Abstract
Oral mucositis is one of the most common toxicities observed during radiotherapy and chemotherapy treatment for cancers. Mucositis results in sore mouth, altered taste sensation, pain and dysphagia leading to malnutrition. Left untreated, oral mucositis leads to ulceration, orodental infection, bleeding and discontinuation of effective radiotherapy or chemotherapy. Frequent hospitalization, enteral or parenteral nutrition, increased demand for analgesics ultimately account for increased cost of healthcare. Quantification of oral mucositis using standardized grading system is important for appropriate evaluation, reporting and management. In the recent past there is a paradigm shift in the pathobiology of cancer therapy related mucositis. Clear understanding of its pathogenesis is essential for the formulation of effective mucositis care. Numerous drug therapies, radiation techniques and oral care protocols have been tried in the past to reduce oral mucositis, None have proven to be consistently effective. Current trends for the prevention and treatment of oral mucositis is multi-targeted treatment supplemented by aggressive oral hygiene, reactive oxygen species (ROS) inhibitors, growth factors and use of specific topical agents to improve treatment of oral mucositis in future.
Keywords: Oral mucositis, radiotherapy, chemotherapy, prevention, treatment
Introduction
Head and neck cancers is an important group of malignancy accounting for 5% of all cancers (1). The conventional treatment is surgery, radiation and/or chemotherapy. Among all modalities, radiotherapy is a frequent option in more than 60% of cases, especially when the disease is relatively advanced. Extensive use of multi-modality approach, newer radiotherapy techniques and concurrent chemoradiotherapy resulted in improved local control and survival. This approach has increased the risk of complications especially mucositis in the oropharyngeal mucosa. There has been no established guideline in managing mucositis. This acute and debilitating side-effect is important and requires attention. The magnitude of mucositis is under-reported by the clinicians and researchers alike in their publications. Mucositis is considered as an inevitable or natural consequence of radiation therapy or chemotherapy. Chemotherapy too resulted in significant mucositis while using specific types of regimens for breast cancer, sarcomas, and conditioning regimen for marrow transplantation in leukemias. Radiation mucositis starts appearing towards 2nd and 3rd week of conventional radiotherapy and reach maximum at a cumulative dose of 50Gy and gradually decline towards 5th to 7th week as it coincide with reduction of field size in classical “shrinking field technique”. Complete resolution of mucositis is seen at around 6-weeks post radiotherapy (1). The problem of mucositis is further complicated by the use of altered fractionation schedule or concurrent chemoradiotherapy regimens. Following interstitial brachytherapy, mucositis begins to appear 7–10 days after removal and maximal at 2-weeks post removal of radioactive sources. The mucositis generally heals by 6-weeks, unless the implanted volume is large, in which case, complete healing may take several months3. Chemotherapy induced mucositis appears towards the end of 10th day and subsides at the end of another week. The above manifestation appears just at the peak time of bone marrow suppression and agranulocytosis as the mucosa and bone marrow cells have similar growth kinetics.
Sites of oral mucositis
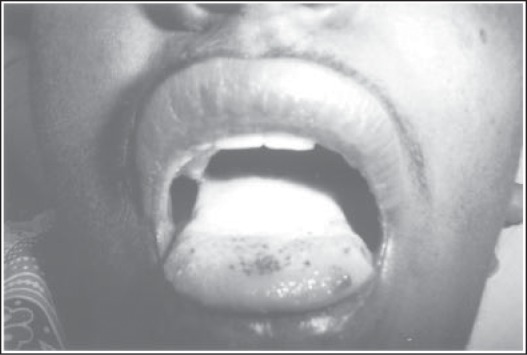
The mucosa of the oral cavity and oropharynx is anatomically and embryologically part of the gut, thus both gut mucosa and oropharyngeal mucositis appear at the same time in systemic chemotherapy induced mucositis (Figure 1). The oral mucosa extends from inner lining of the buccal mucosa, bucco-gingival sulcus, labial folds, undersurface of tongue, floor of the mouth, soft-palate, pharynx, dorsum of the tongue, gingival ridges and hard palate. The gingival ridge, hard palate and dorsum of the tongue are keratinizing and are fixed, while rest mucosae are movable. Chemotherapy induced mucositis preferentially manifest at the movable parts of the mucosa whereas radiation mucositis involve both movable and fixed mucosa of the oral cavity (4). Almost all radiotherapy techniques in this anatomical site involve oral mucosa. Cancers involving nasopharynx, oropharynx and laryngopharynx involve mucosa extending from base of skull to upper esophagus. Mucositis is observed in all subsites. Oral mucosa too is of the preferential target of somatotoxic chemotherapy.
