Summary
This paper is a comprehensive review of hand burn injuries. The different classifications of thermal burns, out- and inpatient care, indications for escharotomies as well as surgical management, skin substitutes, and paediatric hand burns are thoroughly reviewed.
Keywords: burn, thermal injury, hand, treatment
Abstract
Les Auteurs, dans une analyse détaillée de tous les aspects des brûlures de la main, présentent les diverses classifications des lésions thermiques, les soins en régime hospitalier ou ambulatoire, les indications pour les escarrectomies, comme aussi la gestion chirurgicale, les substituts cutanés et les brûlures des mains en âge pédiatrique.
Introduction
Burn injury is a common form of trauma that often involves the upper extremity.1-4 Burns of the hand occur to a disproportionate degree compared with their distribution in body surface area.5 It occurs commonly in a young population of predominantly male victims from accidents sustained either in the workplace or at home6-9 and, as a result, functional outcome is very important for future productivity.3,10,11 Functional loss of the hands has been estimated to make up a 57% loss of function of an individual.12
Achieving optimal results depends almost entirely on the successful management in the acute or subacute phase.5,12 Although late reconstruction of a variety of dysfunctional states and altered anatomy are the subjects of numerous publications in the literature, the final outcome in these late problems is consistently far less than ideal, whereas the consequences are enormous. Over the last two decades there has been a marked improvement in survival rates following major thermal injuries with greater emphasis on morbidity and functional outcome.13,14 An increasing number of badly burned patients are surviving a lengthy hospitalization, multiple surgical procedures, and episodes of life-threatening sepsis. They can be left with a pair of poorly functional hands if little attention is directed towards the prioritization of the upper extremity burns. Little solace can be garnered from such survival if due diligence has not been given to the importance of the rehabilitation of the patient as a fully functional person. The rehabilitation period begins the moment the burn team encounters the patient.
The present is a review of thermal injury to the hand using available evidence from the literature. A Medline, Pubmed and Scopus database search was conducted to identify citations for articles relating thermal injury to the hands in humans, published between 1935 and 2011. The keywords used for the search included "burn", "thermal injury", "hand" and "treatment". Study references were also screened manually in order to identify potential citations not captured by the initial database search. Level of evidence of each article was not taken into account. Inclusion criteria included English-language articles dealing with humans, case reports, review and original articles. Exclusion criteria included reports of only successful cases, articles with unclear results, and guidelines.
Initial assessment
After the initial life-threatening problems of airway, breathing, and circulation have been managed, the evaluation and treatment of the burned extremity becomes necessary. Continuing with the advanced trauma life support (ATLS), algorithms D (Disability) and E (Expose, Examine) relate to the assessment of vascular perfusion in the upper extremity. The hand is scrubbed clear of any soot or dirt, and potentially constricting jewellery, bracelets, and watches are removed.
Triage: in- or out-patient treatment?
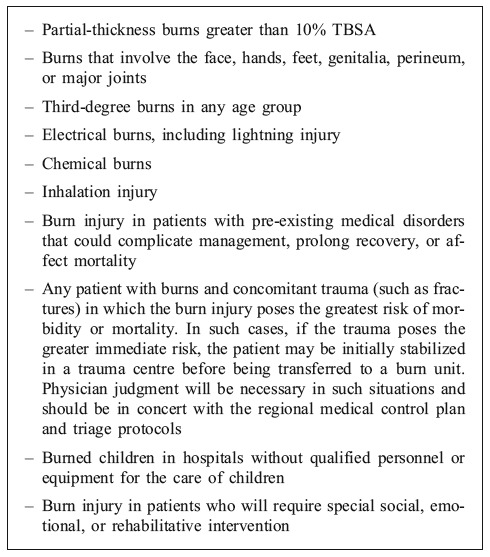
Most upper limb burns are self-treated in the home and do not present for medical care. Only 25% of burns require medical assistance15 and, of these, only a minority requires in-patient care. In a one-year study of 119 hospitals only 8% of the 11,759 patients with burn injuries required admission.2 Determination of whether to admit the patient to a burn centre or not depends on the burn injury, associated injuries, the patients, and their social circumstances.
Table I. American Burn Association burn treatment centre referral criteria16.
Classification of thermal injuries
It is notoriously difficult for even an experienced observer to estimate the actual depth of the initial burn accurately.17 The key feature in classifying burn injuries to the hand is the accurate determination of the depth of injury to the skin, which remains heavily reliant on the judgment of the experienced physician, despite the recognition that the accuracy of prediction is less than 50% based on a single time-point observation of the wounds.18,19,21 Conversely, other reports claim an accuracy of prediction up to 70%, the most common error being overestimation of depth. Therefore a complementary accurate history of the burn is critical and cannot be overstressed. The rationalization behind this is beyond the scope of this review.
Other modalities to estimate the burn depth include burn wound biopsy, ultrasonography, use of vital dyes such as indocyanine green, fluorescein, and methylene blue, laser Doppler flowmetry, thermography, light reflectance and more recently magnetic resonance imaging.18,19,21 Because of the numerous limitations of these techniques, serial assessment of the wounds on a daily basis until re-epithelialized to determine time to healing remains the standard of care, recognizing that the rates of epithelial healing are likely to be non-linear and resemble the logarithmic growth of epithelial cells in culture. This consideration explains the faster rate of healing in terminal phases of wound closure and can be important in predicting the time to healing, particularly in the paediatric age group.10
Admission photographs are taken using standard hand views, facilitated by the advent of digital computerized photography and rendering acquisition, transmission, storage as well as retrieval of images more efficient.22
Out-patient management
After cooling of the wound23 and administration of the appropriate analgesia, the burn wound should be cleaned and debrided of all contamination. Areas of peeled skin also need to be removed. The treatment of blisters remains controversial. Small intact blisters of less than 1 cm in diameter can be left intact and the wound allowed to heal spontaneously over approximately a 7-to-14 day period.24 In the past, large blisters were left intact as a biological dressing protective against infection.25 However, burn blister fluid is rich in inflammatory mediators that may propagate the burn wound injury, increasing the conversion to a zone of necrosis.26 Large blisters should therefore be aspirated or removed by incision, or the blister formally debrided. Dressings can then be applied directly to the burn wound base.
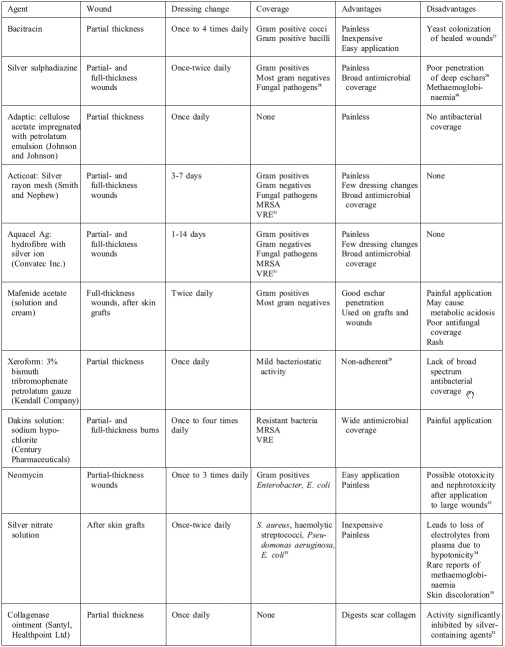
The overall goal of out-patient management is to prevent infection and maintain mobility. Superficial partialthickness burns with erythema only require topical moisturizing creams and immediate active mobilization. The dressing keeps the wound clean and reduces pain. Provided there is no increase in pain or discharge through the dressing suggesting infection, the same dressing can be left intact for 3-5 days.24
A deeper partial-thickness burn should be dressed with a topical antibiotic with more penetrating power such as silver sulphadiazine (SSD) applied twice daily to the wound and then held in place with gauze and bandages. This should be kept as light as possible to allow early active mobilization. Another alternative is to apply SSD to the hands and fingers and then place the hand in a glove. This allows almost unrestricted mobilization but causes maceration. Full-thickness burns will similarly require a topical antibiotic dressing. A topical antibiotic with good eschar penetration should be used.
