Abstract
Bone is a specialised connective tissue and together with cartilage forms the strong and rigid endoskeleton. These tissues serve three main functions: scaffold for muscle attachment for locomotion, protection for vital organs and soft tissues and reservoir of ions for the entire organism especially calcium and phosphate. One of the most unique and important properties of bone is its ability to constantly undergo remodelling even after growth and modelling of the skeleton have been completed. Remodelling processes enable the bone to respond and adapt to changing functional situations. Bone is composed of various types of cells and collagenous extracellular organic matrix, which is predominantly type I collagen (85–95%) called osteoid that becomes mineralised by the deposition of calcium hydroxyapatite. The non-collagenous constituents are composed of proteins and proteoglycans, which are specific to bone and the dental hard connective tissues. Maintenance of appropriate bone mass depends upon the precise balance of bone formation and bone resorption which is facilitated by the ability of osteoblastic cells to regulate the rate of both differentiation and activity of osteoclasts as well as to form new bone. An overview of genetics and molecular mechanisms that involved in the differentiation of osteoblast and osteoclast is discussed.
Keywords: Bone cells, osteoblasts, osteoclasts, regulations
Introduction
Bone is rigid and its architecture arranged to provide maximum strength for the least weight. Most bones have a dense rigid outer shell of compact bone, the cortex and the central medullary or cancellous zone of thin interconnecting narrow bone trabeculae. The space in the medullary bone between trabeculae is occupied by haemopoietic bone marrow.
Bone extracellular matrix comprises of both mineral and organic phases. About 60% of bone net weight is inorganic material, 25% organic material and 5% water. By volume, bone comprises of 36% inorganic, 36% organic and 28% water.
The inorganic/mineral component comprises of calcium and phosphate in the form of needle-like or thin plates of hydroxyapatite crystals [Ca10(PO4)6(OH)2]. These are conjugated to a small proportion of magnesium carbonate, sodium and potassium ions. The organic matrix of bone is composed of collagen and non-collagenous organic materials. Collagen comprises about 90% of the organic bone matrix. Type I collagen is the most abundant form of intrinsic collagen found in the bone that is secreted by osteoblasts. Most of the non-collagenous organic materials are endogenous proteins produced by the bone cells. One group of non-collagenous proteins is the proteoglycans. This incorporates chondroitin sulphate and heparan sulphate glycosaminoglycans. As the proteoglycans bind to collagen, they may help regulate collagen fibril diameters and may play a role in mineralisation. Other components include osteocalcin (Gla protein), involved in binding calcium during the mineralisation process, osteonectin which may serve some bridging function between collagen and the mineral component, sialoproteins (rich in sialic acid) and certain proteins which appear to be concentrated from plasma.
Bone also contains exogenously derived proteins that may circulate in the blood and become locked up in the bone matrix itself. It is a rich source of cytokines (such as interleukin, tumour necrosis factor and colony-stimulating factors) and growth factors (such as transforming growth factors, fibroblast growth factors, platelet-derived growth factors and insulin-like growth factors) produced by variety of cells associated with bone. These proteins play an important role in biological activity of bone cells. When present within the bone, they are inactive but may become mobilised when bone is being resorbed by osteoclasts.
Bone is composed of four different cell types; osteoblasts, osteocytes, osteoclasts and bone lining cells. Osteoblasts, bone lining cells and osteoclasts are present on bone surfaces and are derived from local mesenchymal cells called progenitor cells. Osteocytes permeate the interior of the bone and are produced from the fusion of mononuclear blood-borne precursor cells.
Bone Lining Cells And Osteocytes
When bone surfaces are neither in the formative nor resorptive phase, the bone surface is completely lined by a layer of flattened and elongated cells termed bone-lining cells. These show little sign of synthetic activity as evidenced by their organelle content. They are regarded as post proliferative osteoblasts. By covering the bone surface, they protect it from any osteoclast resorptive activity. They may be reactivated to form osteoblasts.
Osteocytes are cells lying within the bone itself and are ‘entrapped’ osteoblasts. They are post-proliferative, representing the most mature differentiation state of osteoblast lineage. There are about 25,000 osteocytes per mm3 of bone. The osteocytes occupy lacunae, which are regularly distributed, and many fine canals called canaliculi radiate from them in all directions. The canaliculi allow the diffusion of substances through the bone. Numerous cell processes from the osteocytes run in the canaliculi in all directions. The canaliculi of osteocytes are arranged in a more perpendicular than parallel direction to the bone surface direction.
