Abstract
Background:
PI3K/Akt (PKB) pathway has been shown in several cell types to be activated by ligands to cell surface integrins, leading to the metastasis of tumour cells. The signalling pathways involved in the metastatic spread of human scirrhous gastric carcinoma cells have not been defined.
Methods:
The role of the PI3K/Akt pathway in an extensive peritoneal-seeding cell line, OCUM-2MD3 and a parental cell line, OCUM-2M, was investigated by assessing in vitro adhesion and spreading assay, and in vivo peritoneal metastatic model. We also examined the correlation of PI3K/Akt pathway with integrin signals by immunoprecipitations, using cells by transfection with mutant p85 (Δp85).
Results:
Adhesiveness and spreading of OCUM-2MD3 cells on collagen type IV was significantly decreased by PI3K inhibitors and expression of mutant p85, but not by inhibitors of protein kinase C (PKC) or extracellular signal-regulated kinase (ERK). Immunoprecipitation studies indicated that the PI3K/Akt pathway was associated with integrin signalling through Src and vinculin. In an in vivo experimental metastasis model, p85 inhibition reduced peritoneal metastasis of OCUM-2MD3 cells.
Conclusion:
PI3K/Akt signalling may be required for integrin-dependent attachment and spreading of scirrhous gastric carcinoma cells, and would be translated into generating better strategies to optimise their use in cancer clinical trials.
Keywords: PI3 kinase, gastric carcinoma, adhesion, spreading: metastasis, integrin signalling
Gastric carcinoma is one of the most frequent and lethal malignancies in the world (Sowa et al, 1989). Aggressive gastric carcinoma, especially scirrhous type adenocarcinoma, is characterised by a rapid dissemination into the abdominal cavity, which leads to peritoneal metastasis. Such metastasis is a multistep phenomenon, involving detachment of malignant cells from the primary tumour, transfer to the peritoneal cavity, attachment to the peritoneum, and, finally, proliferation to form secondary tumour foci (Fidler, 1991). During the sequential steps of peritoneal metastasis, the direct adhesive interaction between tumour cells and extracellular matrix (ECM) is thought to be critical (Fidler, 1991). Cell-matrix interactions result in a cascade of cellular responses that ultimately promote not only cell binding but also cytoskeletal rearrangements and cell spreading, with a concomitant formation of focal adhesions (Yamada and Geiger, 1997). These interactions are mediated by a diverse class of heterodimeric transmembrane receptors known as integrins (Giancotti and Ruoslahti, 1999). Integrin engagement with the ECM induces activation of multiple intracellular signal transduction pathways, involving protein phosphorylation, that regulate a variety of cellular functions (Lipscomb et al, 2005; Luo et al, 2007; Mahabeleshwar et al, 2007). Although many signalling enzymes are activated following integrin engagement, the signalling pathways involved in the metastatic spread of human scirrhous gastric carcinoma cells have not been defined.
One pathway that has been implicated in integrin-induced signal transduction is PI3K and Akt/PKB (Delcommenne et al, 1998; Engelman, 2009). Phosphatidyl inositol 3-kinases are a family of lipid kinases that propagate intracellular signalling cascades regulating a wide range of cellular processes, including metabolism, growth, survival, and motility (Samuels et al, 2004; Courtney et al, 2010). There are three classes of PI3Ks grouped according to structure and function. Class IA PI3K, which is the one most clearly implicated in human cancer, consists of a regulatory subunit (p85, p55, or p50) and a catalytic subunit (p110). Two mammalian genes, PIK3CA and PIK3R1, encoding p110α and p85α, respectively, are somatically mutated in cancers, and these mutations promote activation of the PI3K pathway (Katso et al, 2001; Samuels et al, 2004; Courtney et al, 2010). Phosphatidyl inositol 3-kinase phosphorylates the 3′-OH group on phosphatidylinositols in the plasma membrane. This leads to recruitment of the protein Ser/Thr-kinase, Akt and other targets of this pathway, to the cell membrane, where it becomes activated (Cantley, 2002; Vivanco and Sawyers, 2002; Engelman, 2009; Vasudevan et al, 2009). Akt/PKB, originally identified by its homology to protein kinases A and C, is the best characterised effector kinase within the PI3K cascade and a mediator of PI3K signalling (Vivanco and Sawyers, 2002; Manning and Cantley, 2007). PI3K/Akt cascade transmits signals from ligand-stimulated receptor tyrosine kinase to effector molecules that control metabolism, proliferation, survival, and motility (Vivanco and Sawyers, 2002). PI3K/Akt pathway has also been shown in several cell types to be activated by ligands to cell surface integrins. Thus, PI3K/Akt signalling might contribute to cancer cell progression through its effects on integrin signalling in scirrhous gastric carcinoma. With the development of inhibitors targeting the PI3K pathways, it will be pivotal to elucidate the role of PI3K/Akt dependent and independent pathways in cancer progression in specific cancer types.
The cell line OCUM-2MD3, which was established from a human scirrhous gastric tumour, readily develops peritoneal metastases with bloody ascites after peritoneal inoculation in immunodeficient mice (Yashiro et al, 1996b). OCUM-2MD3 cells displayed higher surface expression of the α2β1- and α3β1-integrin, exhibited ECM-mediated adhesion and invasion (Nishimura et al, 1996). Whether PI3K/Akt pathway mediates adhesion and spreading, and in vivo metastasis of scirrhous gastric carcinoma is the focus of the present report.
Materials and methods
Cell line and cell culture
An extensively peritoneal-seeding human scirrhous gastric cancer cell line, OCUM-2MD3, and parental cell line, OCUM-2M, were used (Yashiro et al, 1996b). Other scirrhous gastric carcinoma cell line, OCUM-12, was provided by Dr Kosei Hirakawa, Osaka City University, Osaka, Japan. MKN-45(poorly differentiated gastric carcinoma cell line) and MKN-74 (well-differentiated gastric carcinoma cell line) were obtained from JCRB cell bank (Osaka, Japan). The cell lines were maintained in Dulbecco’s modified Eagle medium (DMEM) (Life Technologies, Grand Island, NY, USA), supplemented with 10% heat-inactivated fetal calf serum at 5% CO2.
