for some of the underconnectivity phenomena in autism, as well as other facets of brain activity and behavioral performance.
5.1. Previous computational modeling of autism
Many of the previous models are neural network or connectionist models that have explored the possibility that autism is characterized by abnormalities at the level of individual connectionist units and weights in a neural network. For example, in such models, poor generalization in autism has been variously attributed to inadequate numbers of hidden units (Cohen, 1994), excessive conjunction coding (McClelland, 2000), and most often, to excessive inhibition (Gustafsson, 1997; O’Laughlin and Thagard, 2000), described as “underaroused depression” in the amygdala, “hypervigilant learning” in temporal and prefrontal cortices, and “failure of adaptive timing” in hippocampal and cerebellar areas. Brock et al. (2002) attributed weak central coherence to an impairment of temporal binding between local networks, whereas temporal binding within local networks was presumed to be intact or possibly even enhanced. Very few of the models have attempted to account for specific experimental data, with Bjorne and Balkenius’ (2005) attempt at explaining attention-switching deficits (attributed to a failure to engage a reinforcement system) being an exception. The most complex connectionist model of autism (Grossberg and Seidman, 2006), which is assessed in more detail in the final discussion, proposes an imbalance of parameters among three component subsystems.
Because the lowered functional connectivity in autism is a recently-discovered phenomenon, only one previous computational model of autism has attempted to provide an account of it, at least at a general level. The developmental connectionist model of Lewis and Elman (2008) proposed that the deviant brain growth trajectory (estimated from head circumference) in autism could affect conduction delays that ultimately favor short-distance connections over long-distance connections. The functional underconnectivity in children with autism that is predicted by the model has not yet been reported. Moreover, it remains unclear whether a mechanism based on head or brain size can account for functional underconnectivity in adult autism, where head size is not reliably different.
5.2. The 4CAPS neuroarchitecture
The 4CAPS cognitive neuroarchitecture models cortical function in terms of a set of collaborating computational centers intended to correspond to cortical centers (Just and Varma, 2007). We provide a brief description of 4CAPS here and a more complete specification in the Appendix. Each 4CAPS center is a hybrid symbolic-connectionist processing system with its own specializations and computational resources. Productions, or if-then rules, implement these processes. 4CAPS productions do their work by incrementally increasing or decreasing the activation levels of representational elements, activating or inhibiting them. Each center possesses a finite amount of resources paralleling the biological and informational constraints of cortical areas. 4CAPS models account not only for behavioral phenomena (such as patterns of errors and response times), but also the pattern of brain activity in a number of cortical areas, as measured by fMRI. The relation between the cortical and cognitive levels of functioning is that the amount of cognitive activity in each 4CAPS center – the proportion of its resources currently being used, called the Capacity Utilization (CU) – is intended to correspond to the amount of brain activity observed in the corresponding cortical center (Just and Varma, 2007).
The granularity of 4CAPS models is at the level of brain centers each consisting of tens of millions of neurons, a good match to the theory’s proposal that underconnectivity between frontal and posterior centers accounts for the performance and brain activation of people with autism. 4CAPS models account for aggregate neural information processing and communication: large populations of neurons implement cognitive functions such as goal management, and large numbers of axons, running collinearly in white matter tracts, implement communication channels between centers. 4CAPS models do not attempt to account for information processing at the much finer level of individual neurons nor for neural communication at the level of the individual axons. (The finer-level modeling is the domain of connectionist models of the type described above.)
One important property of a 4CAPS model is that the computational centers communicate with each other by having access to and being able to operate on some of each others’ representational elements (partial products and outputs). This property provides a medium for modeling inter-center collaboration and communication. A bandwidth limitation would constrain the number of representational elements that can be shared between centers in a given time period.
A second property of 4CAPS models that is relevant for modeling functional underconnectivity in autism is that they provide a moment-by-moment measure of the amount of activity occurring in each center, making it possible to compute the correlation between the activity levels of two centers over some time interval. This correlation of the activity time series of two cortical centers corresponds to functional connectivity measures based on fMRI activation. The correspondence can be made closer by first transforming the 4CAPS measures of activity with a mathematical function that closely resembles the hemodynamic response function, sampling this function at the same frequency as the fMRI are acquired, and then computing the correlation between the two activation time series functions, as described below.
A central theme of our proposal is that resource constraints shape cognition in general, and that underconnectivity shapes the cognition in autism. This is the simple consequence of the fact that the brain, like all biological systems, is subject to hard constraints on bioenergetic and structural resources. If cognition is the emergent product of computation in a network of collaborating brain areas, and the communication between brain areas is dependent on resource availability (such as communication bandwidth), then a critical prediction is that resource constraints shape cortical information processing and hence cognitive information processing. The patterns of cortical and cognitive activity observed in autism may constitute an adaptation to underconnectivity, resulting in some of the characteristic “strategies” or “cognitive styles” that are observed in the disorder.
5.3. Previous modeling of the Tower of London task in typical controls
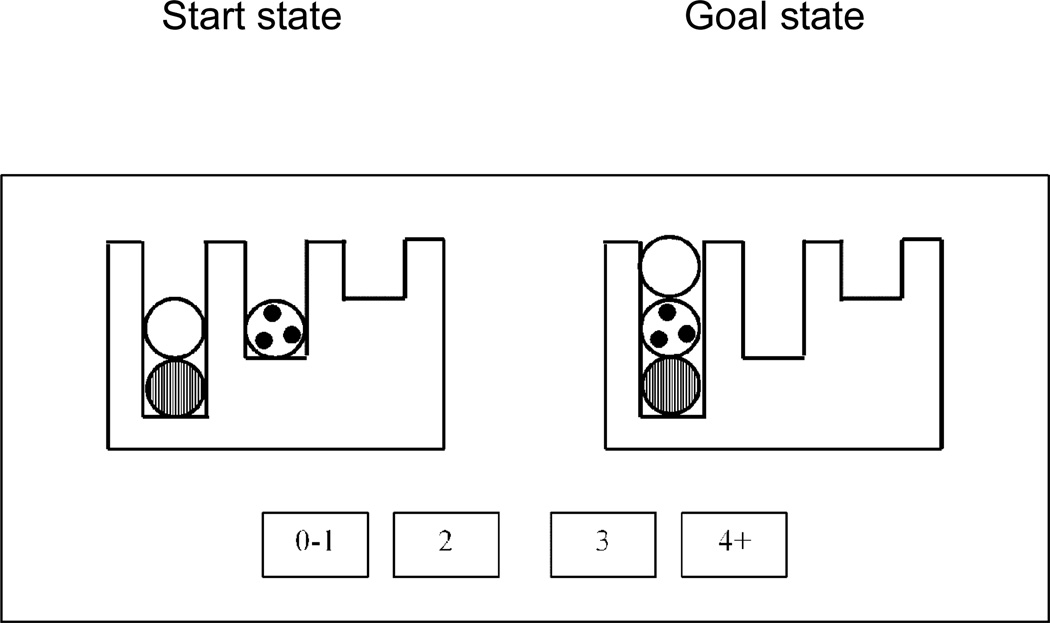
A previous 4CAPS model of TOL problem-solving in healthy college-aged participants provided a reasonably good account of the behavioral performance and the brain activation as modulated by problem difficulty. (The task required re-arranging the positions of three distinctive balls in three suspended pool pockets until they matched a specified goal (or ending) configuration, as shown in Figure 4). In that model, described in more detail by Newman et al. (2003), four cortical centers were pivotal in the problem solving, with two frontal centers contributing more strategic functions and two parietal centers contributing more visuospatial functions. The RH (Right Hemisphere)-Spatial center (corresponding to a posterior parietal region around and superior to the intraparietal sulcus area) proposes moves using a perceptual strategy, namely one that attempts to make the current state of the display look more similar to the desired end state. These perceptually-based moves are effective in some simpler problems, but they do not necessarily lead to optimal solutions for more difficult problems in which it is necessary to temporarily move away from the goal in order to fulfill a subgoal. The LH (Left Hemisphere)-Spatial center maintains a representation of the current problem-solving state and initiates the performance of the moves on this state, transforming it to produce a new current state. RH-Executive (corresponding to right DLPFC, or middle frontal gyrus) proposes moves of a more strategic nature. RH-Executive is indispensable when it is necessary to make a move that temporarily moves the problem state away from (i.e., makes it less similar to) the ending state, a move that is in effect a necessary cognitive detour. Such moves are made under the direction of goals proposed by RH-Executive. LH-Executive selects between the perceptual moves proposed by RH-Spatial and the strategic moves proposed by RH-Executive. The functional specializations of these centers were assigned on the basis of previous research as well as the effects of experimental manipulations observed in the Newman et al. (2003) data.
Figure 4.
A sample Tower of London problem. This display shows a 3-move problem, and a schematic diagram showing the response buttons to indicate the number of moves required.
It is important to note that in 4CAPS, higher-level cognition is the result of collaboration among cortical centers. Communication between centers occurs as a result of the creation, activation, and inhibition of shared representational elements. Some of the representational elements created within a center are made available to other centers, so that when one center has created a new element (such as a newly proposed TOL move), the other centers can operate on it. RH-Spatial typically initiates TOL problem solving by generating a set of possible perceptually-based moves, namely those moves that would make the current perceptual state (the configuration of the balls in the pockets) more visually similar to the ending state. LH-Executive selects the most preferred of these moves by means of a competition/selection mechanism. If this move can be performed, then the LH-Spatial center updates the current problem state representation. If the move cannot be executed, and problem solving reaches an impasse, then RH-Executive formulates a strategy (i.e., articulates goals) for resolving this impasse, and subsequently proposes moves that achieve these goals. The Newman et al. model was used as a typical control model; it serves as a starting point for our explorations of functional connectivity in autism.
6. The 4CAPS model of frontal-posterior underconnectivity in autism
The new model takes as its point of departure the 4CAPS model of TOL problem-solving in healthy adults (Newman et al., 2003), and modifies it to account for the fMRI findings of lower functional connectivity in autism in this task (Just et al., 2007). The first modification is the enforcement of bandwidth constraints on communication between frontal and posterior centers. The second modification, which is assumed to arise as an adaptation to the bandwidth constraints, is increased autonomy of the posterior centers.
6.1. Tower of London task
Eighteen adults with high-functioning autism and 18 matched controls performed the TOL task (Just et al., 2007). The standard TOL task was modified for use in the scanner, such that the participants did not physically move any ball, but instead mentally made the moves to solve a problem, kept track of the number of moves made, and indicated that number using a forced-choice response. The left side of the display showed the initial state and the right side showed the goal state, as illustrated in Figure 4. In this example, the first move of the shortest-path solution is to place the white ball in the rightmost pocket, then to move the spotted ball to the left pocket, and finally to move the white ball to the left pocket. Thus, three moves are required to solve this problem. The participant indicated the number of moves by pressing the appropriate button in the response panel (“3”) and the next problem was then presented. The study was implemented as a “block design” with each block containing problems of similar difficulty. (Neither the experimental design nor the results nor the modeling below allow a clear contrast between the easier versus the harder problems.) Although some of the easier problems can be solved via straightforward perceptual processing, the harder problems require more executive processing, such as planning several moves ahead in order to satisfy various goals and subgoals.
6.2. Frontal-parietal bandwidth constraint
The first modification of the typical model was the enforcement of a bandwidth limitation on inter-center communication. Recall that the functional connectivity between frontal and parietal pairs of regions in the autism group was reliably lower than in a control group (Just et al., 2007). Such findings and findings of white matter abnormalities suggest an impairment of neural communication. The goal of the modeling was to provide a precise account of the mechanism that gives rise to the lower frontal-posterior functional connectivity (synchronization).
