Abstract
Deltamethrin is a widely used insecticide belonging to the class of pyrethroid. Although the neurotoxicity of pyrethroids including deltamethrin is well established, it is still unclear whether exposure to deltamethrin during neonatal period has any deleterious effect on the survival of the Purkinje cells in the cerebellum. In the study, we investigated the total number of Purkinje cells in experimental rats exposed to deltamethrin using a stereological method, the fractionator. Deltamethrin in a dose of 1 mg/kg/day (corresponds to 20% of LD50 ) was administered through oral gavage to male pups from 2nd to 5th postnatal day (PND). At PND 21 the animals were sacrificed and their cerebelli were removed. The cerebelli were systematically sampled using the fractionator method and stained with cresyl fast violet. The number of the Purkinje cells was counted for each cerebellum. The results showed that there was no significant difference in the total number of Purkinje cells in the deltamethrin-treated group as compared to the control animals. This suggests that deltamethrin exposure at the current dosage during the neonatal period do not have any significant effect on the survival of the Purkinje cells in the cerebellum.
Keywords: pyrethroid, deltamethrin, stereology, fractionator neonatal, postnatal, nucleolus, rat
Introduction
Pyrethroid is considered to be among the safest class of insecticides with a relatively high insect/mammalian toxicity ratio (1). Pyrethroids are widely used in the agricultural sector to protect the crops against a wide range of insect pests. In domestic environment, they are often used as active ingredients against various household insects such as mosquitoes (2). In tropical countries, where the use of mosquito repellents is widespread, a large number of populations including the children are exposed to pyrethroids throughout the year, especially during the night (3). An investigation in Germany revealed that a large portion of the general population is homogeneously exposed to pyrethroids, most likely through the daily diet (4). Because of their widespread use, their potential health effects have become major public concern particularly in the children (5, 6).
Neurotoxicity of pyrethroids on the brain in experimental animals is well established (7, 8). In general, neonatal animals are more vulnerable to the toxic effects of pyrethroids. It is estimated that neonatal rat is 17 times more vulnerable to acute toxicity of pyrethroids than the adult (9). This vulnerability is possibly due to the age-related differences in pharmacokinetics such as immaturity of hepatic metabolic detoxification (9, 10). Studies have revealed that exposure to pyrethroids during the neonatal period affected the behaviour and motor activites later in life (11, 12, 13, 14). Moreover, neonatal exposure to pyrethroids even at relatively low doses, disrupt the normal development and migration of neurons in the brain (15, 16). For instance, the administration of 0.7 mg/kg deltamethrin (< 1 % of adult median lethal dose, LD50) to the neonatal rats from postnatal day (PND) 9 to 13 was found to delay the migration of granule cells and appearance of radial glial fibers in the cerebellum. In addition, the radial glial fibers were found to be disorganized and hypertrophic after the exposure (16). Moreover, neonatal pyrethroid exposure increases the permeability of the bloodbrain barrier which may predispose the developing brain to other damaging neurotoxicants (3).
Purkinje cells are located in a single layer separating the outer molecular from the inner granule cell layer of the cerebellar cortex. In rats, the Purkinje cells are formed during day 13–16 of gestation. At birth, the Purkinje cell layer is about 6-cell thick and by the PND 5, the layer reduces in thickness to form a single cell layer (17, 18). The Purkinje cells play an important role in normal development and maturation of the cerebellum and have an inductive effect on the development of the cerebellar granule cells (19).
Many studies have attempted to determine the number of Purkinje cells after exposure to certain chemical substances such as alcohol (20) and nicotine (21), and after exposure to certain conditions such as x-ray irradiation (22) and malnutrition (23). A number of studies revealed that exposure to certain pyrethroids significantly affected the survival of Purkinje cells in the adult rats (24, 25, 26). While there have been a few studies on the effect of pyrethroids on the developing brain, none of the studies focused their investigation on the Purkinje cells, specifically on the number of the Purkinje cells after the exposure. Therefore, the present study aimed to investigate the effect of a type II pyrethroid, the deltamethrin, administered during neonatal period on the total number of Purkinje cells using design-based stereological method - the fractionator. Deltamethrin is a widely used pyrethroid especially in the agricultural sector and has actions against a wide range of insects (27).