Figure 1:
Oral cavity showing RTOG grade-IV micositis
Pathogenesis of mucositis
Traditionally it was believed that somatotoxic dose of radiation and chemotherapy induce mucositis due to the depletion of basal cell layer stem cells of the oral mucosa. Due to loss of basal cell layer stem cells, the uppermost layers of the mucosal epithelium slough off leading to breach in the continuity of the mucosa as ulceration. Ulceration breaches the barrier to the invading toxin producing bacteria leading to septicaemia. Exposure of mucosa causes irritation of the nerve endings leading to oral and pharyngeal pain. Recently we have witnessed several important developments in the pathogenesis of mucositis. Careful assembly of basic science data resulted in a defined complement cascade pathway. Sonis et al (5) put forth a putative 5-step pathogenesis of chemotherapy and radiation induced mucositis that explain well in the etiology of mucositis also it helps in the formulation of effective management in future. The pathways are as follows :
1. Phase of Initiation
Radiotherapy and chemotherapy induce DNA strand break and cause instant basal cell injury. At the same time, the primary initiator in a cascade of complimentary events contributing to oral mucositis appears to be the product of oxidative stress and of reactive oxygen species (ROS). The later could directly damage cells, tissues and blood vessels. Studies have shown that ROS are consistently produced when somatotoxic agents are applied and when inhibited reduce the mucosal damage (figure 2). In addition ROS also affect other tissues to stimulate transcription factors in the next phase
Figure 2:
Illustration showing phase of initiation: Adopted from Sonis ST. Pathobiology of micositis. Nat Rev Cancer 2004; 4: 227–284.
2. Phase of Primary damage response
Following DNA damage and release of ROS, transcription factors are activated. Among them the nuclear factor-kB (NF-kB) appears to be the most prominent. It is activated by both radiation and chemotherapy and could upregulate genes that lead to the production of a group of proinflammatory cytokines, including tumor necrosis factor a (TNF-a), interleukin 1b (IL 1b) and interleukin 6 (IL 6). These putative cytokines exert cellular damage and induce apoptosis. Other non-DNA injury could lead to mucosal damage. Both radiation and cytotoxic agents hydrolyze cell membrane lipid sphingomyelin by stimulating sphingomyelinase and ceramide synthetase, thus activate ceramide pathways leading to apoptosis. Fibronectin degradation products activate macrophages leading to stimulation of matrix metalloproteinsases, which cause direct tissue injury or an increase in production of TNF-a.
3. Phase of Signal amplification
TNF-a activate ceramide and capase pathways leading to tissue damage and activate the transcription pathways mediated through NF-kB. In a feedback loop, these process results in further production of TNFa, IL 1b, and IL 6. They also activate matrix metalloproteinases leading to direct tissue injury as described earlier.
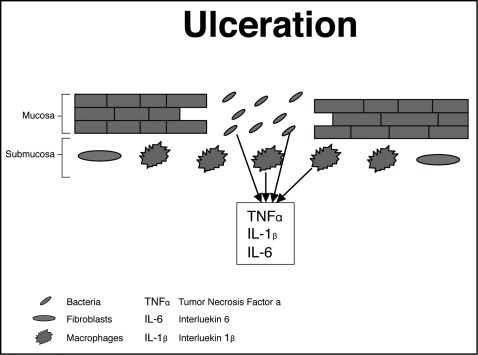
4. Phase of Ulceration
The summation of all metabolic insult results in ulceration, which is the most significant pathology in the host following radiotherapy and chemotherapy. As these patients are often neutropenic, ulcerated mucosa allows invasion of micro-organisms from the mouth into the systemic circulation causing life-threatening septicaemia. In addition in this stage, products from the colonizing bacteria invade the submucosal tissues. This in turn activates macrophages that release further pro-inflammatory cytokines. Damaging enzymes are then produced by inflammatory cells that move to the base of the ulcerated tissue (figure 3).
Figure 3:
Illustration showing phase of ulceration: Adopted from Sonis ST. Pathobiology of micositis. Nat Rev Cancer 2004; 4: 227–284.
5. Phase of Healing
The extra-cellular matrix initiates the healing process of oral mucositis by signaling the renewal of the epithelial cell proliferation and differentiation. Oral microbial flora is re-established, and white cell count returns to normal. However epithelial tissue changes secondary to radiotherapy and chemotherapy remain, and there is residual angiogenesis, increasing the patient’s risk of oral mucositis with subsequent courses of anti-mitotic therapy.
Impact of mucositis
In conventional radiotherapy schedule about 25% of patients receiving radiotherapy to head and neck cancer suffer from symptomatic mucositis requiring treatment. In altered fractionation schedules the mucositis incidence increases to 25–50%. In the current practice of concurrent chemoradiotherapy schedules, the mucositis incidence could be as high as 60% and in toxic schedules of chemotherapy the mucositis can be up to 80% (6, 7). Mucositis leads to ulceration and painful swallowing requiring narcotic analgesics. Modern radiotherapy technique like intensity modulated radiotherapy (IMRT) produce patchy mucositis near the primary tumor similar to interstitial brachytherapy. Conditioning regimens using whole body radiotherapy or high dose melphalan result in severe mucositis in 90% of cases as previously reported, requiring total parenteral nutrition. Higher grades of mucositis are associated with loss of taste, decreased intake of food and fluids, increased bleeding, pain, malnutrition, loss of voice and ultimately lower quality of life. More importantly mucositis is a dose limiting toxicity necessitating dose reduction of chemotherapy in 25% of patients and in the worst situation discontinuation of chemotherapy. Discontinuation of radiotherapy or increased overall duration of radiotherapy course is known to decrease local control rate and ultimately survival (8, 9). Alteration of chemotherapy schedule or sub-optimal dose of drug can lead to the development of drug resistance and sub-optimal treatment outcome. Thus in general mucositis increases the cost of health care resources and personnel attention (10).