In the normal control TOL model, the bandwidth between all pairs of centers is effectively unlimited. To constrain the frontal-parietal communication in the autism model, a limitation was placed on this bandwidth. This constraint was implemented by limiting the total amount of activation that can be consumed at any one time by the representational elements being communicated between the Executive (frontal) and Spatial (parietal) centers. There is no explicit transmission or receipt of elements in this implementation; rather, representational elements are placed in a communication channel by some center such that they are accessible by other centers. The bandwidth constraint implemented here effectively limits the size of the communication channel containing the representational elements posted by either an Executive or a Spatial center for the other centers’ use. This constraint limits the rate of Executive-Spatial communication, simulating the hypothesized anatomical underconnectivity between frontal and posterior regions. Implementing a bandwidth constraint by limiting the capacity of a communication channel is consistent with the more general focus on resource constraints in 4CAPS. However, other implementations are possible. For example, consistent with our definition of bandwidth, adding noise to a channel is an alternative way of decreasing its bandwidth. The question of how best to model bandwidth limitations on interregional communication is a promising one for future research.
6.3. Increased parietal autonomy as an adaptation to bandwidth constraints
By definition, the communications infrastructure in a distributed computational architecture, particularly its bandwidth, constrains the amount of information that can be transferred per unit time among processing centers. Decreased bandwidth should impact system performance if the communications needs are not met by the available bandwidth. An important prediction is that a bandwidth constraint could result in an adaptation of the network, such as a shift to a different mode of processing that makes the network’s performance less susceptible to the bandwidth constraint. In particular, the nodes of the network could adapt by relying less on collaboration with other nodes and instead functioning more autonomously. This would be an example of a resource limitation shaping the behavior of the network.
In the model below, we propose that parietal areas function more autonomously in autism, adapting to the constraint on the communication with frontal regions by acting without frontal input in those problem-solving circumstances where frontal input is not essential. For example, instead of waiting for a top-down response from a frontal center, a parietal center might instead do the processing using only the information that is available posteriorly, reducing the coupling between regions. This strategy may arise as an adaptation to the structural constraints, and its effectiveness would depend on the nature of the task.
Increased parietal autonomy is implemented in the autism model by making a second modification to the normal 4CAPS TOL model, which gives the Spatial centers more autonomy under some circumstances. In the normal control model, every move requires the coordination of several centers when moves are being proposed (by RH-Spatial and RH-Executive), selected (LH-Executive), and performed (LH-Spatial). Under the second modification, the Spatial centers have increased autonomy in two circumstances which require little planning or strategizing about the problem. The first circumstance occurs during the last move of a problem: if a perceptual move proposed by the RH-Spatial center would transform the current state into the ending state, thus solving the problem, then the RH-Spatial performs that move directly (i.e., sends a signal to the motor system to make the move) without requiring the input from the frontal Executive centers that would normally collaborate in selecting this move. The second circumstance where the Spatial centers possess greater autonomy occurs after a subgoal has been satisfied and a move that had previously met an impasse can now be performed. (Recall that when a desired move cannot be made because its destination pocket is occupied by another ball or because another ball is on top of the ball to be moved, a subgoal is established to remove the ball that constitutes the impediment.) This instance of autonomy allows Spatial centers to perform the previously blocked move without further input from Executive centers.
The choice of these two circumstances as the instantiation of parietal autonomy was based on parsimony or minimality: they are the only circumstances when there is no real choice to be made, i.e., there is no need to compare and select between multiple possible moves. It seems plausible that parietal areas should wait for input from frontal areas beyond some threshold duration only when such inputs are essential for adequate accuracy. More generally, we regard parietal autonomy as plausible and effective whenever there is a strong perceptual basis for an action and there is not a need for a conceptual evaluation of the action.
6.4. Models
We consider three models. The intact normal control model is just the model described by Newman et al. (2003). The two modifications of the autism model were introduced sequentially. The reduced bandwidth model implements the first modification, reduced frontal-parietal communication bandwidth. The full autism model additionally implements the second modification, the increased autonomy of parietal areas. Because we view parietal autonomy as an adaptation to reduced communication bandwidth, and because a model without a bandwidth constraint fails to correspond to white matter alterations in autism, we did not consider a model with increased parietal autonomy but unconstrained frontal-parietal communication.
6.5. Incorporating the hemodynamic response
To relate the time course of the fMRI activation to the time course of the 4CAPS model’s processing, the activity of each model center is transformed using a gamma function that provides a good approximation to the hemodynamic response function in the brain that fMRI measures. The time series of the capacity utilizations of each model center i, CUi(t), was first sampled at the same rate that fMRI images were acquired in the study (once every 3 sec). This capacity utilization time series was then convolved with a hemodynamic response function h(t) to generate a predicted activation time series fMRIi(t), as described in the Appendix.
To model functional connectivity, the correlation between the predicted activation time series of pairs of model centers (such as LH-Executive and LH-Spatial) was computed for the normal control model and the full and reduced autism models and compared to the fMRI-based functional connectivity group difference.
7. Modeling results
The control and autism models were run on several blocks of the stimuli used in the Just et al. (2007) study. The models solved the problems making the same moves as the human participants (using the shortest solution path), generating a predicted fMRI time series for each model center. Correlations between these time series were computed for pairs of model centers in each model, corresponding to the functional connectivity measures of the human data.
7.1. Group differences in frontal-posterior functional connectivity
First, consider the contrast between the normal control model and the reduced bandwidth autism model. The effect of imposing a bandwidth constraint on frontal-parietal communication was to slow down the processing without making a qualitative change in the processing. The same sequence of computations occurred in each center at the lower bandwidth, but the computations were drawn out over more time, so the solution times increased by some proportion. However, because all centers were slowed similarly, their degree of synchronization (functional connectivity) did not change very much. Decreasing the inter-center communication bandwidth has this effect because the centers are closely coupled in the original model: every move requires the coordination of several centers to propose, select and perform it. Thus, reduced bandwidth alone did not lead to functional underconnectivity (hyposynchronization).
Next, consider the contrast between the normal control model and the full autism model. Recall that the full autism model has not only reduced bandwidth, but also the adaptation increasing the autonomy of the Spatial centers (corresponding to parietal regions). With the addition of this second modification, the autism model shows decreased Executive-Spatial functional connectivity compared to the original model. The full autism model also shows increased response times. The decrease in functional connectivity was common to all four Executive-Spatial pairs, but was strongest in the pairs including the LH-Executive center and either Spatial center.
The left panel of Figure 5 shows how closely the predicted activation levels of LH-Executive and LH-Spatial track each other in the normal control model, before any modifications are made. The right panel shows the lower correlation between the same centers of the full autism model, after the communication bandwidth between Executive and Spatial centers is reduced and the autonomy of Spatial centers is increased. The difference in the functional connectivity patterns between the two models closely resembles the difference in functional connectivity in the fMRI data of two participants with and without autism from the Just et al. (2007) study (shown in Figure 3). In that study, the mean frontal-parietal functional connectivities were reliably lower in the autism group than the control group. The pairs of centers involving left dorsolateral prefrontal cortex (DLPFC) showed the largest decrease in functional connectivity, a result the full autism model reproduces.
Figure 5.
Upper panel: Lower frontal-parietal functional connectivity (time series correlation) in the autism model (right-hand panel) in the Tower of London task than in the control model (left-hand panel). Lower panel: Higher frontal-parietal functional connectivity between the two activation time series in a control participant in the Tower of London task than in an autism participant (from Just et al., 2007) (same as Figure 3).
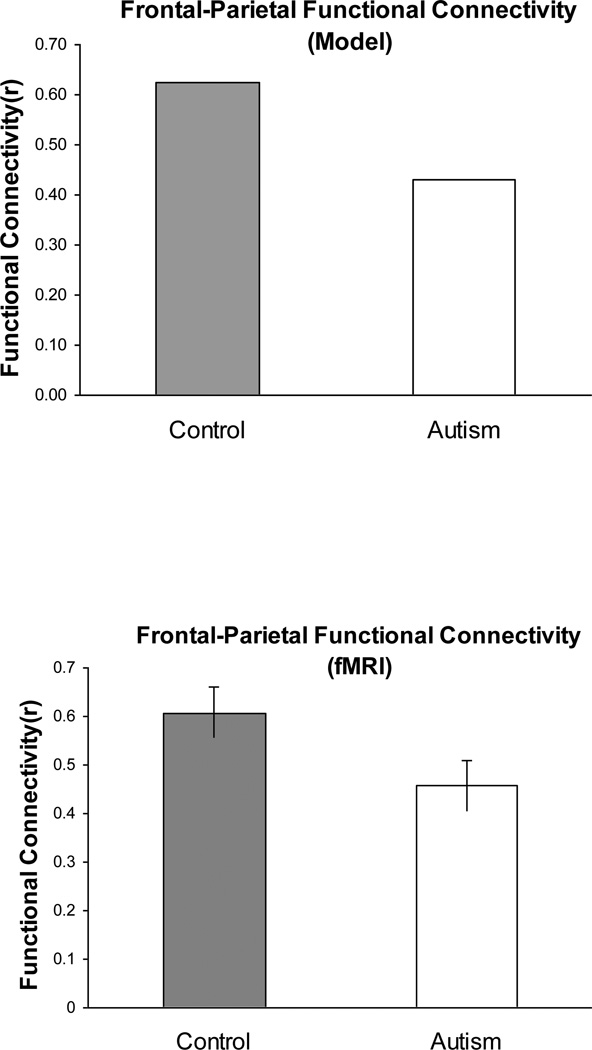
Figure 5 presented the time series for one pair of model centers. When the correlations of the time series for all possible pairs of Executive centers and Spatial centers are averaged for the control model and the full autism model, the resulting difference in functional connectivity, shown in Figure 6, strongly resembles the corresponding group difference found in the fMRI data (shown in Figure 2a). The functional connectivities of both models (autism model: .62; control model: .43) match the functional connectivities of their respective participant groups (autism group: .61; control group: .46) quite well, both qualitatively and quantitatively. Thus the full autism model provides a good account of the lower frontal-posterior functional connectivity observed in autism.
Figure 6.
Upper panel: Lower frontal-parietal functional connectivity in the autism model than in the control model of the Tower of London task. Lower panel: Lower frontal-parietal functional connectivity in autism in the Tower of London task (from Just et al., 2007) (same as Figure 2a).