Antibodies and reagents
Type IV collagen and matrigel were purchased from Becton Dickinson. Bovine serum albumin ((BSA (fraction V)) was from ICN. LY294002, wortmannin (PI3K inhibitor), PD98059 (extracellular signal-regulated kinase (ERK) inhibitor), and calphostin C (protein kinase C (PKC) inhibitor) were from Cabiochem. Protease inhibitors were from Sigma or Roche Molecular Biochemicals. Dimethyl pimelimidate (DMP) was from Pierce. Primary antibodies were from Upstate Biotechnology (anti-p85, anti-phospho Src, and anti-vinculin). Anti-phosphorylated Akt Ser472/473/474, anti-total Akt, anti-phospho paxillin and anti-phospho FAK were from New England Biolabs, and anti-paxillin and anti-actin was from Santa Cruz Biotechnology, Inc. (anti-paxillin and anti-actin).
Plasmids and transfection
To obtain cell lines bearing the wild-type (Wp85) or mutant (Δp85) regulatory subunit of PI3K, OCUM-2M, and OCUM-2MD3 cells were transfected with 10 μg of HA-tagged Wp85 or Δp85 expression plasmids (under the control of the SR promoter; kindly provided by Dr Wataru Ogawa, Kobe University, Kobe, Japan) (Hara et al, 1994; Kotani et al, 1995), using Lipofectamine (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The empty vector was used as a control. Stable transfectants were then selected by incubation with geneticin (G418; Life Technologies) and subcloned by limiting dilution. All transfectants were maintained in medium containing 500 μg ml−1 geneticin. HA-tagged Wp85 and Δp85 proteins in the transfectants were detected by immunoblotting using mouse monoclonal anti-HA antibodies (Santa Cruz Biotechnology, Inc.).
Cell attachment and spreading assay
The attachment of cells was assessed by MTT assay. Tissue culture 96-well clusters were coated with 100 μl of type IV collagen (6.4 μg ml−1), polylysine (32 μg ml−1), or 2% BSA for 2 h at room temperature. Cells were preincubated in suspension for 30 min with wortmannin, and for 1 h with other inhibitors. Then OCUM-2MD3 cells, and the other scirrhous gastric carcinoma cell lines OCUM-12, MKN-45, and MKN-74 were suspended in serum-free DMEM, placed on coated plates at 7.5 × 104 cells per well and incubated at 37 °C for 30 min. The results were normalised, assuming the adhesion to polylysine-represented attachment of 100% of the added cells. Cell spreading was determined as follows. OCUM-2MD3 cells were detached and resuspended to 1 × 105 ml−1 in warm DMEM with DMSO or PI3K inhibitors. Aliquots of cell suspensions (100 μl) were added to substrate-coated wells and incubated for 75 min at 37 °C. Cells were fixed by the addition of 100 μl of 6% (w/v) glutaraldehyde. Wells were then aspirated, and CMF-PBS, containing 0.02% (w/v) sodium azide, was added until an inverted meniscus was formed at the top of each well. The percentage of cells spread in each well was determined using phase-contrast microscopy.
Immunoblotting and immunoprecipitation
Immunoblotting and immunoprecipitation were performed as described previously (Schneider et al, 1982; Nakatani et al, 1999). Briefly, OCUM-2M and 2MD3 cells were seeded on 60 mm dishes coated with type IV collagen (6.4 μg ml−1), or polylysine (32 μg ml−1) at the same concentrations as in the adhesion assay and allowed to attach at 37 °C for the indicated times. Proteins were subjected to SDS–PAGE and transferred to polyvinylidene difluoride membrane. The membrane was blocked and incubated with respective antibody, followed by incubation with secondary antibody. The specific proteins were detected with enhanced chemiluminescence. When using LY294002, cells were pretreated with LY294002 or DMSO for 1 h and then treated with type IV collagen in serum-free medium for 1 h. For immunoprecipitations, 500 μg of total protein were incubated with antibodies to anti-p85 at room temperature for 2 h, followed by 1 h incubation with protein A-Sepharose beads (Upstate Biotechnology). After 3 washes in lysis buffer, the immunocomplexes were resolved by SDS–PAGE, and analysed by immunoblotting with anti-phospho FAK (1 : 1000), anti-phospho Src (1 : 1000), anti-phospho paxillin (1 : 500), or anti-vinculin (1 : 1000), using the chemiluminescence system for detection. In some experiments, equal amounts of lysates were analysed directly by immunoblotting.
In vivo metastatic model
Mice were maintained in microisolator cages in a pathogen-free isolation facility and studies were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. For experimental metastasis, OCUM-2MD3 cells expressing Wp85, Δp85, or empty vector were inoculated intraperitoneally into 4-week-old female athymic nude mice (NCr-nu/nu), obtained from Charles River Laboratories (Portage, MI, USA). To obtain the stable transfected cell lines, wild type and mutant p85-expressed OCUM-2MD3 cells were selected and subcloned. Experimental metastasis was assayed, as described previously, with some modifications (Yashiro et al, 1996b). Briefly, the cells were harvested from subconfluent cultures and collected by centrifugation, washed once, and resuspended in serum-free DMEM at 1 × 107 cells ml−1. Mice were injected intraperitoneally, with OCUM-2MD3 transfectants, 2 × 106 cells in 200 μl, and observed for an additional 4 weeks. The mice were weighed at weekly intervals. For in vivo treatment with LY294002, the mice (n=5 per group) were randomised into PBS-, DMSO-, and LY294002-treated groups. OCUM-2MD3 cells were pretreated for 1 h with 20 μmol l−1 of LY294002, PBS containing 8% (v/v) DMSO, or PBS only. Cells (2 × 106) were injected intraperitoneally in nude mice, and subsequently the mice were given 25 mg kg−1 or 50 mg kg−1 of LY294002 3 times a week for 2 weeks. Mice were killed 2 and 4 weeks after peritoneal injection in the dose of 25 mg kg−1 treatment group, and 4 weeks in 50 mg kg−1 treatment group. At necropsy, body weights were recorded and the extent of macroscopic peritoneal metastasis was assessed. The maximum diameters (L) and perpendicular diameters (W) of metastatic nodules were measured with a vernier caliper. The volume of nodules was calculated by the formula:
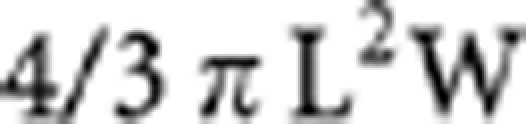 |
Statistical analysis
The data were analysed using Student’s t-test. A P-value of less than 0.05 was considered statistically significant.