Mucositis assessment tools
There are many mucositis assessment scales available and some are still under development worldwide. As the scales have been used by radiation oncologists, medical oncologists, head and neck surgeons, hematologists, somatologists, dentists and nurses, the variability of the use of a particular scale is inevitable. However a unified mucositis tool could be of immense value in a multidisciplinary set up or for inter-institutional comparison. The most popular mucositis scales are radiation therapy oncology group (RTOG) for radiotherapy, World Health Organization (WHO) for chemotherapy, common toxicity criteria of NCI for chemotherapy and radiotherapy. Oral Mucositis Assessment Scale (OMAS) is a recent tool developed to evaluate detail anatomical sites of mucositis and may be appropriate for multidisciplinary healthcare teams (6), (Table-1). While assessing mucositis one must evaluate head and neck cancer specific quality of life scale. The most commonly used specific QoL tool is EORTC QLQ H&N 35 questionnaire. The other parameters worth mention are overall radiotherapy period, duration of mucositis, duration of hospitalization, requirement for TPN or tube feeding, and weight loss. The above secondary endpoints influence health care expenses (10). Future assessment scale should be user friendly, easy to understand, and practicable during clinical assessment.
Management perspectives
There are extensive literature exist in the management of mucositis. It is one of the side effect where there are more than 500 trials conducted for its management (11). The management options could be grouped for simplicity as improvement of orodental hygiene, radiotherapy technique, and drug therapies. The drug therapy could be summarized as antibiotics, anti-inflammatory agents, growth factors, free radical scavengers, laser therapy and topical agents. The individual options are described as follows:
Improvement of oral care
Orodental status improvement is one of the hallmarks of optimal head and neck cancer care. In advanced oncology centers participation of dentists in the multidisciplinary team is a norm (12). However their participation in mucositis management is rarely observed in practice. Most head and neck cancers show sign of orodental infection at the outset. Several studies have shown a reduction in oral mucositis in patients who received oral care to remove source of infection before and during their cancer treatment, whereas other studies have not encountered such changes (13, 14). Two different studies have shown that standardized oral care protocols reduced mucositis in one group (15) and oral pain in other16. Many oncologists feel that oral care with cancer therapy is beneficial but the effect on mucositis is questionable.
Mucosal sparing radiation therapy technique
Radiotherapy technical measures should not be underestimated for the prevention of mucositis. Simple radiotherapy beam modifying devices namely mouth bite, gauze packing, palatal shield, retractors etc can protect part of the mucosa from the radiation (17). Translational research on radiobiology has shown that dose per fraction, total dose, interfraction interval and overall treatment duration affect the extent of mucositis. Altered fractionation techniques like continuous hyperfractionated accelerated radiotherapy (CHART), continuous accelerated 7-days-a-week radiotherapy (CAIR), concomitant boost and hyperfractionation schemes increases the incidence of mucositis (18). Hence lower dose per fraction, 5-fractions per week, optimal interfraction interval and conservative field size could reduce mucositis. Modern radiotherapy techniques using IMRT could spare ∼30% of mucosa during radical radiotherapy to head and neck areas where the dose objectives were applied to mucosal volume (19).
Mucositis pain control
Control of pain is a frustrating experience for both patients and physician alike. In severe form of mucositis, aggressive analgesia with intravenous opoids is required. A modification of patient controlled analgesia, where individual pharmacokinetic profile for morphine were used to tailor for infusion rate of each patient was compared to traditional patient controlled analgesia. The pharmacokinetically based patient controlled analgesia was superior to conventional patient controlled analgesia in term of relief of oral mucositis pain and even though more morphine was used by the former group, there was no increase in the side-effects of morphine. When morphine is combined with alfentanil, the pain control was more effective then alfentanil alone. Capsiacin an active ingredient of chili peppers act by desensitizing some neurons to provide temporary pain relief. However the evidence is not enough to support above claim. The use of aspirin mucilage and xylocaine viscous gurgle and oral rinse are being used in practice in mucositis related pain.
Infection control
Infection is an important component in the pathogenesis of oral mucositis especially in the ulcerative phase. This aspect has been explored extensively in the literature. Two reports have demonstrated the use of topical antibiotic lozenge containing polymyxin-B, tobramycin and amphotericin-B reduced oral mucositis due to radiotherapy (20, 21). Chlorhexidine mouthwash too shown to reduce oral mucositis especially among patient receiving hematopoietic stem cell transplantation (HSCT) and radiation therapy (22, 23). However similar study by other workers did not show any difference (24, 25). Though mucositis believed to encourage systemic streptococcal infection among neutropenic patients however clindamycin prophylaxis did not show any benefit in HSCT patients (26). Candida organisms do not appear to involve in the etiology of mucositis but should be borne in mind to lessen the potential for the spread of systemic infection through ulcerated tissue. Fluconazole and clotrimazole prophylaxis have been shown to reduce candidiasis in cancer patients (27, 28).