7.2. Modeling individual differences in structural and functional connectivity
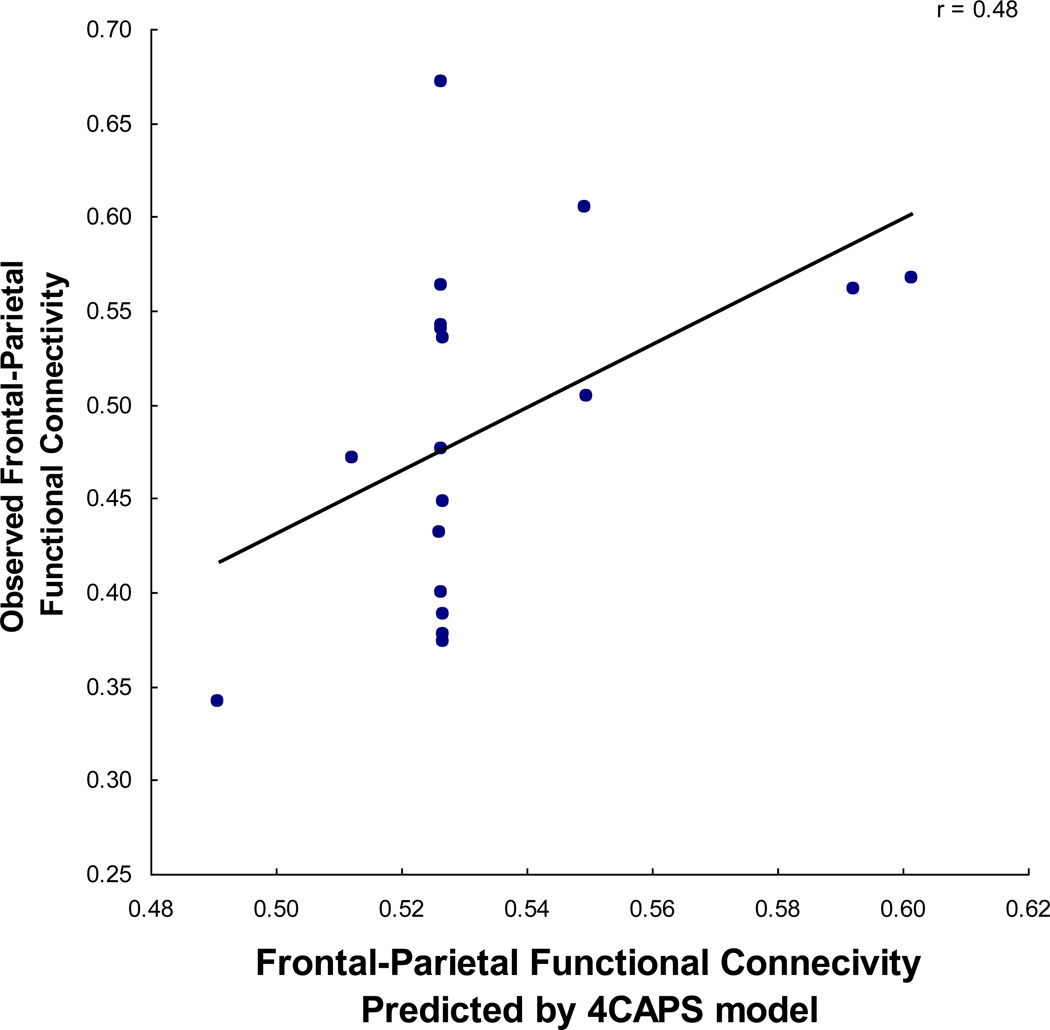
In the autism model, the size of the reduced bandwidth can be parameterized for the individual participants with autism. In several previous fMRI studies of autism, including a study of the resting state, the frontal-posterior functional connectivity of an individual participant with autism was correlated with the size of the relevant portion of the corpus callosum (Cherkassky et al., 2006; Just et al., 2007; Kana et al., 2006; Kana et al., 2009). (There was no such correlation among control participants, presumably because anatomical connectivity was not a limiting factor affecting their functional connectivity.) To account for the relation between corpus callosum size and functional connectivity in the autism model, the communication bandwidth parameter was made linearly proportional to the size of each autistic participant’s genu area, the callosum area that connects contralateral frontal areas. The model can thus make a prediction about the functional connectivity of individual participants. The resulting correlation between predicted and observed functional connectivity is 0.48, as shown in Figure 7. The model provides a good account of the functional connectivity of the autism participants whose genu size is particularly small or large; for the participants with an intermediate genu size, the model predicts the mean functional connectivity for the subgroup, but does not account for the fact that these participants vary considerably among themselves. In sum, the computational model of autism can predict some of the individual differences in the frontal-parietal connectivity using an anatomical parameter obtained from measurement of individual participant’s brains.
Figure 7.
Scatterplot relating individual differences in functional connectivity in participants with autism as predicted by the 4CAPS TOL model (based on simulation runs parameterized to measurements of individuals’ genu area) and observed functional connectivity.
7.3. Additional predictions of the model
Although this article has focused on the model’s decreased-bandwidth account of the decreased frontal-posterior functional connectivity in autism, the model accounts for a considerably wider range of phenomena. The two phenomena that are central in this article are the reduced frontal-posterior functional connectivity and the relation between the white matter properties and the functional connectivity. In addition, the model also accounts for this relation at the level of individual participants with autism. A third phenomenon addressed by the model is a behavioral effect, namely that the response times were reliably longer for both groups for harder problems (those requiring more moves), but more so for the participants with autism. This result is consistent with the claim of lowered bandwidth in autism, which impacts the speed of cortical communication and therefore has a larger cumulative impact in problems with more moves. The model produces this phenomenon. A fourth phenomenon is that the activation levels in frontal and parietal regions increased with problem difficulty (number of moves), effects observed in the model and described in detail in Newman et al. (2003).
The model also makes some predictions for which currently published data are not available. One such prediction (mentioned below in the context of another theory) is that in perceptual tasks that entail minimal or no frontal participation, the functional connectivity among posterior areas may actually be higher in autism because the lower amount of frontal-posterior traffic may result in an increase in posterior-posterior bandwidth in autism relative to controls. The model also predicts that in an fMRI resting-state study, the functional connectivity should be lower in low-functioning autism than in controls, and perhaps lower than in high-functioning autism.
In sum, the autism model provides an integrated account for a number of disparate brain activation and behavioral findings in the TOL task. In addition, the model can account for findings in other tasks, such as decreased frontal and increased posterior activation in many tasks (as shown in Table 1). Thus the model’s account for the varied phenomena associated with autism in the TOL task generalizes to many other tasks that have a substantial frontal involvement.
Table 1.
Summary of domain-general and domain-specific autism findings
| DOMAIN | TASK | Domain-General Brain Phenomena in Autism |
Domain-Specific Behavioral Phenomena in Autism |
||||
|---|---|---|---|---|---|---|---|
| Lower Frontal -Posterior synchronization |
Smaller Corpus callosum |
Correlation between Corpus Callosum and synchronization |
Less frontal activation |
More Posterior activation |
Domain-Specific Behavioral Phenomena in Autism |
||
| Executive | Tower of London puzzle (Just et al., 2007) |
yes | yes | yes | MFG | LG | Difficulty with more complex problems |
| Executive | Inhibition of high-level response (Kana et al., 2007) |
yes | R IFG | Impaired high-level inhibition | |||
| Executive | Inhibition of high-level response (Solomon et al., 2009) |
yes | MFG, R SFG | Impaired high-level inhibition | |||
| Language | Visual imagery in sentence comprehension (Kana et al., 2006) |
yes | yes | yes | L IFG, L MFG | L IPS, R SPL | Imagery activation even for more abstract sentences ("thinking in pictures") |
| Language | Sentence comprehension (Just et al., 2004) |
yes | L IFG, MFG | L STG | Difficulty with complex syntax; good word-reading |
||
| Memory | Working memory for alphabetic letters (Koshino et al., 2005) |
yes | L IFG, L MFG | L IT | Letters are coded relatively more visually than verbally |
||
| Social | Theory of Mind: animation (Kana et al., 2009) |
yes | yes | MFG | R TPJ | Theory of mind difficulties | |
| Social | Face memory (Koshino et al., 2008) |
yes | yes | yes | L IFG, MFG | Faces are treated as relatively more visual than social objects |
|
| Perception | Embedded Figures Test (Damarla et al., 2010) |
yes | yes | yes | MFG | R IPS, R SPL | Less interference from higher-order information |
| Resting State | Fixation (Cherkassky et al., 2006) |
yes | yes | yes | |||
| Resting State | Fixation (Kennedy and Courchesne, 2008) |
yes | yes | yes | |||
| Resting State | Fixation (Monk et al., 2009) |
yes | yes | yes | |||
|
Diffusion Tensor Imaging |
No Task (Alexander et al., 2007; Barnea-Goraly et al., 2004; Keller et al., 2007) |
Lower integrity |
|||||
7.4. Application of the approach to other tasks
Several other laboratory tasks besides TOL have shown performance differences between autism and control participants, such as tasks that tap Theory of Mind processing (the Sally-Anne false belief task), local perceptual processing in the Embedded Figures Task (Shah and Frith, 1983) and the Block Design task (Shah and Frith, 1993), complex language comprehension in the Detroit Test of Oral Directions (Goldstein, et al., 1994), processing face stimuli (Boucher and Lewis, 1992), and ambiguity processing in a homograph task (Frith and Snowling, 1983). Although 4CAPS models of these tasks have not yet been developed, the approach used in the TOL model here should generalize to them. This is because all of these tasks involve some degree of frontal involvement. To model autism in a particular task model, a normal control model for the task would be altered in the same way as the full autism TOL model has been, by (1) decreasing the frontal-posterior bandwidth and (2) increasing the autonomy of posterior centers so they can proceed in simpler cases without the benefit of the frontal input.
7.5. Impact on processing style
There exist informal accounts of people with autism favoring a visual processing style, or “thinking in pictures.” One fMRI study showed the increased activation of parietal areas associated with visual imagery in autism in a task requiring participants to judge whether a sentence was true or false (Kana et al., 2006). Describing the psychological processing in autism as being of a different “processing style” is probably accurate, but it is scientifically unsatisfying. The description simply begs the question of why people with autism should gravitate towards a given style of processing. It is surely not simply a matter of preference or taste or even of choice, but rather an emergent consequence of atypical neural circuitry that results in atypical brain activation and atypical psychological processing. The question then is why a given “style” of processing should emerge in the disorder of autism. Underconnectivity theory proposes that a visual processing style may emerge in autism because of decreased availability of frontal processing resources, leading to increased reliance on posterior processing, particularly visuospatial processing.
7.6. How frontal-posterior underconnectivity might impact frontal processing
In some cases, such as an embedded figures task, the autism model might be expected to perform better without the “benefit” of the frontal input. However, in many other cases, the autism model, with its diminished frontal input, should show deficits in higher-level abstraction (thus exhibiting weak central coherence) and in Theory of Mind tasks.
Given that we have proposed that lowered bandwidth between frontal and posterior areas leads to increased parietal autonomy in autism in this type of task, one may ask why we do not also propose increased frontal autonomy. First, it is certain the frontal processing is different in autism in at least some tasks. Many studies (such as Just et al., 2004) have found decreased frontal activation in autism. We implicitly construe the frontal difference as a lower degree of involvement in performing the TOL task, for which “autonomy” is not an accurate label. In addition, findings of increased radiate white matter in the frontal lobes (Herbert et al., 2004) suggest that the local connectivity within the frontal lobe is different in autism. Even though underconnectivity theory focuses on the communication among brain areas, it seems undeniable that the centers themselves are different in some ways in autism. We offer no general account of the nature of these differences in specific centers, aside from their being less collaborative with other centers. In the case of the superior parietal area, we propose an altered relation with frontal areas. It remains for future research to determine how individual cortical areas such as the prefrontal cortex are different in autism.
8. Discussion of the model
Formal modeling of a complex process constitutes a theoretical account to provide added value beyond a verbal description of the underlying mechanism. A verbal description of a dynamic process cannot capture the detail of the processing or the unfolding of events over time. Modeling enforces a specific, precise computational implementation of the theory, shedding light on both the sufficiency and necessity of its proposals. The ability of the models to solve TOL problems also demonstrates the sufficiency of the cognitive functions attributed to left and right prefrontal and parietal areas and their proposed pattern of collaboration. Underconnectivity theory proposes that decreased functional connectivity in people with autism emerges as an interaction between two factors: decreased communication bandwidth between frontal and posterior areas and increased posterior autonomy. The computational modeling supports this claim. Decreased communication bandwidth alone is insufficient. Increased parietal autonomy must also be posited, as demonstrated by the superior ability of the full model (versus the reduced model) to account for the observed group and individual differences. We propose that increased posterior autonomy is an adaptation to decreased communication bandwidth, an adaptation that helps deal with a different (i.e., reduced) pattern of connectivity with the frontal cortex.