As a result of their widespread distribution and interconnections osteocytes are obvious candidates to detect stresses induced in bone and are therefore regarded as the main mechanoreceptors of bone. It has been shown that mechanical stress can be sensed by osteocytes and these cells secrete paracrine factors such as insulin-like growth factor-I (IGF-I) and express c-fos in response to mechanical forces (1).
At the structural level, the appearance of the osteocyte may vary according to its position in relation to the surface layer. Osteocytes which are newly incorporated into bone matrix from the osteoblast layer have high organelle content, similar to osteoblasts. However, as they become more deeply situated with continued bone formation, they appear to be less active. The cell is then seen to have a nucleus with a thin ring of cytoplasmic processes extending from the osteocyte into the canaliculi in the matrix.
The processes of one cell are joined to those of another by gap junctions. These allow cell-to-cell communication and co-ordination of activity. In this feature, they are lack of processes and are isolated. A pericellular space (which might represent a shrinkage artefact) is usually seen to intervene between the cell membrane and the surrounding bone and contains unmineralised matrix and a few collagen fibrils. Osteocytes are also in communication with osteoblasts at the surface.
Osteoblasts
Osteoblasts are specialised fibroblast-like cells of primitive mesenchymal origin called osteoprogenitor cell that originate from pluripotent mesenchymal stem cells of the bone marrow. The evidence of mesenchymal stem cells as precursors for osteoblasts is based on the capacity of bone to regenerate itself both in vivo and in vitro by using cell populations (2). It has been shown that the bone marrow stroma have the capacity to differentiate into osteoblasts, chondroblasts, fibroblasts, adipocytes and myoblasts (3).
In active form, osteoblasts are cuboidal in shape and found on a bone surface where there is active bone formation. Osteoblasts are in contact with each other by means of adherens and gap junctions. These are functionally connected to microfilaments and enzymes (such as protein kinase) associated with intracellular secondary messenger systems. This complex arrangement provides for intercellular adhesion and cell to cell communication.
The principle function of osteoblasts is to synthesize the components that constitute the extracellular matrix of bone. These include structural macromolecules, such as type I collagen, which accounts for about 90% of the organic matrix, as well as numerous proteoglycans, non-collagenous and cell attachment proteins.
Osteoblasts also promote mineralisation of the organic matrix by matrix vesicles, extracellular organelles found in osteoid and associated with matrix calcification (4). Matrix vesicles contain alkaline phosphatase, adenosine triphosphatase (ATPase) and inorganic pyrophosphatase as well as proteinases such as plasminogen activator. They act as seeding sites for hydroxyapatite crystal formation through localized enzymatic accumulation of calcium and phosphate (5). Crystal growth proceeds from these initial foci in matrix vesicles to form spheroids, which gradually coalesce to form a network of apatite crystals. Type I collagen provides an additional mineralisation mechanism by binding and orientating proteins, such as osteonectin, that also nucleate hydroxyapatite.
Regulation of osteoblast differentiation
The systematic and logical study of many mouse mutants generated led to establishment of genetic control in osteoblast differentiation. Many genes have been identified as regulators of cell differentiation.
A. Transcriptional factor
1. Core-binding factor alpha-1
Core-binding factor alpha-1 (Cbfa-1) is an osteoblast-specific gene whose expression is essential for osteoblast differentiation and skeletal patterning (6–8). Deletion of Cbfa-1 in mice leads to mutant animals in which the skeleton comprises only of chondrocytes producing a typical cartilaginous matrix without evidence of bone formation (6, 8, 9). Even, patients with Cbfa-1 mutations develop cleidocranial dysplasia (10). Cbfa-1 function is not only limited to osteoblast cell differentiation. In vivo study has shown that Cbfa-1 also acts as a maintenance factor for differentiated osteoblasts by regulating the level of bone matrix deposited by already differentiated osteoblasts (11).
B. Secreted molecules factor
1. Bone Morphogenetic Proteins (BMPs)
Osteoblasts are cells responsible for the secretion and deposition of bone morphogenetic proteins (BMPs) into the extracellular matrix during bone formation. BMPs, except BMP-1, belong to the transforming growth factor-β (TGF-β) superfamily, members of which are known to regulate the proliferation, differentiation and death of cells in various tissues (12).