Elevation is an important part of the acute management of the burned upper limb. Out-patients elevate a burnt upper limb on a pillow when asleep and use a high-arm sling during the day. This decreases oedema, pain, and analgesic requirements. Patients with minor upper limb burns should be educated by a dedicated hand therapist to carry out their own exercises with active exercises every 2 to 4 hours and gentle stretching during the day. Compliance should be monitored by the hand therapist to ensure that function does not become impaired with documentation of baseline measurements of hand function and joint movement. An out-patient hand therapy programme should be instigated involving active and gentle passive mobilization, as well as ongoing measurements to monitor progress.
Patients should be warned of the signs of incipient infection and told to report immediately if they become apparent. A patient who was borderline between in- and outpatient care may need to be followed up on a daily basis in order to prevent complications which compromise care, such as infection. Out-patient hand burns should ideally be reviewed on the second day of treatment. Superficial partial thickness burns can be treated with dressings, hand therapy, and can subsequently be seen at up to weekly intervals.
Table II. Enumeration of most commonly used different types of burn dressings and topical treatment agents with their characteristics.
Indications for escharotomy
Patients with burns to upper extremities and other regions require a careful assessment on admission by experienced members of a team of professionals that includes nursing, physiotherapy, occupational therapy, and social workers as well as a hand surgeon or burn surgeon knowledgeable in hand anatomy and function and the pathophysiology of burn injuries.3,10,11,35,37 This has been shown to greatly improve the outcome of burned upper extremities. 12,38 The first decision point in determining need for escharotomy is clinical assessment of the area affected by the burn married to the depth of injury. Circumferential burn is the leading factor to lower the threshold for escharotomy. The classic characteristics of partial- and fullthickness injury are well known. Partial-thickness injuries are moist, pink, and blistering; they blanch with pressure and are quite painful. Full-thickness injuries are dry, pale, whitish to brown, or non-blanching "cherry" red, and insensate to light touch. Unfortunately, many injuries fall somewhere between and may progress with time to a deeper injury. These fall into Jackson's description of the "zone of stasis" that may covert to the "zone of coagulation" with ensuing oedema, ischaemia, desiccation, or infection.39 If a clear-cut full-thickness injury is present on admission, and is circumferential, then an escharotomy can be performed as soon as the patient is assessed and stabilized.3,12 In addition, second-degree burns may produce considerable oedema, acutely amplifying the concern about compartment syndrome.40 Escharotomy may be done in a preventive sense, before there are any clinical signs of altered perfusion. If the injury is not clearly full-thickness, then other clinical signs are relied upon. Clinical signs associated with vascular compromise include deep muscle pain (especially on passive stretching), resistance to passive straightening of the fingers, decreased capillary filling, decreased pulses, cyanosis, and neurological changes - the earliest sign - with diminished distal sensation.3,41-43
Loss of pulses is a late finding which indicates compression of even larger arteries as well as smaller more compressible intramuscular vessels. Intrinsic muscle ischaemic damage can occur in the presence of palpable45 or Doppler pulses.46 Ischaemic necrosis of intrinsic muscles can result in persistent contracture, joint stiffness, and poor function. Detection of peripheral pulses, even with Doppler ultrasonography, does not correlate well with direct intramuscular compartment measurements.47
At any point in the progression of oedema, if the skin feels tense and unyielding, and is unable to "give" with the underlying oedema, an escharotomy should be performed, despite normal pulse examinations. If escharotomies are not indicated initially, hourly vascular observation of the elevated limb should be made for at least the first 24 h until the vascular integrity of the limb is no longer in doubt.24
There is a disagreement about the usefulness of compartment pressure measurements. Smith et al. found them to be "erratic and unreliable"24 while others have noted that clinical signs are subjective and may require a degree of cooperation that the acutely injured patient may not be able to give.42 A variety of techniques are available with which to perform the direct percutaneous measurement of tissue pressure.48-51 Whitesides et al. maintained that effective tissue perfusion generally ceases at 40 mm Hg tissue pressure, varying with systolic blood pressure and the degree of peripheral vasoconstriction.51 Luce advocates continuous monitoring of interstitial tissue pressure by placing a subcutaneous catheter in the volar forearm.5 An increase in the tissue pressure greater than 30 cm H2O (or mm Hg) requires removal of dressing and forearm and hand escharotomy. Distal extremity perfusion can also be monitored hourly by examination of distal pulses through palpation or with the use of digital Doppler ultrasonography.10
Escharotomy and fasciotomy techniques
The chief purpose of both escharotomy and fasciotomy is to relieve pressure from underlying structures resulting from circumferential deep burns or high-voltage electrical injury. Escharotomy by definition is a full-thickness incision through the eschar exposing the subcutaneous fat of full-thickness circumferential burns of the extremity.21,52,53 Fasciotomy is defined as an incision through skin, fat, and muscle fascia, exposing the underlying muscle compartment, performed to prevent and treat compartment syndrome developing from high-voltage electrical injuries or deep thermal burns involving muscle.
Escharotomies are usually carried out in the operating room either with the use of unipolar diathermy or by scalpel.3,12 Diathermy enables immediate haemostasis if required. After the area to undergo escharotomy has been appropriately prepped and draped, full-thickness incisions are made in the lateral and medial mid-axis lines.37 Maintaining the incisions longitudinally aids in avoiding damage to sensory nerves (superficial radial sensory nerve, medial and lateral antebrachial cutaneous nerves)54 and preserves subcutaneous veins. Care must also be taken to avoid injury to the ulnar nerve at the ulnar groove as it passes between the medial epicondyle and the olecranon process, which is why it is recommended to run the incision anterior to the medial epicondyle. The escharotomies should extend 1-2 cm beyond the area of full-thickness burn.