The fractionator is one of the design-based stereological methods to count particles such as neurons in tissue sections. It is statistically and economically more efficient than the other stereological method, so called the ‘Vref × Nv’ method in counting particles (28). The fractionator allows neurons to be estimated directly without having to make any assumptions about their size, shape or orientation. Moreover, the knowledge of organ volume is not required to calculate the total number of neurons. The major and the only requirement is that the neuron being counted can be identified unequivocally on all sections throughout the organ. The method offers another advantage, tissue shrinkage suffered during tissue processing will not interfere with the validity of the method (29, 30).
Methods
Animal
Virgin female Sprague Dawley rats were mated and housed singly in standard plastic cages. They were maintained on standard pellet diet and water ad libitum and were checked each morning for delivery. The day of delivery was designated as PND 0. At birth, the litters were standardized to contain 8 pups with as many males as possible. The pups remained with their respective dams throughout the experimental period, except during experimental procedures.
The male pups of each litter were equally divided into treated and control groups, each group consisting of 10 pups. Pups assigned to the treated group were given deltamethrin at a dose of 1 mg/kg body weight from PND 2 to PND 5 via oral gavage. The dosage of 1 mg/kg corresponds to about 20% of the median lethal dose (LD50) of deltamethrin at this age (10). Deltamethrin (99 % pure) was procured from SIGMA and prepared by dissolving it in corn oil vehicle immediately prior to the start of the experiment. Deltamethrin was administered through a polyethylene tube (1.5 mm diameter) by a hypodermic syringe. In the control group, an equal volume of corn oil was administered during the corresponding period. In both groups, the body weights were taken at PND 2, 7, 14 and 21.
The present study was approved by the Animal Ethics Committee, Universiti Sains Malaysia, Health Campus, Kubang Kerian, Kelantan.
Tissue Preparation
At PND 21, the animals were deeply anesthetized with anesthetic ether and immediately perfused via intracardiac route with physiological saline followed by fixative. The brain was dissected out immediately after perfusion and then weighed. The cerebellum of each brain was separated from the cerebral hemispheres by cutting between the superior and inferior colliculi in the transverse fissure. The weight of the individual cerebellum was then determined. The cerebelli were kept in 10% formalin for 48 hours before they were systematically sampled with the fractionator method.
Fractionator Method
Estimation of Purkinje cell number in the cerebellum was determined by the fractionator method. The method utilizes a number of systematic sampling stages as described below.
In stage 1 of the fractionator, each cerebellum was cut into 2 mm parasagittal slices. The position of the first slice was random in the interval of 0–2 mm. This ensures that all regions of the cerebellum had an equal chance of being sampled. All of these slices were then cut into strips of about 2 mm wide. The strips were left in the order they were cut. Every 4th strip was sampled systematically with a random start between 1 and 4. A new random number was chosen for each cerebellum. Therefore, for each cerebellum, the probability of selecting any individual strip was 1 in 4 (fl = 4).
In stage 2 of the fractionator, strips sampled at stage 1 were cut into smaller pieces about 2 mm3 in size. The tissue pieces were again arranged according to the order of cutting. A systematic random sampling procedure, similar to that just described, was used to select 1 in 6 (f2 = 6) of these pieces.
In stage 3, tissue sampled at stage 2 was processed for routine paraffin histology. Pieces from the same cerebellum were embedded together in the same mould. These tissue blocks were cut with a microtome (Leica) at a nominal thickness of 4 um. A sample of 1 in 30 (f3 = 30) of the sections was selected in a systematic fashion until the block had been exhaustively serially sectioned. Each selected section was picked up on glass slides and then stained with cresyl fast violet technique. To minimize experimenter biases, each of the slide was given a secret code number. The secret codes were only revealed after the completion of the counting studies.
The selected slides were examined under a Nikkon (Eclipse E600) light microscope which was attached to a videocamera (CoolSnap-Pro, Media Cybernetics). Images projected from the videocamera were visualized and displayed onto a monitor screen interfaced with a computer (Dell). In the present study, the Purkinje cell nucleolus was used as the counting item. All Purkinje cell nucleoli visible in the screen were counted under the 20x objective.