Anti-inflammatory agents
As described in the pathobiology, inflammation is an important component of mucositis. Hence inflammation control is one of the measures to control mucositis. Prostaglandin agent dinoprostone (prostaglandin E2), misoprostol (Prostaglandin E1) and prednisolone have been used; however the results are not encouraging. Another agent pentoxifylline has been known to antagonize TNF-a, but was unsuccessful to control mucositis. Benzydamine, a non-steroidal anti-inflammatory agent that inhibit TNF-a, shown to be effective to control oral mucositis and pain due to radiotherapy (29).
Reactive Oxygen Species (ROS) inhibitors
ROS production is the first step of initiation of mucositis following radiotherapy and chemotherapy. Hence inhibition of the ROS could be a promising option of mucositis prevention. Amifostine; a thiol compound and a potent ROS scavenger found effective to prevent DNA damage following radiotherapy. Amifostine may protect endothelium, salivary glands and connective tissues and found to reduce IL6 and TNF-a in the blood of cancer patients (30, 31). There are conflicting data available in favor and against the use of amofostine for mucositis treatment(32–35). Amifostime might protect tumors from the effects of radiation in addition to protection of the normal tissues. Hence FDA has approved amifostine for the prevention of xerostomia in head and neck cancer radiotherapy and prevention of nephrotoxicity in cisplatinum based chemotherapy in ovarian cancer. Other ROS inhibitors like N-acetylcystine (NAS), manganese superoxide dismutase and benzydamine might have promising role in prevention of radiation mucositis.
Salivary function modifiers
Some of the chemotherapeutic agents secreated through saliva leading to mucositis. Etoposide and 5-fluorouracil are known to secreate through saliva. Propanthelin, an anti-cholinergic agent has been shown to reduce oral mucositis among patients receiving etoposide chemotherapy. Contrary to the above concept, salivary gland stimulation enhances mucosal healing by the production of epidermal growth factor (EGF). Azelastine an agent reducing cytokine release from lymphocyte has shown to reduce mucositis. Oral cooling therapy with ice cubes induces vasoconstriction of the oral mucosa results in decrease drug delivery of 5-fluorouracil to the mucosa, thereby reduced 5-FU induced mucositis in two studies (36, 37). Glutamine is an essential amino acid mediate various metabolism including nitrogen metabolism has shown to reduce mucositis in animal studies. A glutamine analogue AES014 have shown to reduce symptomatic mucositis among patients undergoing chemotherapy (38).
Topical agents
The idea of applying topical agents for the control of mucositis is because of its simplicity. An ideal agent should come in contact with the oral and pharyngeal mucosa for a prolonged period. Sucralfate is a coating agent being used extensively in peptic ulcer thereby encourages ulcer healing. Many studies using sucralfate have failed to demonstrate any benefit (39–42). Sodium alginate is another coating agent also used in esophagitis and gastritis could be used as a coating agent to act as a barrier on the oral ulcers. A study from Japan shown benefit in oral mucositis (43). Hydroxpropyl cellular gel provides good adherence and coverage to localized areas of ulceration. It was evaluated as a coating agent in oral mucositis secondary to chemotherapy. Oral pain was reduced, and duration of adhesion was commonly seen for at least 3 hours (44). Similarly polyvinylpyrrolidone/sodium hyaluronate gel has been shown to reduce oral mucositis in two preliminary studies (45, 46). Topical application of pure natural honey is a natural product being explored for the treatment and prevention radiation mucositis. It is an antibiotic, a nutritional supplement, an ROS scavenger, and suppressor of proinflammatory cytokine IL6 and TNF-a. A comparative clinical study has shown significant reduction in the RTOG grade-3 and 4 mucositis in addition to positive gain in the body weight during radiotherapy (47). Further multicenter studies are ongoing to prove the benefit of this simple and cost-effective treatment.
Laser therapy
Low energy Helium/Neon (He/Ni) laser therapy was tried for the control of symptomatic mucositis induced by chemotherapy. It has been postulated that laser exert its effect on mitochondria and on ROS that could protect against chemotherapy induced mucositis. The initial studies are encouraging(48–50).