Digital escharotomies have been shown to improve the rate of survival of the digits. Salisbury et al.55 observed that only 7.1% of digits with escharotomies developed necrosis compared to 20.8% in the control group without escharotomies. Others see little benefit from them and feel that digital escharotomy commits the treatment plan to later excision and grafting.3,5
Digital escharotomies should be made in the safe zone between the neurovascular bundle and the extensor apparatus in the mid-axial line, ulnarly in the index middle and ring fingers and radially in the thumb.38 This allows preservation of the sides of the thumb and other digits that would be the opposing surfaces for the use of pinch grip, provided the digits survive. The side of the little finger to undergo escharotomy is less clear. The medial escharotomy of the upper limb can be simply continued along the ulnar border of the little finger allowing a continuous release. This may be most appropriate in a hand expected to recover full prehensile function. In an injury where simple grasp is the probable outcome, it may be better to preserve the ulnar border of the little finger and to do the digital escharotomy on the radial side of the little finger.24 The intrinsic muscles of the hand are very sensitive to ischaemic necrosis following thermal injury.45 The intrinsic compartments should be released in severe hand burns or if there is evidence of intrinsic tightness or pain on stretching the intrinsic muscles. Release of the intrinsic compartment reduces the risk of ischaemic necrosis and subsequent intrinsic minus deformities, which would result in a crippling hand deformity with extension at the MCP joint and flexion at the IP joints. This is tested by extending the MCP joint and feeling the resistance to passive flexion of the PIP and DIP joints. In such instances, escharotomies should be made on the dorsum of the hand to enable access to the fascia of the intrinsic compartment. Longitudinal incisions are placed in the spaces between the 2nd, 3rd, 4th, and 5th metacarpals to reduce the risk of extensor tendon exposure.3 Alternatively, two incisions can be made directly over the 2nd and 4th metacarpals providing access to the adjacent intermetacarpal spaces.35,37
Fasciotomies are most commonly indicated following high-voltage electrical injury (>1000 V). If the hand or forearm is involved in a high-voltage electrical injury, decompression of the median nerve at the wrist and proximal hand should also be performed. Fasciotomies may be indicated in other types of burns if adequate escharotomies have failed to restore perfusion.27,37 This may be indicated by symptoms or elevated pressure measurements.
Surgical management
Luce5 described the management of the burned hand of indeterminate depth as a negotiation between the "rock" of healing by excessive contracture and the "hard place" of needless excision. The timing of surgical intervention remains the most hotly disputed aspect of the management of the burned hand. Since a prospective, randomized series taking all variables into account has not been performed, the evidence must necessarily be historical and anecdotal. Early excision of localized deep hand burns was proposed as early as the 1940s56,57 and 1950s.58 Advocates of this approach15,24,38,59-77 feel that if the condition of the patient allows, excision and grafting of the upper limb burn wound need to begin as soon as the depth of the wound is clear; on day 2 or 3 post-burn24 or between days 2 and 5 post-burn.70,71 Belliappa et al. advocate a brief period of observation until the depth of injury is well demarcated to avoid excising living tissue unnecessarily.15
A recent randomized controlled trial on early versus late excision of deep hand burns included 50 patients and found no statistical significance between the groups regarding "function, scar formation, daily activity limitation and overall satisfaction"78 while another trial studying 40 patients with similar burn characteristics concluded that early burn excision and grafting gave statistically significant better results in hand function.79 Maslauskas et al. also found significantly better hand function as measured by hand grip and pinch in patients with early excision when compared to patients who underwent delayed excision and grafting.80 Tambuscio et al. found a statistically significant increase in secondary surgery on patients with hand burns who underwent late excision and grafting as opposed to early surgical treatment.11
Proponents of early excision point to the following advantages:
Reduces risk of abnormal scarring (hypertrophic and keloid)60,75,83
Reduces the number of reconstructive procedures38,60,68,69,71,73,81
Reduces length and consequently cost of hospital stay60-62,65-67,78,84,85
Reduces pain, reduces complications associated with prolonged immobilization,59,86 and allows patients to feed themselves sooner86
There are surgeons who prefer to wait 3-6 weeks for spontaneous sloughing before autografting. Advocates of this "wait and see" approach87-95 point to the flowing advantages to late excision and grafting:
Early excision is logistically more complex and time-consuming65
There is no significant difference in final hand function between burns treated with early excision and those allowed to heal spontaneously65,93-96
Late excision preserves all residual, viable dermal elements87,95,97
However, both sides agree on the critical importance of early hand therapy in determining hand function, on the role of topical antimicrobials in reducing the risk of infection, 90,91 and, in large burns, on giving priority to stabilizing the critically ill patient.42,98
Prevention of secondary injury
Following thermal injury, progressive loss of hand function may be a consequence of several factors including direct effects of heat, secondary effects of immobilization, disuse atrophy, soft tissue loss, contracture formation, bacterial wound colonization, decreased circulation, inadequate or inappropriate splinting, or the formation of oedema in connective tissues.42,45,96,99,100 The magnitude of these changes is in direct proportion to the time required to complete wound healing,99 and many such changes may be reduced or prevented by the judicious institution of early motion of the joints involved.42,59 Peacock et al. observed that "successful rehabilitation of injured hand is often the result of maintaining, not correcting, small joint function."59 The need for secondary reconstructive procedures is decreased by the proper attention to positioning and ranging of joints during the acute phase of wound care. Moreover, the success rates of such procedures increase in direct proportion to the degree of motion preserved before reconstruction.59,101,102 Achauer noted that "burn reconstruction is not an isolated phenomenon that occurs after the burn is covered"102 and that reconstructive goals are more easily attained when "all members of the burn team have a clear idea of reconstructive goals."102
The acute care of the burned upper extremity should counteract these factors, and this involves a balanced programme of elevation, exercise, splinting, and topical chemotherapy to minimize the sequelae of the burn injury. Early wound closure is a major additional key factor to maximize functional results.42
Hand function is mainly adversely affected by oedema. The latter is maximal on the first or second day following thermal injury. During the first 24 h following injury, a leakage of heavy proteinaceous fluid disperses into the tissue spaces, along the tendons, and around the supporting structures of the joints. This exudate becomes organized and may persist to produce structural thickening, adherence, and loss of elasticity in the moving structures of the hand.40,42
Effective control of oedema can be achieved by early motion and elevation of the extremity. The entire hand and arm should be kept above the level of the right atrium in order to achieve maximum passive drainage. Circumferential dressings or slings in order to achieve elevation should be used cautiously, because they may have additive restrictive effects by slippage or tightening (thus worsening the oedema),42 exert tension on the brachial plexus, or compress the ulnar nerve at the elbow.103 A well-defined exercise programme can maintain the normal muscle-pump mechanism for returning venous blood and lymph to the heart. The vigour of passive motion exercises is disputed. Levine argues that forceful motion is necessary to mobilize fluid from the interstitial spaces and move it towards the heart, minimizing adhesions and contraction of the joints and tendons.42 Others advocate gentle passive motion exercises to the point of resistance, warning against the tearing of different structures associated with vigorous motion.104,105
The adopted rehabilitation plan is determined by the patient's medical status, level of consciousness, and ability to actively participate in therapy, by the extent of the burn injury, and, by current burn wound therapy. Luce advocates the application of splints within the first hour of resuscitation, in the emergency department with the initiation of therapy, before the onset of any oedema.5 The position f comfort for the burned hand is metacarpophalangeal extension, proximal interphalangeal joint flexion, wrist flexion, and thumb adduction. These are also the positions of deformity and will lead to "claw hand".37,43,45,79
Splinting depends on the phase of wound healing and the area of the burned extremity. The severely burned hand should be positioned opposite to the anticipated deformity. During the inflammatory phase, early splinting of the burned hand attempts to prevent contracture of joint capsules, collateral ligaments, and intrinsic/extrinsic tendons, maximizing the functional potential of the hand. The optimal position for the hand with dorsal burns is achieved with an intrinsic- plus hand splint (wrist in extension, MCP joints in maximal flexion as tolerated, IP joints in full extension, and thumb abduction and opposition). The dorsal skin tightens in this position, leaving little room for oedema.103,106
When the hand has been burned on the dorsal surface, care must be taken to protect the extensor tendons, especially over the PIP joints. With exposed extensor tendons or suspected exposure, range of movement (ROM) should be restricted to isolated and short arc joint motion to prevent damage to the extensor tendon mechanism. Failure to position the extensor mechanism on slack can lead to disruption of the central slip, resulting in a boutonnière deformity. A finger gutter splint holding the PIP joint in full extension can be used if the damage is limited to the extensor hood. A 6-week immobilization period is advised for sufficient healing. DIP gliding exercises should be performed during the immobilization period to prevent contracture of the lateral bands.