In the fractionator method, the particle number (ie Purkinje cell) is estimated in a known fraction of the reference space (ie the cerebellum). The total number of particles in the structure is derived by multiplying the number of objects counted in the volume sampled (ie profile of the Purkinje cells) by the inverse of the sampling fractions of the structure (29, 30). Thus the estimated total number of Purkinje cells for each cerebellum (N) is calculated by multiplying the corrected total number of Purkinje cells counted (Q) with the sampling fractions at stage 1 (f1), stage 2 (f2) and stage 3 (f3) of the fractionator. Thus,
where Q is the corrected total number of Purkinje cell counted for each cerebellum, f1 is the stage 1 sampling fraction, f2 is the stage 2 sampling fraction, f3 is the stage 3 sampling fraction.
In the present study, the total number of Purkinje cells counted were corrected by multiplying the number of counted neurons with the mean ratio of nucleolus/cell . The correction is necessary as Purkinje cells may have double nucleoli per nucleus which may lead to over counting of the cell (31).
Statistics
All values were expressed as mean ± standard error of mean (SEM). Statistical analysis was performed with SPSS (v12) software. Statistical significance between group means was analysed by the unpaired two-tailed Student’s t-test at p<0.05.
Results
Body weight
Mean body weights at PND 2, 7, 14 and 21 are shown in table 1. Statistical analysis showed no significant difference in the body weights except at PND 7. At PND 7, the mean body weight of the rats treated with deltamethrin was 10.8 % lower compared to the respective control group.
Table 1 :
Effect of deltamethrin on the average body weight (g) during early postnatal development
|
Postnatal Age |
||||
|---|---|---|---|---|
| 2 | 7 | 14 | 21 | |
| Control | 8.32 ± 0.10 | 14.82 ± 0.24 | 27.08 ± 0.56 | 46.54 ± 0.61 |
| Deltamethrin-treated | 8.50 ± 0.08 | 13.22 ± 0.30* | 26.04 ± 0.35 | 45.50 ± 1.02 |
Values are expressed as mean ± SEM
p < 0.01
Cerebellum weight
Mean cerebellum weights are shown in table 2. There was no significant difference in cerebellum weight between the control and treated groups (197.7 ± 1.78 vs 198.6 ± 2.41 mg).
Table 2 :
Effect of deltamethrin on the average cerebellum weight and Purkinje cell number
| Control | Deltamethrin-treated | |
|---|---|---|
| Cerebellum weight (mg) | 197.7 ± 1.78 | 198.6 ± 2.41 |
| Purkinje cell number (×103) | 386 ± 21 | 379 ± 26 |
Values are expressed as mean ± SEM
Estimated Purkinje Cell Number
Estimates of Purkinje cell numbers are shown in table 2. The study found no significant differences in the estimated total number of Purkinje cells between the control (386 × 103 ± 21 × 103) and the deltamethrin-treated groups (379 × 103 ± 26 × 103).
Precision
The precision of the estimates can be expressed by the coefficient of error (CE) (SEM/mean). In the present study, CE of the Purkinje cell estimate was calculated according to the method described by Gundersen et al. (32) and following the example shown by Fabricius et al. (33). The average CE for the number of Purkinje cells sampled from the control and treatment groups were 0.056 and 0.069 respectively, which were still below the recommended limit of 0.1 (34).
Discussion
In rats, the neonatal period is the period of critical brain development where there are active neuronal reorganization, differentiation and proliferation occurring in the brain including the cerebellum (17, 40). Drugs or toxicants which are considered relatively safe to adult may not be so during this brain growth spurt of the neonatal period. A previous study showed that exposure to certain toxicants such as alcohol during this period significantly affected the survival of neurons in certain parts of the brain and hence their number (35).
Animal studies have shown that the pyrethroids have the potential to produce neuronal death in the adult. For instance, neuronal loss has been described in the cerebral cortex, hippocampal formation and cerebellum of adult rats after subchronic dermal exposure to permethrin at a dose that produced no gross clinical signs (24). Another study revealed neuronal loss in the hippocampus and cerebral cortex in adult rats after they were given a single intraperitoneal injection of deltamethrin at a dose of 12.5 mg/kg (36). In sharp contrast, several studies revealed that exposure to relatively high doses of pyrethroids did not produce significant neuronal loss in the brain. For instance, it was reported that the administration of a single dose of 20mg/kg deltamethrin (25 % of LD50) failed to induce neuronal loss in the areas of the brain examined (37). Similarly, the administration of type 1 pyrethroid, bifenthrin at doses sufficient to produce 30 hours of tremor failed to produce any detectable neuronal loss (38). In another study, the administration of near lethal dosage of type II pyrethroid, fenvalerate to rats and mice, failed to induce any pathological changes in the brain, although peripheral nerve damage was observed (39). The reasons for the discrepant nature of these findings are unknown.