The unique activity of BMPs suggests that they regulate osteoblast and chondrocyte differentiation during skeletal development. Identification of skeletal abnormalities in animals and patients with mutations in BMPs genes has been reported (13, 14). However, it is still unclear whether BMPs are involved in bone and cartilage formation after birth. The biological effects of recombinant BMP proteins on osteoblast differentiation have been studied in vitro using cell lines.
In cultures of osteoblast lineage cells, Yamaguchi et al., 1991 (15) determined differential effects of BMP-2 on osteoblasts at various stages of differentiation in vitro. They indicated that BMP-2 preferentially stimulates proliferation and differentiation of osteoprogenitor cells into mature osteoblasts with the ability to synthesize osteocalcin. In MC3T3-E1 cells, BMP-2 and BMP-4 enhance the expression of alkaline phosphatase activity (16, 17). BMP-2 and BMP-3 were significantly found to stimulate collagen synthesis (16).
In mesenchymal cell lines, cultures of C3H10T1/2 cells were used to investigate the role of BMPs. Studies indicated that BMP-2 and BMP-7 enhanced osteoblast-related markers in C3H10T1/2 cells (18, 8). On the other hand, in bone marrow stromal cell cultures, Yamaguchi et al., 1996 (19) demonstrated the effects of BMP-2 on osteoblastic differentiation differ among cell types. The osteogenic potency of each BMP might depend on the cell lineage, the stage of differentiation of the cells and the dose of each BMP.
BMPs originally were identified as an activity that induces ectopic bone formation in muscular tissue, suggesting that BMPs regulate the pathway of differentiation of myogenic cells. Katagiri et al., 1994 (20) examined this and found that BMP-2 inhibited myogenic differentiation of C2C12 myoblasts, and converted their differentiation pathway into osteoblasts.
2. Ihh
Indian hedgehog (Ihh) is one member of the Hedgehog family of growth factors that is expressed in the developing skeleton (21). St Jacques et al., 1999 (22) reported that Ihh mutant mice that survived after birth had a markedly reduced proliferation of chondrocytes result in a failure of osteoblast development in endochondral bones. There was no cortical or trabecular structures in the long bones could be detected histologically and there was no detectable osteocalcin expressed. Thus, Ihh signalling is essential for maturation of the chondrocyte. However, there is no evidence whether this is a direct or indirect consequence of the absence of Ihh signalling in regulation of osteoblast differentiation.
Osteoclasts
Osteoclasts are large multinucleated phagocytic cells derived from the macrophage-monocyte cell lineage (23). They migrate from bone marrow to a specific skeletal site. They may fuse either with existing multinucleate osteoclasts or with each other to form de novo multinucleate osteoclasts, or remain as mononuclear cells to constitute a precursor pool for future recruitment.
The bone microenvironment plays an important role in osteoclast formation and function and is dependent upon local signals from other cells and growth factors sequestrated in the bone matrix. Osteoclasts express the enzyme tartrate resistant acid phosphatase (TRAP), calcitonin receptors, vacuolar proton ATPase and vitronectin receptors (24).
Osteoclasts are involved in bone resorption that contributes to bone remodelling in response to growth or changing mechanical stresses upon the skeleton. Osteoclasts also participate in the long-term maintenance of blood calcium homeostasis. During bone resorption, the osteoclasts resorb the bone surface forming depressions known as Howship’s lacunae.
Resorbing osteoclasts are highly polarized cells containing four structurally and functionally distinct membrane domains. In vitro studies revealed the domains are the ruffled border, the sealing zone, the basal membrane and a new functional plasma membrane domain (25, 26). At sites of active resorption the organic and inorganic components of bone are endocytosed at the ruffled border, transcytosed through the cell in vesicles and liberated into the extracellular space via the plasma membrane domain (25, 26). The ruffled border secretes several organic acids by maintaining sufficiently low pH in the microenvironment at the bone surface, which dissolves the mineral component. The organic matrix is degraded by lysosomal proteolytic enzymes, especially the matrix metalloproteinases (MMPs) including collagenase and gelatinase B and cysteine proteinases (CPs) such as Cathepsin B, L and K (27–29) These extensive exchanges between the cell and bone are effectively sealed off from the extracellular environment by the sealing zone (30).
Regulation of osteoclast differentiation
The systematic and logical study of many mouse mutants generated led to the establishment of genetic control in osteoclast differentiation. Many genes have been identified as regulators of cell differentiation.