Growth factors
Following the explosion of knowledge on basic science on treatment related mucositis, the researchers are trying to use specific targeted agents to attack specific pathway of the mucositis cascade pathways. The targeted agents must inhibit at multiple site, as the process of mucositis is multi-targeted. So far there is no ideal growth factor that is 100% effective against mucositis. However there are numerous specific agents being developed to target mucositis. The first growth factor to be used in mucositis was granulocyte colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF). Both agents have been used as parenteral and topical agents. The drugs stimulate neutrophil production. It was felt that by reducing neutropenia with these growth factors during chemotherapy, infection is reduced thereby limit mucositis as infection might be a co-factor in mucositis. However the results are conflicting (51–54). In a randomized trial comparing human placental extract with aspirin gargle showed a decrease in the oral pain and progression of grade-3 mucositis (55). Human placental extract was considered as a growth factor for oral epithelium. Fibroblast growth factor, Transforming growth factor beta-3 and interleukin-11 are being used in mucositis with marginal benefit (56,57). Keratinocyte growth factor is the most successful growth factor being studies extensively for the management of mucositis. It is the first such agent being approved by FDA for the treatment of chemotherapy induced mucositis. Recent findings indicate that KGF signaling induces expression of keratinocyte, endothelial cells and fibroblasts. So activation could be involved in the protection against mucositis. Paliformin a keratinocyte growth factor has been shown to reduce mucositis in patients receiving hematopoietic stem cell transplantation. It reduced grade 3 and 4 mucositis significantly and in addition, decreases the duration of mucositis, reduced oral pain, decreased use of narcotic analgesia and requirement for TPN (58, 59). Two other FGFs, FGA10 and FGF20 have been under evaluation in animal and clinical trials.
Though there are lots of improvements in the appropriate management of oral mucositis. Further preclinical and clinical trials are ongoing to develop new agents to improve therapy further. The dipeptidyl peptidase resistant analog of glucagons-like peptide-2 teduglutide is trophic for the intestinal epithelium. Booth et al reported that the subcutaneous administration of teduglutide before irradiation protected clonogenic stem cells in mice (60). In contrast, van’t Land et al studied lactoferrin an inhibitor of GLP-2-mediated cell proliferation, reduced methotrexate-induced intestinal injury in a rat model (61). Mesna, an uroprotector thiol compound. When administered concurrently with alkylating ifosfamide, mesna ameliorated intestinal apoptosis, hypoproliferation and mucosal atrophy (62). Beck et al studied the role of intestinal trefoil factor in intestinal damage induced by chemotherapy and radiotherapy (63). They reported that mice deficient in intestinal trefoil factor were more susceptible to irradiation and chemotherapy-mediated injury. Results of in vitro studies suggest that trefoil factor has potential value for intervention in future.
In summary management of cancer treatment related mucositis is rapidly evolving. The causes of mucositis is multifactorial, hence the management option must be designed to achieve maximum mucositis control by manipulating multiple cascade pathways. No one targeted treatment is successful hence probably a combination of approach is the trend of care in future. Orodental care, repeated salt soda mouth rinse, use of growth factors and topical therapy could be the answer at present. A collaborative approach with multi-center randomized trial is necessary to test a particular type of new agent used for the management of mucositis.
References
- 1.Mohanti BK, Bahadur S, Lal P, et al. Cancer of the Head and Neck. In: Rath GK, Mohanti, editors. Text Book of Radiation Oncology: Principles and Practice. BI Churchill Livingstone; New Delhi: 2001. pp. 131–199. [Google Scholar]
- 2.Loprinzl CL, Gastineau DA, Foote RL. Oral complications. In: Abeloff MD, Armitage JO, Niederhuber JE, et al., editors. Clinical Oncology. Elsevier Churchill Livingstone; Philadelphia: 2004. pp. 775–792. [Google Scholar]
- 3.Parsons JF. The effect of radiation tissue of the head and neck. In: Million RR, Cassisi NJ, editors. Management of Head and Neck Cancer. A Multidisciplinary Approach. Philadelphia: JB Lippincoat; 1984. pp. 175–207. [Google Scholar]
- 4.Redding SW. Cancer Therapy Related Oral Mucositis. J Dental Education. 2005;69:919–929. [PubMed] [Google Scholar]