Currently, the data on the effect of pyrethroid exposure during neonatal period on the survival of Purkinje cells is very limited, although reasonable amounts of data exist for the adult brain. For instance, subchronic exposure to dermal alpha-cypermethrin at half of the LD50 caused pyknotic changes to the Purkinje cells in the adult rats (41). Another study described morphological changes in Purkinje cells after exposure to 7 mg/kg of oral deltamethrin (42). However, quantitative analysis of the Purkinje cells was not performed in both of the studies. Abdel-Rahman and his colleagues (24) attempted to quantify the Purkinje cell loss after dermal exposure to type 1 pyrethroid, permethrin, and they found a significant reduction in the density of Purkinje cells in the cerebellum after the exposure. However, unbiased stereological method was not employed in the above study. Moreover the report of density may lead to erroneous conclusion as dehydration of the specimens or sections can result in falsely higher neuronal density, so called the “reference trap”. This bias in both the control and treated groups are unknown and cannot be assumed to be the equal (31). Hence the present study employed the state of the art stereological counting technique, the fractionator, to obtain the unbiased estimate of the Purkinje cell count in the cerebellum.
The present study failed to detect any significant effect of deltamethrin exposure during the neonatal period on the number of the Purkinje cells in the cerebellum. The dosage of deltamethrin used in the present study represents about 20 % of the LD50 at this age. The LD50 of oral deltamethrin in corn oil vehicle at PND 11 is 5.1 mg/kg (10). A previous study revealed that the administration of 0.7 mg/kg (i.p) deltamethrin during PND 9–13 of the neonatal period affected the cytogenesis and morphogenesis of the neurons in the cerebellar cortex. In addition, the developing vasculatures were damaged, leading to haemorrhage, thrombus formation and focal degeneration of the brain tissue (15). Another study revealed that the same amount of deltamethrin administered during PND 10–16 affected motor activity which lasted for several months (13). However, in both of the studies, no attempt was made to directly assess the effect of deltamethrin exposure on the Purkinje cells.
In conclusion, our results do not provide evidence to support the notion that exposure to deltamethrin during the neonatal period causes a significant loss of Purkinje cell number in the cerebellum.
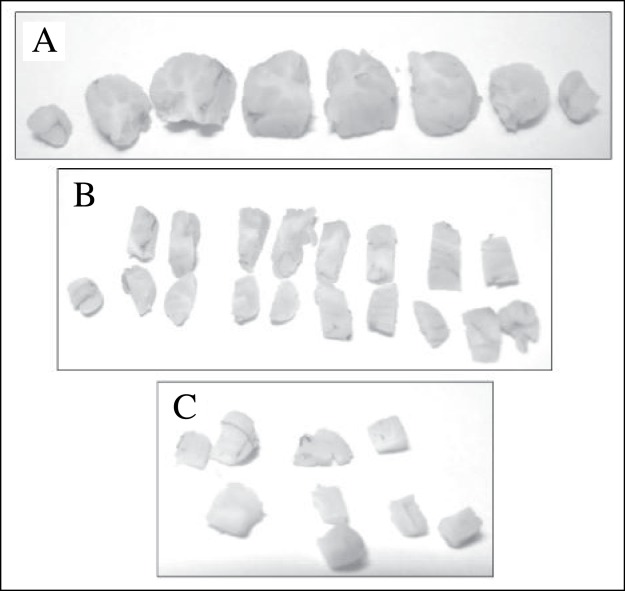
Figure 1 :
Photographs of a cerebellum that has been sectioned according to the fractionator method. In stage 1 of the fractionator, the cerebellum was cut into 2 mm parasagittal slices (A), and all of these slices were then cut into longitudinal strips of about 2 mm wide (B). Every 4th strip was sampled systematically. In stage 2, strips sampled at stage 1 were cut into smaller pieces about 2 mm3 in size (C) and again every 6th tissue piece was sampled systematically. In stage 3, tissues selected at stage 2 were embedded together in the same mould to produce a tissue block which were then sectioned with a microtome. Every 30th section was systematically sampled.