Results
Signalling through PI3K/Akt pathway is necessary for the attachment and spreading of metastatic gastric cancer cells to type IV collagen
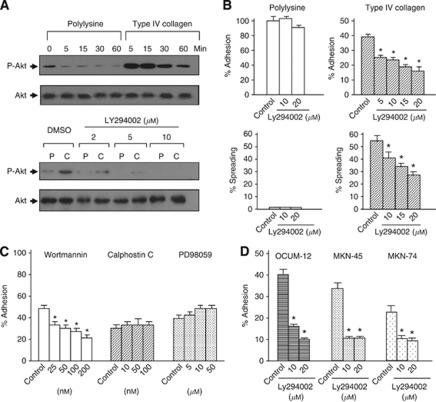
We examined whole-cell extracts from type IV collagen, which is primary collagen component of the basement membrane, attached human scirrhous gastric carcinoma cells for PI3K/Akt activity. Attachment of OCUM-2MD3 cells to type IV collagen increased Akt activation within 5 min of treatment, and was sustained for 30 min (Figure 1A). In contrast, Akt showed only minimal phosphorylation even 30 min after cell adhesion to polylysine, which occurs by nonspecific binding. Adhesive ability of OCUM-2MD3 cells to ECM was increased compared with their parental cell line, OCUM-2M (Nishimura et al, 1996; Yashiro et al, 1996a). The flavonoid derivative, LY294002, is a dual PI3KmTOR inhibitor (Gharbi et al, 2007). Here, we asked whether LY294002 influenced gastric cancer cell adhesion to ECM. As shown in Figure 1B, 10 μℳ LY294002 was sufficient to completely block the activation of PI3K/Akt pathway, as judged by immunoblotting with antibody to the phosphorylated form of Akt. We found that LY294002-treated OCUM-2MD3 cells decreased adhesion to type IV collagen in a dose-dependent manner (Figure 1B). Growth assay showed no influence on the proliferation by LY294002 treatment within 20 μℳ (data not shown), suggesting that reduced adhesion was not caused by cytotoxicity. In contrast, attachment of OCUM-2MD3 cells to polylysine was not altered by treatment with LY294002, suggesting that activity of the PI3K/Akt pathway is required for the attachment of OCUM-2MD3 cells to type IV collagen.
Figure 1.
PI3K/Akt pathway is necessary for the attachment and spreading of metastatic gastric cancer cells to type IV collagen. (A) Upper panel: attachment of cells to type IV collagen induced Akt/PKB phosphorylation shown by immunoblotting. Control is shown on the lower panel with polyclonal antibody to AKT (0.5 μg ml−1). Lower panel: pretreatment with LY294002 attenuated Akt/PKB phosphorylation following attachment of cells to type IV collagen. (B) Effect of LY294002 on the attachment and spreading of OCUM-2MD3 cells to type IV collagen. (C) Adhesion of OCUM-2MD3 cells to type IV collagen was performed in the presence of PI3K inhibitor (wortmannin), PKC inhibitor (calphostin C), and MAPK inhibitor (PD98059). OCUM-2MD3 cells were pretreated with/without inhibitors for 30 minutes with wortmannin, 60 min with calphostin C and PD98059, respectively. (D) OCUM-12, MKN-45, and MKN-74 cells were treated with DMSO (conrol) or 20 μℳ LY294002. Adhesion to Type IV collagen-coated membranes was assessed after 30 min. *P<0.01 vs control. Values shown are mean±s.d. (n=4).
Next, we examined the effect of wortmannin, another kind of PI3K inhibitor. From 25 to 200 nℳ wortmannin significantly reduced the attachment of OCUM-2MD3 cells to type IV collagen (Figure 1C). To further examine the specificity of the role of PI3K/Akt pathway in the attachment of gastric carcinoma cells, we tested whether inhibition of PKC and ERK activity, other major signalling pathways regulating tumour progression (Boulton et al, 1991; Palmantier et al, 1996), with a specific PKC inhibitor, calphostin C, and ERK inhibitor, PD98509, respectively, altered the binding ability of OCUM-2MD3 cells to type IV collagen. Neither inhibitor influenced the adhesion of OCUM-2MD3 cells. LY294002 also decreased adhesion of other scirrhous gastric carcinoma cells, OCUM-12, MKN-45, and MKN-74 to type IV collagen-coated membranes (Figure 1D).
The spreading ability of OCUM-2MD3 cells to type IV collagen, with or without treatment of LY294002, was assessed. As shown in Figure 1B and Supplementary Figure 1, 10 μℳ LY294002 significantly impaired the spreading of OCUM-2MD3 cells to type IV collagen. In contrast, most cells attached to polylysine did not spread within 75 min incubation and LY294002 did not affect spreading ability on polylysine, suggesting that spreading of OCUM-2MD3 cells is dependent on an integrin signalling pathway, and that PI3/Akt activity is required for spreading of gastric carcinoma cells.
PI3K/Akt pathway has an important role in the acquisition of metastatic phenotype
Recent studies have described that p85/PI3K was closely related with gastric carcinoma progression (Liu et al, 2010; Michl and Downward, 2005). In line with this, the level of expression of the PI3K p85 regulatory subunit in OCUM-2MD3 cells was compared with that in its parental cell line, OCUM-2M which has low metastatic ability. Interestingly, p85 expression was markedly higher in metastatic cell line than in the non-metastatic cells (Supplementary Figure 2A). We next compared the Akt activity between OCUM-2M and OCUM-2MD3 cell lines. We did not detect the Akt activation by the OCUM-2M attachment to type IV collagen, although even suspended OCUM-2MD3 cells displayed phosphorylated Akt (Supplementary Figure 2B). However, the expression of total Akt protein was not different between the two cell lines. These results indicate that increased p85 expression may contribute to enhanced activity of Akt and may be required for cells to acquire metastatic phenotype including high adhesive capacity.