Deep burns to the palmar surface of the hand should be monitored carefully. Functional use of the hand and exercise are crucial to maintain ROM.4,107 When the patient has difficulty achieving full composite extension of the MCP and IP joints, a forearm-based extension splint is fabricated to prevent cupping of the hand and to preserve the palmar arch.108,109 Alternating between an intrinsic-plus hand splint and an extension splint could be necessary with circumferential hand burns.103,110 During the proliferative stage of wound healing, collagen deposition along with oedema is responsible for strength and rigidity of scar tissue. Gliding planes can be obliterated by a continuous mass scar. Treatment should maximize each structure's capacity to glide and minimize the formation of scar adhesions. During this phase, static splints must be monitored and modified to accommodate decreased oedema or increased ROM. Static progressive splints are initiated in the proliferative phase to place low-load, progressive stretch to contracting joints and tissues. A static progressive MCP flexion splint is commonly used to increase MCP joint flexion if limitations are noted.
During the inflammatory and proliferative phases, continuous passive motion (CPM) devices can be used to maintain joint mobility. They do not replace the therapist; they are an adjunct to therapy. It is difficult for a hand CPM to take all of the finger joints through the full range of motion.96,111-113
In severe burns, it may not be possible to place the hand in the anticlaw position with splints. Small Kirschner wires may be placed across the PIP joint to fix these joints in extension.3,12,114 With these joints under control, metacarpophalangeal flexion is easy to obtain. This can usually be accomplished with a splint. Obviously, the Kirschner wires increase the danger of infection in the hand. Meticulous wound care with washing and antibiotic ointment must be done twice daily.43
Skin substitutes
Lou and Hickerson have outlined the different types of skin substitutes available for acute and reconstructive procedures on burned hands.115 Options available for temporary coverage of superficial burns include the porcinederived EZ Derm (Brennen Medical, Inc., MN, USA.) as well as Biobrane (Bertek Pharmaceuticals Inc., Morgantown, Wva), which is a biosynthetic dressing made of silicon film with embedded nylon and porcine type I collagen. Biobrane is applied to a wound after it has been debrided of all nonviable tissue and adheres to it until epithelialization has occurred. It is semi-occlusive, allowing the drainage of wound secretions and permeability of topical agents; it is also transparent, allowing the wound bed to be visualized. Biobrane has been fashioned as a glove for use in hand burns to ease its application and subsequent motion of the extremity, and it has been found to decrease pain, increase mobility, and promote faster healing compared to topical agents.37,115,116 It is recommended for use in children due to decreased dressing changes, decreased pain, and excellent functional results.17,37,117
Cadaveric allografts are used with subsequent complete excision and replacement with a patient's autograft or partial excision and split-thickness skin grafting on top of the allograft dermis (this is possible due to the low immunogenicitiy of the dermis).115 Several other skin substitutes also exist for the treatment of full-thickness/deep burn wounds requiring excision. AlloDerm (LifeCell, Branchburg, NJ, USA) is an acellular cadaveric dermal matrix that can be used in a one-step procedure with an autograft STSG allowing excellent take of the split-thickness graft. Integra® (Integra LifeSciences, Plainsboro, NJ, USA) is a synthetic skin substitute made of two layers to act as epidermis and dermis. A thin silicone film mimics the body's epidermis while a matrix of bovine collagen cross-linked with chondroitin-6-sulphate acts as a dermal layer. Integra® can be used in the acute coverage of a wound bed after burn excision, and the dermal layer can be used as a base for STSG after silicone exfoliation. Biobrane can be used as a dressing on top of Integra to visualize its take. Integra Single Layer™ (Integra LifeSciences, Plainsboro, NJ, USA) was recently introduced - this consists of the same components as Integra but without the top silicone layer, allowing the use of this skin substitute in onestep procedures with autogenic STSG.118 Matriderm (De Suwelack Skin and Health Care AG, Billerbeck (Germany), Plainsboro, NJ (USA) is a dermal substitute made of collagen and elastin that serves as a base for STSG in a onestep procedure with excellent graft take results and allows for increased elasticity.
Paediatric hand burns
Children are one of the most common populations that suffer from hand burns as they use their hands for exploration and discovery of their environment.29,37 The main principles of the treatment of hand burns are the same regardless of age, but the paediatric population requires emphasis in certain areas of care due to the inherent anatomical, physiological, and psychological idiosyncrasies of this population. Paediatric skin is thinner than adult skin, resulting in deeper burns in children at lower temperatures, 35,37,107 and the slower withdrawal response in children also contributes to longer contact time with the burn source.104 However, children have a thicker subcutaneous adipose layer, protecting and sparing tendon injuries even in deep burns.35,37,107 The thicker adipose layer also makes excision of full-thickness burns easier in children than in the adult population.37
It is estimated that around 10% of all paediatric hand burns are caused by abuse.35,107 Therefore, health care providers must have a high index of suspicion for this aetiology, especially if the given history is not consistent with the type of burn,107 or if the child and his guardian/parent give significantly different histories. Suspicion of abuse should also be raised by burns with a glove distribution.107
Control of oedema, topical wound care, and monitoring for compartment syndrome as well as the indications for escharotomies and fasciotomies are similar in adult and paediatric populations. Although most paediatric hand burns do not require surgical intervention,37,41 the timing of surgical treatment, should it be indicated, is debatable, with some authors advocating early excision and grafting35,107 and others preferring late surgical treatment.37
When surgical management is advocated, special care must be taken in the operating room to avoid hypothermia and blood loss in the paediatric patient as this age group is more susceptible to these complications. Ease and frequency of dressing must be carefully considered, as must the cosmetic result of reconstruction due to the more delicate paediatric psychology.27,119
A review of 174 grafted paediatric hand burns by Chandrasegaram and Harvey41 revealed significantly lower contracture rates with the use of full-thickness skin grafts than with split-thickness skin grafts in paediatric hand burns. Therefore, full-thickness grafts should be used in areas of concern for subsequent contracture formation.35,41,107 In young children, inguinal skin folds can serve as a donor site for full-thickness skin grafts.35 Long-term follow-up of grafts is required due to changes in the graft such as development of contractures that may occur as a result of the patient's growth.35,41,107
In cases where split-thickness skin grafts are used, either the back and or the anterior thigh can be used in paediatric patients, as a comparison between the two sites revealed pigmentation or compliance by the patient".107 When used as a donor site for STSG in the paediatric population, the back has been shown to be associated with less long-term scarring than the anterior thigh.35 The scalp has also been suggested as a donor site for STSG due to faster healing and decreased pain when compared to other donor sites.27 Additionally, any scar or discoloration will be covered by hair, obscuring it from view.27 The soles of the feet can also be used as donor sites, especially for palmar burns as they give a good result due similarity to the palm in texture and colour.27 Reported complications include recontracture of the recipient site, hypertrophic scarring, and the need of an additional full-thickness skin graft from a secondary donor site that is needed to cover the primary donor site.120
Resuming activities after hand burns
It was found that the most significant factors affecting return to work/military duty after a burn injury were the duration of hospitalization and the total body surface area burned.120-123 The presence of a hand burn has not been consistently found to negatively affect return to work/duty, with some studies finding a statistical significance in delay in work resumption following hand burns, and others not yielding such results.120-123 This may however be due to the increased incidence of mild hand burns that dilute the results and show no statistical significance while results for severe hand burns are negatively associated with poorer rates of work resumption.123
Conclusion
Burn injury is a common form of trauma that frequently affects the upper extremity, with a consequent significant functional loss, if not adequately treated in the acute or subacute phase. Initial triage is of primary importance in determining which patients to admit to the burn unit and which to treat as out-patients. This decision must be made following a proper history and physical examination, trying to accurately assess the circumference and depth of the burned areas. Successful out-patient treatment hangs on correct initial debridement, application of the appropriate topical therapy, early mobilization, and regular follow-ups. Decision for escharotomy relies on the accurate initial assessment and a close follow-up by an experienced burn surgeon. Early burn excision and grafting appear to yield better results in hand function than late burn excision. Secondary injury is avoided by careful burn care rehabilitation planning, including topical wound care, reduction of the oedematous process, early mobilization, and burn wound coverage. The endeavour of burn care is to achieve early wound healing, prevent infection, and restore normal functional activity.