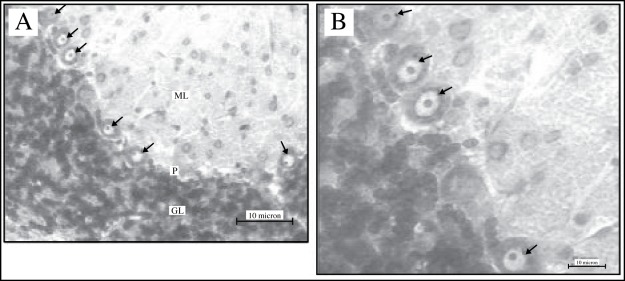
Figure 2 :
Photomicrographs of the cerebellar cortex of the deltamethrin-treated rat which had been stained with cresyl fast violet at lower magnification (A) (20x objective) and at higher magnification (B) (40x objectives). The Purkinje cells (arrows) lie in a monolayer between the molecular (ML) and granular layer (GL). Note the prominent nucleolus in each of the Purkinje cell.
Acknowledgments
This work was supported by Universiti Sains Malaysia Short Term Grant (No. 304/PPSP/6131461). We are very grateful to Mr. Harissal for his technical support.
References
- 1.Aldridge WN. An assessment of the toxological properties of pyrethroids and their toxicity. CRC Rev Toxicol. 1990;21:89–104. doi: 10.3109/10408449009089874. [DOI] [PubMed] [Google Scholar]
- 2.Pauluhn J. Hazard identification and risk assessment of pyrethroids in the indoor environment. Toxicology Letters. 1999;107:193–199. doi: 10.1016/s0378-4274(99)00047-8. [DOI] [PubMed] [Google Scholar]
- 3.Gupta A, Agarwal R, Shukla GS. Functional impairment of blood-brain barrier following pesticide exposure during early development in rats. Hum Exp Toxicol. 1999;18:174–179. doi: 10.1177/096032719901800307. [DOI] [PubMed] [Google Scholar]
- 4.Schettgen T, Heudorf U, Drexler H, Angerer J. Pyrethroid exposure of the general population – is this due to diet? Toxicology Letters. 2002;134:141–145. doi: 10.1016/s0378-4274(02)00183-2. [DOI] [PubMed] [Google Scholar]
- 5.Lu C, Barr DB, Pearson M, Bartell S, Bravo R. A longitudinal approach to assessing urban and suburban children’s exposure to pyrethroid pesticides. Environmental Health Perspectives. 2006;114:1419–1423. doi: 10.1289/ehp.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolaczinski JH, Curtis CF. Chronic illness as a result of low-level exposure to synthetic pyrethroid insecticides: a review of the debate. Food Chem Toxicol. 2004;42:697–706. doi: 10.1016/j.fct.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, Stevens JT, Weiner ML. Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology. 2002;171:3–59. doi: 10.1016/s0300-483x(01)00569-8. [DOI] [PubMed] [Google Scholar]
- 8.Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: Critical review and future research needs. Environmental Health Perspectives. 2005;113(2):123–136. doi: 10.1289/ehp.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantalamessa F. Acute toxicity of two pyrethroids, permethrin and cypermethrin in neonatal and adult rats. Archives of Toxicology. 1993;67:510–513. doi: 10.1007/BF01969923. [DOI] [PubMed] [Google Scholar]
- 10.Sheets LP, Doherty JD, Law MW, Reiter LW, Crofton KM. Age-dependent differences in the susceptibility of rats to deltamethrin. Toxicol Appl Pharmacol. 1994;126:186–190. doi: 10.1006/taap.1994.1106. [DOI] [PubMed] [Google Scholar]
- 11.Ahlbom J, Fredriksson A, Eriksson P. Neonatal exposure to a type-1 pyrethroid (bioallethrin) induces dose-response changes in brain muscarinic receptors and behaviour in neonatal and adult mice. Brain Res. 1994;645:318–324. doi: 10.1016/0006-8993(94)91666-7. [DOI] [PubMed] [Google Scholar]
- 12.Moniz AC, Bernardi MM, Souza-Spinosa HS, Palermo-Neto J. Effects of exposure to a pyrethroid insecticide during lactation on the behaviour of infant and adult rats. Braz J Med Biol Res. 1990;23:45–48. [PubMed] [Google Scholar]
- 13.Eriksson P, Fredriksson A. Neurotoxic effects of two different pyrethroids, bioallethrin and deltamethrin on immature and adult mice: changes in behavoiur and muscarinic receptor variables. Toxicol Appl Pharmacol. 1991;108:78–85. doi: 10.1016/0041-008x(91)90270-o. [DOI] [PubMed] [Google Scholar]