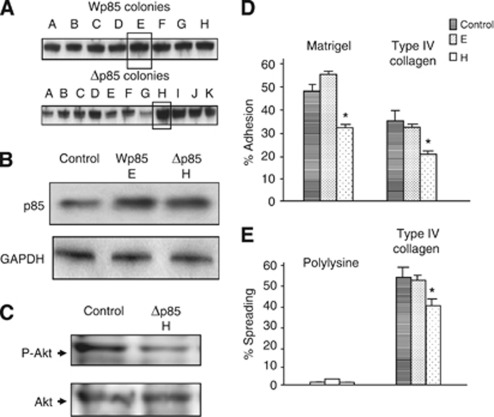
Gastric carcinoma cells transfected with the mutant regulatory subunit of PI3K display reduced adhesion and Akt phosphorylation on type IV collagen
LY294002 and wortmannin are not as specific as we would like, so, to further elucidate the role of PI3K, we established transient subclones from OCUM-2MD3 cells by transfection with the wild-type (Wp85) or mutant (Δp85) regulatory subunit of bovine PI3K. Δp85, a mutant regulatory subunit of PI3K lacking the binding site to the catalytic subunit, was utilised as a dominant negative p85. In these cells, expression of HA-tagged Wp85 and Δp85 proteins in transfectants was detected by immunoblotting (Figures 2A and B). To explore whether these clones alter PI3K signalling activity, the activation of Akt was examined. Although total protein volume was similar, phosphorylation of Akt was decreased in Δp85-transfected OCUM-2MD3 cells compared with cells carrying the empty vector by attachment to type IV collagen for 30 min, as judged by immunoblotting (Figure 2C). Subsequently, we observed that when plated on matrigel and type IV collagen-coated dishes, adhesion of Δp85-transfected OCUM-2MD3 cells was significantly reduced, compared with cells transfected with the empty vector, which was consistent with the result using PI3K inhibitors. Wp85-transfected cells did not show a significant alteration of adhesion (Figure 2D). To examine the spreading ability of these transfected cells, Δp85-transfected cells were seeded onto type IV collagen-coated dishes. Decreased spreading onto type IV collagen was observed when Δp85 was expressed in OCUM-2MD3 cells (Figure 2E). These results suggest that PI3K/Akt pathway may have an important role for attachment of gastric carcinoma cells to ECM.
Figure 2.
(A) Resistant OCUM2MD3 cells overexpressing p85 proteins were screened by immunoblotting with anti-p85alpha antibodies and subcloned by penicillin-cup methods. Clones selected for use in other experiments are shown by a box around the bands on the blot. (B) Expression of cells transfected with empty vector (control), Wp85, and Δp85 by immunoblotting. (C) The activation of Akt of cells with empty vector (control), Δp85 by immunoblotting after attachment to type IV collagen for 30 min. Control is shown on the lower panel with polyclonal antibody to AKT. (D) Adhesiveness of OCUM-2MD3 cells in transfectants with Wp85, Δp85, or the empty vector. (E) The transfectants were investigated for spreading activities. The transfected cells were examined for in vitro adhesion activities as in this figure. *P<0.01 vs control. Values shown are mean±s.d. (n=4).
PI3K associates with focal adhesion proteins related to integrin-dependent signalling
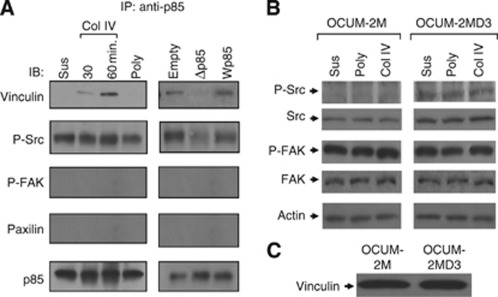
To identify the possible interactions between PI3K and integrin signalling pathways during gastric carcinoma cell adhesion to ECM, we examined whether the proteins involved in integrin signalling were associated with p85. As shown in Figure 3A, vinculin was detected in p85 immunoprecipitates from OCUM-2MD3 cells attached to type IV collagen for 30 or 60 min, but no vinculin was found in precipitates from cells attached to plastic or polylysine. Coimmunoprecipitation of phosphorylated Src with p85 also occurred in extracts from any attached cells and was not altered by attachment to type IV collagen. Attachment to type IV collagen did not induce the coimmunoprecipitation of p85 and either focal adhesion kinase or paxillin (Figure 3A).
Figure 3.
(A) PI3K associates with focal adhesion proteins related to integrin-dependent signalling. Left panel: after attachment to type IV collagen and polylysine, OCUM-2MD3 cells were solubilised with lysis buffer as described in Material and Methods. The lysates were immunoprecipitated with the anti-p85 antibody. Equivalent precipitation of p85 was confirmed and is shown in the lower panel. Right panel: modulation of the association of integrin-associated proteins by p85-transfected OCUM-2MD3 subclones. After attachment to type IV collagen for 60 min, OCUM-2MD3 cells carrying Wp85, Δp85, or empty vector were solubilised with lysis buffer, as described in Materials and Methods. (B) Expression of integrin-related proteins in OCUM-2M and -2MD3 cells. Equivalent volume of protein samples was confirmed and was shown in the lower panel. (C) Vinculin expression in OCUM-2M and -2MD3 cells by immunoblotting.
To explore in more detail, we also examined the extracts for p85-associated proteins using p85-transfected OCUM-2MD3 cells. The level of vinculin in the p85 immunoprecipitates was increased in Wp85-transfected cells and decreased in Δp85-transfected cells compared with that in cells carrying the empty vector (Figure 3A), suggesting that p85 activity is closely related with vinculin. In contrast, the expression of coimmunoprecipitated Src was decreased weakly in dominant negative p85-expressed cells and had no change in wild-type p85-expressed cells, compared with that of empty vector-expressed cells. We did not detect coimmunoprecipitation of focal adhesion kinase (FAK) and paxillin in all three kinds of transfected cells (Figure 3A).