References
- 1.Salisbury RE, Pruitt BA.Epidemiology and general considerations In: Salisbury, RE , Pruitt, BA (eds) "Burns of the Upper Extremity" WB Saunders; Philadelphia: 1-5, 1976 [PubMed] [Google Scholar]
- 2.Pruitt BA, Mason AD.Epidemiological, demographic, and outcome characteristics of burn injury In: Herndon DN (ed) "Total Burn Care" WB Saunders; Toronto: 5-15, 1996 [Google Scholar]
- 3.Sterling J, Gibran NS, Klein MB. Acute management of hand burns. Hand Clin. 2009;25:453–459. doi: 10.1016/j.hcl.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Kowalske KJ, Greenhalgh DG, Ward SR. Hand burns. J Burn Care Res. 2007;28:607–610. doi: 10.1097/BCR.0B013E318093E4B9. [DOI] [PubMed] [Google Scholar]
- 5.Luce EA. The acute and subacute management of the burned hand. Clin Plast Surg. 2000;27:49–63. [PubMed] [Google Scholar]
- 6.Tredget EE, Shankowsky HA, Taerum TV, et al. The role of inhalation injury in burn trauma: A Canadian experience. Ann Surg. 1990;212:720–727. doi: 10.1097/00000658-199012000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Consumer Safety Unit. The Home Accident Surveillance System. London: Dept of Trade and Industry; 2000. [Google Scholar]
- 8.Amar-Inalsingh CH. An experience in treating 501 patients with keloids. Johns Hopkins Med J. 1974;134:284–290. [PubMed] [Google Scholar]
- 9.Leung PC, Ng TK. Occupational hand injuries - the pattern and the causes. J Western Pacific Orthopaedic Assoc. 1979;16:1–12. [Google Scholar]
- 10.Tredget EE. Management of the acutely burned upper extremity. Hand Clin. 2000;16:187–203. [PubMed] [Google Scholar]
- 11.Tambuscio A, Governa M, Caputo G, et al. Deep burn of the hands: Early surgical treatment avoids the need for late revisions? Burns. 2006;32:1000–1004. doi: 10.1016/j.burns.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Keyerman PA, Andres LA, Lucas HD, et al. Reconstruction of the burned hand. Plast Reconstr Surg. 2011;127:752–759. doi: 10.1097/PRS.0b013e3181fed7c1. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence J. The mortality of burns. Fire Safety J. 1991;17:205–215. [Google Scholar]
- 14.Ryan CM, Schoenfeld DA, Thorpe WT, et al. Objective estimates of the probability of death from burn injuries. N Engl J Med. 1998;338:362–366. doi: 10.1056/NEJM199802053380604. [DOI] [PubMed] [Google Scholar]
- 15.Belliappa PP, McCabe SJ. The burned hand. Hand Clin. 1993;9:313–324. [PubMed] [Google Scholar]
- 16.American College of Surgeons Committee on Trauma. American College of Surgeons; Chicago: 1990. Guidelines for the Operation of Burn Units: Resources for Optimal Care of the Injured Patient. [Google Scholar]
- 17.Lang EM, Eiberg CA, Brandis M, et al. Biobrane in the treatment of burn and scald injuries in children. Ann Plast Surg. 2005;55:485–489. doi: 10.1097/01.sap.0000182652.88669.a6. [DOI] [PubMed] [Google Scholar]
- 18.Hlava P, Moserova J, Königovà R. Validity of clinical assessment of the depth of a thermal injury. Acta Chir Plast. 1983;25:202–208. [PubMed] [Google Scholar]
- 19.Heimbach D, Engrav L, Grube B, et al. Burn depth: A review. World J Surg. 1992;16:10–15. doi: 10.1007/BF02067108. [DOI] [PubMed] [Google Scholar]
- 20.Atiyeh BS, Gunn SW, Hayek SN. State of the art in burn treatment. World J Surg. 2005;29:131–148. doi: 10.1007/s00268-004-1082-2. [DOI] [PubMed] [Google Scholar]
- 21.American Burn Association. Advanced Burn Life Support Provider's Manual. Assessment and fluid resuscitation of the burn patient. Chicago: [Google Scholar]
- 22.Rice S, Gomez M. The role of digital imaging in the treatment of the burn patient.; American Burn Association Meeting; Orlando. 1999. [Google Scholar]
- 23.Raine TJ, Heggers JP, Robson MC, et al. Cooling the burn wound to maintain microcirculation. J Trauma. 1981;21:394–397. doi: 10.1097/00005373-198105000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Smith MA, Munster AM, Spence RJ. Burns of the hand and upper limb - a review. Burns. 1998;24:493–505. doi: 10.1016/s0305-4179(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 25.Heggers JP, Ko F, Robson MC, et al. Evaluation of burn blister fluid. Plast Reconstr Surg. 1980;65:798–804. doi: 10.1097/00006534-198006000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Robson MC, Del Becarro EJ, Heggers JP. The effect of prostaglandins on the dermal microcirculation after burning, and the inhibition of the effect by specific pharmacological agents. Plast Reconstr Surg. 1979;63:781–787. [PubMed] [Google Scholar]
- 27.Choi M, Armstrong MB, Panthaki ZT. Pediatric hand burns: thermal, electrical, chemical. J Craniofac Surg. 2009;20:1045–1048. doi: 10.1097/scs.0b013e3181abb25f. [DOI] [PubMed] [Google Scholar]
- 28.Spann CT, Taylor SC, Weinberg JM. Topical antimicrobial agents in dermatology. Dis Mon. 2004;50:407–421. doi: 10.1016/j.disamonth.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 29.McKee DM. Acute management of burn injuries to the hand and upper extremity. J Hand Surg Am. 2010;35:1542–1544. doi: 10.1016/j.jhsa.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Fuller FW. The side effects of silver sulfadiazine. J Burn Care Res. 2009;30:464–470. doi: 10.1097/BCR.0b013e3181a28c9b. [DOI] [PubMed] [Google Scholar]
- 31.Khundar R, Malic C, Burgev T. Use of Acticoat dressings in burns: What is the evidence? Burns. 2010;36:751–758. doi: 10.1016/j.burns.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Monafo WW, West MA. Current treatment recommendations for topical burn therapy. Drugs. 1990;40:364–373. doi: 10.2165/00003495-199040030-00004. [DOI] [PubMed] [Google Scholar]