- 5.Sonis ST. The patholobiology of mucositis. Nat Rev Cancer. 2004;4:277–284. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 6.Trotti A. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253–262. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 7.Al-Saaraf M, LeeBlanc M, Giri PG, et al. Chemoraditherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: a phase-III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–1317. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 8.Cox JD, Pajak TF, Marcial VA, et al. Interruption adversely affect local control and survival with hyperfractionation radiation therapy of carcinoma of the upper respiratory and digestive tracts: New evidence for accelerated proliferation from Radiation Therapy Oncology Group Protocol 8313. Cancer. 1992;69:2744–2748. doi: 10.1002/1097-0142(19920601)69:11<2744::aid-cncr2820691119>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Pajak TF, Laramore GE, Marcial VA, et al. Elapsed treatment days-a critical item for radiotherapy quality control review in head and neck trials: RTOG report. Int J Radiat Oncol Biol Phys. 1991;20:13–20. doi: 10.1016/0360-3016(91)90132-n. [DOI] [PubMed] [Google Scholar]
- 10.Elting LS, Cooksley C, Chambers M, et al. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy based mucositis. Cancer. 2003;98:31–539. doi: 10.1002/cncr.11671. [DOI] [PubMed] [Google Scholar]
- 11.Worthington HV, Clarkson JE, Eden OB. Intervention for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2006;(issue-3):1–32. [Google Scholar]
- 12.Sonis ST, Fey EG. Oral complications of cancer therapy. Oncology. 2002;16:680–686. [PubMed] [Google Scholar]
- 13.Dudjak L. Mouth care for mucositis due to radiation therapy. Cancer Nurs. 1987;10:131–140. [PubMed] [Google Scholar]
- 14.McGuire D, Owen D, Peterson D. Nursing interventions for acute oral pain and mucositis. Oncol Nurs Forum. 1998;25:341. [Google Scholar]
- 15.Levy-Polack MP, Sebelli P, Polack NL. Incidence of oral complications and application of a preventive protocol in children with acute leukemia. Spec Care Dentist. 1998;18:189–193. doi: 10.1111/j.1754-4505.1998.tb01738.x. [DOI] [PubMed] [Google Scholar]
- 16.Cheng KK, Molassiotis A, Cheng AM, et al. Evaluation of an oral care protocol intervention in the prevention of chemotherapy induced oral mucositis in pediatric cancer patients. Eur J Cancer. 2001;37:2056–2063. doi: 10.1016/s0959-8049(01)00098-3. [DOI] [PubMed] [Google Scholar]
- 17.Kaanders JH, Fleming TJ, Ang KK, et al. Devices valuable in had and neck radiotherapy. Int J Radiat Oncol Biol Phys. 1992;23:639–645. doi: 10.1016/0360-3016(92)90023-b. [DOI] [PubMed] [Google Scholar]
- 18.Skladowski K, Maciejewski B, Golen M, et al. Continuous accelerated 7-days-a-week radiotherapy for head-and-neck cancer: Long-term results of phase-III clinical trial. Int J Radiat Oncol Biol Phys. 2006;66:706–713. doi: 10.1016/j.ijrobp.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Sanguineti G, Endres EJ, Gun BG, Parker B. Is there a “mucosa-sparing” benefit of IMRT for head-and-neck cancer? Int J Radiat Oncol Biol Phys. 2006;66:931–938. doi: 10.1016/j.ijrobp.2006.05.060. [DOI] [PubMed] [Google Scholar]
- 20.Okuno SH, Foote RL, Loprinzi CL, et al. A randomized trial of a non-absorbable antibiotic lozenge given to alleviate radiation-induced mucositis. Cancer. 1997;79:2193–2199. doi: 10.1002/(sici)1097-0142(19970601)79:11<2193::aid-cncr18>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 21.Symonds RP, Mcllroy P, Khorrami J, et al. The reduction of radiation mucositis by selective decontamination of antibiotic pastille: A placebo controlled double-blind trial. Br J Cancer. 1996;74:312–317. doi: 10.1038/bjc.1996.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferretti GA, Ash RC, Brown RT, et al. Control of oral mucositis and candidiasis in marrow transplantation: a prospective double-blind trial of chlorhexidine digluconate of oral rinse. Bone Marrow Transplant. 1988;3:483–493. [PubMed] [Google Scholar]
- 23.Samaranayake LP, Robertson AG, MacFarlane TW, et al. The effect of chlorhexidine and benzedamine mouthwashes on mucositis induced by therapeutic irradiation. Clin Radiol. 1988;39:291–294. doi: 10.1016/s0009-9260(88)80538-5. [DOI] [PubMed] [Google Scholar]
- 24.Epstein JB, Vickers L, Spinelli J, et al. Efficacy of chlorhexidine and nystatin rinses in prevention of oral complications in leukemia and bone marrow transplantation. Oral Surg Oral Med Oral Pathol. 1992;73:692–699. doi: 10.1016/0030-4220(92)90009-f. [DOI] [PubMed] [Google Scholar]
- 25.Dodd MJ, Larson PJ, Dibble SL, et al. Randomized clinical trial of chlorhexidine versus placebo for prevention of oral mucositis in patients receiving chemotherapy. Oncol Nurs Forum. 1996;23:921–927. [PubMed] [Google Scholar]