Src expression is increased in metastatic cell lines
Observations that Src was constitutively expressed in metastatic carcinoma cells prompted us to investigate whether this expression pattern would change in non-metastatic cells. Interestingly, we found that Src expression was increased in OCUM-2MD3 cells compared with that in OCUM-2M cells (Figure 3B). FAK activity was also constitutively found in OCUM-2MD3 cell lines adhered to plastic dishes, poylysine, and type IV collagen. However, FAK activity was not altered between these two cell lines. These results led us to investigate the expression of vinculin. As shown in Figure 3C, there was no difference in the expression of vinculin between these cell lines.
Inhibition of the PI3K/Akt pathway reduced peritoneal metastasis in nude mice
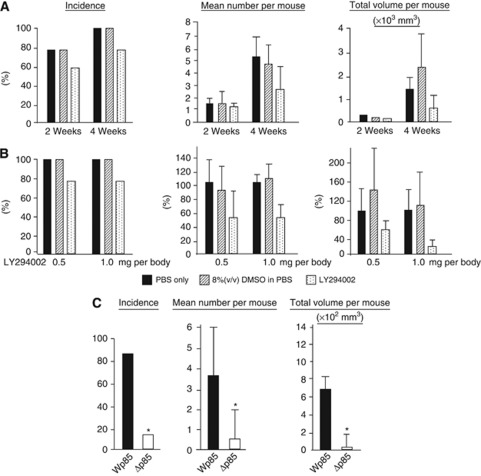
The results of mice treated with LY294002, DMSO, or PBS, respectively, are summarised in Table 1 and Figure 4A. During the experiment, LY294002-treated mice did not show any sign of general toxicity, including loss of weight or skin lesions associated with high doses of the compound (Hu et al, 2002). Peritoneal metastases were recognised as white nodules in the peritoneal cavity. With respect to the incidence of metastatic nodules, frequency of metastasis in LY294002-treated group was decreased compared with PBS-treated group, DMSO-treated group, but difference was not statistically significant among the 3 groups both in groups treated for 2 and 4 weeks. Whereas, the total volume of metastatic nodules was significantly suppressed in LY294002-treated groups compared with PBS only and 8% (v/v) DMSO in PBS-treated ones (P<0.05).
Table 1. Effect of PI3K inhibitor on grossly visible peritoneal metastatic nodules from OCUM-2MD3 human gastric cancer cells injected into nude mice.
|
2 weeks
|
4 weeks
|
|||||
|---|---|---|---|---|---|---|
| Treated time | PBS | DMSO | LY294002 25 mg per body | PBS | DMSO | LY294002 25 mg per body |
| Incidence | 4/5 (80%) | 4/5 (80%) | 3/5 (60%) | 5/5 (100%) | 5/5 (100%) | 4/5 (80%) |
| Mean no. per mouse | 1.4±0.4a | 1.6±0.4a | 1.1±0.2a | 5.6±0.9a | 4.4±0.9a | 2.8±0.9a |
| Total volume per mouse (mm3) | 183±57a | 107±31a | 78±34a | 1482±289a | 2193±904a | 693±307a,b |
| Body weight | 17.2±0.9a | 16.9±1.0a | 17.8±1.0a | 16.2±1.0a | 17.1±1.2a | 17.1±0.8a |
Values are means±s.e.m.
Significantly different from DMSO group, P<0.05.
Figure 4.
Inhibition of the PI3K/Akt pathway reduced peritoneal metastasis in nude mice. (A) The frequency of metastasis, number and total volume of nodules/mouse were identified. Upper lane: after cells were injected 2 weeks and 4 weeks with the treatment of 25 mg kg−1 LY294002. Lower lane: after cells were injected 4 weeks with the treatment of 25 mg kg−1 and 50 mg kg−1 of LY294002. *P<0.05 vs control. (DMSO treatment). Values shown are mean±s.d. (n=5). (B) Effect of p85 expression on incidence of grossly visible peritoneal metastatic nodules. *P<0.05 vs Wp85-transfected subclones. Values shown are mean±s.d. (n=10).
These data led us to hypothesise that the inhibition of PI3K might not be complete, given the use of low doses of LY294002, and that a more complete inhibition of PI3K activity might yield more effective inhibition of metastasis. Thus, we tested the p85 transfectants of OCUM-2MD3 cells for peritoneal metastatic ability (Table 2 and Figure 4B). Peritoneal dissemination was markedly less frequent in mice inoculated with cells expressing Δp85 (40.0%) than with cells expressing Wp85 (80.0%). Furthermore, the number and total volume of tumours, per mouse, were also significantly less in Wp85-transfected cells (3.4±1.2 tumours per mouse, and 446±287, respectively) compared with Δp85-transfected cells (1.5±1.0, and 34±12, respectively) (P<0.05).
Table 2. Effect of p85 expression on incidence of grossly visible peritoneal metastatic nodules from OCUM-2MD3 human gastric cancer of nude mice.
| Metastasis | Wp85 | Δp85 |
|---|---|---|
| Incidence | 8/10 (80%) | 4/10 (40%) |
| Mean no. per mouse | 3.4±1.2a | 1.5±1.0a,b |
| Total volume per mouse (mm3) | 446±287a | 34±12a,b |
| Body weight | 18.3±1.5a | 16.7±0.5a |
Values are means±s.e.m.
Significantly different from Wp85 group, P<0.05.