- 14.Husain R, Malaviya M, Seth PK, Husain R. Effect of deltamethrin on regional brain polyamines and behaviour in young rats. Pharmacol Toxicol. 1994;74:211–215. doi: 10.1111/j.1600-0773.1994.tb01100.x. [DOI] [PubMed] [Google Scholar]
- 15.Patro N, Mishra SK, Chattopadhyay M, Patro IK. Neurotoxicological effects of deltamethrin on the postnatal development of cerebellum of rat. J Biosci. 1997;22:177–130. [Google Scholar]
- 16.Patro N, Patro IK. Effects of deltamethrin on granule cell migration during postnatal development of rat cerebellum. Indian Journal of Experimental Biology. 2005;43:158–162. [PubMed] [Google Scholar]
- 17.Altman J. Postnatal development of the cerebellar cortex in the rat. The external germinal layer and the transition molecular layer. J Comp Neurol. 1972;145:353–398. doi: 10.1002/cne.901450305. [DOI] [PubMed] [Google Scholar]
- 18.Altman J, Anderson WJ. Experimental reorganization of the cerebellar cortex 1. Morphologically effects of elimination of all microneurons with prolonged X-irradiation started at birth. J Comp Neurol. 1972;146:355–406. doi: 10.1002/cne.901460305. [DOI] [PubMed] [Google Scholar]
- 19.Wetts R, Herrup K. Direct correlation between Purkinje and granule cell number in the cerebella of lurcher chimeras and wild-type mice. Brain Res. 1983;312:41–47. doi: 10.1016/0165-3806(83)90119-0. [DOI] [PubMed] [Google Scholar]
- 20.Pauli J, Wilce P, Bedi KS. Acute exposure to alcohol during early postnatal life causes a deficit in the total number of cerebellar Purkinje cells in the rat. J Comp Neurol. 1995;360:506–12. doi: 10.1002/cne.903600311. [DOI] [PubMed] [Google Scholar]
- 21.Chen WJA, Edwards RB, Romero RD, Parnell SE, Monk RJ. Long-term nicotine exposure reduces Purkinje cell number in the adult rat cerebellar vermis. Neurotoxicology and Teratology. 2003;25:329–334. doi: 10.1016/s0892-0362(02)00350-1. [DOI] [PubMed] [Google Scholar]
- 22.Li HP, Miki T, Gu H, Satriotomo I, Mastumoto Y, Kuma H, Gonzalez D, Bedi KS, Suwaki H, Takeuchi Y. The effect of the timing of prenatal X-irradiation on Purkinje cell numbers in rat cerebellum. Developmental Brain Research. 2002;139:159–166. doi: 10.1016/s0165-3806(02)00542-4. [DOI] [PubMed] [Google Scholar]
- 23.Warren MA, Bedi KS. The effects of a lengthy period of undernutrition from birth and subsequent nutritional rehabilitation on the granule-to-Purkinje cell ratio in the rat cerebellum. J. Anat. 1988;159:147–153. [PMC free article] [PubMed] [Google Scholar]
- 24.Abdel-Rahman A, Shetty AK, Abou-Donia MB. Subchronic dermal application of N,N-diethyl m-toluamide (DEET) and permethrin to adult rats, alone or in combination, causes diffuse neuronal cell death and cytoskeletal abnormalities in the cerebral cortex and the hippocampus, and Purkinje neuron loss in the cerebellum. Experimental Neurology. 2001;172:153–171. doi: 10.1006/exnr.2001.7807. [DOI] [PubMed] [Google Scholar]
- 25.Abdel-Rahman A, Dechkovskaia AM, Goldstein LB, Bullman SH, Khan W, El-Masry EM, Abou-Donia MB. Neurological deficits induced by malathion, DEET, and permethrin, alone or in combination in adult rats. J Toxicol Environ Health. 2004;67:331–56. doi: 10.1080/15287390490273569. [* PICT is empty or cannot be processed. | In-line Graphic * [DOI] [PubMed] [Google Scholar]
- 26.Latuszyñska J, Luty S, Raszewski G, Tokarska-Rodak M, Przebirowska D, Przylepa E, et al. Neurotoxic effect of dermally-applied chlorpyrifos and cypermethrin in Wistar rats. Ann Agric Environ Med. 2001;8:163–170. [PubMed] [Google Scholar]