- 26.Donnelly JP, Muus P, Horrevorts AM, et al. Failure of clindamycin to influence the course of severe oromucositis associated with streptococcal bacteremia in allogenic bone marrow transplant recipient. Scand J Infect Dis. 1993;25:43–50. doi: 10.1080/00365549309169668. [DOI] [PubMed] [Google Scholar]
- 27.Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation: a prospective randomized double-blind study. J Infect Dis. 1995;171:1545–1552. doi: 10.1093/infdis/171.6.1545. [DOI] [PubMed] [Google Scholar]
- 28.Cuttner J, Troy KM, Funaro L, et al. Clotremazole treatment for prevention of oral candidiasis in patients with acute leukemia undergoing chemotherapy. Am J Med. 1986;81:771–774. doi: 10.1016/0002-9343(86)90342-6. [DOI] [PubMed] [Google Scholar]
- 29.Epstein JB, Silverman S, Paggiarino DA, et al. Benzydamine HCL for prophaylaxis of radiation-induced oral mucositis: results from a multicenter, randomized, double blind, placebo-control trial. Cancer. 2001;92:875–885. doi: 10.1002/1097-0142(20010815)92:4<875::aid-cncr1396>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 30.Koukourakis MI. Amifostine in clinical oncology: current use and future applications. Anticancer Drugs. 2002;13:181–209. doi: 10.1097/00001813-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Mantovani G, Maccio A, Madeddu C, et al. Reactive oxygen species, anti-oxidant mechanisms, and serum cytokine levels in cancer patients; impact of antioxidant treatment. J Cell Mol Med. 2002;6:570–582. doi: 10.1111/j.1582-4934.2002.tb00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komaki R, Lee JS, Kaplan B, et al. Randomized phase-III study of chemoirradiation with or without amifostine for patients with favoubable performance status inoperable stage II–III non-small cell lung cancer: preliminary results. Semin Radiat Oncol. 2002;12(suppl 1):46–49. doi: 10.1053/srao.2002.31363. [DOI] [PubMed] [Google Scholar]
- 33.Brizel D, Wasserman T, Henke M, et al. Phase-III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18:3339–3345. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 34.Leong SS, Tan EH, Fong KW, et al. Randomized double-blind trial of combined modality treatment with or without amifostine in unresectable stage-III non-small-cell lung cancer. J Clin Oncol. 2003;21:1767–1774. doi: 10.1200/JCO.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Liu T, Liu Y, He S, et al. Use of radiation with or without WR-272 in advanced rectal cancer. Cancer. 1992;69:2820–2825. doi: 10.1002/1097-0142(19920601)69:11<2820::aid-cncr2820691130>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 36.Mahood DJ, Dose AM, Laprinzi CL, et al. Inhibition of fluoracil-induced stomatitis by oral cryotherapy. J Clin Oncol. 1991;9:449–452. doi: 10.1200/JCO.1991.9.3.449. [DOI] [PubMed] [Google Scholar]
- 37.Sprinzl GM, Galvan O, de Vries A, et al. Topical application of granulocyte-macrophase colony stimulating factor (GM-CSF) for the treatment of oral mucositis. Eur J Cancer. 2001;37:2003–2009. doi: 10.1016/s0959-8049(01)00170-8. [DOI] [PubMed] [Google Scholar]
- 38.Peterson D, Petit R. Phase-III study:AES-14 in chemotherapy patients at risk for mucositis (abstract 2917) Prog Proc Am Soc Clin Oncol. 2003;22:725. [Google Scholar]
- 39.Epstein JB, Wong FL. The efficacy of sucralfate suspension in the prevention of oral mucositis due to radiation therapy. Int J Radiat Oncol Biol Phys. 1994;28:693–698. doi: 10.1016/0360-3016(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 40.Makkonen TA, Bostrom P, Vilja P, et al. Sucralfate mouth washing in the prevention of radiation-induced musositis due to radiation therapy. Int J Radiat Oncol Biol Phys. 1994;30:177–182. doi: 10.1016/0360-3016(94)90533-9. [DOI] [PubMed] [Google Scholar]
- 41.Meredith R, Salter M, Kim R, et al. Sucralfate for radiation mucositis; results of a double-blind randomized trial. Int J Radiat Oncol Biol Phys. 1997;37:275–279. doi: 10.1016/s0360-3016(96)00531-7. [DOI] [PubMed] [Google Scholar]
- 42.Loprinzi CL, Ghosh C, Camoriano j, et al. Phase-III controlled evaluation of sucralfate to alleviate stomatitis in patients receiving fluorouracil based chemotherapy. J Clin Oncol. 1997;15:1235–1238. doi: 10.1200/JCO.1997.15.3.1235. [DOI] [PubMed] [Google Scholar]
- 43.Oshitani T, Okada k, Kushima T, et al. Clinical evaluation of sodium alginate on oral mucositis associated with radiotherapy. Int J Mol Med. 1998;2:675–679. [PubMed] [Google Scholar]
- 44.Leveque FG, Parzuchowski JB, Farinacci GC, et al. Clinical evaluation of MGI 209 an anesthetic film-forming agent for relief of painful oral ulcer associated with chemotherapy. J Clin Oncol. 1992;10:1963–1968. doi: 10.1200/JCO.1992.10.12.1963. [DOI] [PubMed] [Google Scholar]