8.1. Cause, effect, and adaptation
The modeling serves as a reminder that the observed brain functioning in autism is likely to be a resultant combination of the brain change introduced by autism and the brain’s natural adaptive plasticity. This complex interaction is further complicated by the developmental trajectory of both of these factors. In this view of the dynamic system of the brain developing over many years, the proposed theory postulates that autism arises because of some brain connectivity disorder. In this view, the greater parietal autonomy and greater reliance on posterior brain areas in autism is interpreted not as part of the primary physiological basis of autism, but a functional consequence. The modeling also serves as a reminder that the adapted system will not always succeed as well as an intact system might. In the TOL domain, the adapted system is less effective in cases where the greater executive ability of the frontal systems is essential, such as when the solution to a TOL problem requires the management of deeply embedded goals. Lower performance might also occur when certain frontal processes (such as Theory of Mind processing of the medial frontal area) cannot be compensated by a posterior center. On the other hand, the adapted system may be more effective in some situations, described below, in which the frontal contributions may do more harm than good, such as in the search for embedded figures.
It is interesting to note that a phenomenon that is similar to the compensatory autonomy of cortical centers occurs in other complex systems, such as the design of communications protocols in delay-tolerant computer networks (Fall, 2003). Such networks handle transient connectivity problems not by pausing when an expected response to a message fails to arrive, but by continuing to process or by moving on to another task. To adapt to the longer delay times caused by the bandwidth constraint, the parietal centers in the full autism model do not halt until a frontal center’s input is received; instead, they continue to solve problems using perceptual processing. Thus the impact of poor connectivity on network performance is minimized.
The issue of adaptation highlights the fact that we have not modeled the proposed adaptation. The additional parietal autonomy of the full autism model was a part of the model. In a future formal account, it would be desirable to have this autonomy evolve on its own, perhaps as a function of learning over a training set of problem-solving experiences in which inputs from frontal areas are delayed and not essential.
8.2. Summary of previous connectivity-related findings: Commonalities across domains of thought
The model can be extended to account for brain characteristics in other types of tasks besides TOL and other types of thinking besides executive processing. A summary of the findings from 10 types of tasks indicates a number of commonalities as well as specificities. One robust finding is the frontal-posterior undersynchronization, which to date has been observed in every high-level thinking task that has been examined, including tasks of executive functioning, language, memory, social processing, high-level perception, as well as in a resting state, as shown in the third column of Table 1. The frontal-posterior undersynchronization can be explained by reduced frontal-posterior bandwidth. In many of these tasks, the corpus callosum of the participants with autism was reliably smaller (fourth column), and the corpus callosum size was correlated with the degree of synchronization (fifth column). (In addition, areas around the corpus callosum showed lower structural integrity in autism, as measured by fractional anisotropy in diffusion tensor imaging studies.) Many of these studies also showed decreased activation in a frontal area, again attributed by the model to decreased frontal-posterior bandwidth. Moreover, many of the studies indicate increased activation in a posterior association area, consistent with the model’s account of greater posterior autonomy in autism. Thus there are commonalities across domains in the frontal-posterior undersynchronization, in the relation (in several studies) between the undersynchronization and the properties of the white matter, and in the way that the activation is distributed in the brain (less frontal and more posterior).
8.3. Domain-specific behavioral findings in autism
The commonalities of the brain characteristics of autism manifest themselves behaviorally in ways that are task-specific. For example, in the pilot studies of the Tower of London puzzle, participants with autism who were matched on IQ with control participants had relatively more difficulty with problems with more complex solutions, resulting in an fMRI study design that excluded the most difficult problems. Some observations of the behavioral manifestations of autism, described in the right-hand column of Table 1, are not necessarily based on the corresponding brain imaging study, but draw on larger behavioral studies. For example, the poorer comprehension of complex syntax in autism was observed in the Detroit Test of Oral Directions (Minshew et al., 1997). The good performance at word recognition was observed by Newman et al. (2007). In general, the tendency in each task is for the autism group to use a processing style that draws less on functions that have a vital frontal component (e.g. executive functioning, complex language, theory of mind, face and social processing) and more on posterior functions (visual, imaginal, configurational processing). The greater reliance in autism on posterior areas may lead to processing that is more autonomous of frontal areas, more visual or configural in content, and in some circumstances, more effective.
The extensibility of the model to a range of other tasks provides an account of the diverse types of thinking that are affected in autism. The new theoretical account provides an ability to predict some of the characteristics of thinking in autism in new situations, so that studies of autism do not have to be performed for every possible type of task. If the brain activity and thought processes in people without autism are understood well enough, then the autism model can then provide a first-order prediction of what will occur in autism.
9. Relation to Other Theories of Autism
The concreteness of the computational model facilitates the comparison between underconnectivity theory and predecessor approaches to autism, such as the theories of weak central coherence (Frith, 1989), impaired complex information processing (Minshew et al., 1997), enhanced perceptual functioning (Mottron et al., 2006), mindblindness (Baron-Cohen, 1995), impaired social processing and motivation (Dawson et al., 2002), and longer-distance cortical communication. All of these theories capture some fundamental aspect of autism, and all of them are at least partially correct. Can underconnectivity theory provide a framework that unifies them? These predecessor theories were typically formulated on the basis of behavioral data, before extensive brain imaging studies of autism had been performed, so they tend not to be grounded in a biological substrate. We propose that it is the biological substrate that provides the unifying elements, accounting for the diversity of symptoms that give rise to diverse theories of autism. At the same time, there are systematicities in the diversity, which underconnectivity theory begins to account for, in both biological and psychological terms. We consider below how seven predecessor theories can be related to the underconnectivity theory perspective. We do not suggest that these other theories are wrong, but we do comment on the limitations of their comprehensiveness or precision, limitations that were often unavoidable at the time that the theories were proposed. Moreover, some of these theories continue to have clinical usefulness.
9.1. Weak Central Coherence (WCC) theory
Frith’s (1989) theory of weak central coherence deals with a tendency in autism to focus on details and narrower perspectives at the expense of broader integrative information processing. Frith offered a compelling analogy between the flow of normal thought and the flow of a river which imposes coherence among contributing streams or inputs, with autism having weaker central coherence among the contributing streams of thought. Although WCC theory provided a useful framework, it is fundamentally an analogy between mental processes and phenomena in a hydraulic system. The excellence of the analogy distracts from that theory’s absence of a plausible underlying mechanism. In more recent neuroimaging studies, Frith and her co-workers have attempted to relate the concept of weak central coherence to brain activity. For example, Castelli et al. (2002) report that the average PET-measured activity levels in two involved brain regions is less correlated across autistic participants than across control participants. Note that this is a much coarser measure of correlation between cortical areas than the time series correlation provided by fMRI, and the mechanism underlying even this coarse correlation is not specified. By contrast, underconnectivity theory and the computational model in particular squarely target abnormalities of underlying biological structures (i.e., white-matter tracts) and cortical communication processes that use them, and link them to psychological processes (i.e., inter-area communication and the sharing of representational elements). Like WCC theory, underconnectivity also predicts weak coherence among ongoing processes, but specifically when the processes would normally include frontal areas. Underconnectivity theory attributes the weak central coherence in such cases to poorer communication between frontal and posterior areas, including both peer-to-peer and controller-to subordinate-nodes communication. Another difference between the two approaches is that WCC theory implicitly posits the existence of a mechanism that is responsible for arranging coherence, such as an unspecified central controller. By contrast, underconnectivity theory interprets weak central coherence as an emergent system property arising from poorer frontal-posterior communication bandwidth. Moreover, the 4CAPS model suggests that the reduced bandwidth may foster the development of more autonomous and less integrated processing centers. Underconnectivity theory goes on to predict impairments in motor function, memory, and expressive nonverbal language (such as hand gestures and facial expressions), and to virtually all functions involving a frontal contribution, particularly when a large processing load is being imposed on the system (i.e., a load that consumes the limited communication bandwidth). In sum, WCC theory provides a useful organizing analogy that is applicable to a broad range of phenomena in autism. Underconnectivity theory makes the same clinical sense as WCC theory, but additionally proposes a set of underlying neurobiological mechanisms that relate the biological and psychological levels, providing the theory with greater generative and integrative power.
9.2. Autism as a complex information processing disorder
Across several studies, Minshew and her colleagues (Minshew and Goldstein, 1998; Minshew et al., 1997; Williams et al., 2006), using a battery of neuropsychological tests, observed impairment in autism in a wide range of domains (attention, memory, spatial processing, language, reasoning, motor) as a function of the level of information processing. Lower level processes are spared or even enhanced, but impairment is observed in all these domains as a function of complex information processing demand. Complexity is never rigorously defined in the statement of the theory. A comparison between the complex tasks in which the autism deficit occurs and the simpler tasks in which it does not occur suggests that “complexity” in this context refers to a higher level of abstraction (which itself is not rigorously defined here). That is, the complex tasks require dealing with abstract concepts at a higher level than individual concrete entities. Underconnectivity theory links the complex information processing deficits to a specific neurobiological and functional substrate, frontal-posterior brain connectivity. According to the underconnectivity theory, the difficulties in complex information processing may occur when frontal involvement is mandatory for normal performance (presumably to support the high levels of abstraction), but in autism, the poor frontal-posterior connectivity (abnormally limited bandwidth) undermines the frontal contributions. In the TOL data modeled here, there was no substantial performance deficit in autism, although the autism group responded more slowly to the more difficult problems. However, the items in that task included only easier TOL problems chosen to be performed by the autism group without difficulty. It is likely that some of the frontal-lobe roles (such as generating and maintaining goal hierarchies for guiding strategic processing) can be assumed with only moderate success by the parietal regions.
Related to the complex information processing disorder approach is the executive dysfunction theory of autism (Hughes et al., 1994; Pennington and Ozonoff, 1996). Executive dysfunction tends to be observed primarily in more complex tasks. According to underconnectivity theory, executive dysfunction can be viewed as arising from poor connectivity between the frontal areas that perform executive functions with other cortical regions.
9.3. Enhanced Perceptual Functioning (EPF)
Mottron and his colleagues (2006) have argued that autism is marked by a relative sparing and perhaps enhancement of certain types of perceptual processing. They characterize the perceptual processing in autism as being locally oriented, enhancing low-level discrimination and perception of simple static stimuli, and entailing greater use of posterior (as opposed to frontal) brain regions in complex visual tasks, compared to control participants. There is also diminished perception of complex movement in autism, and autonomy of low-level information processing from higher-order operations. This extensive list of the perceptual characteristics in autism bears a good correspondence to the connectivity theory position, particularly the theory’s postulation of increased autonomy of posterior centers. The greater autonomy of perceptual processing from higher-order (frontal) processing is attributed by the 4CAPS autism model to a reduction in the frontal-posterior communication bandwidth.
The bias in autism towards local rather than global processing noted by the EPF approach is attributed by underconnectivity theory to a lower frontal-posterior communication bandwidth which degrades the top-down or global influences. An additional prediction of underconnectivity theory is that in some perceptual tasks, the functional connectivity among posterior areas may actually be higher in autism because the lower amount of frontal-posterior traffic may eventually lead to an increase in both posterior autonomy and posterior-posterior bandwidth. In sum, the enhancements in autism in some lower-level perceptual functions on which EPF focuses may arise due to poorer connectivity between frontal and more posterior (perceptual) areas. These enhancements are second order effects or sequelae from the underconnectivity perspective. Moreover, underconnectivity theory provides a biologically-based account for some of the perceptual enhancements.
9.4. Mindblindness
The mindblindness theory of autism (Baron-Cohen, 1995) posits that a deficit in Theory of Mind (ToM) processing is centrally causal to autism. Many studies have produced evidence of the existence of such a deficit (e.g. Baron-Cohen, 1989; Happe, 1995; Tager-Flusberg, 1992). Moreover, brain imaging studies in particular have indicated what the biological basis of the deficit might be. ToM processing is underpinned by the activity of at least two brain areas, the right posterior superior temporal sulcus and the nearby temporo-parietal junction, and the medial frontal area. Underconnectivity theory posits that bandwidth limitations restrict the communication between these two areas, attributing the ToM deficit to the impaired communication between the frontal and posterior components of the ToM cortical network.