Discussion
In this study, we demonstrated that engagement of type IV collagen receptors in OCUM-2MD3 cells resulted in the phosphorylation of Akt. This Akt phosphorylation seems to be specific for integrin-dependent adhesion, because attachment of OCUM-2MD3 cells to polylysine did not induce Akt phosphorylation. In contrast, we observed no phosphorylation of Akt in OCUM-2M cells, and attachment of OCUM-2M cells to ECM did not lead to an increase in phosphorylation of Akt (Supplementary Figure 1B). These data suggest that increased phosphorylation of Akt in OCUM-2MD3 cells may be a specific property of the metastatic form of these cells. Although previous reports (Hippo, 2001; #1508) have performed global analysis on differential gene expression of OCUM-2M and OCUM-2MD3 cells, PI3K/Akt pathway has never been the target of genetic differences between two cell lines. The binding ability of OCUM-2MD3 cells to type IV collagen was significantly inhibited by PI3K inhibitors LY294002 and wortmannin, but not by a PKC inhibitor, or an ERK inhibitor. These results indicate that PI3K/Akt pathway may be a specific mediator of integrin-dependent cell attachment in OCUM-2MD3 cells. To confirm that the response to PI3K inhibitor is common in gastric carcinoma cells, we next sought to address whether other gastric carcinoma cell lines had the similar response to LY294002. Interestingly, three cell lines, OCUM-12, MKN-45, and MKN-74, treated with LY294002 showed a significant impairment of adhesive ability, indicating that effect of PI3K inhibitor might be common in gastric carcinoma. Our current data also showed that LY294002 reduced the spreading ability of OCUM-2MD3. Cell spreading is dependent on cytoskeletal organisation and focal adhesion complex formation in response to the engagement of integrins to ECM. These data suggest that the PI3K/Akt pathway would be a candidate of signalling component involved in the mechanism of cell spreading in gastric carcinoma cells. However, our data do not directly show how PI3K associates with cytoskeletal components, required for adhesion of gastric carcinoma cell, because these chemical components have some off-target effects and spreading, and cannot rule out the involvement of PI3K in integrin-independent signalling. To address this, we next established cell lines overexpressing the wild-type p85 and a mutant defective in P85 binding site.
Interestingly, p85 expression was markedly greater in extensively metastatic cell lines than in the parental cell lines (Supplementary Figure 2A). We showed that the P85 binding-deficient mutant reduced phosphorylation of Akt and cell adhesion to ECM substances. Wp85 expressing cells failed to further increase the adhesion ability, indicating that OCUM-3MD3 cell had already sufficient p85 activity for metastasis. The fact that the endogenous expression of p85 in OCUM-2M cells was much lower than that of OCUM-2MD3 cells prompted us to examine the adhesive property of p85-expressed OCUM-2M cells. Wp85 expression induced increased adhesion of OCUM-2M cells (data not shown). These results suggest that PI3K activation may be one of an important step in the acquisition of properties consistent with the metastatic ability.
The observation that PI3K activity was associated with integrin signalling led us to investigate which proteins, consisting the integrin signalling, might be a target of PI3K. Focal adhesion kinase and Src are two of the most important members of focal adhesion complexes (Chen and Guan, 1994; Giancotti and Ruoslahti, 1999; Reiske et al, 1999; Mitra and Schlaepfer, 2006). First, we examined the expression of FAK in immunoprecipitates of p85. However, we did not detect any interaction of FAK and PI3K in p85 immunoprecipitates, indicating that FAK activation was likely to be independent of PI3K regulation for adhesion of gastric carcinoma cell lines. Several reports demonstrated that PI3K facilitated FAK-promoted cell migration (Reiske et al, 1999; Sieg et al, 1999). And we showed FAK phosphorylation was detected in suspended OCUM-2MD3 cells and not affected by cell attachment to type IV collagen (Figure 3B). When cells were kept in suspension in serum-free media, dephosphorylation of FAK was observed within 1 h (data not shown), indicating that FAK activity may be constitutively induced in harvested OCUM-2MD3 cells by various stimuli, such as growth factor or cytokines. Kahana et al (2002) reported that dysregulation of the FAK contributed to the malignancy of melanoma cells. So introduction of constitutively active FAK may lead to transformation, and the suppression of apoptosis of scirrhous gastric carcinoma. Immunoprecipitation results also indicated that a signalling complex containing at least PI3K, Src, and vinculin was formed after OCUM-2MD3 cell attachment to ECM. To the best of our knowledge, it is the novel signalling combination identified in cell adhesion and spreading process in human gastric carcinoma. Attachment to type IV collagen induced this interaction in a time-dependent manner, suggesting that this interaction is integrin dependent. Increased expression of vinculin in the p85 immunoprecipitates within 1 h indicates that vinculin is a downstream target of PI3K, because induced phosphorylation of Akt occurred within 5 min. Already there is evidence that vinculin has a critical role in cytoskeletal rearrangement (Ezzell et al, 1997; Xu et al, 1998; Bakolitsa et al, 2004). Vinculin is believed to negatively regulate cell motility, as vinculin (−/−) cells are more motile and show higher levels of focal adhesion kinase, p130Cas and paxillin (Xu et al, 1998). This observation is consistent with our data, because p85 was not associated with paxillin in OCUM-2MD3 cells, indicating that spreading of cells modulated by PI3K through integrin signalling is dependent on vinculin, but not paxillin. Src kinase has many cellular functions such as growth factor-induced proliferation, invasion, and protection from apoptosis (Brown and Cooper, 1996; Chiang et al, 2005). In OCUM-2MD3 cells, constitutive activation of Src may cause usual interaction of PI3K, and these activations and associations may be both necessary for cell adhesion to ECM, but Src contribution may be partial in OCUM-2MD3 cell adhesion, because specific Src inhibitor, PP2, significantly inhibited the adhesion of OCUM-2MD3 cells to type IV collagen, but less compared with PI3K inhibitor (Supplementary Figure 3B). Recent studies have been described that activity of Src kinase was required for integrin-matrix adhesion (Kaplan et al, 1995; Li et al, 2002). Kaplan et al reported that Src may serve as an adaptor protein to localise specific proteins to adhesive structures, involved in cell adhesion, which raise the possibility that Src has a role as an adaptor protein to localise PI3K in OCUM-2MD3 cells. Interestingly, the expression of p85 and Src in OCUM-2MD3 cells were higher than in OCUM-2M (Figure 3B and Supplementary Figure 2A), indicating that p85 and Src activity may have an important role in acquisition of metastatic potential of this cell lines. We also suggest the evidence that activity of Akt was increased in metastatic cell lines, which was not shown in non-metastatic cells (Figure 3B) indicates that Akt/PKB pathway may contribute to form metastasis in peritoneal cavity through PI3K by another biological function, the most likely candidate being cell survival (Engelman, 2009).