- 33.Klasen HJ. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns. 2000;26:131–138. doi: 10.1016/s0305-4179(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 34.Aldo JE, King JW. The management of burns with silver nitrate solution. J Natl Med Assoc. 1966;58:165–166. [PMC free article] [PubMed] [Google Scholar]
- 35.Palmieri TL. Initial management of acute pediatric hand burns. Hand Clin. 2009;25:461–467. doi: 10.1016/j.hcl.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Ramundo J, Graw M. Collagenase for enzymatic debridement: A systematic review. J Wound Ostomy Continence Nurs. 2009;36:S4–11. doi: 10.1097/WON.0b013e3181bfdf83. [DOI] [PubMed] [Google Scholar]
- 37.Feldmann ME, Evans J, Seung Jun O. Early management of the burned pediatric hand. J Craniofac Surg. 2008;19:942–950. doi: 10.1097/SCS.0b013e318175f38d. [DOI] [PubMed] [Google Scholar]
- 38.Sheridan RL, Hurley J, Smith MA, et al. The acutely burned hand: Management and outcome based on a 10-year experience with 1047 acute hand burns. J Trauma. 1995;38:406–411. doi: 10.1097/00005373-199503000-00022. [DOI] [PubMed] [Google Scholar]
- 39.Jackson DM. The diagnosis of the depth of burning. Br J Surg. 1953;40:588–596. doi: 10.1002/bjs.18004016413. [DOI] [PubMed] [Google Scholar]
- 40.Demling RH. The burn edema process: Current concepts. J Burn Care Rehabil. 2005;26:207–227. [PubMed] [Google Scholar]
- 41.Chandrasegaram MD, Harvey JE. Full-thickness vs split-skin grafting in pediatric hand burns - a 10-year review of 174 cases. J Burn Care Res. 2009;30:867–871. doi: 10.1097/BCR.0b013e3181b48610. [DOI] [PubMed] [Google Scholar]
- 42.Levine NS, Buchanan RT. The care of burned upper extremities. Clin Plast Surg. 1986;13:107–118. [PubMed] [Google Scholar]
- 43.Parry SW. Reconstruction of the burned hand. Clin Plast Surg. 1989;16:577–586. [PubMed] [Google Scholar]
- 44.Wong L, Spence RJ. Escharotomy and fasciotomy of the burned upper extremity. Hand Clin. 2000;16:165–174. [PubMed] [Google Scholar]
- 45.Salisbury RE, McKeel DW, Mason AD jr. Ischemic necrosis of the intrinsic muscles of the hand after thermal injuries. J Bone Joint Surg Am. 1974;56:1701–1707. [PubMed] [Google Scholar]
- 46.Halpern AA, Nagel DA. Compartment syndromes of the forearm: Early recognition using tissue pressure measurements. J Hand Surg Am. 1979;4:258–263. doi: 10.1016/s0363-5023(79)80160-4. [DOI] [PubMed] [Google Scholar]
- 47.Saffle JR, Zeluff GR, Warden GD. Intramuscular pressure in the burned arm: Measurement and response to escharotomy. Am J Surg. 1980;140:825–831. doi: 10.1016/0002-9610(80)90126-9. [DOI] [PubMed] [Google Scholar]
- 48.Wells HS, Youmans JB, Miller DG jr. Tissue pressure (intracutaneous, subcutaneous, and intramuscular) as related to venous pressure, capillary filtration and other factors. J Clin Invest. 1938;17:489–499. doi: 10.1172/JCI100976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swann HG, Montgomery AV, Davis JC jr, et al. A measure of intrarenal pressure. Tex Rep Biol Med. 1950;8:262–270. [PubMed] [Google Scholar]
- 50.Swann HG, Montgomery AV, Davis JC jr, et al. A method for rapid measurement of intrarenal and other tissue pressures. J Exp Med. 1950;92:625–636. doi: 10.1084/jem.92.6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitesides TE, Haney TC, Morimoto K, et al. Tissue pressure measurements as a determining for the need of fasciotomy. Clin Orthop Relat Res. 1975;113:43–51. doi: 10.1097/00003086-197511000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Larkin JM, Moylan JA. Tetanus following a minor burn. J Trauma. 1975;15:546–548. [PubMed] [Google Scholar]
- 53.Moylan JA jr, Inge WW jr, Pruitt BA jr. Circulatory changes following circumferential extremity burns evaluated by the ultrasonic flowmeter and analysis of 60 thermally injured limbs. J Trauma. 1971;11:763–770. doi: 10.1097/00005373-197109000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Blount WP. Volkmann's ischemic contracture (editorial) Surg Gynecol Obstet. 1950;90:244–246. [Google Scholar]
- 55.Salisbury RE, Levine NS.The early management of upper extremity thermal injury In: Salisbury, RE , Pruitt, BA (eds) "Burns of the Upper Extremity" WB Saunders; Philadelphia: 36-46, 1976 [Google Scholar]
- 56.McCorkle HJ, Silvani H. Selection of the time for grafting of skin to extensive defects resulting from deep thermal burns. Ann Surg. 1945;121:285–290. doi: 10.1097/00000658-194503000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cope O, Langohr JL, Moore FD, et al. Expeditious care of fullthickness burn wounds by surgical excision and grafting. Ann Surg. 1947;125:1–22. [PMC free article] [PubMed] [Google Scholar]
- 58.Moncrief JA. Third-degree burns of the dorsum of the hand. Ann J Surg. 1958;96:535–544. doi: 10.1016/0002-9610(58)90971-1. [DOI] [PubMed] [Google Scholar]
- 59.Peacock EE jr, Madden JW, Trier WC. Some studies on the treatment of burned hand. Ann Surg. 1970;171:903–914. doi: 10.1097/00000658-197006010-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mahler D, Hirchowitz B. Tangential excision and grafting for burns of the hands. Br J Plast Surg. 1975;28:189–192. doi: 10.1016/0007-1226(75)90128-9. [DOI] [PubMed] [Google Scholar]
- 61.Jackson DM. Second thoughts on the burn wound. J Trauma. 1969;9:839–862. doi: 10.1097/00005373-196910000-00003. [DOI] [PubMed] [Google Scholar]
- 62.Janzekovic Z. A new concept in the early excision and immediate grafting of burns. J Trauma. 1970;10:1103–1108. [PubMed] [Google Scholar]
- 63.Janzekovic Z. Excision of burns. Burns. 1977;4:61–66. [Google Scholar]
- 64.Wexler MR, Rousso M. The immediate treatment of the burned hand. Prog Surg. 1978;16:165–179. doi: 10.1159/000402261. [DOI] [PubMed] [Google Scholar]