- 27.Hijzen TH, Slangen JL. Effects of type I and type II pyrethroids on the startle response in rats. Toxicol Lett. 1988;40:141–152. doi: 10.1016/0378-4274(88)90155-5. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz C, Hof PR. Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. Journal of Chemical Neuroanatomy. 2000;20:93–114. doi: 10.1016/s0891-0618(00)00066-1. [DOI] [PubMed] [Google Scholar]
- 29.Gundersen HJG. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. Journal of Microscopy. 1986;143:3–45. [PubMed] [Google Scholar]
- 30.Mayhew TM. A review of recent advances in stereology for quantifying neural structure. Journal of Neurocytology. 1992;21:313–328. doi: 10.1007/BF01191700. [DOI] [PubMed] [Google Scholar]
- 31.von Bartheld CS. Counting particles in tissue sections: Choices of methods and importance of calibration to minimize biases. Histol Histopathol. 2002;17:639–648. doi: 10.14670/HH-17.639. [DOI] [PubMed] [Google Scholar]
- 32.Gundersen HJG, Jensen EBV, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology-reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- 33.Fabricius K, Wörtwein G, Pakkenberg B. The impact of maternal separation on adult mouse behaviour and on the total neuron number in the mouse hippocampus. Brain Struct Funct. 2008;212:403–416. doi: 10.1007/s00429-007-0169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West MJ, Gundersen HJG. Unbiased stereological estimation of the number of neurons in the human hippocampus. J. Comp. Neurol. 1990;196:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- 35.Thomas JD, Goodlett CR, West JR. Alcohol-induced Purkinje cell loss depends on developmental timing of alcohol exposure and correlates with motor performance. Developmental Brain Research. 1998;105:159–166. doi: 10.1016/s0165-3806(97)00164-8. [DOI] [PubMed] [Google Scholar]
- 36.Wu A, Liu Y. Apoptotic cell death in rat brain following deltamethrin treatment. Neuroscience Letters. 2000;279:85–88. doi: 10.1016/s0304-3940(99)00973-8. [DOI] [PubMed] [Google Scholar]
- 37.Ray DE, Fry JR. A reassessment of the neurotoxicity of pyrethroid insecticides. Pharmacology & Therapeutics. 2006;111:174–193. doi: 10.1016/j.pharmthera.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Holton JL, Nolan CC, Burr SA, Ray DE, Cavanagh JB. Increasing or decreasing nervous activity modulates the severity of the glio-vascular lesions of 1,3-dinitrobenzene in the rat: effects of the tremorgenic pyrethroid, bifenthrin and of anaesthesia. Acta Neuropathol. 1997;93:159–165. doi: 10.1007/s004010050597. [DOI] [PubMed] [Google Scholar]
- 39.Parker CM, Albert JR, Van Gelder GA, Patterson DR, Taylor JL. Neuropharmacologic and neuropathologic effect of fenvalerate in mice and rats. Fundam Appl Toxicol. 1985;5:278–286. doi: 10.1016/0272-0590(85)90075-2. [DOI] [PubMed] [Google Scholar]
- 40.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Human Development. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 41.Luty S, Latuszynska J, Halliop J, Tochman A, Obuchowska D, Przylepa E, Korczak E. Toxicity of dermally applied alpha-cypermethrin in rats. Ann Agric Environ Med. 1998;5:109–115. [PubMed] [Google Scholar]
- 42.Husain R, Husain R, Adhami VM, Seth PK. Behavioral, neurochemical and neuromorphological effects of deltamethrin in adult rats. J Toxicol Environ Health. 1996;48:515–526. doi: 10.1080/009841096161212. [DOI] [PubMed] [Google Scholar]