These observations led us to investigate whether inhibition of PI3K could contribute to therapy for scirrhous gastric carcinoma, because several PI3K pathway inhibitors have been developed and are being evaluated in preclinical studies and in early clinical trials (Courtney et al, 2010). In the experimental metastatic model, LY294002 reduced total volume of metastatic nodule per mouse of OCUM-2MD in peritoneal cavity as well as in vitro study. And high-dose administration of LY294002 contributed only the total volume of metastatic nodules per mice, whereas the effect on the incidence of metastasis and number of nodules per mouse did not show any significant change. There have been some reports on experiments using LY294002 in vivo model (Hu et al, 2002; Nakanishi et al, 2002). Accordingly, inhibition of PI3K by LY294002 has recently been shown to sensitise ovarian cancer cells to chemotherapy-induced apoptosis (Hu et al, 2002) and suppress colony formation and haematogenous metastasis of hepatic carcinoma cells (Nakanishi et al, 2002). These findings led us to propose that in vivo condition, inhibition of PI3K activity, may not only impair the adhesion of gastric carcinoma cells but also block the apoptosis and growth factor signalling pathway (Dudek et al, 1997; Engelman, 2009). Our results from these early studies indicated that PI3K inhibitor administration might prevent the peritoneal metastasis, but it is likely that treatment with LY294002 was insufficient to use alone. These findings gave us a hint to attempt a genetic approach. Stable Wp85 and Δp85 transfectants were administrated intraperitoneally in nude mice. In particular, Δp85 transfection dramatically reduced the incidence of peritoneal metastasis compared with Wp85, and this effect was markedly greater than the treatment with LY294002. The important finding may be the expression change of p85 showed the potential alteration of frequency of metastasis. The one probable explanation for this difference may be that constitutive suppression of p85 activity might be more effective on the treatment of peritoneal metastasis by inhibiting the biological response mediated by PI3K, highly suggesting that gene therapy with dominant negative p85 is a possible target for treatment of peritoneal metastasis of gastric carcinoma; otherwise, continuous administration of PI3K inhibitor might be effective. Our data suggest that PI3K/Akt signalling mediates attachment and spreading of scirrhous gastric carcinoma cells on ECM by associating with integrin signalling through Src and vinculin, and have an important role in acquisition of metastatic property. Inhibition of this activity contributed to a decrease in peritoneal metastasis. These findings would be translated into generating better strategies to optimise their use in cancer clinical trials.
Acknowledgments
We thank Dr Wataru Ogawa for providing wild-type and mutant p85 expression plasmids under the control of the SR promoter. We thank Dr Steven K. Akiyama for helpful discussions, and Dr Ron Cannon and Dr Richard DiAugustine of NIEHS for a careful review of the manuscript. This work was supported by the Intramural Research Program of the NIH and NIEHS.
Footnotes
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Bakolitsa C, Cohen DM, Bankston LA, Bobkov AA, Cadwell GW, Jennings L, Critchley DR, Craig SW, Liddington RC (2004) Structural basis for vinculin activation at sites of cell adhesion. Nature 430: 583–586 [DOI] [PubMed] [Google Scholar]
- Boulton TG, Nye SH, Robbins DJ, Ip NY, Radziejewska E, Morgenbesser SD, DePinho RA, Panayotatos N, Cobb MH, Yancopoulos GD (1991) ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell 65: 663–675 [DOI] [PubMed] [Google Scholar]
- Brown MT, Cooper JA (1996) Regulation, substrates and functions of src. Biochim Biophys Acta 1287: 121–149 [DOI] [PubMed] [Google Scholar]
- Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296: 1655–1657 [DOI] [PubMed] [Google Scholar]
- Chen HC, Guan JL (1994) Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA 91: 10148–10152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang GJ, Billmeyer BR, Canes D, Stoffel J, Moinzadeh A, Austin CA, Kosakowski M, Rieger-Christ KM, Libertino JA, Summerhayes IC (2005) The src-family kinase inhibitor PP2 suppresses the in vitro invasive phenotype of bladder carcinoma cells via modulation of Akt. BJU Int 96: 416–422 [DOI] [PubMed] [Google Scholar]
- Courtney KD, Corcoran RB, Engelman JA (2010) The PI3K pathway as drug target in human cancer. J Clin Oncol 28: 1075–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S (1998) Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA 95: 11211–11216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME (1997) Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 275: 661–665 [DOI] [PubMed] [Google Scholar]
- Engelman JA (2009) Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 9: 550–562 [DOI] [PubMed] [Google Scholar]
- Ezzell RM, Goldmann WH, Wang N, Parasharama N, Ingber DE (1997) Vinculin promotes cell spreading by mechanically coupling integrins to the cytoskeleton. Exp Cell Res 231: 14–26 [DOI] [PubMed] [Google Scholar]
- Fidler IJ (1991) Cancer metastasis. Br Med Bull 47: 157–177 [DOI] [PubMed] [Google Scholar]
- Gharbi SI, Zvelebil MJ, Shuttleworth SJ, Hancox T, Saghir N, Timms JF, Waterfield MD (2007) Exploring the specificity of the PI3K family inhibitor LY294002. Biochem J 404: 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E (1999) Integrin signaling. Science 285: 1028–1032 [DOI] [PubMed] [Google Scholar]
- Hara K, Yonezawa K, Sakaue H, Ando A, Kotani K, Kitamura T, Kitamura Y, Ueda H, Stephens L, Jackson TR, Hawkins PT, Dhand R, Clark AE, Holman GD, Waterfield MD, Kasuga M (1994) 1-Phosphatidylinositol 3-kinase activity is required for insulin- stimulated glucose transport but not for RAS activation in CHO cells. Proc Natl Acad Sci USA 91: 7415–7419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Hofmann J, Lu Y, Mills GB, Jaffe RB (2002) Inhibition of phosphatidylinositol 3'-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res 62: 1087–1092 [PubMed] [Google Scholar]