A recent fMRI study of ToM explicitly measured the functional connectivity between these two regions (Kana et al., 2009). The study presented the animations of interacting geometric figures provided by Castelli that had been used in her PET study of this task. The participants viewed an interaction between the figures, particularly in a ToM condition in which the intentionality of the figures could be inferred. In the original PET study, Castelli et al. (2002) found that the average PET-measured activity levels in frontal and posterior components of the ToM network were less correlated with each other across autistic participants than across control participants. The newer fMRI study found a reliable reduction in the synchronization of the activation (across points in time) between the frontal Theory of Mind areas (medial frontal, orbitofrontal) and posterior ToM areas (right middle and superior temporal gyri, temporoparietal junction) in the group with autism, relative to controls during the ToM task, as the underconnectivity theory predicted. In this perspective, the ToM or mindblindness deficit can be viewed as a special case of a deficit of frontal-posterior connectivity. The specific role of the frontal and temporal components of ToM is still debated; however, our studies suggest that it is the integrated functioning of these regions that accomplishes the ToM processing and that this integration is disrupted in autism.
9.5. Impaired social processing and motivation
The deficits in social interaction are among the most evident in autism, and are often accorded a central or even causal role in the disorder (Dawson et al., 2002; Schultz et al., 2003). Contemporary brain imaging studies have succeeded in determining the neural substrates of some of the abnormal social processing, finding for example, that one of the key brain areas involved in the processing of faces (the fusiform face area, or FFA) activates abnormally (hypoactivation with some displacement of location) (Schultz, 2005; Schultz et al., 2000). But when this abnormal activation is viewed from the perspective of the cortical network that is involved in such tasks (rather than just a region), the abnormality can be seen as an outcome of a connectivity problem. For example, in addition to the abnormal FFA activation in the n-back faces working memory task, Koshino et al. (2008) also found several other network disturbances. First, the functional connectivity between the FFA and frontal areas was lower in the autism group than in the control group. Thus the abnormal FFA activation could be an effect of autism rather than a cause. An additional abnormality was the reliably lower level of activation in the right posterior Theory of Mind areas (posterior middle and superior temporal area) in the autism group, suggesting that the social processing associated with this area was evoked to a lower degree in autism. These additional characteristics of the abnormal activation suggest that autism is a neural systems disorder, involving abnormal interaction among multiple areas in the processing of social stimuli such as faces.
A related proposal attributes the impairment in social processing in autism to atypical development of the ability to initiate joint attention (IJA) (Mundy et al., 1994, 2009). According to this proposal, the initiation of joint attention, which requires close collaboration between temporo-parietal and frontal areas, is foundational for social referencing and social learning. This proposal is of course consistent with the underconnectivity theory offered here. Underconnectivity claims that the integrity of the frontal-posterior communication system is compromised in autism, hindering communication between frontal and posterior components of the attention network, predicting an impairment of IJA, and a relative preservation of joint attention capabilities that are not subserved by frontal areas.
A second type of abnormal social processing in autism that can be interpreted as a connectivity problem was investigated in a study of the processing of the gaze of an avatar, who looked either towards or away from a visual stimulus (Pelphrey et al., 2005). The posterior superior temporal sulcus (STS) activated differentially to an appropriate versus an inappropriate gaze shift (by the avatar) in control participants. However, there was no differential response in participants with autism. The account of the abnormal social processing in right posterior STS offered by Pelphrey et al. is that “[i]n individuals with autism, the connection between higher level [frontal] systems and the STS region may be broken, and thus the higher level systems do not engage and maintain activation in the STS region.” This is of course consistent with the theory of frontal-posterior underconnectivity offered here.
More generally, social processing is a complex task involving many brain regions, particularly frontal ones. For these regions to function together normally as an ensemble requires intact connectivity among them, enabling effective communication of information among them. Although social processing is sometimes not thought of in such informational terms (perhaps because of the central inclusion of affective information), the complexity of social thought requires no less of a communication infrastructure than any other type of thought. In fact, it is possible that social processing is even more reliant on intact frontal-posterior connectivity than cognitive processing, and that the greater reliance makes the social deficits particularly apparent in autism.
9.6. Long-distance connectivity
Some researchers have suggested that long-range brain connectivity may be disrupted in autism (Belmonte et al., 2004; Lewis and Elman, 2008). The enlargement of brain size in autism during the early stages of development (Aylward et al., 2002; Courchesne et al., 2001, 2003; Piven et al., 1995; Sparks et al., 2002) increases the distance between key processing centers in the cortex, suggesting the hypothesis that distant interregional communication is compromised. More specifically, the long-distance hypothesis predicts that in an analysis of functional connectivities in an fMRI study, there should be an interaction between the group variable (autism vs. control) and the distance variable (the distance between two areas of activation), with the autism group showing lower functional connectivity than the control group only for long-distance pairs.
The functional connectivity data in the Just et al. (2007) Tower of London study described above were re-analyzed to test this hypothesis. The 105 pair-wise measures of z′-transformed functional connectivity (arising from 15 functional ROIs) for each participant were categorized as long- or short-distance pairs as defined by a median split of the Euclidean distances between the centroids of the two ROIs in the pair. Contrary to the long-distance hypothesis, the data showed no evidence of an interaction between the group difference in functional connectivity and the distance between two areas of activation (F(1, 34) = 0.27, p = .61). The functional connectivity between two ROIs decreased with Euclidean distance (F(1, 34) = 324.85, p < .0001), but it did so similarly for both groups. By contrast, underconnectivity theory posits that the connection distance per se is not the critical variable, but instead focuses on whether the connection is between a frontal area and a posterior cortical area (primarily parietal, in this task), and correctly predicts the reliable interaction (F(1,34) = 6.30, p < .02) between the group variable (autism vs. control) and the frontal-parietal vs. other-pairs variable, with the autism group showing reliable functional underconnectivity only for frontal-parietal pairs (Just et al., 2007).
How could a neurobiological factor selectively affect frontal-posterior tracts but not other long-distance connections? The frontal lobes are particularly implicated in many of the abnormal developmental mechanisms discussed above, including glial activation and neuroinflammation (Vargas et al. 2005), minicolumnopathy (Casanova et al., 2006), and early brain overgrowth (Carper et al., 2002, 2005; Herbert et al., 2004), and given the protracted maturation of frontal lobe circuitry, such abnormalities could plausibly result in underconnectivity that is relatively specific to frontal-posterior tracts (Courchesne and Pierce, 2005a, 2005b). The fMRI studies of functional connectivity and the DTI studies of structural connectivity reviewed here clearly show that connections involving the frontal lobes are affected more than others.
9.7. The iSTART computational model
The most complex and difficult to assess neural network model of autism proposes parameter alterations in several subsystems, leading to an imbalance between various proclivities (Grossberg and Seidman, 2006). This model, called iSTART, is a combination of three existing models: the Adaptive Resonance Theory (ART) of how prefrontal cortex interacts with medial temporal lobe regions to learn to recognize objects and events; the Cognitive-Emotional-Motor (CogEM) model of how orbitofrontal cortex interacts with the amygdala, sensory and motor cortices, and the cerebellum to attend to motivationally important events, engender affective states, and produce motivated behavior; and the Spectral Timing model of how the cerebellum encodes expectations of time-delayed rewards, and how expectation violations can cause the shifting of attention and resetting of working memory. The range of impairments associated with autism is understood as arising from “imbalances” in the parameter values of these models. For example, ART emphasizes the role of prefrontal cortex in directing attention to features in a top-down fashion (Grossberg, 1999). Low “vigilance,” or attending to relatively few features, results in the learning of abstract prototypes. High vigilance, or attending to relatively many features, results in less abstract exemplar encodings. iSTART proposes that one parametric imbalance in autism results in “hypervigilance,” or attention to (and thus encoding) of every stimulus feature. The resulting exemplars are “hyperspecific”. To take another example, CogEM learns associations between the objects in the world and the internal emotional states that give those objects value (Dranias et al., 2008; Grossberg et al., 2008). According to iSTART, parametric imbalance in autism causes the threshold for emotional response by the amygdala to be abnormally high, resulting in underarousal depression. Because the amygdala provides inputs to orbitofrontal cortex, underarousal depression can reduce prefrontal responses (plans and actions) to emotionally important events.
iSTART has a number of strengths. Like the underconnectivity theory, it builds on existing models of typical adult functioning, and thus potentially offers a continuous account of the autism spectrum. In addition, iSTART is quantitatively precise. Finally, iSTART is broad in scope, attempting to account for a range of behaviors: cognitive, emotional, motivational, timing, and motor.
iSTART has certain latent compatibilities with the underconnectivity theory. Both construe brain function as inherently interactive, the product of collaboration between cortical and subcortical areas. Both assign executive functions to prefrontal cortex, including the direction of attention and selection among alternatives. Finally, both explain autism as a consequence (in part) of the failure of these executive functions. In the underconnectivity theory, this is because bandwidth limitations on communication between prefrontal and posterior areas slow processing to the point where posterior areas must often proceed without top-down guidance. In iSTART, a parameter imbalance causes attention to be broadly spread over all features, resulting in hypervigilance and other atypical states.
Although both theories are in their infancy, the underconnectivity theory has three types of advantages over iSTART. First, it has been directly evaluated against data collected from individuals with autism, and shown to fit that data. iSTART, by contrast, has thus far provided only a schematic account of the autism data. Grossberg and Seidman (2006) argue that imbalancing its parameters should produce enhanced perceptual processing, for example, but report the results of no simulations. Therefore, iSTART’s ability to account for the data on autism remains speculative. Second, the underconnectivity theory is better positioned to account for the language impairments seen in autism. There exists a comprehensive 4CAPS model of language comprehension (Just and Varma, 2007). It explains how Broca’s area, Wernicke’s area, and their right-hemisphere homologs collaborate to comprehend sentences. The model has been shown to be consistent with a broad range of behavioral and brain imaging data. It is a straightforward task for future research to decrease the communication bandwidth between its frontal and posterior components, record the resulting impairments, and see how they compare with those observed in people with autism. By contrast, the iSTART account of parameter imbalances leading to language deficiencies remains to be developed, although the ARTWORD model of speech perception could perhaps serve as a foundation for such an effort (Grossberg and Myers, 2000). The final advantage of the underconnectivity theory is that it addresses the new functional connectivity and DTI data on autism. These methods are opening new windows into this syndrome, and it is critical that theories address the resulting data. In this regard, iSTART is currently silent.
10. Questions raised by the theory
Although the underconnectivity perspective provides a useful theoretical framework for investigating autism, the theory does not currently provide the answers to some remaining central questions about autism. We view the current form of the underconnectivity theory as preliminary, and anticipate that future research will result in considerable refinement, expansion, and modification. Below we raise some of the key questions that are currently framed by the theory but which have not yet been empirically investigated.
10.1. Other types of connectivity disturbances besides those measured by fMRI
We expect that alterations in connectivity among neural systems and in local connectivity are likely to emerge with further study, including the possibility of increased connectivity between some areas. Moreover, other technologies in addition to fMRI are being brought to bear on issues of neural connectivity in autism, such as histology, electrophysiology, morphometry, and diffusion imaging. Among the uncertainties to be addressed by some combination of these approaches concerns the relation between white matter and gray matter abnormalities, and the relation between functional and anatomical abnormalities in autism.