- 65.Goodwin CW, Maguire MS, McManus WF, et al. Prospective study of burn wound excision of the hands. J Trauma. 1983;23:510–517. doi: 10.1097/00005373-198306000-00012. [DOI] [PubMed] [Google Scholar]
- 66.Nielsen AB, Sommer J. Surgical treatment of the deeply burned hand. Burns. 1983;9:214–217. doi: 10.1016/0305-4179(83)90041-4. [DOI] [PubMed] [Google Scholar]
- 67.Stone PA, Lawrence JC. Healing of tangentially excised and grafted burns in man. Br J Plast Surg. 1973;26:20–31. doi: 10.1016/s0007-1226(73)80031-1. [DOI] [PubMed] [Google Scholar]
- 68.Pegg SP, Gregory JJ, Hogan PG, et al. Epidemiological pattern of adult burn injuries. Burns. 1979;5:326–334. [Google Scholar]
- 69.Wang XW, Sun YH, Zhang GZ, et al. Tangential excision of eschar for deep burns of the hand: Analysis of 156 patients collected over 10 years. Burns. 1984;11:92–98. doi: 10.1016/0305-4179(84)90130-x. [DOI] [PubMed] [Google Scholar]
- 70.Meeker IA jr, Snyder WH jr. Dermatome debridement and early grafting of extensive third-degree burns in children. Surg Gynecol Obstet. 1956;103:527–534. [PubMed] [Google Scholar]
- 71.Macmillan BG. Sixth National Burn Seminar. Wound management. Early excision. J Trauma. 1967;7:75–79. [PubMed] [Google Scholar]
- 72.Burke JF, Bondoc CC, Quinby WC. Primary burn excision and immediate grafting: A method of shortening illness. J Trauma. 1974;14:389–395. doi: 10.1097/00005373-197405000-00005. [DOI] [PubMed] [Google Scholar]
- 73.Burke JF, Bondoc CC, Quinby WC jr, et al. Primary surgical management of the deeply burned hand. J Trauma. 1976;16:593–598. doi: 10.1097/00005373-197608000-00001. [DOI] [PubMed] [Google Scholar]
- 74.Bondoc CC, Quinby WC, Siebert S, et al. Management of acute thermal hand injuries In: Tubiana, R (ed) "The Hand", WB Saunders; Philadelphia: 766-774, 1988 [Google Scholar]
- 75.Deitch EA, Wheelahan TM, Rose MP, et al. Hypertrophic burn scars: Analysis of variables. J Trauma. 1983;23:895–898. [PubMed] [Google Scholar]
- 76.Fraulin FO, Tredget EE. Subcutaneous instillation of donor sites in burn patients. Br J Plast Surg. 1993;46:324–326. doi: 10.1016/0007-1226(93)90013-2. [DOI] [PubMed] [Google Scholar]
- 77.Tredget EE, Nedelec B, Scott PG, et al. Hypertrophic scars, keloids, and contractures: The cellular and molecular basis for therapy. Surg Clin North Am. 1997;77:701–730. doi: 10.1016/s0039-6109(05)70576-4. [DOI] [PubMed] [Google Scholar]
- 78.Mohammadi AA, Bakhshaeekia AR, Marzban S, et al. Early excision and skin grafting versus delayed skin grafting in deep hand burns (a randomized clinical controlled trial) Burns. 2011;37:36–41. doi: 10.1016/j.burns.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 79.Omar MT, Hassan AA. Evaluation of hand function after early excision and skin grafting of burns versus delayed skin grafting (a randomized clinical trial) Burns. 2011;37:707–713. doi: 10.1016/j.burns.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 80.Maslauskas K, Rimdeika R, Rapoliene J, et al. Analysis of burned hand function (early versus delayed treatment.) Medicina (Kaunas) 2005;41:846–851. [PubMed] [Google Scholar]
- 81.Bondoc CC, Quinby WC, Burke JF. Primary surgical management of the deeply burned hand in children. J Pediatr Surg. 1976;11:355–362. doi: 10.1016/s0022-3468(76)80189-3. [DOI] [PubMed] [Google Scholar]
- 82.Frist W, Ackroyd F, Burke J, et al. Long-term functional results of selective treatment of hand burns. Am J Surg. 1985;149:516–521. doi: 10.1016/s0002-9610(85)80049-0. [DOI] [PubMed] [Google Scholar]
- 83.Magliacani G, Bormioli M, Cerruti V. Late results following treatment of deep burns of the hand. Scand J Plast Reconstr Surg. 1979;13:137–139. doi: 10.3109/02844317909013041. [DOI] [PubMed] [Google Scholar]
- 84.Nuchtern JG, Engrav LH, Nakamura DY, et al. Treatment of fourth-degree hand burns. J Burn Care Rehabil. 1995;16:36–42. doi: 10.1097/00004630-199501000-00007. [DOI] [PubMed] [Google Scholar]
- 85.Malfeyt GA. Burns of the dorsum of the hand treated by tangential excision. Br J Plast Surg. 1976;29:78–81. doi: 10.1016/0007-1226(76)90098-9. [DOI] [PubMed] [Google Scholar]
- 86.Hunt JL, Sato R, Baxter CR. Early tangential excision and immediate mesh autografting of deep dermal hand burns. Ann Surg. 1979;189:147–151. doi: 10.1097/00000658-197902000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Labandter H, Kaplan I, Shavitt C. Burns of the dorsum of the hand: Conservative treatment with intensive physiotherapy versus tangential excision and grafting. Br J Plast Surg. 1976;29:352–354. doi: 10.1016/0007-1226(76)90021-7. [DOI] [PubMed] [Google Scholar]
- 88.Sykes PJ, Bailey BN. Treatment of hand burns with occlusive bags: A comparison of three methods. Burns. 1976;2:162–168. [Google Scholar]
- 89.Barillo DJ, Harvey KD, Hobbs CL, et al. Prospective outcome analysis of a protocol for the surgical and rehabilitative management of burns to the hands. Plast Reconstr Surg. 1997;100:1442–1451. doi: 10.1097/00006534-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 90.Hermans RP. Topical treatment of serious infections with special reference to the use of a mixture silver sulphadazine and cerium nitrate: Two clinical studies. Burns. 1984;11:59–62. doi: 10.1016/0305-4179(84)90164-5. [DOI] [PubMed] [Google Scholar]
- 91.Boeckx W, Blondeel PN, Vandersteen K, et al. Effect of cerium nitrate-silver sulphadiazine on deep dermal burns: A histological hypothesis. Burns. 1992;18:456–462. doi: 10.1016/0305-4179(92)90177-v. [DOI] [PubMed] [Google Scholar]
- 92.Van Zuijlen PP, Kreis RW, Vloemans AF, et al. The prognostic factors regarding long-term functional outcome of full-thickness hand burns. Burns. 1999;25:709–714. doi: 10.1016/s0305-4179(99)00067-4. [DOI] [PubMed] [Google Scholar]