- 45.Innocenti M, Moscatelli G, Lopez S. Efficacy of gelclair in reducing pain in palliative care patients with oral lesions; preliminary findings from an open pilot study. J Pain Sympt Manage. 2002;24:456–457. doi: 10.1016/s0885-3924(02)00524-9. [DOI] [PubMed] [Google Scholar]
- 46.Buchsel PC. Gelclair oral gel. Clin J Oncol Nurs. 2003;7:109–110. doi: 10.1188/03.CJON.109-112. [DOI] [PubMed] [Google Scholar]
- 47.Biswal BM, Zakaria A. Nik Min. A. Topical application of honey in the management of radiation mucositis: a preliminary study. Support Care Cancer. 2003;11:242–248. doi: 10.1007/s00520-003-0443-y. [DOI] [PubMed] [Google Scholar]
- 48.Cowen D, Tarieu C, Schubert M, et al. Low energy helium-neon laser in the prevention of oral mucositis in patients undergoing bone marrow transplant: results of a double blind randomized trial. Int J Radiat Oncol Biol Phys. 1997;38:697–703. doi: 10.1016/s0360-3016(97)00076-x. [DOI] [PubMed] [Google Scholar]
- 49.Bensadoun RJ, Franquin JC, Ciais G, et al. Low energy He/Ne laser in the prevention of radiation induced mucositis. Support Care Cancer. 1999;33:359–363. doi: 10.1007/s005200050256. [DOI] [PubMed] [Google Scholar]
- 50.Migliorati C, Massumoto C, Eduardo FP, et al. Low energy laser therapy in oral mucositis. J Oral Laser Applications. 2001;1:97–101. [Google Scholar]
- 51.Bez C, Demarosi F, Sardella A, et al. GM-CSF mouth-rinses in the treatment of severe oral mucositis: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endo. 1999;88:311–315. doi: 10.1016/s1079-2104(99)70034-x. [DOI] [PubMed] [Google Scholar]
- 52.Sprinzl GM, Galvan O, deVaris H, et al. Local application of granulocyte macrophase coloney stimulating factor (GM-CSF) for the treatment of oral mucositis. Eur J Cancer. 2001;37:2003–2009. doi: 10.1016/s0959-8049(01)00170-8. [DOI] [PubMed] [Google Scholar]
- 53.Cartee L, Petros WP, Rosnner GL, et al. Evaluation of GM-CSF mouthwash for the prevention of chemotherapy-induced mucositis: a randomized, double-blind, dose ranging study. Cytokine. 1995;7:471–477. doi: 10.1006/cyto.1995.0064. [DOI] [PubMed] [Google Scholar]
- 54.Nicolatou O, Sotirropoulou-Lontou A, Skariatos J, et al. A pilot study of the effect of granulocyte-macrophase-colony-stimulating-factor on oral mucositis in head-and-neck cancer patients during x-radiation therapy: a preliminary report. Int J Radiat Oncol Biol Phys. 1998;42:551–556. doi: 10.1016/s0360-3016(98)00253-3. [DOI] [PubMed] [Google Scholar]
- 55.Kaushal V, Verma K, Manocha S, et al. Clinical evaluation of human placental extract (placentrex) in radiation induced oral mucositis. Int J Tissue Reaction. 2001;23:105–110. [PubMed] [Google Scholar]
- 56.Wymenga AN, van der Graff WT, Hofstra LS, et al. Phase-I study of the transforming growth factor beta3 mouthwashes for prevention of chemotherapy-induced mucositis. Clin Cancer Res. 1999;5:1363–1368. [PubMed] [Google Scholar]
- 57.Foncuberta MC, Cognoni PJ, Brandts CH, et al. Topical transforming growth factor-beta3 in the prevention or alleviation of chemotherapy-induced oral mucositis in patients with lymphomas and solid tumors. J Immunother. 2001;24:384–388. doi: 10.1097/00002371-200107000-00014. [DOI] [PubMed] [Google Scholar]
- 58.Meropol NJ, Somer RA, Gutheil J, et al. Randomized phase-I trial of recombinant human keratinocyte growth factor plus chemotherapy: Potential role as mucosal protectant. J Clin Oncol. 2003;21:1452–1458. doi: 10.1200/JCO.2003.10.079. [DOI] [PubMed] [Google Scholar]
- 59.Spelberger R, Stiff P, Bensinger W, et al. Paliformin for oral mucositis after intensive therapy for hematologic cancers. New Engl J Med. 2004;351:2590–2597. doi: 10.1056/NEJMoa040125. [DOI] [PubMed] [Google Scholar]
- 60.Booth C, Booth D, Williamson S, et al. Teduglutide ([Gly 2] GLP-2) protects small intestinal stem cells from radiation damage. Cell Prolif. 2004;37:385–400. doi: 10.1111/j.1365-2184.2004.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van’t Land B, Van Beek NM, Van den Berg JJ, et al. Lactoferrin reduces methotrexate-induced small intestinal damage, possibly through inhibition of GLP-2 mediated epithelial cell proliferation. Dig Dis Sci. 2004;49:425–433. doi: 10.1023/b:ddas.0000020497.35250.93. [DOI] [PubMed] [Google Scholar]
- 62.Ypsilantis P, Tentes I, Assimakoulos SF, et al. Mesna ameliorates intestinal mucosa damage after ifosfamide administration in the rabbit at a dose-related manner. J Surg Res. 2004;121:84–91. doi: 10.1016/j.jss.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 63.Beck PL, Wong JF, Li Y, et al. Chemotherapy-and-radiotherapy-induced intestinal damage is regulated by intestinal trefoil factor. Gastroenterology. 2004;126:796–808. doi: 10.1053/j.gastro.2003.12.004. [DOI] [PubMed] [Google Scholar]