A. Transcriptional control
1. op/op
Osteopetrosis (op) is a skeletal condition where there is failure of bone resorption to keep in balance with bone formation. This results in an excessive amount of mineralised bone. Osteopetrotic (op/op) is the classical mouse mutation that controls osteoclast differentiation (31). Mice homozygous for this recessive mutation lack osteoclasts and macrophages. The osteopetrotic phenotype of these mice is not cured by bone marrow transplantation.
2. PU.1
Specific DNA binding proteins regulate the transcription of eukaryotic gene. Many of these DNA binding proteins are unique in their expression and probably serve a general role in gene transcription. Others are restricted in their expression to one or a few cell types. PU box revealed a region containing a purine-rich sequence (5′-GAGGAA-3′). PU.1 is a binding protein, that code for this specific DNA enhancer activity. PU.1 belongs to the member of the family proteins that exhibit tyrosine-specific (ets) domain-containing transcription factor that is expressed specifically in the macrophage and B lymphoid lineages (32). Deletion of PU.1 results in a multilineage defect in the generation of progenitors for B and T lymphocytes, monocytes, and granulocytes (33).
3. c-fos
Another transcription factor that plays a critical role during osteoclast differentiation is c-fos. This factor is the cellular homolog of the v-fos oncogene and is a major component of the AP-1 transcription factor. Deletion of c-fos in mice led to an early arrest of osteoclast differentiation without any overt consequences on osteoblast differentiation (34). Grigoriadis et al., 1994 (35) also showed that mice lacking c-fos factor develop osteopetrosis but have normal macrophage differentiation.
4. Nuclear factor kappa B
Nuclear factor kappa B (NF-κB) is a transcription factor that is composed of five polypeptide subunits; p50, p52, p65, c-Rel, and RelB (36). Mice deficient with both p50 and p52 subunits of NF-κB have impaired macrophages functions that failed to generate mature osteoclasts and B cells and developed osteopetrosis (37). NF-κB plays a critical role in expression of a variety of cytokines involved in early osteoclast differentiation, including interleukin-1 (IL-1), tumour necrosis factor-α(TNF-α), interleukin-6(IL-6) and other growth factors.
5. c-Src
c-Src plays a critical role in the activation of quiescent osteoclasts to become bone-resorbing osteoclasts. Animals lacking this gene developed osteopetrosis although the osteoclast formation was normal. However, it has shown that mature osteoclasts could not form a ruffled border and therefore failed to resorb bone (38).
6. Microphthalmia
This transcription factor was identified by searching for the gene mutated in the microphthalmia (mi) mouse. Heterozygous mi mice have the following defects; loss of pigmentation, reduced eye size and failure of secondary bone resorption (osteopetrosis). In mi mice, osteoclasts differentiate normally, but they fail to resorb bones (39).
B. Secreted molecules factor
1. Macrophage colony-stimulating factor
The gene mutated in osteopetrotic (op/op) mice encodes the growth factor, macrophage colony-stimulating factor (M-CSF). M-CSF plays an important role in osteoclast development. Mutation in M-CSF gene showed a severe osteopetrosis due to absence of osteoclasts (40). Fuller et al., 1993 (41) also identified the role of M-CSF in maintaining the survival and chemotactic behaviour of mature osteoclasts. They showed that M-CSF prevented apoptosis of osteoclasts, enhanced osteoclast motility and inhibited bone resorption.
2. Osteoprotegerin
Simonet et al., 1997 (42) identified a protein which belongs to a member of the tumour necrosis factor (TNF) receptor superfamily that regulated osteoclast differentiation. This molecule, osteoprotegerin (OPG) contained no hydrophobic transmembrane-spanning sequence, indicating that it is a soluble factor. This molecule is identical to osteoclastogenesis inhibitory factor (OCIF). It strongly inhibits osteoclast formation in vitro and in vivo (43).
The OPG/OCIF-deficient mice develop osteoporosis due to an increase in osteoclast number (44, 45). Recombinant of OPG/OCIF blocks osteoclast differentiation from precursor cells in vitro; due to its ability to bind and neutralize osteoprotegerin ligand (OPGL) produced by activated osteoblasts or stromal cells (43).
Recombinant OPG has been used to screen for OPGL on the surface of various cell lines. OPGL has been shown to directly stimulate bone resorption dose-dependently in vitro, and OPG blocked its action in vitro and in vivo (46). Previously, this protein (47) had been cloned and found to be identical to tumour necrosis factor (TNF)-related activation-induced cytokine (TRANCE), RANK-ligand (RANKL) or osteoclast differentiation factor (ODF) (48–49).