- Kahana O, Micksche M, Witz IP, Yron I (2002) The focal adhesion kinase (P125FAK) is constitutively active in human malignant melanoma. Oncogene 21: 3969–3977 [DOI] [PubMed] [Google Scholar]
- Kaplan KB, Swedlow JR, Morgan DO, Varmus HE (1995) c-Src enhances the spreading of src−/− fibroblasts on fibronectin by a kinase-independent mechanism. Genes Dev 9: 1505–1517 [DOI] [PubMed] [Google Scholar]
- Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD (2001) Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol 17: 615–675 [DOI] [PubMed] [Google Scholar]
- Kotani K, Hara K, Yonezawa K, Kasuga M (1995) Phosphoinositide 3-kinase as an upstream regulator of the small GTP- binding protein Rac in the insulin signaling of membrane ruffling. Biochem Biophys Res Commun 208: 985–990 [DOI] [PubMed] [Google Scholar]
- Li L, Okura M, Imamoto A (2002) Focal adhesions require catalytic activity of Src family kinases to mediate integrin-matrix adhesion. Mol Cell Biol 22: 1203–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb EA, Simpson KJ, Lyle SR, Ring JE, Dugan AS, Mercurio AM (2005) The alpha6beta4 integrin maintains the survival of human breast carcinoma cells in vivo. Cancer Res 65: 10970–10976 [DOI] [PubMed] [Google Scholar]
- Liu JF, Zhou XK, Chen JH, Yi G, Chen HG, Ba MC, Lin SQ, Qi YC (2010) Up-regulation of PIK3CA promotes metastasis in gastric carcinoma. World J Gastroenterol 16: 4986–4991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo BH, Carman CV, Springer TA (2007) Structural basis of integrin regulation and signaling. Ann Rev Immunol 25: 619–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabeleshwar GH, Feng W, Reddy K, Plow EF, Byzova TV (2007) Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ Res 101: 570–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129: 1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl P, Downward J (2005) Mechanisms of disease: PI3K/AKT signaling in gastrointestinal cancers. Z Gastroenterol 43: 1133–1139 [DOI] [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer DD (2006) Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol 18: 516–523 [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Sakamoto M, Yasuda J, Takamura M, Fujita N, Tsuruo T, Todo S, Hirohashi S (2002) Critical involvement of the phosphatidylinositol 3-kinase/Akt pathway in anchorage-independent growth and hematogeneous intrahepatic metastasis of liver cancer. Cancer Res 62: 2971–2975 [PubMed] [Google Scholar]
- Nakatani K, Sakaue H, Thompson DA, Weigel RJ, Roth RA (1999) Identification of a human Akt3 (protein kinase B gamma) which contains the regulatory serine phosphorylation site. Biochem Biophys Res Commun 257: 906–910 [DOI] [PubMed] [Google Scholar]
- Nishimura S, Chung YS, Yashiro M, Inoue T, Sowa M (1996) Role of alpha 2 beta 1- and alpha 3 beta 1-integrin in the peritoneal implantation of scirrhous gastric carcinoma. Br J Cancer 74: 1406–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmantier R, Roberts JD, Glasgow WC, Eling TE, Olden K (1996) Regulation of the adhesion of a human breast carcinoma cell line to type IV collagen and vitronectin: roles for lipoxygenase and protein kinase C. Cancer Res 56: 2206–2212 [PubMed] [Google Scholar]
- Reiske HR, Kao SC, Cary LA, Guan JL, Lai JF, Chen HC (1999) Requirement of phosphatidylinositol 3-kinase in focal adhesion kinase- promoted cell migration. J Biol Chem 274: 12361–12366 [DOI] [PubMed] [Google Scholar]
- Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE (2004) High frequency of mutations of the PIK3CA gene in human cancers. Science 304: 554. [DOI] [PubMed] [Google Scholar]
- Schneider C, Newman RA, Sutherland DR, Asser U, Greaves MF (1982) A one-step purification of membrane proteins using a high efficiency immunomatrix. J Biol Chem 257: 10766–10769 [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Schlaepfer DD (1999) Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci 112: 2677–2691 [DOI] [PubMed] [Google Scholar]
- Sowa M, Kato Y, Nishimura M, Yoshino H, Kubo T, Umeyama K (1989) Clinico-histochemical studies on type 4 carcinoma of the stomach – with special reference to mucopolysaccharides and sialic acid in tumor tissue. Jpn J Surg 19: 153–162 [DOI] [PubMed] [Google Scholar]
- Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ, Hennessy BT, Tseng H, Pochanard P, Kim SY, Dunn IF, Schinzel AC, Sandy P, Hoersch S, Sheng Q, Gupta PB, Boehm JS, Reiling JH, Silver S, Lu Y, Stemke-Hale K, Dutta B, Joy C, Sahin AA, Gonzalez-Angulo AM, Lluch A, Rameh LE, Jacks T, Root DE, Lander ES, Mills GB, Hahn WC, Sellers WR, Garraway LA (2009) AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell 16: 21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2: 489–501 [DOI] [PubMed] [Google Scholar]
- Xu W, Coll JL, Adamson ED (1998) Rescue of the mutant phenotype by reexpression of full-length vinculin in null F9 cells; effects on cell locomotion by domain deleted vinculin. J Cell Sci 111: 1535–1544 [DOI] [PubMed] [Google Scholar]
- Yamada KM, Geiger B (1997) Molecular interactions in cell adhesion complexes. Curr Opin Cell Biol 9: 76–85 [DOI] [PubMed] [Google Scholar]
- Yashiro M, Chung YS, Inoue T, Nishimura S, Matsuoka T, Fujihara T, Sowa M (1996a) Hepatocyte growth factor (HGF) produced by peritoneal fibroblasts may affect mesothelial cell morphology and promote peritoneal dissemination. Int J Cancer 67: 289–293 [DOI] [PubMed] [Google Scholar]
- Yashiro M, Chung YS, Nishimura S, Inoue T, Sowa M (1996b) Peritoneal metastatic model for human scirrhous gastric carcinoma in nude mice. Clin Exp Metastasis 14: 43–54 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.