- 93.Edstrom LE, Robson MC, Machiaverna JR, et al. Prospective randomized treatments for burned hands: Non-operative vs. operative". Scand J Plast Reconstr Surg. 1979;13:131–135. doi: 10.3109/02844317909013040. [DOI] [PubMed] [Google Scholar]
- 94.Salisbury RE, Wright P. Evaluation of early excision of dorsal burns of the hand. Plast Reconstr Surg. 1982;69:670–675. doi: 10.1097/00006534-198204000-00017. [DOI] [PubMed] [Google Scholar]
- 95.Kalaja E. Acute excision or exposure treatment? Secondary reconstructions and functional results. Scand J Plast Reconstr Surg. 1984;18:95–99. doi: 10.3109/02844318409057409. [DOI] [PubMed] [Google Scholar]
- 96.Robson MC, Smith DJ.Burned hand In: Jurkiewicz, MJ (ed) "Plastic Surgery: Principles and Practices" Mosby; St Louis: 1990 [Google Scholar]
- 97.Robson MC, Smith DJ jr, VanderZee AJ, et al. Making the burned hand functional. Clin Plast Surg. 1992;19:663–671. [PubMed] [Google Scholar]
- 98.Levine BA, Sirinek KR, Peterson HD, et al. Efficacy of tangential excision and immediate autografting of deep second degree burns of the hand. J Trauma. 1979;19:670–673. doi: 10.1097/00005373-197909000-00006. [DOI] [PubMed] [Google Scholar]
- 99.Beasley RW. Secondary repair of burned hand. Hand Clin. 1990;6:319–341. [PubMed] [Google Scholar]
- 100.Harden NG, Luster SH. Rehabilitation considerations in the care of the acute burn patient. Crit Care Nurs Clin North Am. 1991;3:245–253. [PubMed] [Google Scholar]
- 101.Edstrom LE, Robson MC, Headley BJ. Evaluation of exercise techniques in the burn patient. Burns. 1977;4:113–117. [Google Scholar]
- 102.Achauer BM. "Burn Reconstruction". Thieme Medical Publishers; New York: 1991. [Google Scholar]
- 103.Jordan RB, Daher J, Wasil K. Splints and scar management in acute and reconstructive burn care. Clin Plast Surg. 2000;27:71–85. [PubMed] [Google Scholar]
- 104.Boswick JA jr.Rehabilitation and reconstruction of the burned hand In: Boswick, JA jr (ed) "The Art and Science of Burn Care" Aspen Publishers; Rockville: 1987 [Google Scholar]
- 105.Covey M.Occupational therapy hand In: Boswick, JA jr (ed) "The Art and Science of Burn Care" Aspen Publishers; Rockville: 1987 [Google Scholar]
- 106.Malick MH, Carr JA. "Manual on Management of the Burn Patient Including Splinting, Mold and Pressure Techniques". Harmarville Rehabilitation Center; Pittsburg: 1982. [Google Scholar]
- 107.Birchenoughn SA, Gampper TJ, Morgan RF. Special considerations in the management of pediatric upper extremity and hand burns. J Craniofac Surg, 2008;19:933–941. doi: 10.1097/SCS.0b013e318175f3f6. [DOI] [PubMed] [Google Scholar]
- 108.Howell JW. Management of the acutely burned hand for the nonspecialized clinician. Phys Ther. 1989;69:1077–1090. doi: 10.1093/ptj/69.12.1077. [DOI] [PubMed] [Google Scholar]
- 109.Schwanholt C, Daugherty MB, Gaboury T, et al. Splinting the pediatric palmar burn. J Burn Care Rehabil. 1992;13:460–464. doi: 10.1097/00004630-199207000-00014. [DOI] [PubMed] [Google Scholar]
- 110.McCormack RM.Principles of treatment and reconstruction of the burned hand and fingers In: "Symposium on the Hand" Mosby; St Louis: 1971 [Google Scholar]
- 111.Puddicombe BE, Nardone MA. Rehabilitation of the burned hand. Hand Clin. 1990;6:281–292. [PubMed] [Google Scholar]
- 112.Bentham JS, Brereton WD, Cochrane IW, et al. Continuous passive motion device for hand rehabilitation. Arch Phys Med Rehabil. 1987;68:248–250. [PubMed] [Google Scholar]
- 113.Covey MH, Dutcher K, Marvin JA, et al. Efficacy of continuous passive motion (CPM) devices with hand burns. J Burn Care Rehabil. 1988;9:397–400. [PubMed] [Google Scholar]
- 114.Achauer BM.Extremities In: Achauer, BM (ed) "Management of the Burned Patient" Applegate and Lange; New York: 1987 [Google Scholar]
- 115.Lou RB, Hickerson WL. The use of skin substitutes in burned hands. Hand Clin. 2009;25:497–509. doi: 10.1016/j.hcl.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 116.Lineen E, Namias N. Biologic dressings in burns. J Cranofac Surg. 2008;19:923–928. doi: 10.1097/SCS.0b013e318175b5ab. [DOI] [PubMed] [Google Scholar]
- 117.Whitaker IS, Cantab MA, Prowse S, et al. A critical evaluation of the use of Biobrane as a biologic skin substitute. A versatile tool for the plastic and reconstructive surgeon. Ann Plast Surg. 2008;60:333–337. doi: 10.1097/SAP.0b013e31806bf446. [DOI] [PubMed] [Google Scholar]
- 118.Brusselaers N, Pirayesh A, Hoeksema H, et al. Skin replacement in burn wounds. J Trauma. 2010;68:490–501. doi: 10.1097/TA.0b013e3181c9c074. [DOI] [PubMed] [Google Scholar]
- 119.Dodd AR, Nelson-Mooney K, Greenhalgh DG, et al. The effect of hand burns on quality of life in children. J Burn Care Res. 2010;31:414–422. doi: 10.1097/BCR.0b013e3181db5295. [DOI] [PubMed] [Google Scholar]
- 120.Tanabe HY, Aoyagi A, Tai Y, et al. Reconstruction for palmar skin defects of the digits and hand using plantar dermal grafting. Plast Reconstr Surg. 1998;101:992–998. doi: 10.1097/00006534-199804040-00016. [DOI] [PubMed] [Google Scholar]
- 121.Chapman TT, Richard RL, Hedman TL, et al. Military return to duty and civilian return to work factors following burns with focus on the hand and literature review. J Burn Care Res. 2008;29:756–762. doi: 10.1097/BCR.0b013e3181848b41. [DOI] [PubMed] [Google Scholar]
- 122.Hwang YF, Chen-Sea MJ, Chen CL. Factors related to return to work and job modification after a hand burn. J Burn Care Res. 2009;30:661–667. doi: 10.1097/BCR.0b013e3181abfabf. [DOI] [PubMed] [Google Scholar]
- 123.Tantulla K, Vuola J, Asko-Seljavaara S. Return to employment after burn. Burns. 1997;23:341–344. doi: 10.1016/s0305-4179(97)89876-2. [DOI] [PubMed] [Google Scholar]