3. Receptor activator of NF-κB and its ligand
Receptor activator of NF-κB (RANK) is a membrane bound receptor found on the osteoclast membrane and T cells (48, 50). Transgenic mice expressing RANK develop an osteopetrosis.
The presence of RANK on osteoclasts and their precursors suggested that osteoclast-differentiating factor, residing on stromal cells, may be RANK-ligand (RANKL). RANKL and RANK are members of the TNF and TNF-receptor superfamilies, respectively.
RANKL is present on the membrane of the osteoblast progenitor but also can be found as soluble molecules in the bone microenvironment. The membrane-bound of this protein could be a reservoir of the active molecule. In vitro this protein has all the attributes of a real osteoclast differentiation factor. It favours osteoclast differentiation in conjunction with M-CSF, it bypasses the need for stromal cells and 1, 25 (OH)2 vitamin D3 to induce osteoclast differentiation, and it activates mature osteoclasts to resorb mineralised bone (50).
RANKL is also expressed in abundance by activated T cells, cells that can, in vitro, induce osteoclastogenesis (51, 52). These cells can directly trigger osteoclastogenesis and are probably pivotal to the joint destruction. Indeed, it is the balance between the expression of the stimulator of osteoclastogenesis, RANKL, and of the inhibitor OPG, that dictates the quantity of bone resorbed (53).
RANKL has been shown to activate mature osteoclasts to resorb bone in vitro (46). RANKL-deficient mice lack osteoclasts and develop a severe osteopetrosis and immunological defect (54).
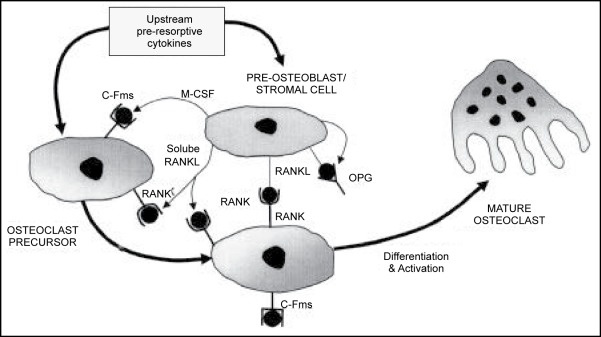
It is possible to summarize the role of OPG-RANK-RANKL in this signal transduction pathway. (Figure 1)
Figure 1.
Relationship of OPG/RANK/RANKL ; The control of osteoclastogenesis that emerged in the relationship of OPG/RANK/RANKL. RANKL, expressed on the surface of preosteoblastic/stromal cells. M-CSF, which binds to its receptor, c-fms, on preosteoclastic cells, appears to be necessary for osteoclast development because it is the primary determinant of the pool of these precursor cells. RANKL, however is critical for the differentiation, fusion into multinucleated cells, activation and survival of osteoclastic cells. OPG put a break on the entire system by blocking the effects of RANKL. Khosla, 2001 (55).
Osteoclast-Osteoblast Relationship
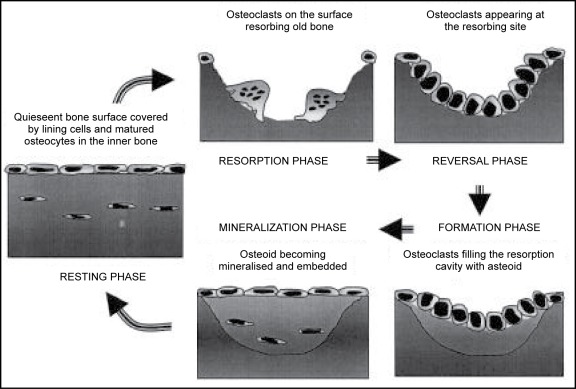
Termination of bone resorption and the initiation of bone formation in the resorption lacunae occur through a coupling mechanism (56). This coupling mechanism ensures that the amount of bone laid down is equivalent to the bone removed during the resorption phase. A model illustrating this ‘coupling’ process is shown in Figure 2.
Figure 2.
Bone Remodelling Process ; Remodelling process is accomplished by cycles of resorption of old bone by osteoclasts and the subsequent formation of bone by osteoblasts. Modified from Manolagas and Jilka, 1995 (57).