10.2. Generality of connectivity disturbances across tasks
Although we have reported functional underconnectivity in a number of different types of thinking that span the autism syndrome (executive function, perception, language, social processing, inhibition), there has been no study of functional connectivity in many other types of relevant tasks. The tasks yet to be so investigated with neuroimaging include tasks without frontal involvement, tasks with non-visual input (auditory, haptic, gustatory, or olfactory) and simple motor tasks. Because underconnectivity theory was developed on the basis of findings in tasks with frontal involvement, it is parsimonious to initially assume that the disruption in the connectivity applies only between frontal and non-frontal regions, as has been observed in these tasks. But ensuing studies could falsify this assumption.
10.3. Extension to lower-functioning and younger participants
Another lacuna in the research is the absence of functional connectivity studies of people with autism who are not high-functioning (and have more severe cases of autism). Although it is more difficult to acquire functional imaging data in low-functioning participants, it seems possible to do so in a study of resting state functional connectivity, where lowered functional connectivity has been demonstrated in high-functioning autism (Cherkassky et al., 2006; Kennedy and Courchesne, 2008; Monk et al., 2009). Another type of extension might examine the ontology of the functional underconnectivity in much younger participants. Finally, structural imaging studies of young children with autism, which are underway in several laboratories, could provide valuable information about the development of the white matter that provides the cortical connectivity.
10.4. Relation to other neurological populations
It would not be surprising if a system as complex as the brain could be afflicted with some form of disconnection in other neurological conditions besides autism, and the comparison among the conditions could be informative about the nature of connectivity and its disturbances. For example, Herbert et al. (2004) found larger (compared to controls) frontal, temporal, and occipital superficial/radiate white matter volumes not only in autism but also in developmental language delay, indicating a non-specificity of these outcomes. However, in the parietal lobe, only the autism group showed the increased volume of superficial/radiate white matter. Such cross-syndrome comparisons of disordered anatomical and functional connectivity may reveal systematic patterns of commonalities and specificities of connectivity symptoms indicating possible branching points in development.
Dyslexia is another disorder that, like autism and developmental language delay, entails a disruption of white matter (e.g., Deutsch et al., 2005). Moreover, the white matter of children who are poor readers (and the cognitive abilities that the white matter underpins) has recently been shown to improve with training (Keller and Just, 2009b). This demonstration of the modifiability of white matter suggests that autism therapies might also be have the potential of modifying a connectivity deficit.
10.5. Etiology of underconnectivity
The theory presented here does not attempt to account for the pathophysiological origins of autism, although it provides guidance for a search for such origins at more molecular levels of analysis. In particular, genomic factors are beginning to be related to brain connectivity disruptions in autism. One tentative hypothesis proposes that autism is caused by developmental dysregulation of interregional brain connectivity (Geschwind and Leavitt, 2007), a proposal that is very congenial to underconnectivity theory. As the pathophysiology of autism becomes better understood, the link to genomic factors will become easier to make. Underconnectivity theory crystallizes and integrates some of the known pathophysiology and neuropsychology of autism, facilitating the linkage to genomics.
11. Summary
Underconnectivity theory proposes that autism be characterized as a neural systems disorder marked by frontal-posterior connectivity abnormalities. Because of lowered bandwidth between frontal and posterior brain networks in autism, the flow of information between frontal and posterior areas is impaired, resulting in deficits in tasks that require substantial frontal contribution and in increased reliance on posterior regions. This increased dependence on more posterior regions could become less effective as task difficulty increases and the need for higher-level strategies and concepts grows. The diversity of symptoms seen in autism may be due to the wide range of activities for which substantial frontal participation is important.
The 4CAPS TOL model provides a sufficiency proof of underconnectivity theory. With lower bandwidth and more autonomy of posterior centers, the model reproduces several key phenomena observed in fMRI studies of autism. The 4CAPS model accounts for the observed reaction times, fMRI activation, group differences and individual differences in functional connectivity in the TOL task. In a more general way, the model accounts for such group differences in a wide variety of tasks in different domains (as shown in Table 1).
Although the biological basis of autism is at least in part associated with white matter differences, there could also be concomitant or even causal gray matter differences in autism. New imaging technologies that can trace white matter tracts in individuals in great detail are rapidly emerging, thereby transforming some of these theoretical issues into empirical issues. A theory of frontal-posterior underconnectivity in autism provides an initial framework to guide such future research.
Highlights.
Our theory attributes autism to impaired frontal-posterior brain connectivity.
We present functional and structural brain imaging and behavioral evidence.
We model underconnectivity with frontal-posterior bandwidth constraints.
We account for language and social deficits and perceptual sparing in autism.
We account for individual differences in functional connectivity in autism.
Acknowledgements
This research was supported by the Autism Centers of Excellence Grant HD055748 from the National Institute of Child Health and Human Development and the Office of Naval Research Grant N00014-07-1-0041.
Abbreviations
- fMRI
functional magnetic resonance imaging
- 4CAPS
Cortical Capacity-Constrained Collaborative Activation-based Production Systems
Appendix
Formal specification of the 4CAPS cognitive neuroarchitecture
This Appendix provides additional details of the mathematics behind the 4CAPS architecture. Readers interested in a more complete treatment are referred to Just and Varma (2007).
Centers are Specialized for Cognitive Functions
Consider a 4CAPS model consisting of M centers, each corresponding to a different brain area. Centers are specialized to varying degrees for each of N cognitive functions. The specialization of center i for function j is denoted Sij ∈ [1,∞]. This indicates the amount of the center’s resources required to perform one “unit” of the function. A value of 1 represents perfect specialization, a value greater than 1 represents less-than-perfect specialization, and a value of ∞ represents no specialization for the function (because centers have finite resource supplies, as described below). For example, in the TOL model, the RH-Executive center has a specialization of 1 for the goal management function (because it performs it perfectly efficiently) but a specialization of ∞ for the visuospatial representation function (because it cannot perform it at all); the LH-Spatial center has complementary specializations. More generally, there is a distribution of functions across centers, such that each center is specialized to some degree for multiple functions, and conversely, each function can be performed to varying degrees by multiple centers.
How are cognitive functions implemented? Each center is an encapsulated production system, and each cognitive function is a combination of cognitive representations implemented as declarative memory elements (DMEs) and cognitive processes on those representations implemented as production rules. Each DME is a vector of symbolic features, and is annotated with an activation level. Each production rule has a condition side specifying a pattern of DMEs and an action side specifying processing to be performed if that pattern matches. Returning to the example of the TOL model, the goal management function is implemented by DMEs representing goals (e.g., “unblock the striped ball in the left pocket”) and productions that process these goals (e.g., “if a goal has been satisfied, then suppress its activation”).
At each point in time, called a cycle, the condition sides of productions are matched against the available DMEs, and the actions sides of all matching productions are executed. Actions either excite or suppress the activation levels of DMEs. The total resource consumption of center i at a particular point in time is:
where Aij denotes the amount of function j performed.
Centers Possess Finite Resources
Each center possesses a finite amount of resources with which to perform cognitive functions, reflecting the fact that the brain is a finite biological system. More precisely, the resource capacity of center i is denoted Ci and the following constraint is enforced at all times:
| (1) |
Resource constraints are particularly important when a difficult task is being performed. When a center is well-specialized for a function but lacks sufficient resources to perform it, then two recourses are possible. First, if another center is also specialized for the function (albeit to a lesser degree) and possesses free resources, then the excess processing spills over to that center. Second, if no other centers are specialized for the function, then only a portion of it will be performed on the current cycle, and the remainder will be deferred to subsequent cycles, until sufficient resources become available. It is the second condition that applies to the TOL model. For example, when solving a difficult problem, the resource demands of the goal management can overwhelm the resource supply of the RH-Executive center. Because no other centers are specialized for that function, processing slows down.
The question, then, is how to assign cognitive functions to centers in a way that minimizes resource consumption while maximizing cognitive performance? This can be formulated as follows. Recall that Aij denotes the amount of function j performed by center i. The assignment problem is how to determine the Aij at each point in time.
Recall that we have M constraints on the Aij, one for each center i, that stipulate that no center allocates more resources to cognitive functions than its capacity; these are the (1) above. We also have N constraints on the Aij, one for each function j, that stipulate that as much of the function as possible is performed. These take the form:
| (2) |
where Rj denotes the requested amount of function j to be performed. Finally, we have MxN constraints, one for each Aij, specifying that all activations are non-negative:
| (3) |
Many choices of Aij satisfy the constraints (1), (2), and (3). We select the assignment that maximizes the following objective function:
| (4) |
Defining the weight Wij as 1/Sij results in an assignment that assigns as much of each cognitive function as possible to the center most specialized for it.
(1), (2), (3), and (4) constitute a linear programming problem. 4CAPS formulates the assignment problem in this form on each cycle and solves it using the simplex algorithm (Cormen et al., 2001; Dantzig and Thapa, 1997).
Capacity Utilization Predicts fMRI Activation
The capacity utilization of center i at a point in time is the proportion of its resources that are being consumed.
The key linking hypothesis is that the capacity utilization of a center predicts activation in the corresponding brain area. Capacity utilization is an instantaneous measure. The average capacity utilization in a center over a sequence of stimuli can be used to predict the average activation in the corresponding brain area as would be measured in a study employing a block design.
At a finer temporal grain, the predicted fMRI time series in a center i during the processing of a single stimulus can be computed in the following manner. First, the capacity utilizations in that center are sampled at the same rate at which fMRI images were acquired in the study being modeled. The result is a capacity utilization time series CUi(t). For example, in the Just et al. (2007) study, images were acquired every 3 sec. Second, the capacity utilization time series is convolved with a hemodynamic response function h(t) to generate a predicted activation time series fMRIi(t).
The hemodynamic response function is modeled by a gamma function with a fixed delay, which has previously been shown to provide a good approximation (Aguirre et al.,1998; Boynton et al., 1996).
| (2) |
In the interest of parsimony, 4CAPS uses the published parameter estimates (δ=2.5, τ=1.25, n=3). The predicted fMRI time series for a center can be compared with the observed fMRI time series in the corresponding brain area.
4CAPS models also generate functional connectivity predictions: the correlation between the predicted fMRI time series in two centers can be compared with the correlation between the observed fMRI time series in the corresponding brain areas.
Of course, 4CAPS models also predict behavioral measures such as response time, i.e., the number of cycles required to perform a task.