During resorption the osteoclasts release local factors from the bone which result in two effects; inhibition of osteoclast function and stimulation of osteoblast activity. Finally, when the osteoclast completes its resorptive cycle, it secretes proteins that serve as a substrate for osteoblast attachment (58).
Conclusion
Bone remodelling is required to preserve the functional capacity of bone. The process of bone remodelling involves the resorption of bone by the activity of osteoclasts on a particular surface, followed by a phase of bone formation by osteoblast. The status of the bone represents the net result of a balance between these two processes. Normally during growth there is a balance between bone resorption and formation. In the normal adult skeleton, bone formation equals resorption and this is a constant dynamic process throughout life.
References
- 1.Lean JM, Mackay A, Chow J, Chambers T. Osteocytic expression of mRNA for c-fos and IGF-I; an immediate early gene response to an osteogenic stimulus. American Journal of Physiology. 1996;270:937–945. doi: 10.1152/ajpendo.1996.270.6.E937. [DOI] [PubMed] [Google Scholar]
- 2.Stein GS, Lian JB. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocrine Review. 1993;14:424–442. doi: 10.1210/edrv-14-4-424. [DOI] [PubMed] [Google Scholar]
- 3.Friedenstein AJ. Precursor cells of mechanocytes. International Review of Cytology. 1976;47:327–359. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- 4.Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. Journal of Cell Biology. 1969;41:59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson HC, Reynolds JJ. Pyrophosphate stimulation of calcium uptake into cultured embryonic bones. Fine structure of matrix vescles and their role in calcification. Developmental Biology. 1973;34:211–227. doi: 10.1016/0012-1606(73)90351-5. [DOI] [PubMed] [Google Scholar]
- 6.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted distruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 7.Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IA, Stamp GWH, Beddington RSP, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 8.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 9.Kim IS, Otto F, Zabel B, Mundlos S. Regulation of chondrocyte differentiation by Cbfa1. Mechanisms of Development. 1999;80:159–170. doi: 10.1016/s0925-4773(98)00210-x. [DOI] [PubMed] [Google Scholar]
- 10.Lee B, Thirunavukkarasu K, Zhou L, Pastore L, Baldini A, Hecht J, Geoffroy V, Ducy P, Karsenty G. Missense mutations abolishing DNA binding OSF2/CBFA1 in patients affected with cleidocranial dysplasia. Nature Genetics. 1997;16:307–310. doi: 10.1038/ng0797-307. [DOI] [PubMed] [Google Scholar]
- 11.Ducy P, Starbuck M, Priemel M, Shen J, Pinero G, Geoffroy V, Amling M, Karsenty G. A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes and Development. 1999;13:1025–1036. doi: 10.1101/gad.13.8.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes and Development. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 13.Kingsley DM, Bland AE, Grubber JM, Marker PC, Russell LB, Copeland NG, Jenkins NA. The mouse short ear skeletal morphogenesis locus is associated with defects in a bone morphogenetic member of the TGFβ superfamily. Cell. 1992;71:399–410. doi: 10.1016/0092-8674(92)90510-j. [DOI] [PubMed] [Google Scholar]
- 14.Thomas JT, Kilpatrick MW, Lin K, Erlacher L, Lembessis P, Costa T, Tsipouras P, Luyten FP. Distruption of human limb morphogenesis by a dominant negative mutation in CDMP1. Nature Genetics. 1997;17:58–64. doi: 10.1038/ng0997-58. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi A, Katagiri T, Ikeda T, Wozney JM, Rosen V, Wang EA, Kahn AJ, Suda T, Yoshiki S. Recombinant human bone morphogenetic protein-2 stimulates osteoblastic maturation and inhibits myogenic differentiation in vitro. Journal of Cell Biology. 1991;113:681–687. doi: 10.1083/jcb.113.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takuwa Y, Ohse C, Wang EA, Wozney JM, Yamashita K. Bone morphogenetic protein-2 stimulates alkaline phosphatase activity and collagen synthesis in cultured osteoblastic cells, MC3T3-E1. Biochemical and Biophysical Research Communications. 1991;174:96–101. doi: 10.1016/0006-291x(91)90490-x. [DOI] [PubMed] [Google Scholar]
- 17.Nakase T, Takaoka K, Masuhara K, Shimizu K, Yoshikawa H, Ochi T. Interleukin-1β enhances and tumour necrosis factor-α inhibits bone morpogenetic protein-2 induce alkaline phosphatase activity in MC3T3-E1 osteoblastic cells. Bone. 1997;11:17–21. doi: 10.1016/s8756-3282(97)00038-0. [DOI] [PubMed] [Google Scholar]
- 18.Katagiri T, Yamaguchi, Ikeda T, Yoshiki S, Wozney JM, Rosen V, Wang EA, Tanaka H, Omura S, Suda T. The non-osteogenic mouse pluripotent cell line, C3H1OT1/2 is induced to differentiate into osteoblastic cells by recombinant human bone morphogenetic protein-2. Biochemical and Biophysical Research Communications. 1990;172:295–299. doi: 10.1016/s0006-291x(05)80208-6. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi A, Ishizuya T, Kintou N, Wada Y, Katagiri T, Wozney JM, Rosen V, Yoshiki S. Effects of BMP-2, BMP-4 and BMP-6 on osteoblastic differentiation of bone marrow-derived stromal cell lines, ST2 and MC3T3-G2/PA6. Biochemical and Biophysical Research Communications. 1996;220:366–371. doi: 10.1006/bbrc.1996.0411. [DOI] [PubMed] [Google Scholar]
- 20.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. Journal of Cell Biology. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Developmental Biology. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- 22.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes and Development. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker DG. Osteoporosis cured by temporary parabiosis. Science. 1973;180:875. doi: 10.1126/science.180.4088.875. [DOI] [PubMed] [Google Scholar]
- 24.Lee SK, Goldring SR, Lorenzo JA. Expression of the calcitonin receptor in bone marrow cell cultures and in bone: a specific marker of the differentiated osteoclast that is regulated by calcitonin. Endocrinology. 1995;136:4572–4581. doi: 10.1210/endo.136.10.7664679. [DOI] [PubMed] [Google Scholar]
- 25.Salo J, Lehenkari P, Mulari M, Metsikkö K, Väänänen HK. Removal of osteoclast bone resorption products by transcytosis. Science. 1997;276:270–273. doi: 10.1126/science.276.5310.270. [DOI] [PubMed] [Google Scholar]
- 26.Blair H, Teitelbaum SL, Ghiselli R, Gluck S. Osteoclastic bone resorption by a polarised vacuolar proton pump. Science. 1989;245:855–857. doi: 10.1126/science.2528207. [DOI] [PubMed] [Google Scholar]
- 27.Hill PA, Docherty A, Bottomley K, O’Connell JP, Morphy JR, Reynolds SJ, Meikle MC. Inhibition of bone resorption in vitro by selective inhibitors of gelatinase and collagenase. Biochemical Journal. 1995;308:167–175. doi: 10.1042/bj3080167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill PA, Buttle D, Jones S, Boyde A, Murata M, Reynolds JJ, Meikle MC. Inhibition of bone resorption by selective inactivators of cysteine proteinases. Journal of Cellular Biochemistry. 1994;56:118–130. doi: 10.1002/jcb.240560116. [DOI] [PubMed] [Google Scholar]
- 29.Drake FH, Robert AD, James IE, Conver JR, Debouck CC, Richardson S, Lee-Rykaczewski E, Coleman L, Rieman D, Barthlow R, Hastings G, Gowen M. Cathepsin K but not Cathepsins B, L or S is abundantly expressed in human osteoclasts. Journal of Biological Chemistry. 1996;271:12511–12516. doi: 10.1074/jbc.271.21.12511. [DOI] [PubMed] [Google Scholar]
- 30.Silver IA, Murrills RJ, Etherington DJ. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Experimental Cell Research. 1988;175:266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- 31.Marks SCJ, Lane PW. Osteopetrosis, a new recessive skeletal mutation on chromosome 12 of the mouse. Journal of Heredity. 1976;67:11–18. doi: 10.1093/oxfordjournals.jhered.a108657. [DOI] [PubMed] [Google Scholar]
- 32.Klemsz MJ, McKercher SR, Celada A, Van Beveren C, Maki RA. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell. 1990;61:113–124. doi: 10.1016/0092-8674(90)90219-5. [DOI] [PubMed] [Google Scholar]
- 33.Scott EW, Simon MC, Anastasi J, Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science. 1994;265:1573–1577. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- 34.Johnson RS, Spiegelman BM, Papaioannou V. Pleitropic effects of a null mutation in the c-fos proto-oncogene. Cell. 1992;71:577–586. doi: 10.1016/0092-8674(92)90592-z. [DOI] [PubMed] [Google Scholar]