Bandwidth Limitations on Center Communication
The 4CAPS autism TOL model includes a mechanism for specifying a communication channel between sets of centers. In particular, the TOL model includes a communication channel between Spatial centers and Executive centers. Representations (DMEs) that are shared between centers pass through this channel. Associated with the channel is a parameter that governs the rate of activation flow of representations between Spatial and Executive centers. This is the bandwidth limitation, and it can be parametrically varied to model individual differences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre GK, Zarahn E, D'Esposito M. The variability of human, BOLD hemodynamic responses. NeuroImage. 1998;8:360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, Miller JN, Lu J, Jeong EK, McMahon WM, Bigler ED, Lainhart JE. Diffusion tensor imaging of the corpus callosum in Autism. NeuroImage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Bothell D, Byrne MD, Douglass S, Lebiere C, Qin Y. An integrated theory of the mind. Psychological Review. 2004;111:1036–1060. doi: 10.1037/0033-295X.111.4.1036. [DOI] [PubMed] [Google Scholar]
- Arbib MA, Billard A, Iacoboni M, Oztop E. Synthetic brain imaging: Grasping, mirror neurons and imitation. Neural Networks. 2000;13:975–997. doi: 10.1016/s0893-6080(00)00070-8. [DOI] [PubMed] [Google Scholar]
- Asperger H. Autistic Psychopathy in Childhood. In: Frith U, editor. Translated in Autism and Asperger's Syndrome. Vol. 1991. Cambridge: Cambridge University Press; 1944. pp. 37–92. [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, O'Boyle JG, Schultz RT, Pearlson GD. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 2010;53:247–256. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59:175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. A clinicopathological study of autism. Brain. 1998;121:101–117. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: Preliminary evidence from diffusion tensor imaging. Biological Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Are autistic children ‘behaviorists’? Journal of Autism and Developmental Disorders. 1989;19:579–600. doi: 10.1007/BF02212859. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Mindblindness: An essay on autism and theory of mind. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- Baslow MH. N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem. Res. 2003;28:941–953. doi: 10.1023/a:1023250721185. [DOI] [PubMed] [Google Scholar]
- Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35:866–874. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. Journal of Neuroscience. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Bashat D, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R, Even A, Levy Y, Ben Sira L. Accelerated maturation of white matter in young children with autism: A high B value DWI study. NeuroImage. 2007;37:40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Bird G, Catmur C, Silani G, Frith C, Frith U. Attention does not modulate neural responses to social stimuli in autism spectrum disorders. NeuroImage. 2006;31:1614–1624. doi: 10.1016/j.neuroimage.2006.02.037. [DOI] [PubMed] [Google Scholar]
- Björne P, Balkenius C. A model of attentional impairments in autism: first steps toward a computational theory. Cognitive Systems Research. 2005;6:193–204. [Google Scholar]
- Boucher J, Lewis V. Unfamiliar face recognition in relatively able autistic children. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1992;33:843–859. doi: 10.1111/j.1469-7610.1992.tb01960.x. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. The Journal of Neuroscience. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J, Brown CC, Boucher J, Rippon G. The temporal binding deficit hypothesis of autism. Developmental Psychopathology. 2002;14:209–224. doi: 10.1017/s0954579402002018. [DOI] [PubMed] [Google Scholar]
- Carlsson ML. Hypothesis: Is infantile autism a hypoglutamatergic disorder? Relevance of glutamate-serotonin interactions for pharmacotherapy. Journal of Neural Transmission. 1998;105:525–535. doi: 10.1007/s007020050076. [DOI] [PubMed] [Google Scholar]
- Carper RA, Courchesne E. Localized enlargement of the frontal cortex in early autism. Biological Psychiatry. 2005;57:126–133. doi: 10.1016/j.biopsych.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: Early hyperplasia and abnormal age effects. NeuroImage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- Casanova M, Trippe J. Radial cytoarchitecture and patterns of cortical connectivity in autism. Philos. Trans. R. Soc. Lond. B Biol Sci. 2009;364:1433–1436. doi: 10.1098/rstb.2008.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, van Kooten IA, Switala AE, van Engeland H, Heinsen H, Steinbusch HW, Hof PR, Trippe J, Stone J, Schmitz C. Minicolumnar abnormalities in autism. Acta Neuropathol. (Berl.) 2006;112:287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Changeux J-P, Danchin A. Selective stabilization of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976;264:705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. NeuroReport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Chung MK, Dalton KM, Alexander AL, Davidson RJ. Less white matter concentration in autism: 2D voxel-based morphometry. NeuroImage. 2004;23:242–251. doi: 10.1016/j.neuroimage.2004.04.037. [DOI] [PubMed] [Google Scholar]
- Coben R, Clarke AR, Hudspeth W, Barry RJ. EEG power and coherence in autistic spectrum disorder. Clininical Neurophysiology. 2008;119:1002–1009. doi: 10.1016/j.clinph.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Cohen IL. An artificial neural network analogue of learning in autism. Biological Psychiatry. 1994;36:5–20. doi: 10.1016/0006-3223(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Cormen TH, Leiserson CE, Rivest RL, Stein C. Introduction to Algorithms. Third. Cambridge, MA: MIT Press; 2001. [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. Journal of the American Medical Association. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but long-distance disconnection. Current Opinion in Neurobiology. 2005a;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Brain overgrowth in autism during a critical time in development: implications for frontal pyramidal neuron and interneuron development and connectivity. International Journal of Developmental Neuroscience. 2005b;23:153–170. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Redcay E, Kennedy DP. The autistic brain: birth through adulthood. Current Opinion in Neurolology. 2004;17:489–496. doi: 10.1097/01.wco.0000137542.14610.b4. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Redcay E, Morgan JT, Kennedy DP. Autism at the beginning: microstructural and growth abnormalities underlying the cognitive and behavioral phenotype of autism. Developmental Psychopathology. 2005;17:577–597. doi: 10.1017/S0954579405050285. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Leski ML, Morrison JH. The diverse roles of L-glutamic acid in brain signal transduction. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology, The Fifth Generation of Progress. Philadelphia: Lippincott, Williams, and Wilkins; 2002. pp. 71–79. [Google Scholar]
- Dantzig GB, Thapa MN. Linear Programming 1: Introduction. New York: Springer-Verlag; 1997. [Google Scholar]
- Darmarla SR, Keller TA, Kana RK, Cherkassky VL, Williams DL, Minshew NJ, Just MA. Cortical underconnectivity coupled with preserved visuospatial cognition in autism: evidence from an fMRI study of an embedded figures task. Autism Research. 2010;5:273–279. doi: 10.1002/aur.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb S, Schellenberg GD, Dager S, Friedman S, Aylward E, Richards T. Defining the broader phonotype of autism: Genetic, brain, and behavioral perspectives. Developmental Psychopathology. 2002;14:581–611. doi: 10.1017/s0954579402003103. [DOI] [PubMed] [Google Scholar]
- Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JDE, Wandell B. Children's reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex. 2005;41:354–363. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- Dranias M, Grossberg S, Bullock D. Dopaminergic and non-dopaminergic value systems in conditioning and outcome-specific revaluation. Brain Research. 2008;1238:239–287. doi: 10.1016/j.brainres.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study "resting state" functional connectivity. NeuroImage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall K. A Delay-Tolerant Network Architecture for Challenged Internets. Intel Research Technical Report (IRB-TR-03-003) 2003 [Google Scholar]
- Fletcher PT, Whitaker RT, Tao R, DuBray MB, Froehlich Al, Ravichandran C, Alexander AL, Bigler ED, Lange N, Lainhart JE. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. NeuroImage. 2010;51:1117–1125. doi: 10.1016/j.neuroimage.2010.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SD, Shaw DW, Artru AA, Richards TL, Gardner J, Dawson G, Posse S, Dager SR. Regional brain chemical alterations in young children with autism spectrum disorder. Neurology. 2003;60:100–107. doi: 10.1212/wnl.60.1.100. [DOI] [PubMed] [Google Scholar]
- Frith C. What do imaging studies tell us about the neural basis of autism? In: Bock G, Goode J, editors. Autism: Neural Basis and Treatment Possibilities: Novartis Foundation Symposium. Vol. 251. Chichester, UK: John Wiley & Sons; 2003. pp. 149–176. [PubMed] [Google Scholar]
- Frith U. Autism: Explaining the enigma. Oxford, UK: Blackwell; 1989. [Google Scholar]
- Frith U, Snowling M. Reading for meaning and reading for sound in autistic and dyslexic children. British Journal of Developmental Psychology. 1983;1:329–342. [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Current Opinion in Neurobiology. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Goldstein G, Minshew N, Siegel D. Age differences in academic achievement in high-functional autistic individuals. Journal of Clinical and Experimental Neuropsychology. 1994;16:671–680. doi: 10.1080/01688639408402680. [DOI] [PubMed] [Google Scholar]
- Grossberg S. The link between brain learning, attention, and consciousness. Consciousness and Cognition. 1999;8:1–44. doi: 10.1006/ccog.1998.0372. [DOI] [PubMed] [Google Scholar]
- Grossberg S, Bullock D, Dranias M. Neural dynamics underlying impaired autonomic and conditioned responses following amygdala and orbitofrontal lesions. Behavioral Neuroscience. 2008;122:1100–1125. doi: 10.1037/a0012808. [DOI] [PubMed] [Google Scholar]
- Grossberg S, Myers CW. The resonant dynamics of speech perception: Interword integration and duration-dependent backward effects. Psychological Review. 2000;104:735–767. doi: 10.1037/0033-295x.107.4.735. [DOI] [PubMed] [Google Scholar]
- Grossberg S, Seidman D. Neural dynamics of autistic behaviors: cognitive, emotional, and timing substrates. Psychological Review. 2006;113:483–525. doi: 10.1037/0033-295X.113.3.483. [DOI] [PubMed] [Google Scholar]
- Gustafsson L. Inadequate cortical feature maps: a neural circuit theory of autism. Biological Psychiatry. 1997;42:1138–1147. doi: 10.1016/s0006-3223(97)00141-8. [DOI] [PubMed] [Google Scholar]
- Happé FGE. The role of age and verbal ability in the Theory of Mind task performance of subjects with autism. Child Development. 1995;66:843–855. [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Keshavan MS. Corpus callosum size in autism. Neurology. 2000;55:1033–1036. doi: 10.1212/wnl.55.7.1033. [DOI] [PubMed] [Google Scholar]
- Hartline DK, Colman DR. Rapid conduction and the evolution of giant axons and myelinated fibers. Current Biology. 2007;17:R29–R35. doi: 10.1016/j.cub.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Archives of General Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Bakardjiev A, Hodgson J, Adrien KT, Kennedy DN, Filipek PA, Caviness VS., Jr Larger brain and white matter volumes in children with developmental language disorder. Developmental Science. 2003;6:F11–F22. [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, Sanders HA, Kennedy DN, Caviness VS., Jr Localization of white matter volume increase in autism and developmental language disorder. Annals of Neurology. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Hisaoka S, Harada M, Nishitani H, Mori K. Regional magnetic resonance spectroscopy of the brain in autistic individuals. Neuroradiology. 2001;43:496–498. doi: 10.1007/s002340000520. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Tagamets M-A. Predicting human functional maps with neural net modeling. Human Brain Mapping. 1999;8:137–142. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<137::AID-HBM11>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C, Russell J, Robbins TW. Evidence for executive dysfunction in autism. Neuropsychologia. 1994;32:477–492. doi: 10.1016/0028-3932(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Jacobs RA, Jordan MI. Computational consequences of a bias toward short connections. Journal of Cognitive Neuroscience. 1992;4:323–336. doi: 10.1162/jocn.1992.4.4.323. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Preis S, Steinmetz H. The relation between forebrain volume and midsagittal size of the corpus callosum in children. NeuroReport. 1999;10:2981–2985. doi: 10.1097/00001756-199909290-00020. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Staiger JF, Schlaug G, Huang Y, Steinmetz H. The relationship between corpus callosum size and forebrain volume. Cerebral Cortex. 1997;7:48–56. doi: 10.1093/cercor/7.1.48. [DOI] [PubMed] [Google Scholar]
- Jones TB, Bandettini PA, Kenworthy L, Case LK, Milleville SC, Martin A, Birn RM. Sources of group differences in functional connectivity: an investigation applied to autism spectrum disorder. NeuroImage. 2010;49:401–414. doi: 10.1016/j.neuroimage.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Varma S. Computational modeling of high-level cognition and brain function. Human Brain Mapping. 1999;8:128–136. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<128::AID-HBM10>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: Evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Just MA, Varma S. The organization of thinking: What functional brain imaging reveals about the neuroarchitecture of complex cognition. Cognitive, Affective, and Behavioral Neuroscience. 2007;7:153–191. doi: 10.3758/cabn.7.3.153. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Topographic maps are fundamental to sensory processing. Brain Research Bulletin. 1997;44:107–112. doi: 10.1016/s0361-9230(97)00094-4. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Why is brain size so important: Design problems and solutions as neocortex gets bigger or smaller. Brain and Mind. 2000;1:7–23. [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: Decreased activation and underconnectivity in inhibition networks. Biological Psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Social Neuroscience. 2009;4:135–152. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel Eric R, Schwartz James H, Jessell Thomas M., editors. Principles of Neural Science. fourth. New York: McGraw-Hill; 2000. [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Keller TA, Just MA. Reduced fractional anisotropy and increased radial diffusivity in high-functioning autism: A large-sample whole-brain diffusion tensor imaging study. Poster presented at 15th Annual Meeting of the Organization for Human Brain Mapping; June 2009; San Francisco, CA. 2009a. [Google Scholar]
- Keller TA, Just MA. Altering cortical connectivity: Remediation-induced changes in the white matter of poor readers. Neuron. 2009b;64:624–631. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. NeuroReport. 2007;18:23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. NeuroImage. 2008;39:1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, Greenson J, Dawson G, Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Knaus TA, Silver AM, Kennedy M, Lindgren KA, Dominick KC, Siegel J, Tager-Flusberg H. Language laterality in autism spectrum disorder and typical controls: a functional, volumetric, and diffusion tensor MRI study. Brain and Language. 2010;112:113–120. doi: 10.1016/j.bandl.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. NeuroImage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI investigation of working memory for faces in autism: Visual coding and underconnectivity with frontal areas. Cerebral Cortex. 2008;18:289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Bigler ED, Alexander AL, Lazar M, DuBray MB, Chung MK, Johnson M, Morgan J, Miller JN, McMahon WM, Lu J, Jeong EK, Lainhart JE. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neuroscience Letters. 2007;424:127–132. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Levitt JG, O’Neill J, McCracken JT, Guthrie D, Toga AW, Alger JR. Proton magnetic resonance spectroscopic imaging in childhood autism. Biological Psychiatry. 2003;54:1355–1366. doi: 10.1016/s0006-3223(03)00688-7. [DOI] [PubMed] [Google Scholar]
- Lewis JD, Elman JL. Growth-related neural reorganization and the autism phenotype: A test of the hypothesis that altered brain growth leads to altered connectivity. Developmental Science. 2008;11:135–155. doi: 10.1111/j.1467-7687.2007.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Theilmann RJ, Sereno MI, Townsend J. The relation between connection length and degree of connectivity in young adults: a DTI analysis. Cerebral Cortex. 2009;19:554–562. doi: 10.1093/cercor/bhn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes F, Piven J, Vrancic D, Nanclares V, Plebst C, Starkstein SE. An MRI study of the corpus callosum and cerebellum in mentally retarded autistic individuals. Journal of Neuropsychiatry and Clinical Neuroscience. 1999;11:470–474. doi: 10.1176/jnp.11.4.470. [DOI] [PubMed] [Google Scholar]
- Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- Mason RA, Williams DL, Kana RK, Minshew N, Just MA. Theory of Mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46:269–280. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL. The basis of hyperspecificity in autism: a preliminary suggestion based on properties of neural nets. Journal of Autism and Developmental Disorders. 2000;30:497–502. doi: 10.1023/a:1005576229109. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G. Autism as a disorder of complex information processing. Mental Retardation and Developmental Disabilities Research Reviews. 1998;4:129–136. [Google Scholar]
- Minshew NJ, Goldstein G, Siegel DJ. Neuropsychologic functioning in autism: Profile of a complex information processing disorder. Journal of the International Neuropsychological Society. 1997;3:303–316. [PubMed] [Google Scholar]
- Mitchison G. Neuronal branching patterns and the economy of cortical wiring. Proc. R. Soc. London B. 1991;245:151–158. doi: 10.1098/rspb.1991.0102. [DOI] [PubMed] [Google Scholar]
- Mizuno A, Liu Y, Williams DL, Keller TA, Minshew NJ, Just MA. The neural basis of deictic shifting in linguistic perspective-taking in high-functioning autism. Brain. 2011;134:2422–2435. doi: 10.1093/brain/awr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno A, Villalobos ME, Davies MM, Dahl BC, Muller RA. Partially enhanced thalamocortical functional connectivity in autism. Brain Research. 2006;1104:160–174. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. NeuroImage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulieres I, Hubert B, Burack JA. Enhanced perceptual functioning in autism: An update, and eight principles of autistic perception. Journal of Autism and Developmental Disorders. 2006;36:27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Kasari C. Joint attention, developmental level, and symptom presentation in children with autism. Development and Psychopathology. 1994;6:389–401. [Google Scholar]
- Mundy P, Sullivan L, Mastergeorge AM. A parallel and distributed-processing model of joint attention, social cognition, and autism. Autism Research. 2009;2:2–21. doi: 10.1002/aur.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murias M, Webb SJ, Greenson J, Dawson G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biological Psychiatry. 2007;62:270–273. doi: 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SD, Carpenter PA, Varma S, Just MA. Frontal and parietal participation in problem solving in the Tower of London: fMRI and computational modeling of planning and high-level perception. Neuropsychologia. 2003;41:1668–1682. doi: 10.1016/s0028-3932(03)00091-5. [DOI] [PubMed] [Google Scholar]
- Newman TM, Macomber D, Naples AJ, Babitz T, Volkmar F, Grigorenko EL. Hyperlexia in children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37:760–774. doi: 10.1007/s10803-006-0206-y. [DOI] [PubMed] [Google Scholar]
- Noonan SK, Haist F, Müller RA. Aberrant functional connectivity in autism: evidence from low-frequency BOLD signal fluctuations. Brain Research. 2009;1262:48–63. doi: 10.1016/j.brainres.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Laughlin C, Thagard P. Autism and coherence: a computational model. Mind & Language. 2000;15:375–392. [Google Scholar]
- Otsuka H, Harada M, Mori K, Hisaoka S, Nishitani H. Brain metabolites in the hippocampus-amygdala region and cerebellum in autism: An 1H-MR spectroscopy study. Neuroradiology. 1999;41:517–519. doi: 10.1007/s002340050795. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescence: an in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Piven J, Arndt S, Bailey J, Andreasen N. Regional brain enlargement in autism: a magnetic resonance imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:530–536. doi: 10.1097/00004583-199604000-00020. [DOI] [PubMed] [Google Scholar]
- Piven J, Arndt S, Bailey J, Havercamp S, Andreasen NC, Palmer P. An MRI study of brain size in autism. American Journal of Psychiatry. 1995;152:1145–1149. doi: 10.1176/ajp.152.8.1145. [DOI] [PubMed] [Google Scholar]
- Piven J, Bailey J, Ranson BJ, Arndt S. An MRI study of the corpus callosum in autism. American Journal of Psychiatry. 1997;154:1051–1056. doi: 10.1176/ajp.154.8.1051. [DOI] [PubMed] [Google Scholar]
- Polleux F, Lauder JM. Toward a developmental neurobiology of autism. Mental Retardation and Developmental Disabilities Research Review. 2004;10:303–317. doi: 10.1002/mrdd.20044. [DOI] [PubMed] [Google Scholar]
- Ringo JL. Neuronal interconnection as a function of brain size. Brain, Behavior, and Evolution. 1991;38:1–6. doi: 10.1159/000114375. [DOI] [PubMed] [Google Scholar]
- Ringo JL, Doty RW, Demeter S, Simard PY. Time is of the essence: a conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cerebral Cortex. 1994;4:331–343. doi: 10.1093/cercor/4.4.331. [DOI] [PubMed] [Google Scholar]
- Rippon G, Brock J, Brown C, Boucher J. Disordered connectivity in the autistic brain: challenges for the "new psychophysiology". International Journal of Psychophysiology. 2007;63:164–172. doi: 10.1016/j.ijpsycho.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, Brain, and Behavior. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun CP, Belliveau JW, Soulières I, Schwartz S, Mody M. Neuroimaging of the functional and structural networks underlying visuospatial vs. linguistic reasoning in high-functioning autism. Neuropsychologia. 2010;48:86–95. doi: 10.1016/j.neuropsychologia.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipul SE, Keller TA, Just MA. Inter-regional brain communication and its disturbance in autism. Frontiers in Systems Neuroscience. 2011;5:10. doi: 10.3389/fnsys.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipul SE, Williams DL, Keller TA, Minshew NJ, Just MA. Distinctive neural processes during learning in autism. Cerebral Cortex. doi: 10.1093/cercor/bhr162. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, Skudlarski P, Lacadie C, Dohen DJ, Gore JC. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger Syndrome. Archives of General Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Grelotti DJ, Klin A, Kleinman J, Van der Gaag C, Marois R, Skudlarski P. The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philos. Trans. R. Soc. Lond. B, Biol. Sci. 2003;358:415–427. doi: 10.1098/rstb.2002.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT, Klin A. Genetics of childhood disorders: XLIII. Autism, part 2: neural foundations. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1259–1262. doi: 10.1097/00004583-200210000-00018. [DOI] [PubMed] [Google Scholar]
- Shah A, Frith U. An islet of ability in autistic children: a research note. Journal of Child Psychology and Psychiatry. 1983;24:613–620. doi: 10.1111/j.1469-7610.1983.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Shah A, Frith U. Why do autistic individuals show superior performance on the block design task? Journal of Child Psychology and Psychiatry. 1993;34:1351–1364. doi: 10.1111/j.1469-7610.1993.tb02095.x. [DOI] [PubMed] [Google Scholar]
- Shannon C. Communication in the Presence of Noise. Proceedings of the IRE. 1949;37:10–21. [Google Scholar]
- Solomon M, Ozonoff SJ, Ursu S, Ravizza S, Cummings N, Ly S, Carter CS. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47:2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Stone P, Veloso M. Task decomposition and dynamic role assignment for real-time strategic teamwork. In: Muller JP, et al., editors. ATAL ’ 98 LNAI 1555. 1999. pp. 293–308. [Google Scholar]
- Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cerebral Cortex. 2008;18:2659–2665. doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H. Autistic children's talk about psychological states: deficits in the early acquisition of a theory of mind. Child Development. 1992;63:161–172. [PubMed] [Google Scholar]
- Turner KC, Frost L, Linsenbardt D, McIlroy JR, Müller RA. Atypically diffuse functional connectivity between caudate nuclei and cerebral cortex in autism. Behavioral and Brain Functions. 2006;2:34–45. doi: 10.1186/1744-9081-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Annals of Neurology. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Vidal CN, Nicolson R, De Vito TJ, Hayashi KM, Geaga JA, Drost DJ, Williamson PC, Rajakumar N, Sui Y, Dutton RA, Toga AW, Thompson PM. Mapping corpus callosum deficits in autism: An index of aberrant cortical connectivity. Biological Psychiatry. 2006;60:218–225. doi: 10.1016/j.biopsych.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Muller RA. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. NeuroImage. 2005;25:916–925. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waiter GD, Williams JHG, Murray AD, Gilchrist A, Perrett DI, Whiten A. Structural white matter deficits in high-functioning individuals with autistic spectrum disorder: A voxel-based investigation. NeuroImage. 2005;24:455–461. doi: 10.1016/j.neuroimage.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve. 1980;3:141–150. doi: 10.1002/mus.880030207. [DOI] [PubMed] [Google Scholar]
- Welchew DE, Ashwin C, Berkouk K, Salvador R, Suckling J, Baron-Cohen S, Bullmore E. Functional disconnectivity of the medial temporal lobe in Asperger's syndrome. Biol. Psychiatry. 2005;57:991–998. doi: 10.1016/j.biopsych.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Weng SJ, Wiggins JL, Peltier SJ, Carrasco M, Risi S, Lord C, Monk CS. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Research. 2010;1313:202–214. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Fonlupt P, Hubert B, Tardif C, Gepner B, Deruelle C. Abnormal cerebral effective connectivity during explicit emotional processing in adults with autism spectrum disorder. Social Cognition and Affective Neuroscience. 2008;3:135–143. doi: 10.1093/scan/nsn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Goldstein G, Minshew NJ. Neuropsychologic functioning in children with autism: further evidence for disordered complex information-processing. Child Neuropsychology. 2006;12:279–298. doi: 10.1080/09297040600681190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours A. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life () Oxford: Blackwell; 1967. pp. 3–70. [Google Scholar]