- 35.Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, Wagner EF. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodelling. Science. 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- 36.Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes and Development. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 37.Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U. Requirement for NF-κB in osteoclast and B-cell development. Genes and Development. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boyce BF, Yoneda T, Lowe C, Soriano P, Mundy GR. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. Journal of Clinical Investigation. 1992;90:1622–1627. doi: 10.1172/JCI116032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodgkinson CA, Moore KJ, Nakayama A, Steingrimsson Copelan NG, Jenkins NA, Arnheiter H. Mutations at the mouse micropthalmia are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993;74:395–404. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida H, Hayashi SI, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa SI. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 41.Fuller K, Owens JM, Jagger CJ, Wilson A, Moss R, Chambers TJ. Macrophage colony-stimulating factor stimulates survival and chemotactic behavior in isolated osteoclasts. Journal of Experimental Medicine. 1993;178:1733–1744. doi: 10.1084/jem.178.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliot R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Amgen EST Program. Boyle WJ. Osteoprogeterin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 43.Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M, Kanno T, Murakami A, Tsuda E, Morinaga T, Higashio K. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): A mechanism, by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;139:1329–1337. doi: 10.1210/endo.139.3.5837. [DOI] [PubMed] [Google Scholar]
- 44.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes and Development. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, Sato Y, Nakagawa N, Yasuda H, Mochizuki S, Gomibuchi T, Yano K, Shima N, Washida N, Tsuda E, Morinaga T, Higashio K, Ozawa H. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor /osteoprotegerin. Biochemical and Biophysical Research Communications. 1998;247:610–615. doi: 10.1006/bbrc.1998.8697. [DOI] [PubMed] [Google Scholar]
- 46.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliot R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 47.Wong BR, Josien R, Lee Sy, Sauter B, Li HL, Steinman RM, Choi Y. TRANCE [Tumor Necrosis Factor (TNF)-related activation-induced cytokine], a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. Journal of Experimental Medicine. 1997;186:2075–2080. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, Dubose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 49.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki SI, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitor factor and is identical to TRANCE/RANKL. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burgess TL, Qian YX, Kaufman S, Ring BD, Van G, Capparelli C, Kelley M, Hsu H, Boyle WJ, Dunstan CR, Hu S, Lacey DL. The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. The Journal of Cell Biology. 1999;14:527–538. doi: 10.1083/jcb.145.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horwood NJ, Kartsogiannis V, Quinn JM, Romas E, Martin TJ, Gillespie MT. Activated T lymphocytes support osteoclast formation in vitro. Biochemical and Biophysical Research Communications. 1999;265:144–150. doi: 10.1006/bbrc.1999.1623. [DOI] [PubMed] [Google Scholar]
- 52.Rifas L, Arackal S, Weitzmann MN. Inflammatory T cells rapidly induce differentiation of human bone marrow stromal cells into mature osteoblasts. Journal of Cellular Biochemistry. 2003;88:650–659. doi: 10.1002/jcb.10436. [DOI] [PubMed] [Google Scholar]
- 53.Hofbauer LC, Gori F, Riggs LR, Lacey DL, Dunstan CR, Spelsberg TC, Khosla S. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999;140:4382–4389. doi: 10.1210/endo.140.10.7034. [DOI] [PubMed] [Google Scholar]
- 54.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Caparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penniger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 55.Khosla S. Minireview: The OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 56.Parfitt AM. The coupling of bone formation to bone resorption: a critical analysis of the concept and of its relevance to the pathogenesis of osteoporosis. Metabolic Bone Disease and Related Research. 1982;4:1–6. doi: 10.1016/0221-8747(82)90002-9. [DOI] [PubMed] [Google Scholar]
- 57.Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodelling. Emerging insights into the pathophysiology of osteoporosis. New England Journal of Medicine. 1995;332:305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- 58.McKee MD, Farach-Carson MC, Butler WT, Hauschka PV, Nanci A. Ultrastructural immunolocalization of non-collagenous (osteopontin and osteocalcin) and plasma (albumin and α2HS-Glycoprotein) proteins in rat bone. Journal of Bone and Mineral Research. 1993;8:485–496. doi: 10.1002/jbmr.5650080413. [DOI] [PubMed] [Google Scholar]